On the Variability of Functional Connectivity and Network Measures in Source-Reconstructed EEG Time-Series
Abstract
1. Introduction
2. Material and Methods
2.1. Dataset
2.2. Preprocessing
2.3. Connectivity Metrics
2.4. Network Measures
2.5. Cluster Analysis
2.6. Statistical Analysis
3. Results
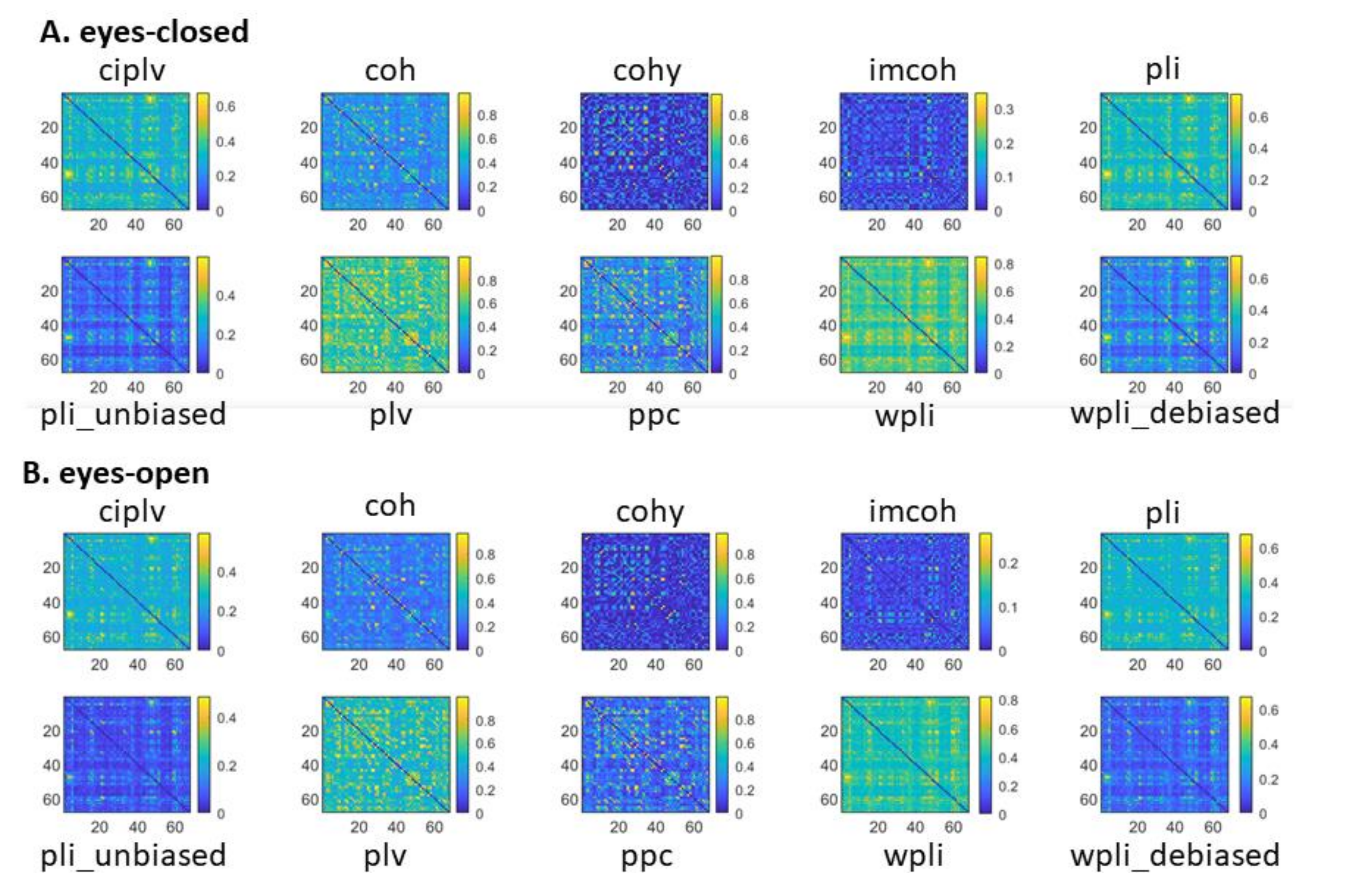
3.1. Global Connectivity Patterns
3.2. Cluster Analysis
3.3. Network Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fingelkurts, A.A.; Fingelkurts, A.A.; Kähkönen, S. Functional connectivity in the brain—Is it an elusive concept? Neurosci. Biobehav. Rev. 2005, 28, 827–836. [Google Scholar] [CrossRef]
- Lee, L.; Harrison, L.M.; Mechelli, A. A report of the functional connectivity workshop, Dusseldorf 2002. Neuroimage 2003, 19, 457–465. [Google Scholar] [CrossRef]
- Sakkalis, V. Review of advanced techniques for the estimation of brain connectivity measured with EEG/MEG. Comput. Biol. Med. 2011, 41, 1110–1117. [Google Scholar] [CrossRef]
- Sizemore, A.E.; Phillips-Cremins, J.E.; Ghrist, R.; Bassett, D.S. The importance of the whole: Topological data analysis for the network neuroscientist. Netw. Neurosci. 2018, 3, 656–673. [Google Scholar] [CrossRef]
- Schoffelen, J.-M.; Gross, J. Source connectivity analysis with MEG and EEG. Hum. Brain Mapp. 2009, 30, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Anzolin, A.; Presti, P.; Van De Steen, F.; Astolfi, L.; Haufe, S.; Marinazzo, D. Quantifying the Effect of Demixing Approaches on Directed Connectivity Estimated Between Reconstructed EEG Sources. Brain Topogr. 2019, 32, 655–674. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Demuru, M.; Hillebrand, A.; Fraschini, M. A comparison between scalp- and source-reconstructed EEG networks. Sci. Rep. 2018, 8, 12269. [Google Scholar] [CrossRef] [PubMed]
- Brookes, M.; Woolrich, M.; Price, D. An Introduction to MEG Connectivity Measurements. Magn. Signals Dyn. Cortical Netw. 2012, 321–358. [Google Scholar] [CrossRef]
- Stam, C.J. Modern network science of neurological disorders. Nat. Rev. Neurosci. 2014, 15, 683–695. [Google Scholar] [CrossRef]
- Hassan, M.; Dufor, O.; Merlet, I.; Berrou, C.; Wendling, F. EEG Source Connectivity Analysis: From Dense Array Recordings to Brain Networks. PLoS ONE 2014, 9, e105041. [Google Scholar] [CrossRef]
- Kida, T.; Tanaka, E.; Kakigi, R. Multi-Dimensional Dynamics of Human Electromagnetic Brain Activity. Front. Hum. Neurosci. 2016, 9, 713. [Google Scholar] [CrossRef] [PubMed]
- Olejarczyk, E.; Marzetti, L.; Pizzella, V.; Zappasodi, F. Comparison of connectivity analyses for resting state EEG data. J. Neural Eng. 2017, 14, 036017. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, L.; Cincotti, F.; Mattia, D.; Marciani, M.G.; Baccala, L.A.; de Vico Fallani, F.; Salinari, S.; Ursino, M.; Zavaglia, M.; Ding, L.; et al. Comparison of different cortical connectivity estimators for high-resolution EEG recordings. Hum. Brain Mapp. 2007, 28, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Mahjoory, K.; Nikulin, V.V.; Botrel, L.; Linkenkaer-Hansen, K.; Fato, M.M.; Haufe, S. Consistency of EEG source localization and connectivity estimates. NeuroImage 2017, 152, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, A.L.; Amaral, L.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef]
- Schalk, G.; McFarland, D.J.; Hinterberger, T.; Birbaumer, N.; Wolpaw, J.R. BCI2000: A general-purpose brain-computer interface (BCI) system. IEEE Trans. Biomed. Eng. 2004, 51, 1034–1043. [Google Scholar] [CrossRef]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006, 31, 968–980. [Google Scholar] [CrossRef]
- Van Diessen, E.; Numan, T.; van Dellen, E.; van der Kooi, A.W.; Boersma, M.; Hofman, D.; van Lutterveld, R.; van Dijk, B.W.; van Straaten, E.C.W.; Hillebrand, A.; et al. Opportunities and methodological challenges in EEG and MEG resting state functional brain network research. Clin. Neurophysiol. 2015, 126, 1468–1481. [Google Scholar] [CrossRef]
- Van Wijk, B.C.; Stam, C.J.; Daffertshofer, A. Comparing Brain Networks of Different Size and Connectivity Density Using Graph Theory. PLoS ONE 2010, 5, e13701. [Google Scholar] [CrossRef]
- Stam, C.J.; Tewarie, P.; Van Dellen, E.; van Straaten, E.C.W.; Hillebrand, A.; Van Mieghem, P. The trees and the forest: Characterization of complex brain networks with minimum spanning trees. Int. J. Psychophysiol. 2014, 92, 129–138. [Google Scholar] [CrossRef]
- De Vico Fallani, F.; Latora, V.; Chavez, M. A Topological Criterion for Filtering Information in Complex Brain Networks. PLoS Comput. Biol. 2017, 13, e1005305. [Google Scholar] [CrossRef] [PubMed]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Goj, R.; Jas, M.; Brooks, T.; Parkkonen, L.; et al. MEG and EEG data analysis with MNE-Python. Front. Neurosci. 2013, 7, 267. [Google Scholar] [CrossRef] [PubMed]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Mognon, A.; Jovicich, J.; Bruzzone, L.; Buiatti, M. ADJUST: An automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology 2011, 48, 229–240. [Google Scholar] [CrossRef]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011, 2011, 879716. [Google Scholar] [CrossRef]
- Gramfort, A.; Papadopoulo, T.; Olivi, E.; Clerc, M. OpenMEEG: Opensource software for quasistatic bioelectromagnetics. Biomed. Eng. Online 2010, 9, 45. [Google Scholar] [CrossRef]
- Hämäläinen, M.S.; Ilmoniemi, R.J. Interpreting magnetic fields of the brain: Minimum norm estimates. Med. Biol. Eng. Comput. 1994, 32, 35–42. [Google Scholar] [CrossRef]
- Fraschini, M.; Demuru, M.; Crobe, A.; Marrosu, F.; Stam, C.J.; Hillebrand, A. The effect of epoch length on estimated EEG functional connectivity and brain network organisation. J. Neural Eng. 2016, 13, 036015. [Google Scholar] [CrossRef]
- Nolte, G.; Bai, O.; Wheaton, L.; Mari, Z.; Vorbach, S.; Hallett, M. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin. Neurophysiol. 2004, 115, 2292–2307. [Google Scholar] [CrossRef]
- Lachaux, J.P.; Rodriguez, E.; Martinerie, J.; Varela, F.J. Measuring phase synchrony in brain signals. Hum. Brain Mapp. 1999, 8, 194–208. [Google Scholar] [CrossRef]
- Bruña, R.; Maestú, F.; Pereda, E. Phase locking value revisited: Teaching new tricks to an old dog. J. Neural Eng. 2018, 15, 056011. [Google Scholar] [CrossRef] [PubMed]
- Vinck, M.; van Wingerden, M.; Womelsdorf, T.; Fries, P.; Pennartz, C.M.A. The pairwise phase consistency: A bias-free measure of rhythmic neuronal synchronization. Neuroimage 2010, 51, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Stam, C.J.; Nolte, G.; Daffertshofer, A. Phase lag index: Assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum. Brain Mapp. 2007, 28, 1178–1193. [Google Scholar] [CrossRef] [PubMed]
- Vinck, M.; Oostenveld, R.; van Wingerden, M.; Battaglia, F.; Pennartz, C.M.A. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage 2011, 55, 1548–1565. [Google Scholar] [CrossRef]
- Fraschini, M.; Lai, M.; Didaci, L. Comparison of functional connectivity metrics using an unsupervised approach: A source resting-state EEG study. J. Integr. Neurosci. 2018, 17, 393–396. [Google Scholar] [CrossRef]
- Rousseeuw, P.J. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef]
- Barry, R.J.; Clarke, A.R.; Johnstone, S.J.; Magee, C.A.; Rushby, J.A. EEG differences between eyes-closed and eyes-open resting conditions. Clin. Neurophysiol. 2007, 118, 2765–2773. [Google Scholar] [CrossRef]
- Li, L. The Differences among Eyes-Closed, Eyes-Open and Attention States: An EEG Study. In Proceedings of the 2010 6th International Conference on Wireless Communications Networking and Mobile Computing (WiCOM), Chengdu, China, 23–25 September 2010; pp. 1–4. [Google Scholar] [CrossRef]
- Tan, B.; Kong, X.; Yang, P.; Jin, Z.; Li, L. The Difference of Brain Functional Connectivity between Eyes-Closed and Eyes-Open Using Graph Theoretical Analysis. Comput. Math. Methods Med. 2013, 2013. [Google Scholar] [CrossRef]
- Fraschini, M.; Pani, S.M.; Didaci, L.; Marcialis, G.L. Robustness of functional connectivity metrics for EEG-based personal identification over task-induced intra-class and inter-class variations. Pattern Recognit. Lett. 2019, 125, 49–54. [Google Scholar] [CrossRef]
- Rajapandian, M.; Amico, E.; Abbas, K.; Ventresca, M.; Goñi, J. Uncovering differential identifiability in network properties of human brain functional connectomes. Netw. Neurosci. 2020, 4, 698–713. [Google Scholar] [CrossRef] [PubMed]
- Hallquist, M.N.; Hillary, F.G. Graph theory approaches to functional network organization in brain disorders: A critique for a brave new small-world. Netw. Neurosci. 2018, 3, 1–26. [Google Scholar] [CrossRef] [PubMed]

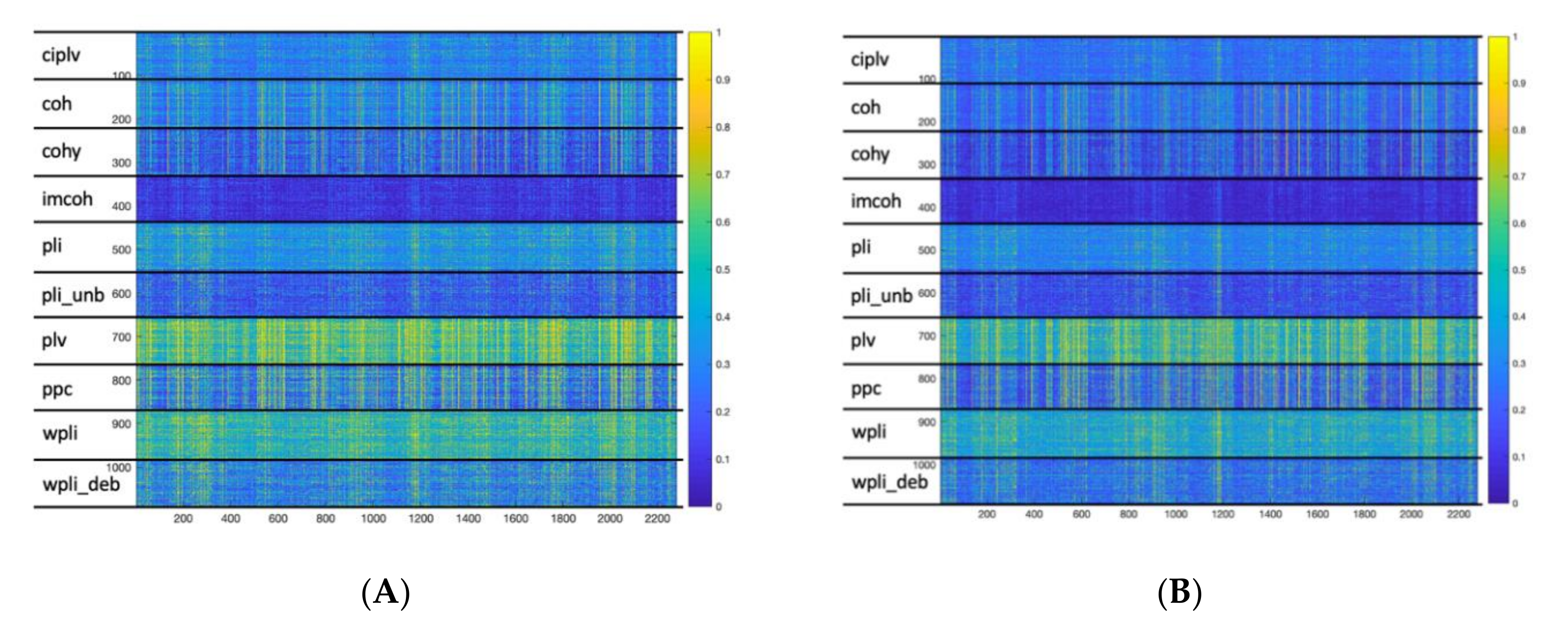
| k | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|
| silhouette (eyes-closed) | 0.413 | 0.314 | 0.343 | 0.305 | 0.271 | 0.232 | 0.221 | 0.191 | 0.177 |
| silhouette (eyes-open) | 0.455 | 0.290 | 0.349 | 0.318 | 0.300 | 0.261 | 0.210 | 0.186 | 0.164 |
| Cluster | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| purity (eyes-closed) | 0.361 | 0.885 | 0.364 | 0.939 | 0.391 | 0.590 | 0.657 | 0.869 | 0.733 | 0.982 |
| purity (eyes-open) | 0.905 | 0.980 | 0.427 | 1.000 | 0.780 | 0.517 | 0.593 | 0.459 | 0.915 | 0.983 |
| k. | Purity | Majority Cluster | Purity | Majority Cluster |
|---|---|---|---|---|
| 2 | 0.854 | [ciplv,coh,cohy,imcoh,pli2,ppc,wpli2] | 0.999 | [ciplv,coh,cohy,imcoh,pli,pli2,ppc,wpli2] |
| 0.756 | [pli,plv,wpli] | 0.709 | [plv,wpli] | |
| 3 | 0.938 | [ciplv,coh,cohy,pli,ppc,wpli2] | 0.956 | [ciplv,coh,cohy,pli,ppc,wpli2] |
| 0.788 | [imcoh,pli2] | 0.867 | [imcoh,pli2] | |
| 0.829 | [plv,wpli] | 0.945 | [plv,wpli] | |
| 4 | 0.841 | [ciplv,pli,wpli2] | 0.856 | [ciplv,pli,wpli2] |
| 0.823 | [coh,cohy,ppc] | 0.934 | [coh,cohy,ppc] | |
| 0.997 | [plv,wpli] | 1 | [plv,wpli] | |
| 0.900 | [imcoh,pli2] | 0.961 | [imcoh,pli2] | |
| 5 | 0.627 | [wpli] | 0.750 | [wpli] |
| 0.800 | [plv] | 0.932 | [plv] | |
| 0.837 | [ciplv,pli,wpli2] | 0.871 | [ciplv,pli,wpli2] | |
| 0.936 | [imcoh,pli2] | 0.994 | [imcoh,pli2] | |
| 0.997 | [coh,cohy,ppc] | 1 | [coh,cohy,ppc] | |
| 6 | 0.649 | [pli,wpli] | 0.755 | [wpli] |
| 0.716 | [plv] | 0.973 | [plv] | |
| 0.807 | [ciplv,pli2,wpli2] | 0.871 | [ciplv,pli,wpli2] | |
| 0.690 | [imcoh] | 0.994 | [imcoh,pli2] | |
| 0.549 | [cohy] | 0.562 | [ppc] | |
| 0.890 | [coh,ppc] | 0.814 | [coh,cohy] | |
| 7 | 0.643 | [pli,wpli] | 0.748 | [wpli] |
| 0.691 | [plv] | 0.973 | [plv] | |
| 0.806 | [ciplv,pli2,wpli2] | 0.871 | [ciplv,pli,wpli2] | |
| 0.690 | [imcoh] | 0.994 | [imcoh,pli2] | |
| 0.766 | [coh,ppc] | 0.897 | ||
| 0.744 | 0.876 | [coh,ppc] | ||
| 0.626 | [cohy] | 0.611 | [cohy] | |
| 8 | 0.658 | [pli,wpli2] | 0.815 | [wpli] |
| 0.967 | [plv] | 0.982 | [plv] | |
| 0.724 | [ciplv,pli2] | 0.955 | [ciplv,pli,wpli2] | |
| 0.900 | [imcoh] | 0.991 | [imcoh] | |
| 0.759 | [coh,ppc] | 0.897 | ||
| 0.764 | 0.876 | [coh,ppc] | ||
| 0.652 | [cohy] | 0.611 | [cohy] | |
| 0.864 | [wpli] | 0.593 | [pli2] | |
| 9 | 0.475 | [pli] | 0.905 | [wpli] |
| 0.967 | [plv] | 0.982 | [plv] | |
| 0.661 | [ciplv,wpli2] | 0.467 | [pli] | |
| 0.939 | [imcoh] | 1 | [imcoh] | |
| 0.759 | [coh,ppc] | 0.897 | ||
| 0.764 | 0.876 | [coh,ppc] | ||
| 0.652 | [cohy] | 0.611 | [cohy] | |
| 0.869 | [wpli] | 0.740 | [ciplv,wpli2] | |
| 0.733 | [pli2] | 0.915 | [pli2] | |
| 10 | 0.475 | [pli] | 0.905 | [wpli] |
| 0.885 | 0.980 | |||
| 0.661 | [ciplv,wpli2] | 0.427 | [pli] | |
| 0.934 | [imcoh] | 1 | [imcoh] | |
| 0.391 | [coh] | 0.780 | [ppc] | |
| 0.560 | [ppc] | 0.517 | [coh] | |
| 0.657 | [cohy] | 0.593 | [cohy] | |
| 0.869 | [wpli] | 0.740 | [ciplv,wpli2] | |
| 0.733 | [pli2] | 0.915 | [pli2] | |
| 0.982 | [plv] | 0.983 | [plv] |
| FWEI | Global Efficiency | CC | Assortativity | Modularity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-Values | ES | D | p-Values | ES | D | p-Values | ES | D | p-Values | ES | D | |
| ciplv | 1.94 × 10−17 | 0.81 | EC < EO | 5.38 × 10−18 | 0.83 | EC > EO | ns | 2.55 × 10−4 | 0.35 | EC < EO | ||
| coh | 1.11 × 10−18 | 0.85 | EC < EO | 6.82 × 10−19 | 0.85 | EC > EO | ns | 7.17 × 10−16 | 0.77 | EC < EO | ||
| cohy | 2.39 × 10−17 | 0.81 | EC < EO | 8.42 × 10−18 | 0.82 | EC > EO | ns | 1.58 × 10−12 | 0.68 | EC < EO | ||
| imcoh | 2.46 × 10−17 | 0.81 | EC < EO | 2.46 × 10−17 | 0.81 | EC > EO | ns | 9.26 × 10−8 | 0.51 | EC < EO | ||
| pli | 3.10 × 10−17 | 0.81 | EC < EO | 8.42 × 10−18 | 0.82 | EC > EO | ns | 9.20 × 10−3 | 0.25 | EC < EO | ||
| pli_unbiased | 1.69 × 10−12 | 0.68 | EC < EO | 7.85 × 10−13 | 0.69 | EC > EO | ns | 2.25 × 10−7 | 0.50 | EC < EO | ||
| plv | 2.73 × 10−17 | 0.81 | EC < EO | 7.38 × 10−18 | 0.82 | EC > EO | ns | 6.26 × 10−15 | 0.75 | EC < EO | ||
| ppc | 4.47 × 10−16 | 0.78 | EC < EO | 6.14 × 10−18 | 0.83 | EC > EO | ns | 3.23 × 10−16 | 0.78 | EC < EO | ||
| wpli | 2.80 × 10−18 | 0.84 | EC < EO | 1.95 × 10−18 | 0.84 | EC > EO | ns | 6.63 × 10−7 | 0.48 | EC < EO | ||
| wpli_debiased | 1.63 × 10−16 | 0.79 | EC < EO | 2.76 × 10−15 | 0.76 | EC > EO | ns | 5.15 × 10−10 | 0.60 | EC < EO | ||
| WEI10 | Global Efficiency | CC | Assortativity | Modularity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-Values | ES | D | p-Values | ES | D | p-Values | ES | D | p-Values | ES | D | |
| ciplv | ns | 1.91 × 10−7 | 0.50 | EC > EO | 6.30 × 10−5 | 0.38 | EC < EO | 5.19 × 10−3 | 0.27 | EC < EO | ||
| coh | ns | 4.51 × 10−11 | 0.63 | EC > EO | ns | ns | ||||||
| cohy | 1.28 × 10−5 | 0.42 | EC < EO | 2.06 × 10−12 | 0.67 | EC > EO | ns | 7.49 × 10−3 | 0.26 | EC < EO | ||
| imcoh | 1.14 × 10−2 | 0.24 | EC < EO | 8.05 × 10−6 | 0.43 | EC > EO | ns | 7.03 × 10−3 | 0.26 | EC < EO | ||
| pli | ns | 1.80 × 10−6 | 0.46 | EC > EO | 1.03 × 10−4 | 0.37 | EC < EO | 2.41 × 10−3 | 0.29 | EC < EO | ||
| pli_unbiased | ns | 7.79 × 10−9 | 0.55 | EC > EO | 1.14 × 10−5 | 0.42 | EC < EO | 3.05 × 10−4 | 0.35 | EC < EO | ||
| plv | ns | 5.69 × 10−5 | 0.39 | EC > EO | ns | ns | ||||||
| ppc | ns | 1.25 × 10−6 | 0.46 | EC > EO | ns | ns | ||||||
| wpli | ns | 1.24 × 10−3 | 0.31 | EC > EO | 1.07 × 10−2 | 0.24 | EC < EO | 1.61 × 10−3 | 0.30 | EC < EO | ||
| wpli_debiased | ns | 2.21 × 10−7 | 0.50 | EC > EO | 4.18 × 10−3 | 0.27 | EC < EO | 8.40 × 10−4 | 0.32 | EC < EO | ||
| WEI15 | Global Efficiency | CC | Assortativity | Modularity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-Values | ES | D | p-Values | ES | D | p-Values | ES | D | p-Values | ES | D | |
| ciplv | 8.20 × 10−3 | 0.25 | EC < EO | 2.10 × 10−8 | 0.54 | EC > EO | 1.45 × 10−5 | 0.42 | EC < EO | 3.71 × 10−4 | 0.34 | EC < EO |
| coh | 3.16 × 10−4 | 0.35 | EC < EO | 2.39 × 10−12 | 0.67 | EC > EO | ns | ns | ||||
| cohy | 3.24 × 10−10 | 0.60 | EC < EO | 5.64 × 10−13 | 0.69 | EC > EO | ns | 1.26 × 10−5 | 0.42 | EC < EO | ||
| imcoh | 3.48 × 10−4 | 0.34 | EC < EO | 5.10 × 10−11 | 0.63 | EC > EO | ns | ns | ||||
| pli | 4.86 × 10−3 | 0.27 | EC < EO | 4.96 × 10−9 | 0.56 | EC > EO | 4.01 × 10−5 | 0.39 | EC < EO | 5.83 × 10−5 | 0.38 | EC < EO |
| pli_unbiased | 9.32 × 10−5 | 0.37 | EC < EO | 7.91 × 10−12 | 0.66 | EC > EO | 2.72 × 10−6 | 0.45 | EC < EO | 3.08 × 10−5 | 0.40 | EC < EO |
| plv | ns | 9.19 × 10−7 | 0.47 | EC > EO | ns | ns | ||||||
| ppc | ns | 1.59 × 10−8 | 0.54 | EC > EO | ns | ns | ||||||
| wpli | ns | 5.81 × 10−6 | 0.43 | EC > EO | ns | 9.26 × 10−4 | 0.32 | EC < EO | ||||
| wpli_debiased | 8.88 × 10−3 | 0.25 | EC < EO | 2.49 × 10−11 | 0.64 | EC > EO | 9.96 × 10−3 | 0.25 | EC < EO | 1.17 × 10−4 | 0.37 | EC < EO |
| WEI50 | Global Efficiency | CC | Assortativity | Modularity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-Values | ES | D | p-Values | ES | D | p-Values | ES | D | p-Values | ES | D | |
| ciplv | 3.10 × 10−17 | 0.80 | EC < EO | <0.0001 | 0.78 | EC > EO | ns | 1.49 × 10−6 | 0.46 | EC < EO | ||
| coh | 1.30 × 10−18 | 0.84 | EC < EO | <0.0001 | 0.79 | EC > EO | 1.84 × 10−5 | 0.41 | EC < EO | 4.25 × 10−10 | 0.59 | EC < EO |
| cohy | 2.46 × 10−17 | 0.81 | EC < EO | <0.0001 | 0.75 | EC > EO | 7.39 × 10−6 | 0.42 | EC < EO | 2.89 × 10−6 | 0.44 | EC < EO |
| imcoh | 2.46 × 10−17 | 0.81 | EC < EO | <0.0001 | 0.77 | EC > EO | 0.0008 | 0.31 | EC < EO | 2.97 × 10−6 | 0.44 | EC < EO |
| pli | 5.07 × 10−17 | 0.80 | EC < EO | <0.0001 | 0.78 | EC > EO | 0.0014 | 0.30 | EC < EO | 0.0001 | 0.36 | EC < EO |
| pli_unbiased | 1.88 × 10−12 | 0.67 | EC < EO | <0.0001 | 0.70 | EC > EO | 0.0004 | 0.33 | EC < EO | 3.15 × 10−7 | 0.48 | EC < EO |
| plv | 2.33 × 10−16 | 0.78 | EC < EO | <0.0001 | 0.68 | EC > EO | 0.0002 | 0.34 | EC < EO | ns | ||
| ppc | 5.19 × 10−16 | 0.77 | EC < EO | <0.0001 | 0.72 | EC > EO | 3.60 × 10−5 | 0.39 | EC < EO | 2.67 × 10−8 | 0.53 | EC < EO |
| wpli | 5.82 × 10−18 | 0.82 | EC < EO | <0.0001 | 0.78 | EC > EO | 0.0081 | 0.25 | EC < EO | 9.26 × 10−6 | 0.42 | EC < EO |
| wpli_debiased | 1.68 × 10−16 | 0.78 | EC < EO | <0.0001 | 0.75 | EC > EO | 0.0070 | 0.25 | EC < EO | 2.91 × 10−7 | 0.49 | EC < EO |
| ECO | Global Efficiency | CC | Assortativity | Modularity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-Values | ES | D | p-Values | ES | D | p-Values | ES | D | p-Values | ES | D | |
| ciplv | ns | ns | 1.45 × 10−4 | 0.36 | EC < EO | ns | ||||||
| coh | ns | 6.72 × 10−3 | 0.26 | EC > EO | ns | ns | ||||||
| cohy | 5.91 × 10−4 | 0.33 | EC < EO | ns | ns | ns | ||||||
| Imcoh | ns | ns | 3.87 × 10−3 | 0.28 | ns | |||||||
| pli | ns | ns | 1.32 × 10−4 | 0.37 | EC < EO | ns | ||||||
| pli_unbiased | ns | ns | 5.28 × 10−4 | 0.33 | EC < EO | ns | ||||||
| Plv | ns | 1.56 × 10−3 | 0.30 | EC > EO | ns | ns | ||||||
| Ppc | ns | 5.44 × 10−3 | 0.27 | EC > EO | 6.91 × 10−3 | 0.26 | ns | |||||
| Wpli | ns | ns | 9.61 × 10−3 | 0.25 | ns | |||||||
| wpli_debiased | ns | ns | ns | ns | ||||||||
| MST | Leaf Fraction | Diameter | Eccentricity | Hierarchy | Kappa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-Values | ES | D | p-Values | ES | D | p-Values | ES | D | p-Values | ES | D | p-Values | ES | D | |
| ciplv | 6.30 × 10−4 | 0.33 | EC > EO | ns | ns | ns | 5.91 × 10−5 | 0.38 | EC > EO | ||||||
| coh | ns | ns | ns | ns | ns | ||||||||||
| cohy | ns | ns | ns | ns | ns | ||||||||||
| imcoh | 4.00 × 10−6 | 0.44 | EC > EO | ns | ns | ns | 3.41 × 10−8 | 0.53 | EC > EO | ||||||
| pli | 7.25 × 10−4 | 0.32 | EC > EO | ns | ns | ns | 4.31 × 10−7 | 0.48 | EC > EO | ||||||
| pli_unbiased | 6.02 × 10−4 | 0.33 | EC > EO | ns | ns | ns | 6.79 × 10−7 | 0.48 | EC > EO | ||||||
| plv | ns | ns | ns | ns | ns | ||||||||||
| ppc | ns | ns | ns | ns | ns | ||||||||||
| wpli | 2.94 × 10−5 | 0.40 | EC > EO | ns | ns | ns | 4.88 × 10−6 | 0.44 | EC > EO | ||||||
| wpli_debiased | 1.92 × 10−3 | 0.30 | EC > EO | ns | ns | ns | 1.99 × 10−5 | 0.41 | EC > EO | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraschini, M.; La Cava, S.M.; Didaci, L.; Barberini, L. On the Variability of Functional Connectivity and Network Measures in Source-Reconstructed EEG Time-Series. Entropy 2021, 23, 5. https://doi.org/10.3390/e23010005
Fraschini M, La Cava SM, Didaci L, Barberini L. On the Variability of Functional Connectivity and Network Measures in Source-Reconstructed EEG Time-Series. Entropy. 2021; 23(1):5. https://doi.org/10.3390/e23010005
Chicago/Turabian StyleFraschini, Matteo, Simone Maurizio La Cava, Luca Didaci, and Luigi Barberini. 2021. "On the Variability of Functional Connectivity and Network Measures in Source-Reconstructed EEG Time-Series" Entropy 23, no. 1: 5. https://doi.org/10.3390/e23010005
APA StyleFraschini, M., La Cava, S. M., Didaci, L., & Barberini, L. (2021). On the Variability of Functional Connectivity and Network Measures in Source-Reconstructed EEG Time-Series. Entropy, 23(1), 5. https://doi.org/10.3390/e23010005






