1. Introduction
The refrigeration machine continues to play a vital role in the field of engineering applications, proven by numerous papers published in international journals, some of them specifically dedicated to this topic.
By examining recently published scientific works regarding reverse cycle machines, one remarks that more papers are devoted to refrigerating machines than to heat pumps [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10], which also corresponds to current applications that are more and more developed; that is, refrigeration freezing and deep-freezing in the food industry, air conditioning, heat pumps, or medical applications.
To meet the consumer requirements regarding the performance and energy expense, the optimization of reverse cycle machines appears as an important task. The optimization procedure could follow two trends depending on the machine operation mode. Thus, the first one is relative to the nominal operation conditions and provides optimal dimensioning of the machine subsystems (design optimization). The second one is associated with control-command of the reverse cycle systems owing to the variability of ambient and operation conditions (dynamic optimization).
Many studies in the literature are concerned with specific improvement of reverse cycle machines, most recently dealing with topical applications, namely, thermoelectric cooler [
11], vortex tube design models [
12], electronic cooling with nanofluids [
13], ground source heat pumps applications [
14], electrocaloric refrigeration [
15], or magnetic refrigeration [
16].
Our approach focuses on a global optimization from a thermodynamic point of view, even if the thermo-economic optimizations [
17,
18,
19,
20,
21,
22,
23,
24] are also important and indispensable from an engineering point of view.
Note that, in terms of the thermodynamic approach, finite time thermodynamics (FTT) optimization and entropy generation minimization [
4,
18] reported important results in the recent past. Nevertheless, these approaches remained limited to endo-reversible thermodynamics tools [
4,
25,
26].
Some insight aiming to get closer to real operation of reverse cycle machines has been developed using linear irreversible thermodynamics [
27].
The purpose of this paper is to complete the previous reviews [
1,
2]. Thus, an extension of a new modified modelling of Carnot irreversible refrigerator recently developed [
4] is proposed, aiming to provide optimal physical dimensions of the refrigerator, mainly optimal allocation of heat transfer characteristics between source (sink) and machine (internal fluid cycled). The model developed in [
4] has considered the coupling between thermostats and machine by an energy balance between source (sink) and cycled medium, but limited only to the endo-reversible case. This methodology has introduced a supplementary physical quantity, namely the heat transfer entropy Δ
S with reference to source for the refrigerator. In the endo-reversible case [
4], the answer is analytical. Furthermore, these results were extended to a refrigerator with internal irreversibility characterized by production of entropy Δ
SI, which was supposed as a
constant parameter [
5]. The proposed modelling in [
5] explored the performance of Chambadal [
28] and Curzon-Ahlborn [
29] cycles transposed from engine in refrigerators by gradually emphasizing the influence of irreversibilities through internal or total entropy productions, and through
irreversibility degrees introduced by Novikov [
30] and
irreversibility ratios introduced by Ibrahim et al. [
31]. The results have shown that Chambadal’s model optimum coincides with the equilibrium thermodynamics one, namely, the non-existence of minimum energy consumption, while the optimization of the Curzon-Ahlborn model relates the external temperatures to the finite dimensions of the machine (
GH,
GC) that have to be optimally allocated. Analytical expressions of the optimal distribution of the finite heat transfer conductances
GH and
GC leading to the minimum energy expense were derived for Δ
S and Δ
SI constant parameters and the total finite dimensions
GT fixed.
The present paper presents the thermodynamics optimization of the Carnot endo-irreversible refrigerator, providing new results regarding the optimal allocation of heat transfer conductances and minimum energy consumption and associated coefficient of performance (
COP) when various forms of entropy production owing to internal irreversibility are considered. It uses the same methodology, but further developed for the case when the minimization of
mean power consumption is sought (
Section 2.2). The new concept of
production entropy action is introduced, being analogous to
energy action used in classical mechanical.
A step further consists of a quasi-Chambadal refrigerator modeling analysis. In this case, the source is considered finite, determining the heat transfer across a temperature gap, while the sink (atmosphere) remains in perfect thermal contact with the cycled fluid. The modeling of the heat transfer at the cold source in the quasi-Chambadal refrigerator is developed for two different cases, once considering the heat transfer coupling constraint, and then without coupling. The effect of various forms of functional dependence of the entropy production on the results reveals new expressions of COP and mean work per cycle.
The Curzon-Ahlborn refrigerator model extends the study achievements. It is more complete and allows modeling without heat transfer coupling at source and sink (classical appraisal), or with coupling. Regarding the energy optimization results, the last case leads to a new optimal allocation of heat transfer conductance, with corresponding
new upper bounds for the
COP and minimum work expense in endo-reversible cycle,
min Wendo-rev (
Section 3.2).
Numerical results are illustrated when the production of entropy depends on temperatures and reference heat transfer entropy. An asymptotic solution is proposed in the low irreversibility case.
2. Thermodynamic Cycles of Two Thermal Reservoirs Machines
2.1. The Standard Reverse Cycles
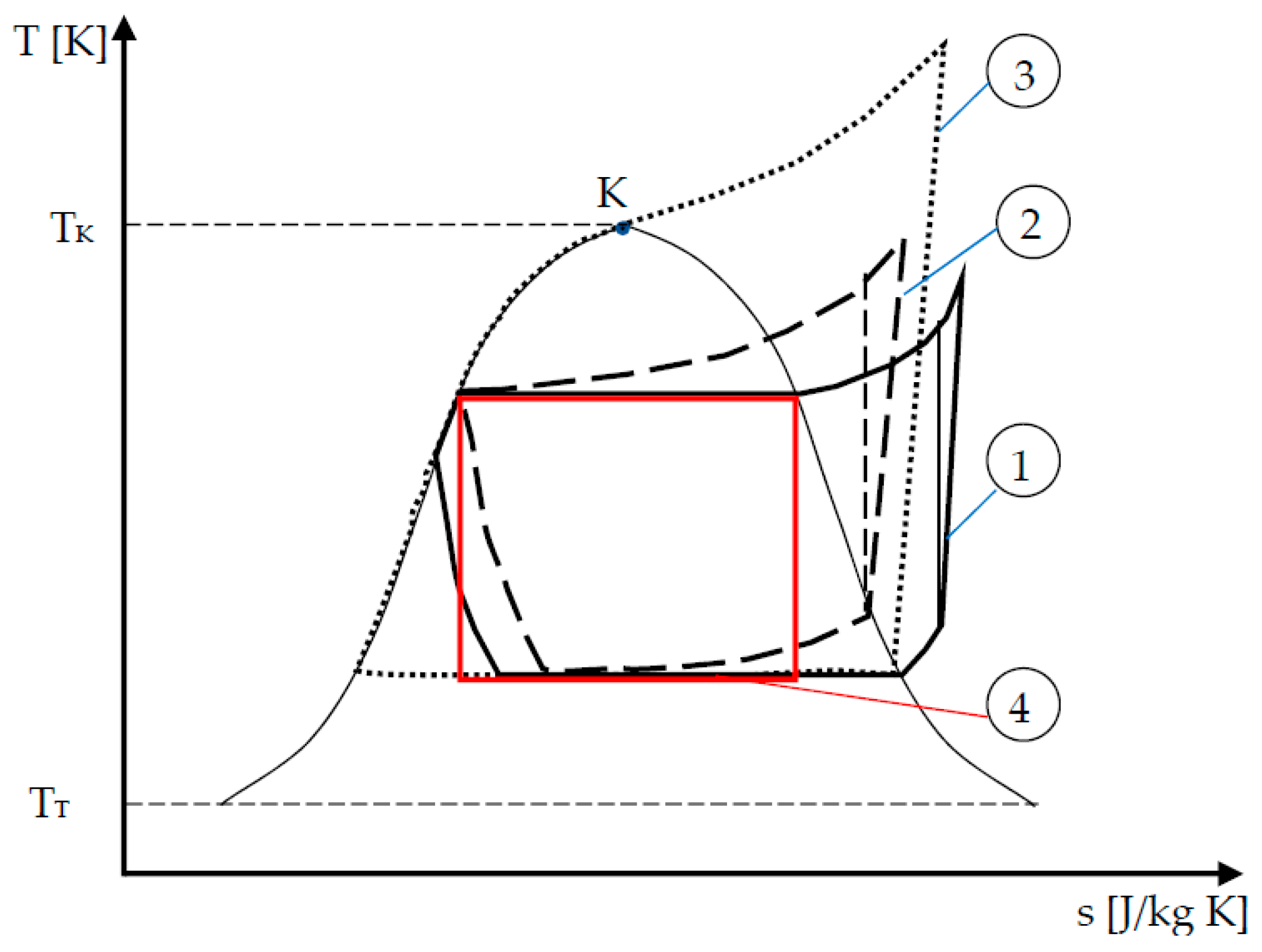
The topic developed here is only concerned with the reverse cycle machines coupled with one heat source and a cold sink. Generally, these machines are vapor compression machines according to cycle 1 illustrated in
Figure 1. Owing to technological constraint, the cycle is composed of a compression of superheated vapor that is followed by desuperheating of vapor. Then, a condensation of the cycled fluid occurs as well as, generally, a subcooling of the saturated liquid before entering the expansion valve, where an isenthalpic transformation occurs. The last transformation closing the cycle consists of fluid vaporization and superheating of vapor. Note that this kind of cycle is efficient only in a specific interval characteristic of a pure fluid, referring here to the temperature variation range [
TT,
TK], with
TT (
TK) triple point (critical point) temperature.
If the fluid is a mixture, the corresponding cycle is subject to a glide of temperature in the liquid vapor domain, thus the corresponding cycle is near to a Lorenz cycle (cycle 2 in
Figure 1).
If a more important variation of temperature is needed, supercritical (or transcritical) cycles are possible (cycle 3). In this configuration, the variation of temperature at the hot side could be huge.
In any case, the problem is to develop the more efficient reverse cycle machines satisfying specific constraints or not. In the paper, only the limiting case of Carnot configuration (cycle 4) aiming at determining performance upper bound of the two reservoirs machines will be considered.
Classically, the equilibrium thermodynamics is concerned with determining the upper bound efficiency of a cycle. For a reverse cycle machine, this efficiency is characterised by the coefficient of performance (COP) relative to the refrigerator COPREF or to the heat pump COPHP.
If the cold source is a thermostat at temperature
TCS, and the hot sink is a thermostat at temperature
THS, one gets the well-known result depending only on these two temperatures:
with Δ
TS =
THS −
TCS.
These formulas determine the upper bound of COPs under reversible operation conditions of the system consisting of source–machine–sink. Nevertheless, these quasi static conditions are associated with infinite time duration of the processes, thus the corresponding heat transfer rate at the cold or hot side of the machine is null. This is the reason for which the modelling hereafter will consider the endo-irreversible cycle condition focused on refrigeration to illustrate the methodology and the obtained results. The same modelling could be adapted for heat pump configuration. It is under development by our research group and is in due course of publication.
2.2. Endo-Irreversible Carnot Refrigerator
The endo-irreversible Carnot machine configuration is represented in
Figure 2 and can also be named the exo-reversible Carnot refrigerator [
5], as there is no heat transfer at finite temperature gap at the cold and hot side of the machine.
The irreversible cycle is identified from state 1 (beginning of the low temperature isotherm), 2 (exit of the low temperature isotherm and beginning of the adiabatic compression), 3 (end of the irreversible adiabatic compression and beginning of the high temperature isotherm), and 4 (exit of the high temperature isotherm and beginning of the irreversible adiabatic expansion, up to state 1).
Figure 2 also shows the internal irreversibility of the cycle by the corresponding production of entropy on the two isothermal transformations Δ
SIC, Δ
SIH and on the adiabatic compression and expansion, Δ
SICo, Δ
SIEx, respectively. The sum of these four terms is the cycle production of entropy, Δ
SI.
In this configuration, one supposes that thermal loss between hot and cold side does not exist. This is a favorable case allowing to estimate the upper bound for the COP corresponding to the minimum of work expense.
The Carnot endo-irreversible model is presented for a cycle with the constraint relative to temperatures that imposes (T0, ambient temperature).
The energy balance over a cycle is expressed as follows:
where
W is the work input in the cycle and
are the heat delivered at the hot sink (heat received at the cold source) by the working fluid. They are expressed as follows:
The entropy balance is expressed as follows:
where
, heat transfer entropy released at the hot side during the cycle;
, heat transfer entropy received at the cold side by the cycle medium;
, the total entropy production during the cycle. It is the sum of four entropy productions corresponding to each transformation of the cycle (see
Figure 2), successively indicated by
such that
By combining Equations (3)–(6) and using as reference the heat transfer entropy chosen at the useful cold side (
) that will be simply denoted by
, one gets the following:
This relation corresponds to the Gouy–Stodola theorem applied to the refrigerator, stating that the minimum of energy expense corresponds to the minimum of entropy production. In fact, this condition is achieved for the reversible or quasi static operation.
Equations (1), (5), and (8) provide the expression of the corresponding
COP as follows:
It results from Equation (9) that the real COP is a decreasing function of two ratios:
Considering the power expense of the refrigerator, one can express its mean value over the cycles as follows:
where
, time duration of the cycle.
The quasi static case is well approximated by an entropy production inversely proportional to
:
Note that Equation (11) provides the important definition of CI, which represents a production entropy action with the unit (J·s/K). It is analogous to energy action used in classical mechanical.
By combining Equations (10) and (11), the mean power appears as a decreasing function of . Contrarily to the engine performance, an optimum of power related to the finite Wrev imposed does not exist.
Nevertheless, when the mean power
is provided for the refrigerating machine, the energy expense becomes the following:
In this case, by considering the second-order equation of (12), a minimum of mechanical energy expense is obtained for
τ*:
and it depends on the square root of the production entropy action:
A paradox appears here, because when , and .
2.3. Quasi-Chambadal Refrigerator
The corresponding cycle focuses on the
useful heat transfer at the cold source and supposes perfect heat transfer at the hot sink, which is the environment at temperature
T0 (
Figure 3).
Thus, the major advance in the modelling compared with the previous case (
Section 2.2) is given by the heat transfer
QC, which is performed at
a temperature gap (
TCS−TC) in Chambadal refrigerator cycle. This leads to two potential approaches, so-called without coupling and with coupling at the cold source. The difference between them is provided by the way the heat transfer
QC is expressed, once using the heat transfer entropy, and then by involving the heat transfer conductance relative to the heat exchanger at the refrigerator cold side.
2.3.1. Modelling without Coupling at the Cold Source
The modelling is analogous to the previous one (
Section 2.2).
The same Δ
SC remains as reference heat transfer entropy, but the cold side temperature at which heat is transferred to the cycled medium is
TC instead of
TCS. Thus, the heat transfer to the cold side is expressed as follows:
The mechanical energy needed becomes the following:
Note that, at this time,
W depends on temperature
TC and not
TCS. As
TC <
TCS, it turns out that the necessary heat transfer involves a higher mechanical expense. In Equation (16),
W appears as a decreasing function of
TC, if
is a considered parameter. The results corresponding to this assumption were reported in [
5]. However,
could also be a function of other properties (temperature, size).
From current knowledge, the production of entropy owing to internal irreversibility of the converter does not have a general analytical expression, as the heat transfer entropy has. Some attempts have been reported [
32,
33] in the finite speed thermodynamics approach of irreversible Stirling machines and Carnot-like refrigerating machines. Nevertheless, the derived analytical expression of the entropy production remains very much dependent on the study assumptions.
The purpose of the present modelling is to emphasize analytically the effect of internal irreversibility on refrigerator performance. Thus, the simplest forms of the functional dependence of were chosen to show the influence of intensity (ΔT) or extensity (ΔS) or the combination of the two. They are considered to hold mainly in the vicinity of the optimum found.
The most common forms for production of entropy used in this analysis are as follows:
- (a)
proportionality to the reference useful heat transfer entropy (
extensity):
with
dI irreversibility degree. Thus,
is a function of the reference extensity.
- (b)
proportionality to the gradient of available
intensity for the converter:
- (c)
the third functional dependence is a combination of the two preceding ones (proportionality to the reference useful reversible energy):
It is easy to show that, for these three general linear forms of the function regarding the entropy production of a refrigerator, this production is decreasing when increases or with the increase in as well as in , and obviously the increase in the three corresponding irreversibility parameters introduced by Equations (17)–(19). Thus, it is obvious that there is no minW in this case.
The general formulation of
COP results from (15) and (16) as follows:
which, compared with
COP for the reversible cycle,
emphasizes a correcting factor depending on temperatures, heat transfer entropy, and production of entropy:
which clearly shows the effect of irreversibility on the Chambadal refrigerator
COP:
Obviously, the increase of TCS and will decrease the COP, while it will increase with TC and .
2.3.2. Modelling with Coupling at the Cold Source (Coupled Chambadal Refrigerator)
The Chambadal’s model introduces a coupling, but only at the cold side of the system. For a linear heat transfer law, we define a new general physical quantity
GC (J/K), a heat transfer conductance relative to the cold heat exchanger introduced by the following:
The consequence of this new constraint is that
changes from an independent variable to a determined one according to the following:
Using the same approach as in
Section 2.3.1, one can show that the consumed mechanical energy W is increasing with
as well as with
, but is decreasing with
, whichever form of the entropy production function is considered.
The coupling at the cold source will modify the
COP (Equation (23)) as well by the new correcting factor F expressed as follows:
In the endo-reversible case (
, one gets the following:
Equation (27) represents a new upper bound of COP of the endo-reversible cycle that results from the coupling at the cold side of Chambadal refrigerator in the modelling based on heat transfer entropy and production of entropy. It clearly shows that the ratio makes the difference from the well-known expression of COP for reversible cycle (Equation (21)) by decreasing the COP when it rises.
3. The Curzon-Ahlborn Refrigerator
This is the more comprehensive modelling that is examined here as it completes the Chambadal approach by considering the heat transfer across a temperature gap to the hot sink as well. Thus, the cycle illustrated in
Figure 3 is modified in
Figure 4 by considering the real heat transfer conditions, namely, different temperature of the source and cycle medium at the hot side, respectively.
3.1. Modelling without Coupling Constraints
The methodology is the same as the one applied in
Section 2.3.1, but, owing to the formal symmetry between hot and cold side of the system (see
Figure 4), it appears this time that the results depend on
TH value. The entropy production laws (18) and (19) become the following:
The energy expense is increasing with irreversibilities parameters , and with ΔS and TH, but it is decreasing with TC.
3.2. Modelling with Coupling Constraints
The same coupling constraint (24) used in the Chambadal model (
Section 2.3.2) is conserved here as well, to which the constraint owing to heat transfer between the hot side of the cycle and heat sink is added:
Combining the entropy balance given by Equation (4) with Equation (30) allows to conserve the same heat transfer reference entropy, thus one gets the following:
Note that, if is no longer a constant parameter, as it is expressed by the functions (28) and (29), appears in Equation (31) as a function of , , and , as well as of , according to Equation (25). This corresponds to a strong coupling and will be developed in the following section.
Nevertheless, we want to detail and comment on the case where
is considered a constant parameter, as well as the importance of the finite physical dimension constraint, owing to the heat transfer limitations of the system:
where
represent the total heat transfer conductance to be optimally allocated between the hot and cold side.
This completes the preceding works done on refrigerators [
5], as well as on engines [
34].
Variational calculus furnishes the following analytical results:
The corresponding
is as follows:
This function that corresponds to the optimal distribution of GH and GC is increasing with ΔS and , and decreasing with . However, one notes that the increase of GT raises the cost of the system, leading to an economic compromise between capital expenditure (designing) and operational cost (energy consumption over the system life).
To complete the results, the combination of Equations (24), (30), (33), and (34) gives the
COP corresponding to
conditions as follows:
where the correction factor expression is much more complex than those derived for Chambadal refrigerator (Equations (22) and (26)) by involving intensities and extensities as well:
If
(case a) of
Section 2.3.1, the result is immediate by substitution in Equation (36). For the two other cases (b and c), the results will be numerical and considered hereafter in
Section 3.3.
An interesting result coming out from Equation (36) is the
COP at
endo-reversible condition, namely, when
:
It is important to note that the correction factor F’ does not vanish in the endo-reversible case when the coupling is considered.
It appears from Equation (38) that, for a given (existence condition of the cycle), the COP at minimum energy expense is strongly influenced by the value of in comparison with GT’s influence. The equilibrium thermodynamics limit is recovered only when /GT is small (or tends to the zero limit).
Equation (38) constitutes a new upper bound for refrigerator COP at minimum energy expense.
A second important consequence concerns the total entropy production of the system
, whose value corresponds to the following:
This function increases with
dI and
, for given
GH and
GC values, but it also presents an extremum when
The comparison of optimal expressions given by Equations (33), (34), (40), and (41) clearly shows that the optimal allocation of the heat transfer conductances differs when the energy expense minimization or minimum entropy production of the system is considered. Thus, in the first case, the dependence on the temperature appears in Equations (33) and (34), while it is missing in the second case from Equations (40) and (41). Consequently, the theorem of entropy production minimization does not hold for refrigerator (or, more precisely, for reverse cycle machine).
The optimum of entropy production is expressed as follows:
The corresponding value for endo-reversible case (
dI = 0) is straight forward:
The value is quadratically increasing with ΔS and decreasing with GT. Note that the equilibrium thermodynamic limit (reversibility) is retrieved when ΔS/GT tends to zero.
3.3. Modelling with Dependence on Temperature of the Production of Entropy
This dependence is defined by Equations (28) and (29).
3.3.1. Production of Entropy Depending only on Temperatures
In this case introduced by Equation (28),
TC remains a simple expression given by Equation (25). For
TH, one gets the following:
with
.
TH appears depending on TC, thus the optimization must be numerical.
Anyway, for low irreversible systems (
), it is possible to derive an acceptable simple approximate solution expressed as follows:
Considering the associated W value to this approximate solution confirms that the energy expenditure is increasing with ΔS and sIL, and decreasing with TC. The optimal distribution of GH and GC remains to be numerically sought (it is in due course).
3.3.2. Production of Entropy Depending both on Intensity and Extensity
When corresponds to Equation (29), the expression of TC remains that given by Equation (25).
It is easy to transpose Equation (44) to obtain the new expression of
TH associated to the new law of entropy production given in Equation (19):
with
.
TH is always correlated to
TC, but for low irreversible systems
, it exists again as a reasonable approximation:
This approximated result corresponds to an energy expense always increasing with and decreasing with TC.
The optimal energy conductance allocation remains a numerical one.
Some results of this numerical optimization are shown in
Figure 5 for the case corresponding to entropy production dependence on both intensity and extensity.
Figure 5 illustrates the variation of the non-dimensional mechanical work expense with the optimum non-dimensional heat transfer conductance at the hot side, when the non-dimensional heat transfer entropy varies. The non-dimensional form of the involved property corresponds to the following:
The curves on the figure clearly show the existence of a minimum energy expense for each value of the optimum non-dimensional heat transfer entropy Δs, and this minimum slightly slides to higher values of (from 0.75 to 0.8) as Δs increases (from 0.19 to 0.22). Moreover, as Δs increases, the variation range of left side values of decreases (from 0.56–0.95 to 0.61–0.95), together with a sharp increase in energy expense . Thus, it is obvious that an operation regime with small values of should be avoided even for small values of Δs.
Figure 5 also shows the strong influence of the reference heat transfer entropy on the minimum energy expense because, for
values near this minimum
, its non-dimensional value increases 2.5 times (from 0.1 to 0.25) for a Δ
s increase of 0.158 (from 0.19 to 0.22).
A detailed study is in due course near to the authors.