Von Willebrand Factor Multimers and the Relaxation Response: A One-Year Study
Abstract
1. Introduction
2. Materials and Methods
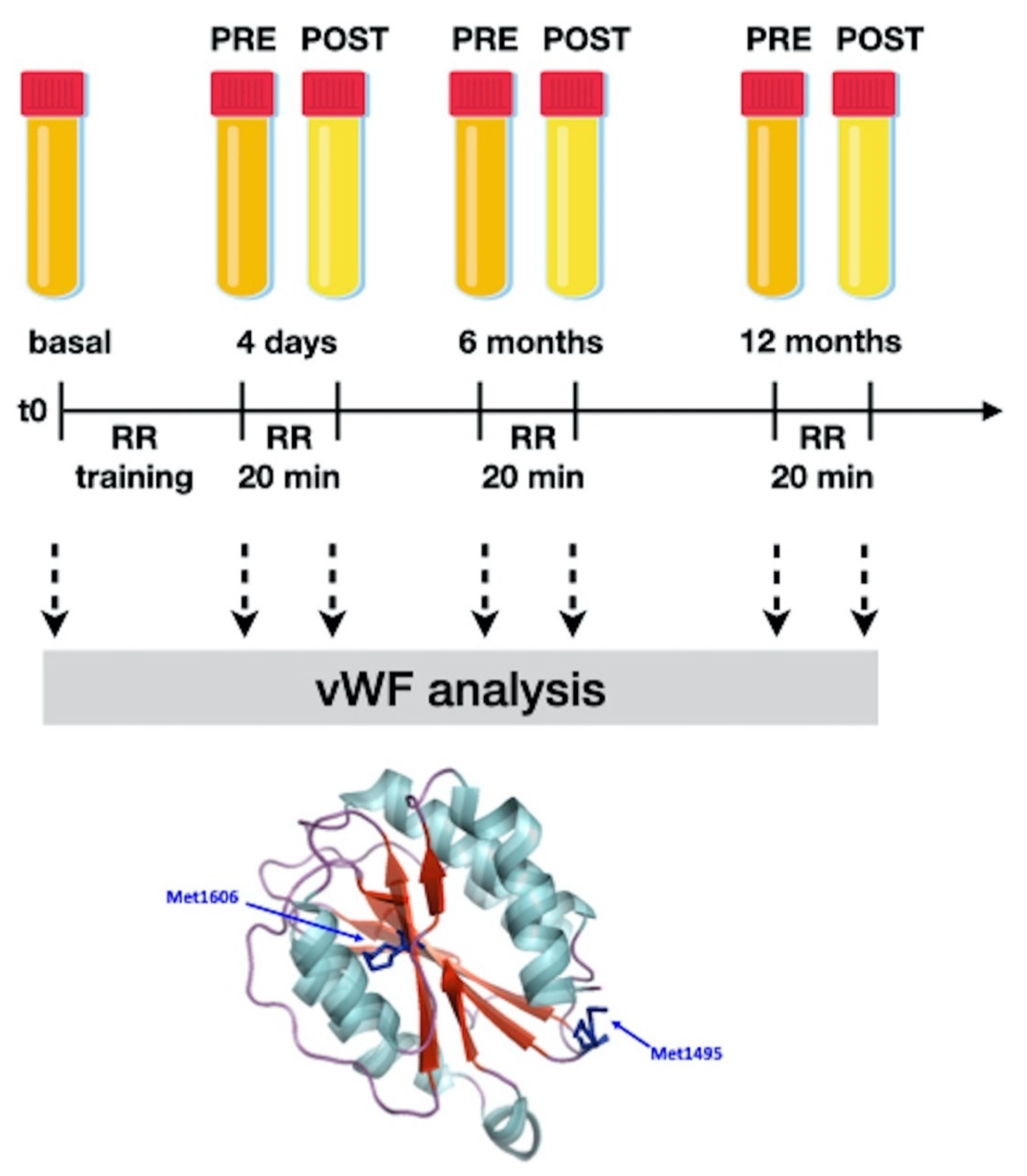
2.1. Biological Samples
2.2. Determination of the Total Carbonyl Content
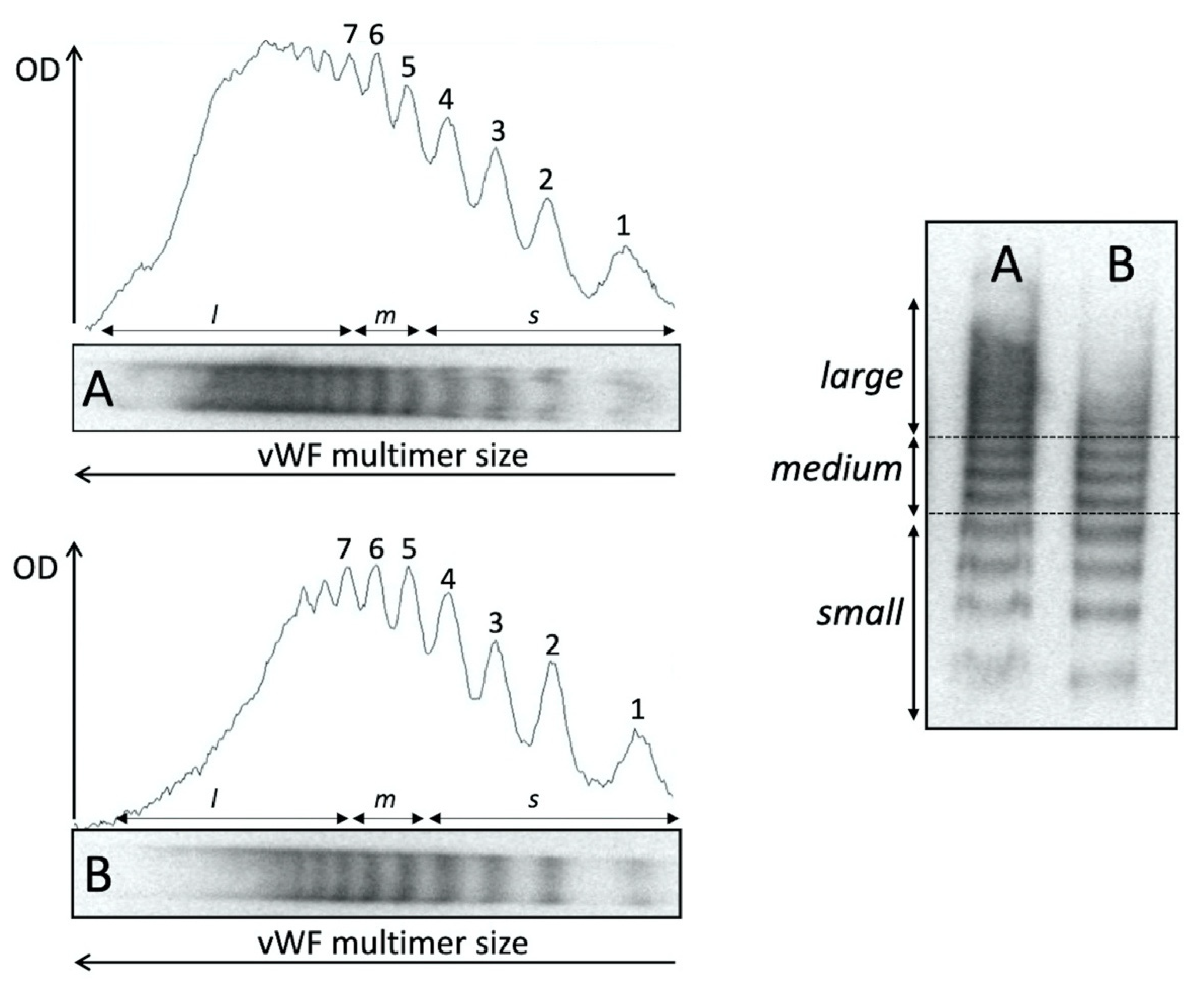
2.3. von Willebrand Factor Multimer Analysis
2.4. Statistical Analysis
3. Results
3.1. Biochemical Markers
3.2. Correlation between vWF Multimers and Total Carbonyl Content with Inflammatory Markers from Our Previous Studies
4. Discussion
5. A Possible Underlying Mechanism According to a Biophysical Perspective: A Molecule Conformational Change and Plasma Coherent States
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Dal Lin, C.; Poretto, A.; Scodro, M.; Perazzolo Marra, M.; Iliceto, S.; Tona, F. Coronary microvascular and endothelial function regulation: Crossroads of psychoneuroendocrine immunitary signals and quantum physics. J. Integr. Cardiol. 2015, 1, 132–209. [Google Scholar] [CrossRef]
- Kawecki, C.; Lenting, P.J.; Denis, C.V. von Willebrand factor and inflammation. J. Thromb. Haemost. 2017, 15, 1285–1294. [Google Scholar] [CrossRef]
- Chen, J.; Chung, D.W. Inflammation, von Willebrand factor, and ADAMTS13. Blood 2018, 132, 141–147. [Google Scholar] [CrossRef]
- Dobrkovska, A.; Krzensk, U.; Chediak, J.R. Pharmacokinetics, efficacy and safety of Humate-P® in von Willebrand disease. Haemoph. Off. J. World Fed. Haemoph. 1998, 4, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Menache, D.; Aronson, D.L.; Darr, F.; Montgomery, R.R.; Gill, J.C.; Kessler, C.M.; Lusher, J.M.; Phatak, P.D.; Shapiro, A.D.; Thompson, A.R.; et al. Pharmacokinetics of von Willebrand factor and factor VIIIC in patients with severe von Willebrand disease (type 3 VWD): Estimation of the rate of factor VIIIC synthesis. Br. J. Haematol. 1996, 94, 740–745. [Google Scholar] [CrossRef]
- Lenting, P.J.; Westein, E.; Terraube, V.; Ribba, A.-S.; Huizinga, E.G.; Meyer, D.; de Groot, P.G.; Denis, C.V. An Experimental Model to Study the in Vivo Survival of von Willebrand Factor. J. Biol. Chem. 2004, 279, 12102–12109. [Google Scholar] [CrossRef]
- Tsai, H.M.; Sussman, I.I.; Nagel, R.L. Shear stress enhances the proteolysis of von Willebrand factor in normal plasma. Blood 1994, 83, 2171–2179. [Google Scholar] [CrossRef]
- Furlan, M.; Robles, R.; Lämmle, B. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood 1996, 87, 4223–4234. [Google Scholar] [CrossRef]
- Tsai, H.M. Physiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ion. Blood 1996, 87, 4235–4244. [Google Scholar] [CrossRef]
- Dal Lin, C.; Marinova, M.; Rubino, G.; Gola, E.; Brocca, A.; Pantano, G.; Brugnolo, L.; Sarais, C.; Cucchini, U.; Volpe, B.; et al. Thoughts modulate the expression of inflammatory genes and may improve the coronary blood flow in patients after a myocardial infarction. J. Tradit. Complement. Med. 2018, 8, 150–163. [Google Scholar] [CrossRef]
- Dal Lin, C.; Gola, E.; Brocca, A.; Rubino, G.; Marinova, M.; Brugnolo, L.; Plebani, M.; Iliceto, S.; Tona, F. miRNAs may change rapidly with thoughts: The Relaxation Response after myocardial infarction. Eur. J. Integr. Med. 2018, 20, 63–72. [Google Scholar] [CrossRef]
- Benson, H.; Proctor, W. Relaxation Revolution. Enhancing Your Personal Health Through the Science and Genetics of Mind Body Healing, 1st ed.; Scribner: New York, NY, USA, 2011. [Google Scholar]
- Benson, H.; Klipper, M.Z. The Relaxation Response; Harper Collins: New York, NY, USA; William Morrow and Comapny, Inc.: New York, NY, USA, 1975. [Google Scholar]
- De Filippis, V.; Lancellotti, S.; Maset, F.; Spolaore, B.; Pozzi, N.; Gambaro, G.; Oggianu, L.; Calò, L.A.; De Cristofaro, R. Oxidation of Met1606 in von Willebrand factor is a risk factor for thrombotic and septic complications in chronic renal failure. Biochem. J. 2012, 442, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Thomazini, C.M.; Soares, R.D.P.S.; da Rocha, T.R.F.; Sachetto, A.T.A.; Santoro, M.L. Optimization of von Willebrand factor multimer analysis in vertical mini-gel electrophoresis systems: A rapid procedure. Thromb. Res. 2019, 175, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Studt, J.D.; Budde, U.; Schneppenheim, R.; Eisert, R.; von Depka Prondzinski, M.; Ganser, A.; Barthels, M. Quantification and facilitated comparison of von Willebrand factor multimer patterns by densitometry. Am. J. Clin. Pathol. 2001, 116, 567–574. [Google Scholar] [CrossRef]
- Dal Lin, C.; Grasso, R.; Scordino, A.; Triglia, A.; Tona, F.; Iliceto, S.; Vitiello, G.; Elia, V.; Napoli, E.; Germano, R.; et al. pH, electric conductivity, and delayed luminescence changes in human sera of subjects undergoing the relaxation response: A preliminary study and theoretical considerations. Org. J. Biol. Sci. 2020, 4, 17–29. [Google Scholar] [CrossRef]
- Ruggeri, Z.M. The role of von Willebrand factor in thrombus formation. Thromb. Res. 2007, 120, S5–S9. [Google Scholar] [CrossRef]
- Wagner, D.D. Cell Biology of von Willebrand Factor. Annu. Rev. Cell Biol. 1990, 6, 217–242. [Google Scholar] [CrossRef]
- Sadler, J.E. von Willebrand factor: Two sides of a coin. J. Thromb. Haemost. 2005, 3, 1702–1709. [Google Scholar] [CrossRef]
- Dong, J.; Moake, J.L.; Nolasco, L.; Bernardo, A.; Arceneaux, W.; Shrimpton, C.N.; Schade, A.J.; McIntire, L.V.; Fujikawa, K.; Lόpez, J.A. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood 2002, 100, 4033–4039. [Google Scholar] [CrossRef]
- Moake, J.L. von Willebrand factor in thrombotic thrombocytopenic purpura. Blood 1986, 65, 1523–1526. [Google Scholar] [CrossRef]
- Moake, J.L.; Rudy, C.K.; Troll, J.H.; Weinstein, M.J.; Colannino, N.M.; Azocar, J.; Seder, R.H.; Hong, S.L.; Deykin, D. Unusually Large Plasma Factor VIII: Von Willebrand Factor Multimers in Chronic Relapsing Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 1982, 307, 1432–1435. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.K.; Starke, R.D.; Lawrie, A.S.; Cohen, H.; Machin, S.J.; Mackie, I.J. The VWF/ADAMTS13 axis in the antiphospholipid syndrome: ADAMTS13 antibodies and ADAMTS13 dysfunction. Br. J. Haematol. 2008, 141, 536–544. [Google Scholar] [CrossRef]
- Petri, B.; Broermann, A.; Li, H.; Khandoga, A.G.; Zarbock, A.; Krombach, F.; Goerge, T.; Schneider, S.W.; Jones, C.; Nieswandt, B.; et al. von Willebrand factor promotes leukocyte extravasation. Blood 2010, 116, 4712–4719. [Google Scholar] [CrossRef]
- Lenting, P.J.; Casari, C.; Christophe, O.D.; Denis, C.V. von Willebrand factor: The old, the new and the unknown. J. Thromb. Haemost. 2012, 10, 2428–2437. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fu, X.; Wang, Y.; Ling, M.; McMullen, B.; Kulman, J.; Chung, D.W.; López, J.A. Oxidative modification of von Willebrand factor by neutrophil oxidants inhibits its cleavage by ADAMTS13. Blood 2010, 115, 706–712. [Google Scholar] [CrossRef]
- Oggianu, L.; Lancellotti, S.; Pitocco, D.; Zaccardi, F.; Rizzo, P.; Martini, F.; Ghirlanda, G.; De Cristofaro, R. The Oxidative Modification of Von Willebrand Factor Is Associated with Thrombotic Angiopathies in Diabetes Mellitus. PLoS ONE 2013, 8, e55396. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.B.; Wiener-Kronish, J.P.; Murray, J.F.; Green, D.R.; Turner, J.; Luce, J.M.; Montgomery, A.B.; Marks, J.D.; Matthay, M.A. Elevated von Willebrand factor antigen is an early plasma predictor of acute lung injury in nonpulmonary sepsis syndrome. J. Clin. Investig. 1990, 86, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, S.; De Filippis, V.; Pozzi, N.; Peyvandi, F.; Palla, R.; Rocca, B.; Rutella, S.; Pitocco, D.; Mannucci, P.M.; De Cristofaro, R. Formation of methionine sulfoxide by peroxynitrite at position 1606 of von Willebrand factor inhibits its cleavage by ADAMTS-13: A new prothrombotic mechanism in diseases associated with oxidative stress. Free Radic. Biol. Med. 2010, 48, 446–456. [Google Scholar] [CrossRef]
- Furlan, M. Von Willebrand factor: Molecular size and functional activity. Ann. Hematol. 1996, 72, 341–348. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Pattison, D.I.; Davies, M.J. Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids 2003, 25, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Chen, J.; Gallagher, R.; Zheng, Y.; Chung, D.W.; López, J.A. Shear stress–induced unfolding of VWF accelerates oxidation of key methionine residues in the A1A2A3 region. Blood 2011, 118, 5283–5291. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Y.-F.; Zhang, C.-Z.; Zhang, X.; Lu, C.; Springer, T.A. Structural specializations of A2, a force-sensing domain in the ultralarge vascular protein von Willebrand factor. Proc. Natl. Acad. Sci. USA 2009, 106, 9226–9231. [Google Scholar] [CrossRef]
- Chung, D.W.; Fujikawa, K. Processing of von Willebrand factor by ADAMTS13. Biochemistry 2002, 41, 11065–11070. [Google Scholar] [CrossRef]
- Pozzi, N.; Lancellotti, S.; De Cristofaro, R.; De Filippis, V. Modeling ADAMTS13-von Willebrand Factor interaction: Implications for oxidative stress-related cardiovascular diseases and type 2A von Willebrand Disease. Biophys. Chem. 2012, 160, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dal Lin, C.; Brugnolo, L.; Marinova, M.; Plebani, M.; Iliceto, S.; Tona, F.; Vitiello, G. Toward a Unified View of Cognitive and Biochemical Activity: Meditation and Linguistic Self-Reconstructing May Lead to Inflammation and Oxidative Stress Improvement. Entropy 2020, 22, 818. [Google Scholar] [CrossRef]
- Dal Lin, C.; Marinova, M.; Brugnolo, L.; Rubino, G.; Plebani, M.; Tona, F. Rapid changes of miRNAs-20, -30, -410, -515, -134, and -183 and telomerase with psychological activity: A one year study on the Relaxation Response and epistemological considerations. J. Tradit. Complement. Med. 2021. ISSN 2225-4110. [Google Scholar] [CrossRef]
- Pavanello; Campisi; Tona; Lin; Iliceto Exploring Epigenetic Age in Response to Intensive Relaxing Training: A Pilot Study to Slow Down Biological Age. Int. J. Environ. Res. Public Health 2019, 16, 3074. [CrossRef]
- Gragnano, F.; Sperlongano, S.; Golia, E.; Natale, F.; Bianchi, R.; Crisci, M.; Fimiani, F.; Pariggiano, I.; Diana, V.; Carbone, A.; et al. The Role of von Willebrand Factor in Vascular Inflammation: From Pathogenesis to Targeted Therapy. Mediators Inflamm. 2017, 2017, 5620314. [Google Scholar] [CrossRef]
- Huck, V.; Schneider, M.; Gorzelanny, C.; Schneider, S. The various states of von Willebrand factor and their function in physiology and pathophysiology. Thromb. Haemost. 2014, 111, 598–609. [Google Scholar] [CrossRef]
- Del Giudice, E.; Voeikov, V.; Tedeschi, A.; Vitiello, G. The origin and the special role of coherent water in living systems. In Fields of the Cell; Fels, D., Cifra, M., Scholkmann, F., Eds.; Research Signpost: Kerala, India, 2015; pp. 95–111. [Google Scholar]
- Dal Lin, C.; Falanga, M.; De Lauro, E.; De Martino, S.; Vitiello, G. Biochemical and biophysical mechanisms underlying the heart and the brain dialog. AIMS Biophys. 2021, 8, 1–33. [Google Scholar] [CrossRef]
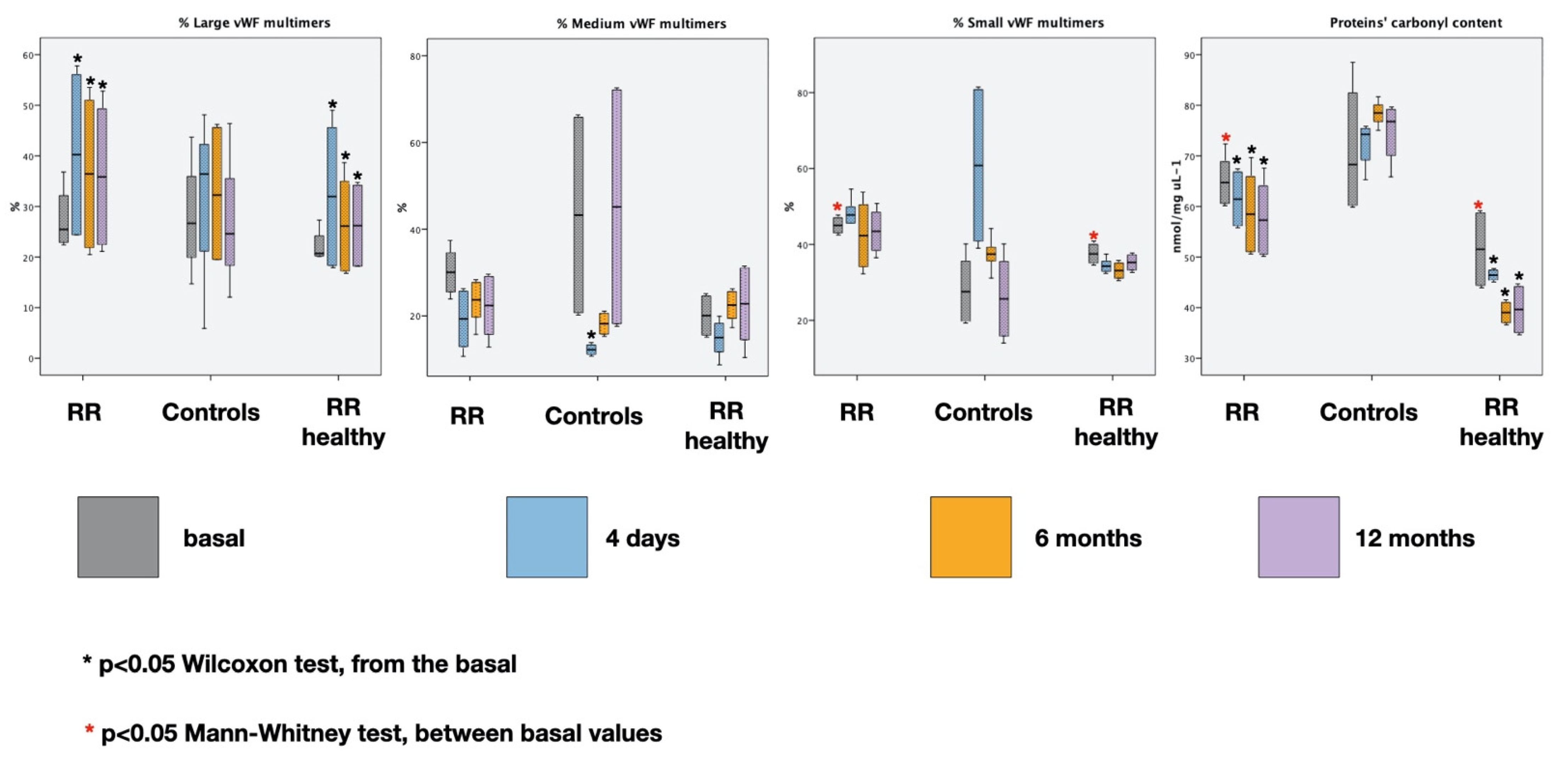
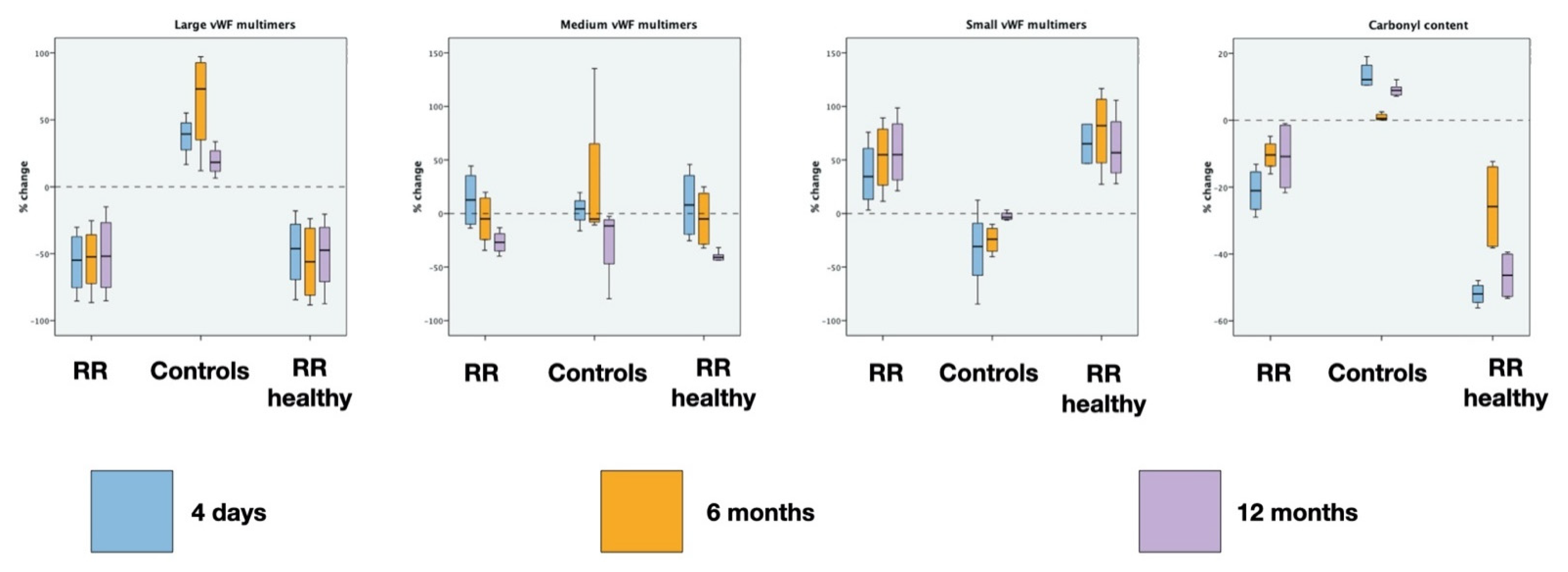
| Groups | Large vWF Basal | Large vWF 4D PRE | Large vWF 4D POST | Large vWF 6M PRE | Large vWF 6M POST | Large vWF 12M PRE | Large vWF 12M POST |
|---|---|---|---|---|---|---|---|
| Relaxation Response | 24.95 (22.4–27.5) | 40.24 (24–56.1) | 12.6 (8.2–17) | 36.42 (21.8–51) | 15.25 (6.9–23.6) | 35.85 (22–49.3) | 14.85 (7.3–22.4) |
| % change | −54.85 (−70.38–−40.83) | −52.37 (−65.31–−41.18) | −51.9 (−70.19–−32.7) | ||||
| Controls | 25.17 (22.55–29.8) | 36.4 (28.78–39) | 48 (44.08–51) | 32.25 (19.5–45) | 40 (34–40.4) | 24.6 (21.4–30) | 31.65 (26.2–39) |
| % change | 39.38 (32.95–44.06) | 73.09 (46.51–90.43) | 18.27 (14–23.46) | ||||
| Relaxation Response Healthy Controls | 20.7 (20.3–21.1) | 31.95 (18–45.6) | 11.05 (7.1–15) | 26.11 (17–35.02) | 8.6 (4.1–13.1) | 26.2 (18–34.2) | 10.3 (4.3–16.3) |
| % change | −46.23 (−61.93–−33.03) | −56.06 (−77.45–−34.68) | −47.43 (−62.68–−35.44) | ||||
| Groups | Medium vWF Basal | Medium vWF 4D PRE | Medium vWF 4D POST | Medium vWF 6M PRE | Medium vWF 6M POST | Medium vWF 12M_PRE | Medium vWF 12M POST |
|---|---|---|---|---|---|---|---|
| Relaxation Response | 30.05 (25.5–34.6) | 19.3 (12.9–25.7) | 23.2 (11.6–34.8) | 23.7 (19.7–27.7) | 23.3 (14.9–31.7) | 22.4 (15.7–29.1) | 16.9 (10.2–23.6) |
| % change | 12.67 (−10.08–35.41) | −4.96 (−24.37–14.44) | −26.97 (−35.03–−18.9) | ||||
| Controls | 43.25 (31.9–54.3) | 13.6 (12.7–13.9) | 14.5 (13.2–14.8) | 26.7 (19.4–32.8) | 31.1 (26.8–35.4) | 31.4 (28.1–41.6) | 23.05 (15.4–30.5) |
| % change | 4.32 (−0.82–8.12) | −5.18 (−6.58–29.97) | −8.58 (−30.62–−2.87) | ||||
| Relaxation Response Healthy Controls | 20.05 (15.5–24.6) | 15 (11.7–18.3) | 17.1 (9.4–24.8) | 22.5 (19.4–25.6) | 22.1 (13.8–30.4) | 22.8 (14.5–31.1) | 13.65 (8.2–19.1) |
| % change | 7.93 (−19.66–35.52) | −5.06 (−28.87–18.75) | −41.02 (−43.45–−38.59) | ||||
| Groups | Small vWF BASAL | Small vWF 4D PRE | Small vWF 4D POST | Small vWF 6M PRE | Small vWF 6M POST | Small vWF 12M_PRE | Small vWF 12M POST |
|---|---|---|---|---|---|---|---|
| Relaxation Response | 45 (43–47) | 47.75 (45.6–49.9) | 64.25 (48.3–80.2) | 42.3 (34.1–50.5) | 61.45 (44.7–78.2) | 43.45 (38.4–48.5) | 65.15 (54–76.3) |
| % change | 34.54 (18.21–53.38) | 54.91 (33.99–73.58) | 55.02 (36.34–76.2) | ||||
| Controls | 27.55 (23.53–31.58) | 49.7 (47.48–57.48) | 34.4 (28.9–37.3) | 43.5 (38.4–47.7) | 30.25 (28.5–35.58) | 39.75 (30.58–44) | 42.9 (41.5–44) |
| % change | −30.78 (−44.22–−19.96) | −24.03 (−32.75–−15.89) | −3.5 (−4.89–−1.07) | ||||
| Relaxation Response Healthy Controls | 37.5 (35–40) | 34.25 (32.9–35.6) | 56.8 (48.3–65.3) | 33.1 (31.1–35.1) | 56.05 (44.7–67.4) | 35.25 (33.2–37.3) | 56.15 (44–68.3) |
| % change | 65.12 (46.81–83.43) | 82.04 (57.35–101.72) | 56.84 (42.96–75.72) | ||||
| Groups | Carbonyl Basal | Carbonyl PRE | Carbonyl POST | Carbonyl PRE6 | Carbonyl POST6 | Carbonyl PRE12 | Carbonyl POST12 |
|---|---|---|---|---|---|---|---|
| Relaxation Response | 64.72 (60.56–68.88) | 61.44 (56.08–66.8) | 48.8 (41.1–56.48) | 58.48 (51–65.92) | 52.16 (47.4–56.88) | 57.3 (50.5–64.08) | 51.72 (40.31–63.12) |
| Controls | 68.28 (60.15–79.42) | 74.24 (71.1–75.44) | 83.36 (81.1–84.26) | 78.48 (77.62–79.28) | 78.56 (77.86–79.86) | 76.76 (72.2–79.2) | 83.64 (77.82–87.04) |
| Relaxation Response Healthy Controls | 51.55 (44.33–58.77) | 46.49 (46.32–46.66) | 22.34 (21.24–23.43) | 39.03 (37.02–41.03) | 29.19 (23.05–35.32) | 39.63 (35.07–44.19) | 20.96 (20.89–21.03) |
| % change | % change | % change | |||||
| Relaxation response | −21.06 (−26.68–−15.45) | −10.38 (−13.71–−7.05) | −10.85 (−20.21–−1.5) | ||||
| Controls | 12.17 (10.5–15.15) | 0.53 (0.1–1.36) | 8.93 (7.77–9.9) | ||||
| Relaxation response healthy controls | −51.95 (−54.48–−49.42) | −25.83 (−37.74–−13.92) | −46.38 (−52.73–−40.03) | ||||
| Groups | Body Temp. | pH | IL-6 ng/L | TNF-alpha pg/mL | TGF-beta μg/L | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | PRE | POST | PRE | POST | PRE | POST | |
| RELAXATION RESPONSE | 36.6 (36.1–36.9) | 36.3 (35.9–36.5) | 7.0 (6.7–7.3) | 7.4 (7.2–7.7) | 3.7 (2.9–4.3) | 2.6 (<2–3.1) | 4.5 (2.8–5.4) | 2.9 (<2–3.6) | 37.9 (35.9–43.8) | 31 (26.4–38.2) |
| CONTROLS | 36.5 (36.1–36.7) | 36.7 (36.3–36.9) | 7.4 (7.2–7.6) | 6.9 (6.7–7.3) | 3.6 (2.8–4.0) | 4.1 (2.6–4.3) | 4.3 (2.6–5.6) | 5.9 (2.8–6.1) | 36.3 (35.4–38.7) | 42.7 (39.5–43.9) |
| RELAXATION RESPONSE HEALTHY CONTROLS | 36.4 (35.9–36.7) | 36.2 (35.7–36.4) | 7.3 (7.0–7.4) | 7.5 (7.3–7.5) | 2.8 (<2–3.2) | <2 (<2–2.4) | 2.4 (<2–3.5) | 2.0 (<2–2.6) | 32.5 (26.5–39.8) | 28.5 (21.7–36.7) |
| RELAXATION RESPONSE | |||||||||
| L vWF | M vWF | S vWF | Carbonyl | Body Temp | pH | IL6 | TNFalpha | TGFbeta | |
| L vWF | 1 | ||||||||
| M vWF | 0.4488212 | 1 | |||||||
| S vWF | −0.8031173 | −0.7695195 | 1 | ||||||
| Carbonyl | 0.54983667 | −0.0934422 | −0.2273933 | 1 | |||||
| Body Temp | 0.4951998 | −0.1058215 | −0.1972022 | 0.13290312 | 1 | ||||
| pH | −0.7568174 | 0.1611795 | −0.0724475 | 0.02068901 | −0.7098822 | 1 | |||
| IL6 | 0.1643193 | −0.0530246 | −0.0165857 | 0.22071129 | 0.35427728 | −0.0521386 | 1 | ||
| TNFalpha | 0.6553398 | 0.24696555 | −0.1065792 | 0.73033795 | −0.6644612 | 0.45628284 | −0.1281666 | 1 | |
| TGFbeta | −0.7298136 | −0.2302344 | 0.28479838 | −0.1833968 | 0.08288911 | 0.21092171 | 0.74492299 | −0.1522784 | 1 |
| CONTROLS | |||||||||
| L vWF | M vWF | S vWF | Carbonyl | Body Temp | pH | IL6 | TNFalpha | TGFbeta | |
| L vWF | 1 | ||||||||
| M vWF | 0.24257875 | 1 | |||||||
| S vWF | −0.5665193 | −0.1410782 | 1 | ||||||
| Carbonyl | −0.5280698 | −0.1795384 | 0.08943854 | 1 | |||||
| Body Temp | 0.59709917 | 0.0075333 | −0.1325308 | −0.4643379 | 1 | ||||
| pH | 0.01243143 | 0.23315709 | 0.25956481 | −0.2490491 | 0.120824 | 1 | |||
| IL6 | −0.0134115 | 0.21426733 | 0.06986446 | 0.06005788 | −0.0399083 | −0.5057946 | 1 | ||
| TNFalpha | 0.13471092 | −0.3923573 | −0.1826808 | 0.7773945 | 0.10633633 | −0.3915101 | 0.14474083 | 1 | |
| TGFbeta | 0.24206284 | 0.09167871 | −0.1731709 | −0.355089 | −0.1506871 | −0.01284 | −0.0760909 | 0.52394815 | 1 |
| RELAXATION RESPONSE HEALTHY CONTROLS | |||||||||
| L vWF | M vWF | S vWF | Carbonyl | Body Temp | pH | IL6 | TNFalpha | TGFbeta | |
| L vWF | 1 | ||||||||
| M vWF | 0.735540032 | 1 | |||||||
| S vWF | −0.7075044 | −0.7317979 | 1 | ||||||
| Carbonyl | −0.2572203 | −0.1531874 | 0.5644646 | 1 | |||||
| Body Temp | 0.15639389 | −0.0084742 | −0.2854408 | −0.0220424 | 1 | ||||
| pH | −0.70486633 | −0.2336897 | −0.0158521 | −0.4094433 | 0.35116455 | 1 | |||
| IL6 | −0.1155365 | 0.0595067 | 0.04987164 | 0.16705885 | 0.07275114 | −0.024814 | 1 | ||
| TNFalpha | 0.6551395 | −0.3787234 | 0.31082905 | 0.75718815 | 0.21356039 | 0.55018354 | −0.0013061 | 1 | |
| TGFbeta | −0.6993715 | −0.2250648 | 0.61388811 | 0.2342013 | 0.00280797 | 0.19113802 | 0.36220604 | −0.6143749 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dal Lin, C.; Acquasaliente, L.; Iliceto, S.; De Filippis, V.; Vitiello, G.; Tona, F. Von Willebrand Factor Multimers and the Relaxation Response: A One-Year Study. Entropy 2021, 23, 447. https://doi.org/10.3390/e23040447
Dal Lin C, Acquasaliente L, Iliceto S, De Filippis V, Vitiello G, Tona F. Von Willebrand Factor Multimers and the Relaxation Response: A One-Year Study. Entropy. 2021; 23(4):447. https://doi.org/10.3390/e23040447
Chicago/Turabian StyleDal Lin, Carlo, Laura Acquasaliente, Sabino Iliceto, Vincenzo De Filippis, Giuseppe Vitiello, and Francesco Tona. 2021. "Von Willebrand Factor Multimers and the Relaxation Response: A One-Year Study" Entropy 23, no. 4: 447. https://doi.org/10.3390/e23040447
APA StyleDal Lin, C., Acquasaliente, L., Iliceto, S., De Filippis, V., Vitiello, G., & Tona, F. (2021). Von Willebrand Factor Multimers and the Relaxation Response: A One-Year Study. Entropy, 23(4), 447. https://doi.org/10.3390/e23040447








