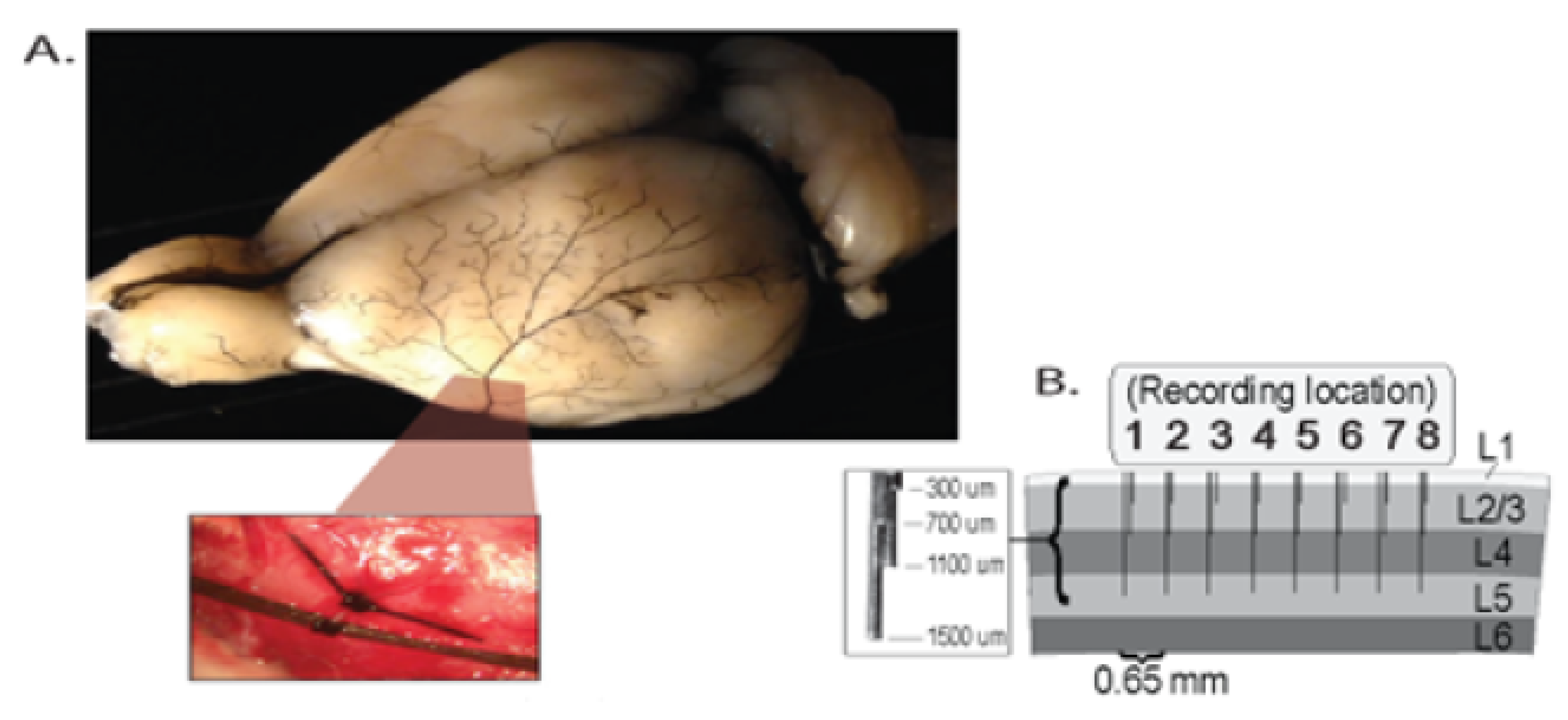
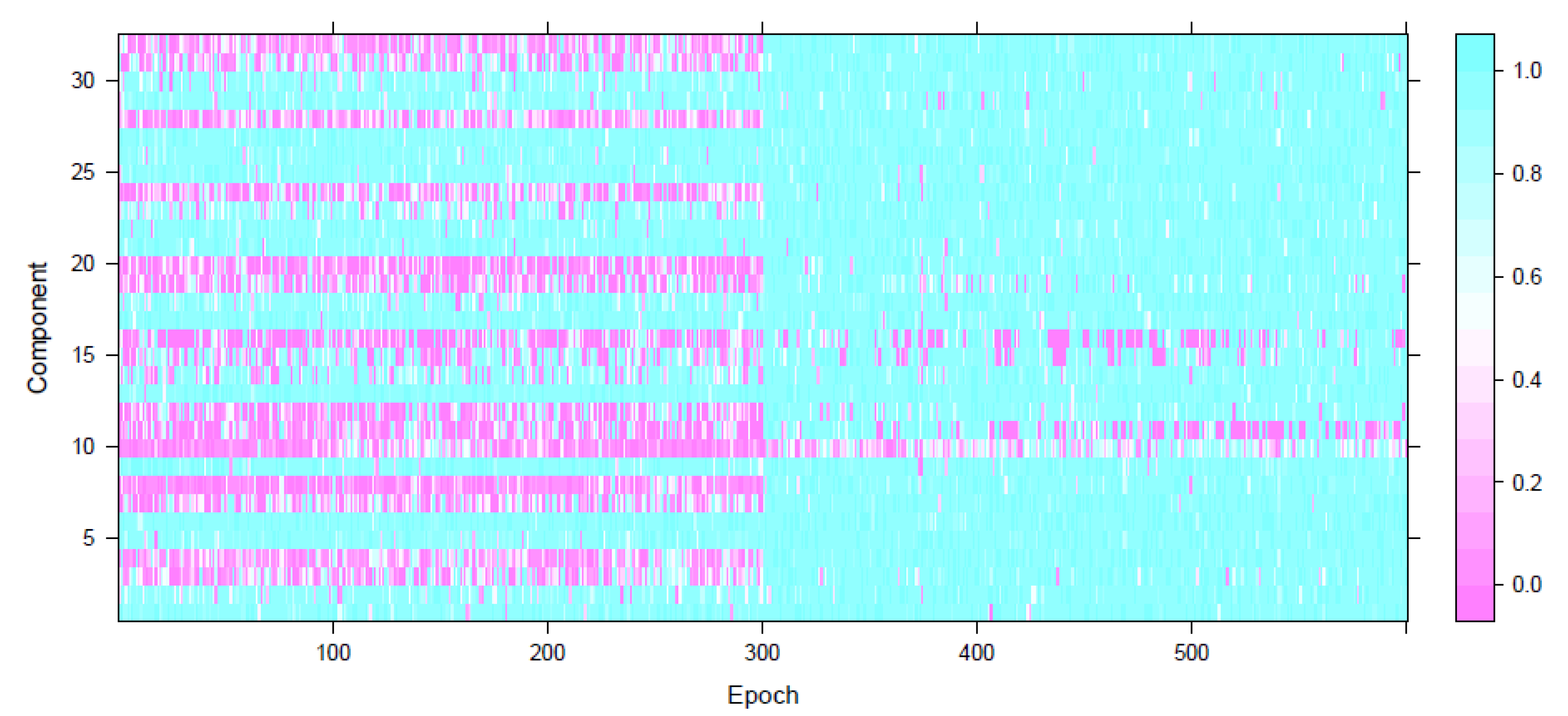
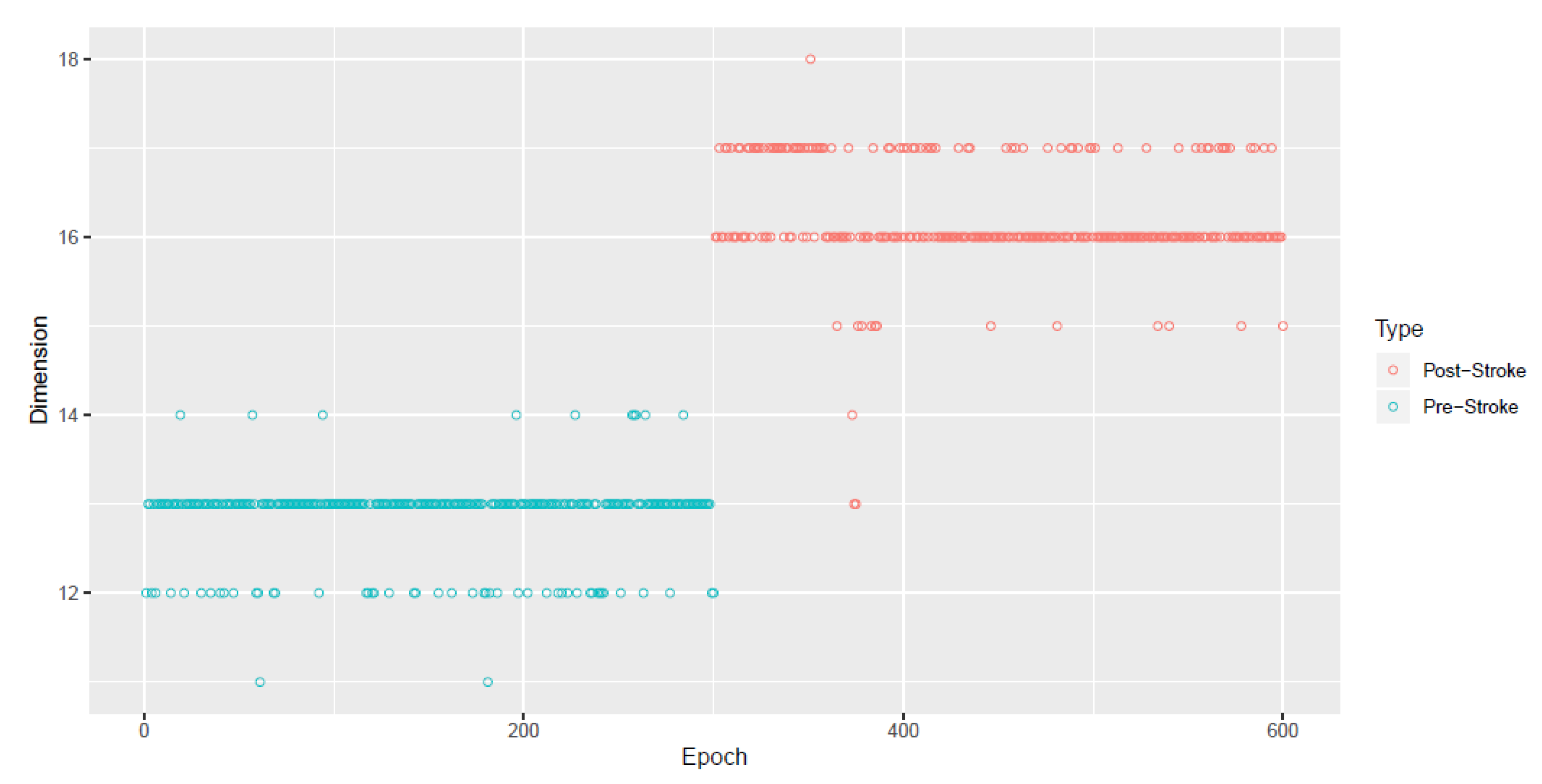
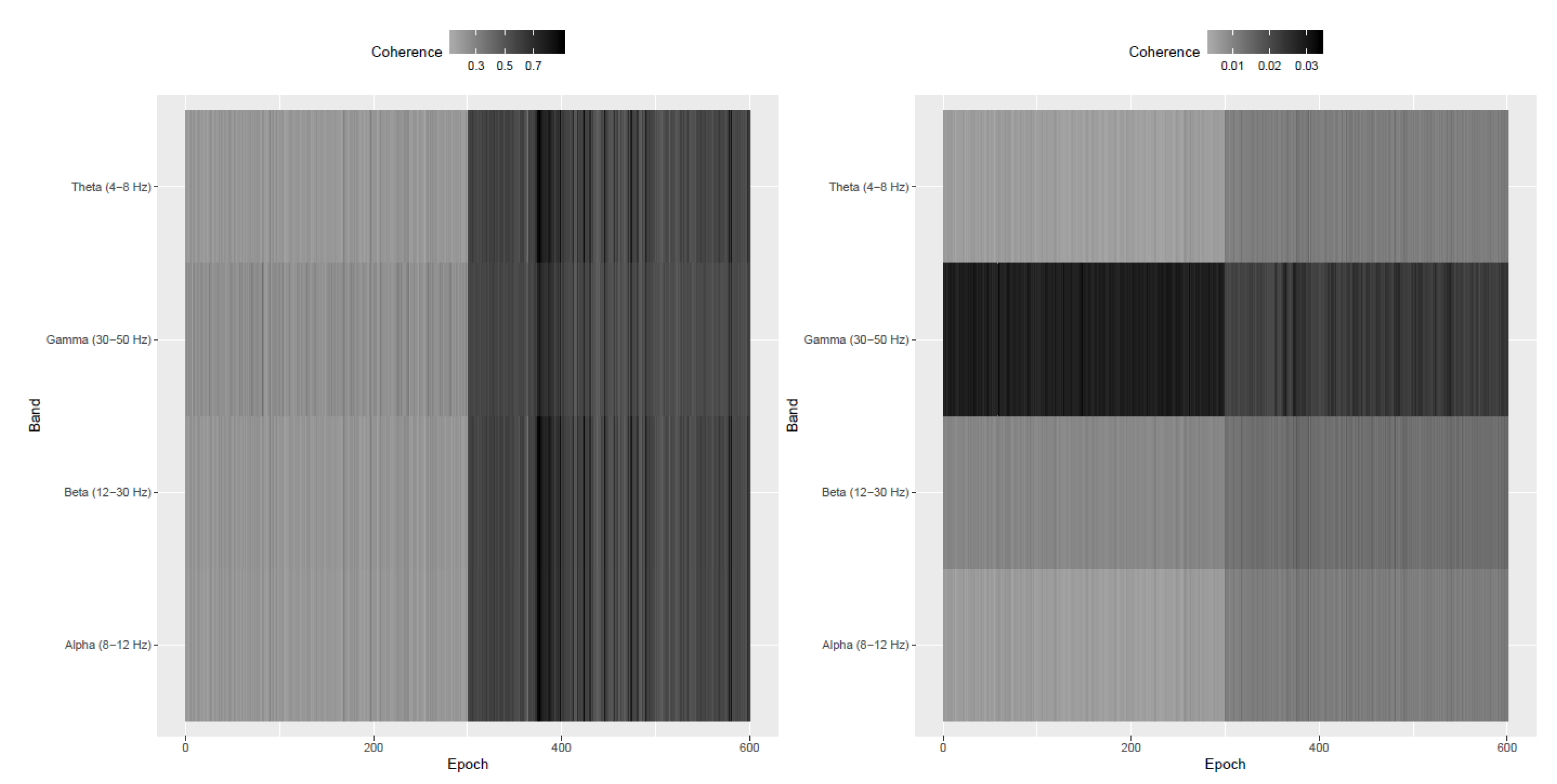
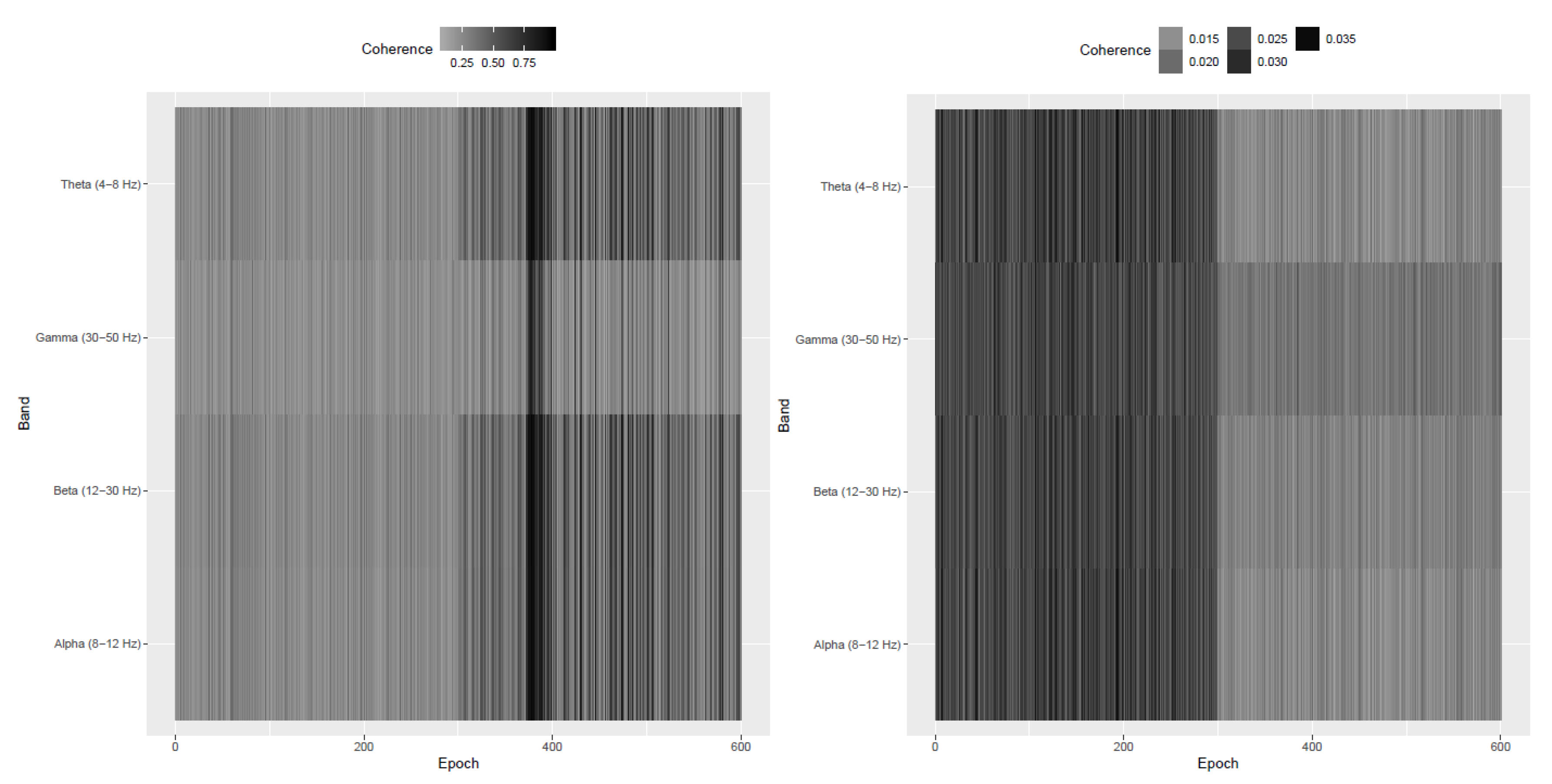
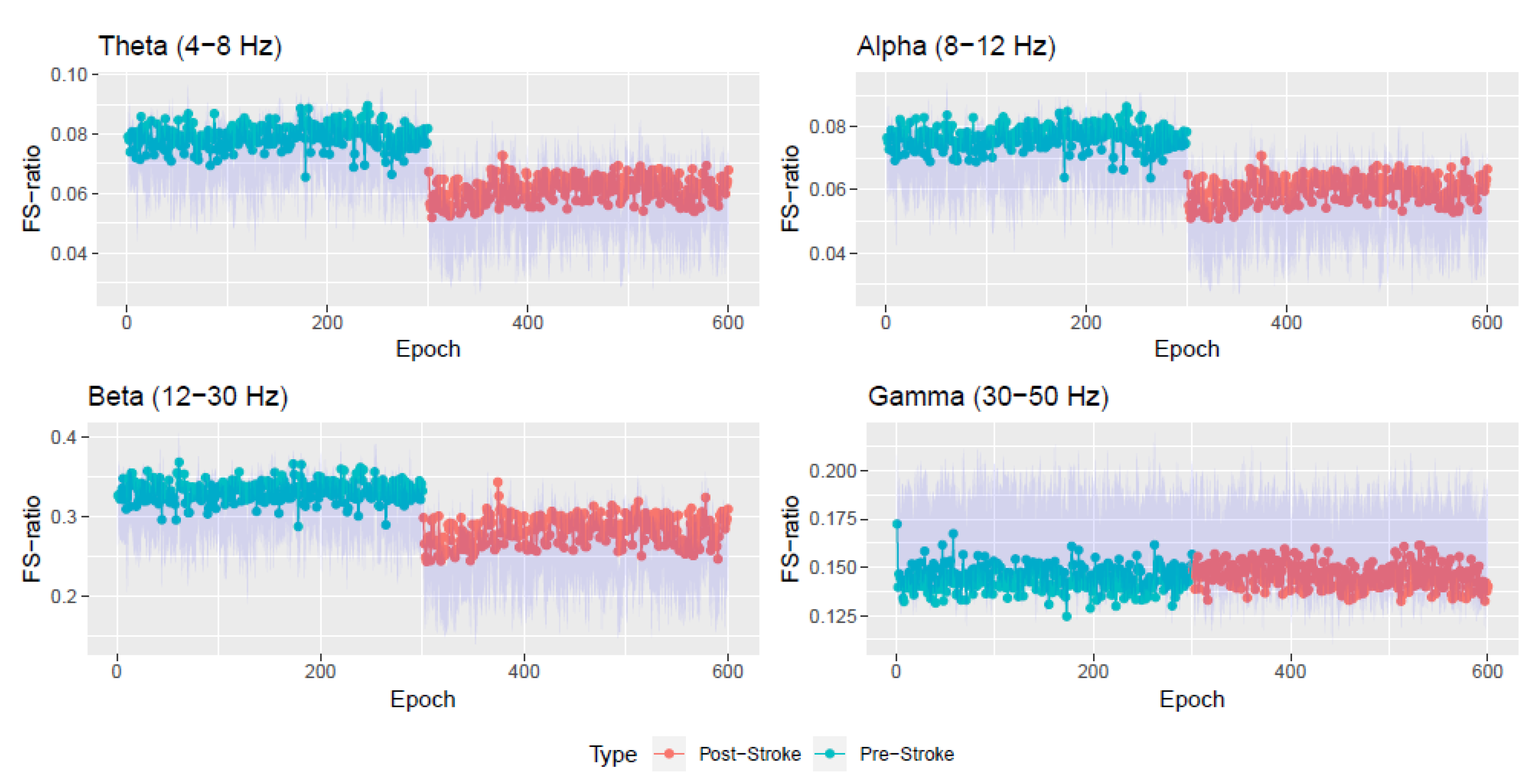
Appendix A. Simulation Study
In this section, we illustrate the performance of the FS-ratio statistic in capturing spread of spectral information using simulated examples. We consider four simulation schemes and report the key summaries of the FS-ratio statistic across repetitions of each of the four schemes. In addition, 95% bootstrap confidence limits for the FS-ratio statistic are computed from
bootstrap replications. Here we use the block bootstrap procedure of Politis and White [
25], Patton et al. [
26]. For an estimate of the spectral matrix defined in (2), the Bartlett-Priestley kernel with bandwidth
and the Daniell kernel, see Example 10.4.1 in Brockwell and Davis [
31] with
were implemented. Similar results were obtained for the two kernel choices and only the results from the latter are presented.
The simulation schemes presented below are designed to mimic the real data situation in
Section 3. There, the entire duration of the neuroscience experiment is divided into non-overlapping successive time segments (
N epochs). Then from these
N epochs, lower dimensional stationary sources of varying dimensions were extracted. Similarly, in our simulations below we shall simulate
N stationary processes with varying dimensions and investigate the evolution of the FS-ratio statistic. We now present 4 simulation schemes to assess the behavior of our FS-ratio statistic. Scheme 1 simulates
N multivariate stationary VAR(2) processes,
where
, with dimensions randomly chosen from
and the error vectors are component-wise i.i.d
. The phase parameter
of the AR components are allowed to vary across epochs i.e two different choices,
and
, are included with
for epochs
and
for epochs
. This causes a shift in the frequency bands of interest as we move across the epochs. Scheme 2 is similar to Scheme 1 but an interaction between the dimension and frequency is included. More precisely, lower dimensional signals simulated in epochs
have a peak at frequency
, and the higher dimensional signals simulated in epochs
have a peak at frequency
. Scheme 3 is also similar to Scheme 1 but the error vectors are allowed to have contemporaneous dependence. The
N multivariate processes in Scheme 4 are first simulated as in Scheme 1 but are then pre-multiplied with a half-orthogonal matrix. This is in line with the model assumption (16) made in
Section 3.
Scheme 1: We simulate
N stationary process
, for
, where the
ith process includes a
-variate process
where each
,
, are independently generated univariate stationary AR(2) process given by
,
,
are i.i.d
and
,
,
. The dimension
for
is randomly chosen from
. Here
and
is given by
Scheme 2: We follow Scheme 1 in generating
N process
, for
and
. Unlike Scheme 1, the dimension
for
is chosen such that
where
is simulated from discrete uniform distribution over
and
is simulated from discrete uniform distribution over
. Observe that in Scheme 2 there is an interaction between the dimension and frequency. The lower dimensional signals simulated in epochs
has a peak at frequency
, and the higher dimensional signals simulated in epochs
has a peak at frequency
.
Scheme 3: Similar to Scheme 1, the
-variate process in the
ith epoch
are where each
are independently generated univariate stationary AR(2) process given by
,
. The
variance matrix of the Gaussian noise
is given by
and
,
,
. The dimension
for
is randomly chosen from
. Here again,
and
is given by
Scheme 4: Here we let and follow Scheme 1 in generating the N process , for and . Then we obtain where and and are two randomly generated orthogonal matrices and is the matrix containing the first rows of the identity matrix . We consider and study the spread of spectral properties across the epochs.
Table A1 and
Table A2 contain the numerical summaries of the FS-ratio statistic over 100 replications of Scheme 1. Please note that the phase parameter
for
in Scheme 1 is at
on a
scale or equivalently at 0.0796 on a
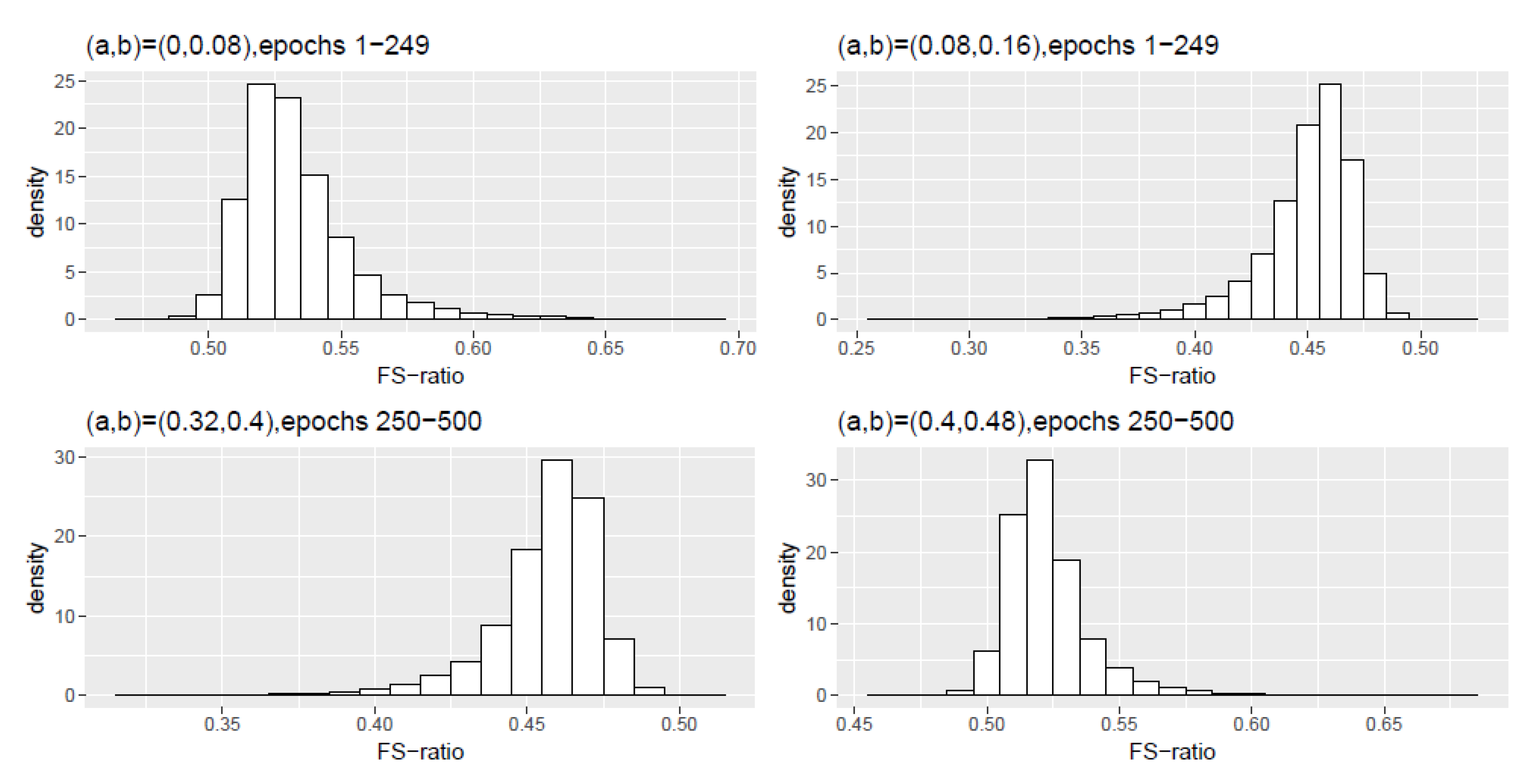
scale. We see from
Table A1 that almost all of the spectral information is contained in the first two chosen frequency ranges around this peak. Similarly for
, the phase parameter is at
on a
scale or equivalently at 0.3981 on a
scale.
Figure A1 plots a histogram density of the FS-ratio statistic from the 100 replications and similar histogram densities for Schemes 2, 3 and 4 can be found in
Figure A2,
Figure A3 and
Figure A4. From
Table A2 we notice that the last two chosen frequency ranges have all of the spectral information.
Figure A1.
Scheme 1: Histogram density of the FS-ratio statistic for different frequency ranges .
Figure A1.
Scheme 1: Histogram density of the FS-ratio statistic for different frequency ranges .
Table A1.
Scheme 1, epochs 1–249: Numerical summaries of FS-ratio statistic for epochs for specified frequency ranges . Here and corresponds to the interval .
Table A1.
Scheme 1, epochs 1–249: Numerical summaries of FS-ratio statistic for epochs for specified frequency ranges . Here and corresponds to the interval .
| Frequency Range | Mean | Median | SD | Lower | Upper |
|---|
| (a,b) | | | | CI | CI |
|---|
| (0,0.08) | 0.5342 | 0.5391 | 0.0221 | 0.4984 | 0.6253 |
| (0.08,0.16) | 0.4486 | 0.4544 | 0.0227 | 0.3566 | 0.4831 |
| (0.16,0.24) | 0.0002 | 0.0002 | 0.0001 | 0.0005 | 0.0019 |
| (0.24,0.32) | 0 | 0 | 0 | 0 | 0.0002 |
| (0.32,0.40) | 0 | 0 | 0 | 0 | 0 |
| (0.40,0.48) | 0 | 0 | 0 | 0 | 0 |
Table A2.
Scheme 1, epochs 250–500: Numerical summaries of FS-ratio statistic for epochs for specified frequency ranges . Here and corresponds to the interval .
Table A2.
Scheme 1, epochs 250–500: Numerical summaries of FS-ratio statistic for epochs for specified frequency ranges . Here and corresponds to the interval .
| Frequency Range | Mean | Median | SD | Lower | Upper |
|---|
| (a,b) | | | | CI | CI |
|---|
| (0,0.08) | 0 | 0 | 0 | 0 | 0 |
| (0.08,0.16) | 0 | 0 | 0 | 0 | 0 |
| (0.16,0.24) | 0 | 0 | 0 | 0 | 0 |
| (0.24,0.32) | 0.0003 | 0.0002 | 0.0002 | 0.0005 | 0.0017 |
| (0.32,0.40) | 0.4561 | 0.4595 | 0.0181 | 0.3786 | 0.4903 |
| (0.40,0.48) | 0.5205 | 0.5210 | 0.0169 | 0.4759 | 0.5826 |
Table A3 and
Table A4 include numerical summaries of the FS-ratio statistic over 100 replications of the model in Scheme 2. Similar to Scheme 1, the phase parameter
for
is at 0.0796 on a
scale for
and at 0.3981 on a
scale for
. The results from
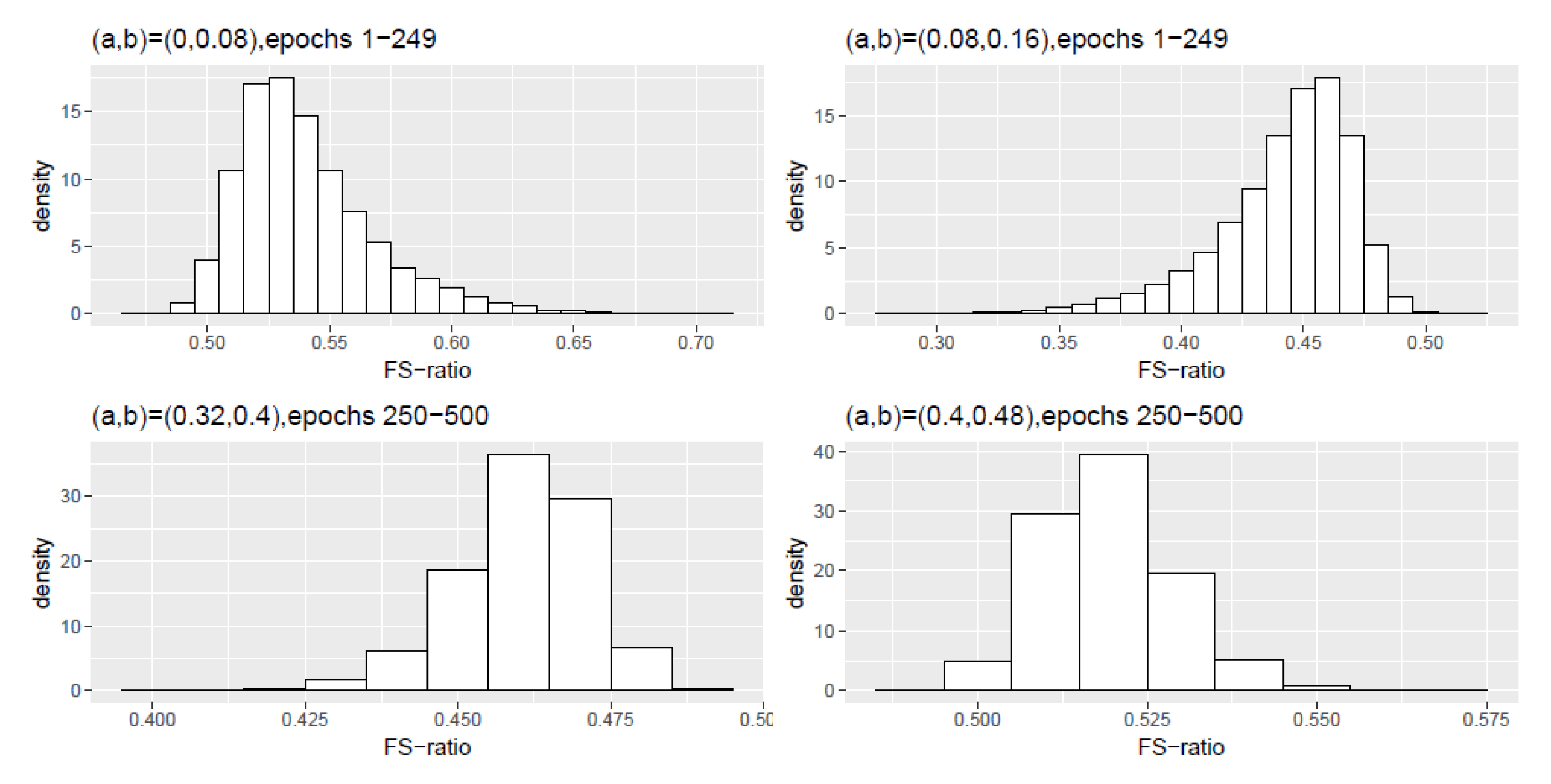
Table A3 indicate most of the spectral information are present in the first two chosen frequency ranges. Similarly for
,
Table A4 shows that the last two chosen frequency ranges have all of the spectral information.
Table A3.
Scheme 2, epochs 1–249: Numerical summaries of FS-ratio statistic for epochs for specified frequency ranges . Here and corresponds to the interval .
Table A3.
Scheme 2, epochs 1–249: Numerical summaries of FS-ratio statistic for epochs for specified frequency ranges . Here and corresponds to the interval .
| Frequency Range | Mean | Median | SD | Lower | Upper |
|---|
| (a,b) | | | | CI | CI |
|---|
| (0,0.08) | 0.5407 | 0.5349 | 0.0286 | 0.5041 | 0.6360 |
| (0.08,0.16) | 0.4423 | 0.4482 | 0.0284 | 0.3475 | 0.4778 |
| (0.16,0.24) | 0.0002 | 0.0003 | 0.0002 | 0.0006 | 0.0022 |
| (0.24,0.32) | 0 | 0 | 0 | 0 | 0.0002 |
| (0.32,0.40) | 0 | 0 | 0 | 0 | 0 |
| (0.40,0.48) | 0 | 0 | 0 | 0 | 0 |
Table A4.
Scheme 2, epochs 250–500: Numerical summaries of FS-ratio statistic for epochs for specified frequency ranges . Here and corresponds to the interval .
Table A4.
Scheme 2, epochs 250–500: Numerical summaries of FS-ratio statistic for epochs for specified frequency ranges . Here and corresponds to the interval .
| Frequency Range | Mean | Median | SD | Lower | Upper |
|---|
| (a,b) | | | | CI | CI |
|---|
| (0,0.08) | 0 | 0 | 0 | 0 | 0 |
| (0.08,0.16) | 0 | 0 | 0 | 0 | 0 |
| (0.16,0.24) | 0 | 0 | 0 | 0 | 0.0001 |
| (0.24,0.32) | 0.0003 | 0.0002 | 0.0001 | 0.0005 | 0.0016 |
| (0.32,0.40) | 0.4605 | 0.4616 | 0.0108 | 0.3862 | 0.4938 |
| (0.40,0.48) | 0.5194 | 0.5186 | 0.0096 | 0.4800 | 0.5863 |
Figure A2.
Scheme 2: Histogram density of the FS-ratio statistic for different frequency ranges .
Figure A2.
Scheme 2: Histogram density of the FS-ratio statistic for different frequency ranges .
Table A5 and
Table A6 contain the numerical summaries of the FS-ratio statistic over 100 replications of the model in Scheme 3. As in Scheme 1, the phase parameter
for
is at 0.0796 on a
scale for
and at 0.3981 on a
scale for
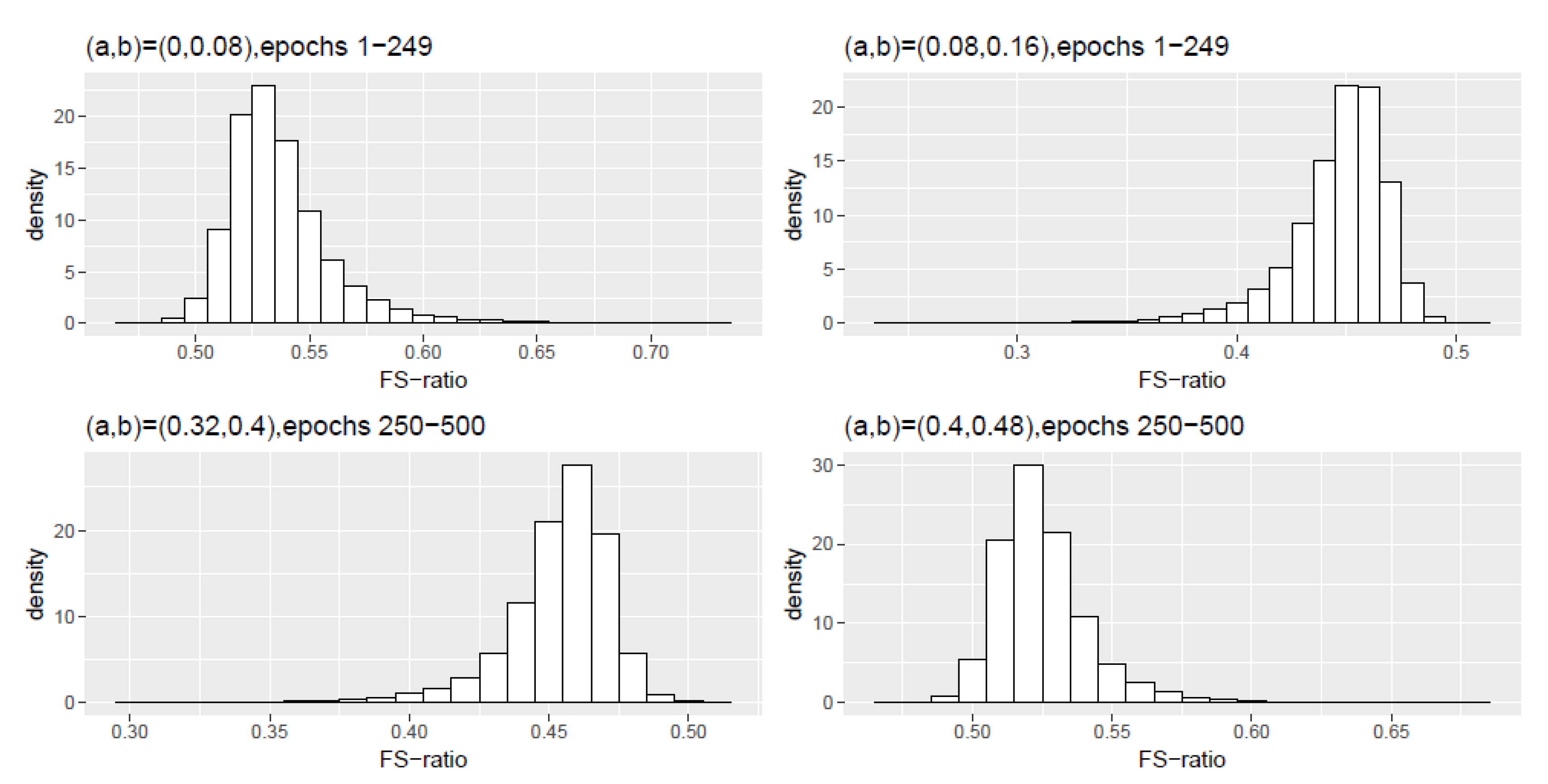
. As in Scheme 1, results from
Table A5 indicate most of the spectral information are present in the first two chosen frequency ranges. Similarly for
,
Table A6 shows that the last two chosen frequency ranges have all of the spectral information.
Table A5.
Scheme 3, epochs 1–249: Numerical summaries of FS-ratio statistic for epochs for specified frequency ranges . Here and corresponds to the interval .
Table A5.
Scheme 3, epochs 1–249: Numerical summaries of FS-ratio statistic for epochs for specified frequency ranges . Here and corresponds to the interval .
| Frequency Range | Mean | Median | SD | Lower | Upper |
|---|
| (a,b) | | | | CI | CI |
|---|
| (0,0.08) | 0.5371 | 0.5327 | 0.0238 | 0.4977 | 0.6284 |
| (0.08,0.16) | 0.4459 | 0.4504 | 0.0239 | 0.3549 | 0.4843 |
| (0.16,0.24) | 0.0002 | 0.0002 | 0.0001 | 0.0005 | 0.0020 |
| (0.24,0.32) | 0 | 0 | 0 | 0 | 0.0002 |
| (0.32,0.40) | 0 | 0 | 0 | 0 | 0 |
| (0.40,0.48) | 0 | 0 | 0 | 0 | 0 |
Table A6.
Scheme 3, epochs 250–500: Numerical summaries of FS-ratio statistic for epochs for specified frequency ranges . Here and corresponds to the interval .
Table A6.
Scheme 3, epochs 250–500: Numerical summaries of FS-ratio statistic for epochs for specified frequency ranges . Here and corresponds to the interval .
| Frequency Range | Mean | Median | SD | Lower | Upper |
|---|
| (a,b) | | | | CI | CI |
|---|
| (0,0.08) | 0 | 0 | 0 | 0 | 0 |
| (0.08,0.16) | 0 | 0 | 0 | 0 | 0 |
| (0.16,0.24) | 0 | 0 | 0 | 0 | 0.0001 |
| (0.24,0.32) | 0.0003 | 0.0003 | 0.0002 | 0.0005 | 0.0018 |
| (0.32,0.40) | 0.4531 | 0.4566 | 0.0196 | 0.3758 | 0.4907 |
| (0.40,0.48) | 0.5252 | 0.5225 | 0.0172 | 0.4810 | 0.5948 |
Figure A3.
Scheme 3: Histogram density of the FS-ratio statistic for different frequency ranges .
Figure A3.
Scheme 3: Histogram density of the FS-ratio statistic for different frequency ranges .
Table A7 and
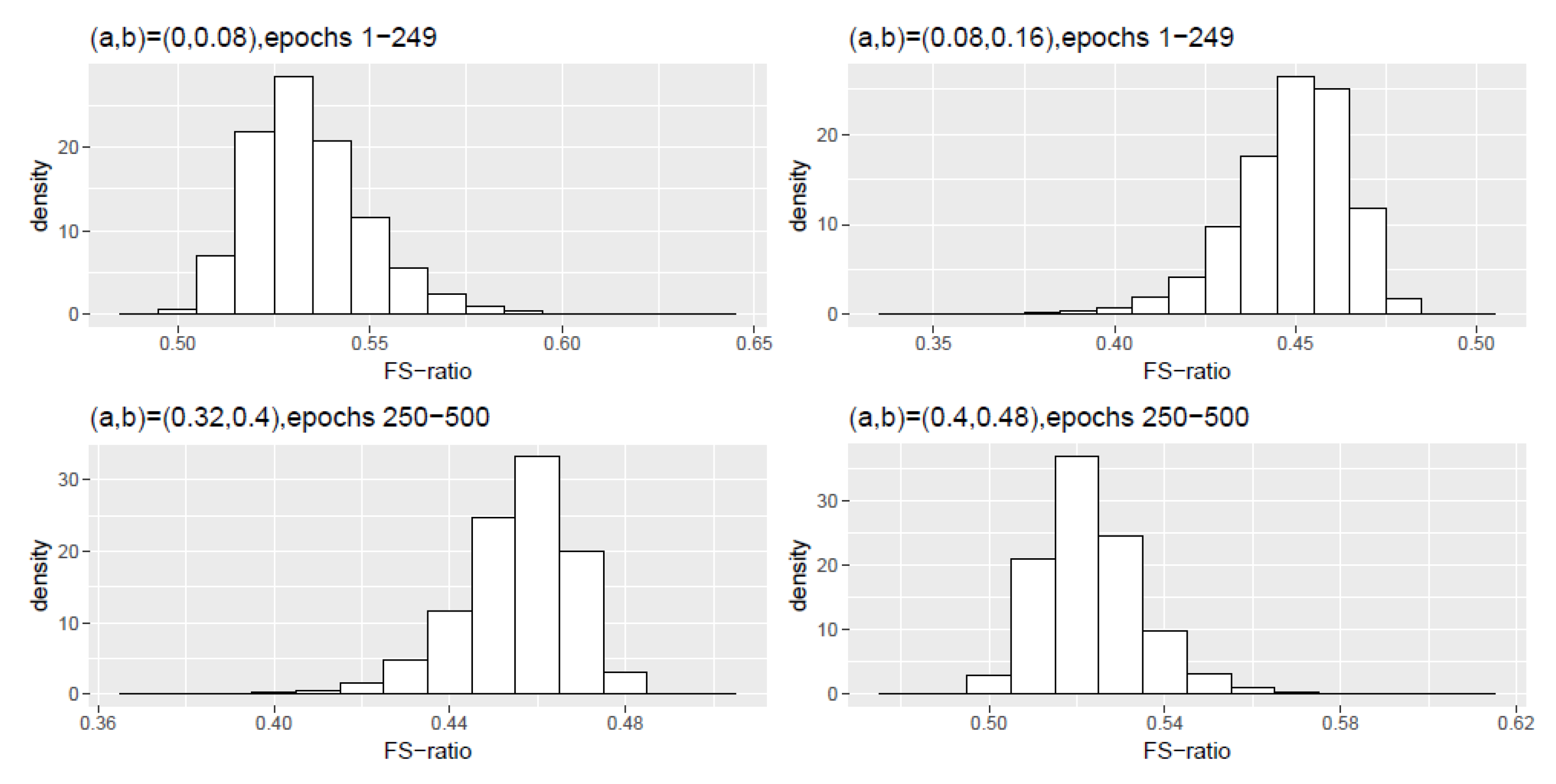
Table A8 contain the numerical summaries of the FS-ratio statistic over 100 replications of the model in Scheme 4. Here we look at
which is a mixture of the components of
generated as in Scheme 1. Please note that the peak of the spectral densities of the components of
is still at the phase parameter
defined in Scheme 1. Hence, the results from
Table A7 and
Table A8 are similar to the results from Scheme 1.
Table A7.
Scheme 4, epochs 1–249: Numerical summaries of FS-ratio statistic for epochs for specified frequency ranges . Here and corresponds to the interval .
Table A7.
Scheme 4, epochs 1–249: Numerical summaries of FS-ratio statistic for epochs for specified frequency ranges . Here and corresponds to the interval .
| Frequency Range | Mean | Median | SD | Lower | Upper |
|---|
| (a,b) | | | | CI | CI |
|---|
| (0,0.08) | 0.5342 | 0.5321 | 0.0155 | 0.4871 | 0.5955 |
| (0.08,0.16) | 0.4489 | 0.4510 | 0.0159 | 0.3872 | 0.4949 |
| (0.16,0.24) | 0.0002 | 0.0002 | 0.0001 | 0.0003 | 0.0011 |
| (0.24,0.32) | 0.0001 | 0.0001 | 0 | 0 | 0.0001 |
| (0.32,0.40) | 0 | 0 | 0 | 0 | 0 |
| (0.40,0.48) | 0 | 0 | 0 | 0 | 0 |
Table A8.
Scheme 4, epochs 250–500: Numerical summaries of FS-ratio statistic for epochs for specified frequency ranges . Here and corresponds to the interval .
Table A8.
Scheme 4, epochs 250–500: Numerical summaries of FS-ratio statistic for epochs for specified frequency ranges . Here and corresponds to the interval .
| Frequency Range | Mean | Median | SD | Lower | Upper |
|---|
| (a,b) | | | | CI | CI |
|---|
| (0,0.08) | 0 | 0 | 0 | 0 | 0 |
| (0.08,0.16) | 0 | 0 | 0 | 0 | 0 |
| (0.16,0.24) | 0 | 0 | 0 | 0 | 0.0001 |
| (0.24,0.32) | 0.0003 | 0.0003 | 0.0001 | 0.0003 | 0.0011 |
| (0.32,0.40) | 0.4553 | 0.4570 | 0.0130 | 0.4021 | 0.4987 |
| (0.40,0.48) | 0.5234 | 0.5219 | 0.0119 | 0.4782 | 0.5738 |
Figure A4.
Scheme 4: Histogram density of the FS-ratio statistic for different frequency ranges .
Figure A4.
Scheme 4: Histogram density of the FS-ratio statistic for different frequency ranges .