Mismatch Negativity and Stimulus-Preceding Negativity in Paradigms of Increasing Auditory Complexity: A Possible Role in Predictive Coding
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
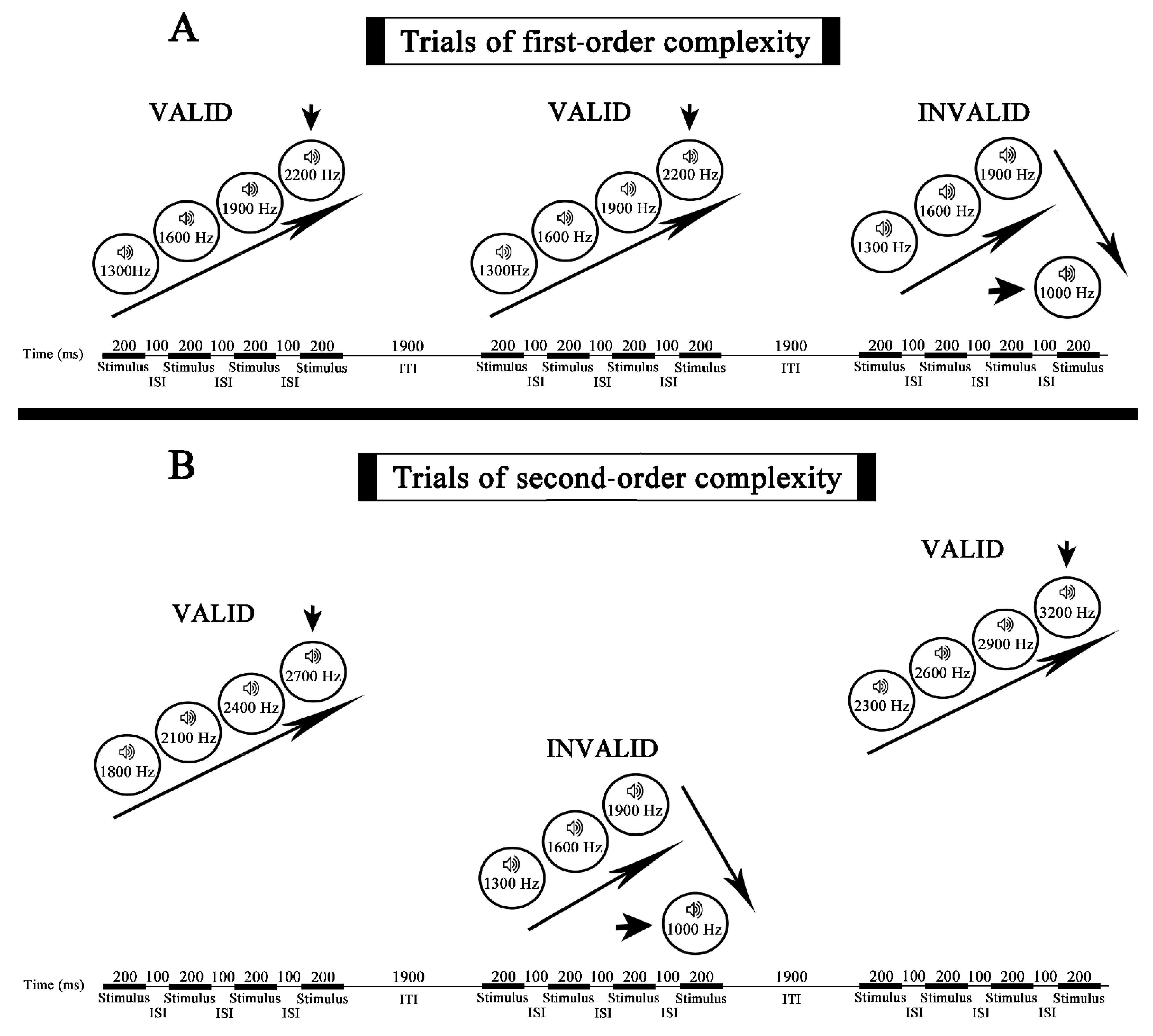
2.2. Stimuli
2.3. EEG Recordings
2.4. Data Analysis
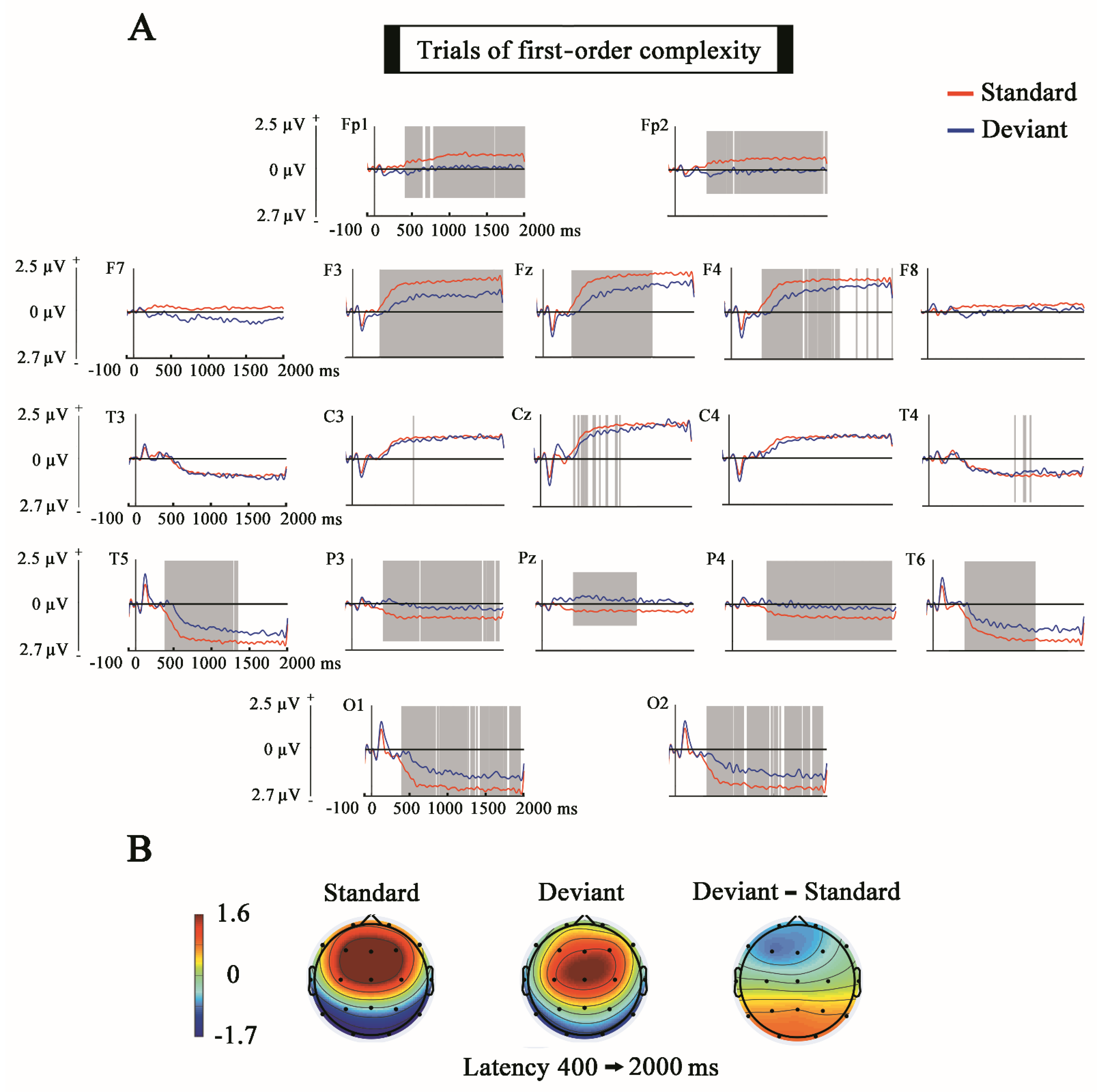
3. Results
4. Discussion
4.1. Mismatch Negativity
4.2. SPN Component
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friston, K.J. The free-energy principle: A rough guide to the brain? Trends Cogn. Sci. 2009, 13, 279–328. [Google Scholar] [CrossRef]
- Knill, D.C.; Pouget, A. The Bayesian brain: The role of uncertainty in neural coding and computation. Trends Neurosci. 2004, 27, 712–719. [Google Scholar] [CrossRef]
- Gómez, C.M.; Flores, A.B. A neurophysiological evaluation of a cognitive cycle in humans. Neurosci. Biobehav. Rev. 2011, 35, 452–461. [Google Scholar] [CrossRef]
- Brodski, A.; Paasch, G.F.; Helbling, S.; Wibral, M. The Faces of Predictive Coding. J. Neurosci. 2015, 35, 8997–9006. [Google Scholar] [CrossRef]
- Heilbron, M.; Chait, M. Great Expectations: Is there Evidence for Predictive Coding in Auditory Cortex? Neuroscience 2018, 389, 54–73. [Google Scholar] [CrossRef]
- Baldeweg, T. ERP repetition effects and mismatch negativity generation: A predictive coding perspective. J. Psychophysiol. 2007, 21, 204–213. [Google Scholar] [CrossRef]
- Wacongne, C.; Labyt, E.; van Wassenhove, V.; Bekinschtein, T.; Naccache, L.; Dehaene, S. Evidence for a hierarchy of predictions and prediction errors in human cortex. Proc. Natl. Acad. Sci. USA 2011, 108, 20754–20759. [Google Scholar] [CrossRef]
- Hickok, G.; Houde, J.; Rong, F. Sensorimotor integration in speech processing: Computational basis and neural organization. Neuron 2011, 69, 407–422. [Google Scholar] [CrossRef]
- Arjona, A.; Escudero, M.; Gómez, C.M. Updating of attentional and premotor allocation resources as function of previous trial outcome. Sci. Rep. 2014, 4, 4526. [Google Scholar] [CrossRef]
- Gómez, C.M.; Arjona, A.; Donnarumma, F.; Maisto, D.; Rodríguez-Martínez, E.I.; Pezzulo, G. Tracking the Time Course of Bayesian Inference With Event-Related Potentials: A Study Using the Central Cue Posner Paradigm. Front. Psychol. 2019, 19, 1424. [Google Scholar] [CrossRef]
- Ylinen, S.; Huuskonen, M.; Mikkola, K.; Saure, E.; Sinkkonen, T.; Paavilainen, P. Predictive coding of phonological rules in auditory cortex: A mismatch negativity study. Brain Lang. 2016, 162, 72–80. [Google Scholar] [CrossRef][Green Version]
- Feldman, H.; Friston, K.J. Attention, uncertainty, and free-energy. Front. Hum. Neurosci. 2010, 4, 215. [Google Scholar] [CrossRef]
- Escera, C.; Corral, M.J. Role of mismatch negativity and novelty-P3 in involuntary auditory attention. Int. J. Psychophysiol. 2007, 21, 251–264. [Google Scholar] [CrossRef]
- Näätänen, R.; Paavilainen, P.; Rinne, T.; Alho, K. The mismatch negativity (MMN) in basic research of central auditory processing: A review. Clin. Neurophysiol. 2007, 118, 2544–2590. [Google Scholar] [CrossRef]
- Näätänen, R. The mismatch negativity: A powerful tool for cognitive neuroscience. Ear Hear. 1995, 16, 6–18. [Google Scholar] [CrossRef]
- Lang, A.H.; Eerola, O.; Korpilahti, P.; Holopainen, I.; Salo, S.; Aaltonen, O. Practical issues in the clinical application of mismatch negativity. Ear Hear. 1995, 16, 118–130. [Google Scholar] [CrossRef] [PubMed]
- May, P.; Tiitinen, H.; Ilmoniemi, R.J.; Nyman, G.; Taylor, J.G.; Näätänen, R. Frequency change detection in human auditory cortex. J. Comput. Neurosci. 1999, 6, 99–120. [Google Scholar] [CrossRef] [PubMed]
- May, P.J.; Tiitinen, H. The MMN is a derivative of the auditory N100 response. Neurol. Clin. Neurophysiol. 2004, 9, 20. [Google Scholar]
- May, P.J.; Tiitinen, H. Mismatch negativity (MMN), the deviance-elicited auditory deflection, explained. Psychophysiology 2010, 47, 66–122. [Google Scholar] [CrossRef]
- Winkler, I. Interpreting the mismatch negativity (MMN). J. Psychophysiol. 2007, 21, 147–163. [Google Scholar] [CrossRef]
- Paavilainen, P.; Jaramillo, M.; Näätänen, R.; Winkler, I. Neuronal populations in the human brain extracting invariant relationships from acoustic variance. Neurosci. Lett. 1999, 265, 179–182. [Google Scholar] [CrossRef]
- Paavilainen, P.; Kaukinen, C.; Koskinen, O.; Kylmälä, J.; Rehn, L. Mismatch negativity (MMN) elicited by abstract regularity violations in two concurrent auditory streams. Heliyon 2018, 4, e00608. [Google Scholar] [CrossRef]
- Korzyukov, O.A.; Winkler, I.; Gumenyuk, V.I.; Alho, K. Processing abstract auditory features in the human auditory corte. NeuroImage 2003, 20, 2245–2258. [Google Scholar] [CrossRef]
- Wacongne, C.; Changeux, J.; Dehaene, S. A Neuronal Model of Predictive Coding Accounting for the Mismatch Negativity. J. Neurosci. 2012, 32, 3665–3678. [Google Scholar] [CrossRef]
- Brunia, C.H.; van Boxtel, G.J. Wait and see. Int. J. Psychophysiol. 2001, 43, 59–75. [Google Scholar] [CrossRef]
- Brunia, C.H.; Damen, E.J. Distribution of Slow-Potentials Related to Motor Preparation and Stimulus Anticipation in a Time Estimation Task. Electroencephalogr. Clin. Neurophysiol. 1988, 69, 234–243. [Google Scholar] [CrossRef]
- Brunia, C.H.; Hackley, S.A.; Boxtel, G.J.M.; Kotani, Y.; Ohami, Y. Waiting to perceive: Reward or punishment? Clin. Neurophysiol. 2011, 122, 858–868. [Google Scholar] [CrossRef]
- Böcker, K.B.; Brunia, C.H.; van den Berg-Lenssen, M.M. A spatiotemporal dipole model of the stimulus preceding negativity (SPN) prior to feedback stimuli. Brain Topogr. 1994, 7, 71–88. [Google Scholar] [CrossRef]
- Walter, W.G.; Cooper, R.; Aldridge, W.J.; McCallum, W.C. Contingent negative variation: An electrophysiological sign of sensorimotor association and expectancy in the human brain. Nature 1964, 203, 380–384. [Google Scholar] [CrossRef]
- Rockstroh, B.; Elbert, T.; Birbaumer, N.; Lutzenberger, W. Slow Brain Potentials and Behavior; Urban & Schwarzenberg: Baltimore, MD, USA, 1982. [Google Scholar]
- Arjona, A.; Gómez, C.M. Sequential Effects in the Central Cue Posner Paradigm: On-line Bayesian Learning. In Cognitive Electrophysiology of Attention; Academic Press: Cambridge, MA, USA, 2014; pp. 45–57. [Google Scholar] [CrossRef]
- Röhricht, J.; Jo, H.; Wittmann, M.; Schmidt, S. Exploring the maximum duration of the contingent negative variation. Int. J. Psychophysiol. 2018, 128, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Näätänen, R.; Kujala, T.; Winkler, I. Auditory processing that leads to conscious perception: A unique window to central auditory processing opened by the mismatch negativity and related responses. Psychophysiology 2011, 48, 4–22. [Google Scholar] [CrossRef]
- Maris, E.; Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 2007, 164, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Fields, E.C.; Kuperberg, G.R. Having your cake and eating it too: Flexibility and power with mass univariate statistics for ERP data. Psychophysiology 2019, 57, e13468. [Google Scholar] [CrossRef]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.M. Fieldtrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 2011, 156869. [Google Scholar] [CrossRef]
- Winkler, I.; Lehtokoski, A.; Alku, P.; Vainio, M.; Czigler, I.; Csépe, V.; Aaltonen, O.; Raimo, I.; Alho, K.; Lang, H.; et al. Preattentive detection of vowel contrasts utilizes both phonetic and auditory memory representations. Cogn. Brain Res. 1999, 7, 357–369. [Google Scholar] [CrossRef]
- Winkler, I.; Kujala, T.; Tiitinen, H.; Sivonen, P.; Alku, P.; Lehtokoski, A.; Czigler, I.; Csépe, V.; Ilmoniemi, R.J.; Näätänen, R. Brain responses reveal the learning of foreign language phonemes. Psychophysiology 1999, 36, 638–642. [Google Scholar] [CrossRef]
- Amenedo, E.; Escera, C. The accuracy of sound duration representation in the human brain determines the accuracy of behavioral perception. Eur. J. Neurosci. 2000, 12, 2570–2574. [Google Scholar] [CrossRef]
- Tervaniemi, M.; Maury, S.; Näätänen, R. Neural representations of abstract stimulus features in the human brain as reflected by the mismatch negativity. NeuroReport 1994, 5, 844–846. [Google Scholar] [CrossRef]
- Ono, K.; Yamasaki, D.; Altmann, C.F.; Mima, T. The effect of illusionary perception on mismatch negativity (MMN): An electroencephalography study. Hear. Res. 2017, 356, 87–92. [Google Scholar] [CrossRef]
- Garrido, M.I.; Kilner, J.M.; Stephan, K.E.; Friston, K.J. The mismatch negativity: A review of underlying mechanisms. Clin. Neurophysiol. 2009, 120, 453–463. [Google Scholar] [CrossRef]
- Romani, G.L.; Williamson, S.J.; Kaufman, L. Tonotopic organization of the human auditory cortex. Science 1982, 216, 1339–1340. [Google Scholar] [CrossRef]
- Pantev, C.; Hoke, M.; Lehnertz, K.; Lutkenhöner, B.; Anogianakis, G.; Wittkowski, W. Tonotopic organization of the human auditory cortex revealed by transient auditory evoked magnetic fields. Clin. Neurophysiol. 1988, 69, 160–170. [Google Scholar] [CrossRef]
- Bertrand, O.; Perrin, F.; Echallier, J.; Pernier, J. Topography and model analysis of auditory evoked potentials: Tonotopic aspects. In Functional Brain Imaging; Pfurtscheller, G., Lopes da Silva, F.H., Eds.; Hans Huber: Toronto, ON, Canada, 1988; pp. 75–82. [Google Scholar]
- Lauter, J.L.; Herschovitch, P.; Formby, C.; Raichle, M.E. Tonotopic organization in the human auditory cortex revealed by positrón emission tomography. Hear. Res. 1985, 20, 199–205. [Google Scholar] [CrossRef]
- Howard, M.A.; Volkov, I.O.; Abbas, P.J.; Damasio, H.; Ollendick, M.C.; Granner, M.A. A chronic microelectrode investigation of the tonotopic organization of human auditory cortex. Brain Res. 1996, 724, 260–264. [Google Scholar] [CrossRef]
- Jääskeläinen, I.P.; Ahveninen, J.; Bonmassar, G.; Dale, A.M.; Ilmoniemi, R.J.; Levänen, S.; Lin, F.H.; May, P.; Melcher, J.; Stufflebeam, S.; et al. Human posterior auditory cortex gates novel sounds to consciousness. Proc. Natl. Acad. Sci. USA 2004, 101, 6809–6814. [Google Scholar] [CrossRef]
- Samson, F.; Zeffiro, T.A.; Toussaint, A.; Belin, P. Stimulus complexity and categorical effects in human auditory cortex: An Activation Likelihood Estimation meta-analysis. Front. Psycho. 2011, 1, 241. [Google Scholar] [CrossRef]
- Schröger, E.; Giard, M.H.; Wolff, C. Auditory distraction: Event-related potential and behavioral indices. Clin. Neurophysiol. 2000, 111, 1450–1460. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, X.; Yuan, P.; Zhang, D.; He, S. The effect of visuospatial attentional load on the processing of irrelevant acoustic distractors. NeuroImage 2006, 33, 715–724. [Google Scholar] [CrossRef]
- Otten, L.J.; Alain, C.; Picton, T. Effects of visual attentional load on auditory processing. NeuroReport 2000, 11, 875–880. [Google Scholar] [CrossRef]
- Mangun, G.R.; Hillyard, S.A. Modulations of sensory-evoked brainpotentials indicate changes in perceptual processing during visual-spatial priming. J. Exp. Psychol. 1991, 17, 1057–1074. [Google Scholar] [CrossRef]
- Gómez, C.M.; Flores, A.B.; Digiacomo, M.R.; Ledesma, A.; González-Rosa, J.J. P3a and P3b components associated to the neurocognitive evaluation of deviantly cued targets. Neurosci. Lett. 2008, 430, 181–185. [Google Scholar] [CrossRef]
- Mathys, C.; Daunizeau, J.; Friston, K.J.; Stephan, K.E. A bayesian foundation for individual learning under uncertainty. Front. Hum. Neurosci. 2011, 5, 39. [Google Scholar] [CrossRef]
- Van Boxtel, G.J.; Böcker, K.B. Cortical measures of anticipation. J. Psychophysiol. 2004, 18, 61–76. [Google Scholar] [CrossRef]
- Bianco, V.; Perri, R.L.; Berchicci, M.; Quinzi, F.; Spinelli, D.; Di Russo, F. Modality-specific sensory readiness for upcoming events revealed by slow cortical potentials. Brain Struct. Funct. 2020, 225, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Gómez, C.M.; Vaquero, E.; López-Mendoza, D.; González-Rosa, J.; Vázquez-Marrufo, M. Reduction of EEG power during expectancy periods in humans. Acta Neurobiol. Exp. 2004, 64, 143–151. [Google Scholar] [CrossRef]
- Bennett, D.; Murawski, C.; Bode, S. Single-trial event-related potential correlates of belief updating (1, 2, 3). eNeuro 2015, 2, 6–13. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Martínez, F.J.; Arjona, A.; Gómez, C.M. Mismatch Negativity and Stimulus-Preceding Negativity in Paradigms of Increasing Auditory Complexity: A Possible Role in Predictive Coding. Entropy 2021, 23, 346. https://doi.org/10.3390/e23030346
Ruiz-Martínez FJ, Arjona A, Gómez CM. Mismatch Negativity and Stimulus-Preceding Negativity in Paradigms of Increasing Auditory Complexity: A Possible Role in Predictive Coding. Entropy. 2021; 23(3):346. https://doi.org/10.3390/e23030346
Chicago/Turabian StyleRuiz-Martínez, Francisco J., Antonio Arjona, and Carlos M. Gómez. 2021. "Mismatch Negativity and Stimulus-Preceding Negativity in Paradigms of Increasing Auditory Complexity: A Possible Role in Predictive Coding" Entropy 23, no. 3: 346. https://doi.org/10.3390/e23030346
APA StyleRuiz-Martínez, F. J., Arjona, A., & Gómez, C. M. (2021). Mismatch Negativity and Stimulus-Preceding Negativity in Paradigms of Increasing Auditory Complexity: A Possible Role in Predictive Coding. Entropy, 23(3), 346. https://doi.org/10.3390/e23030346





