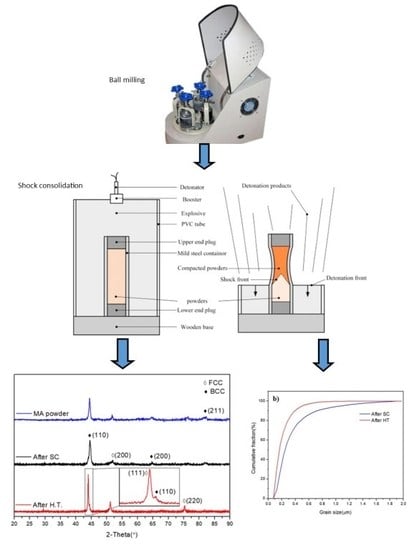
Fabrication of Nanocrystalline AlCoCrFeNi High Entropy Alloy through Shock Consolidation and Mechanical Alloying
Abstract
1. Introduction
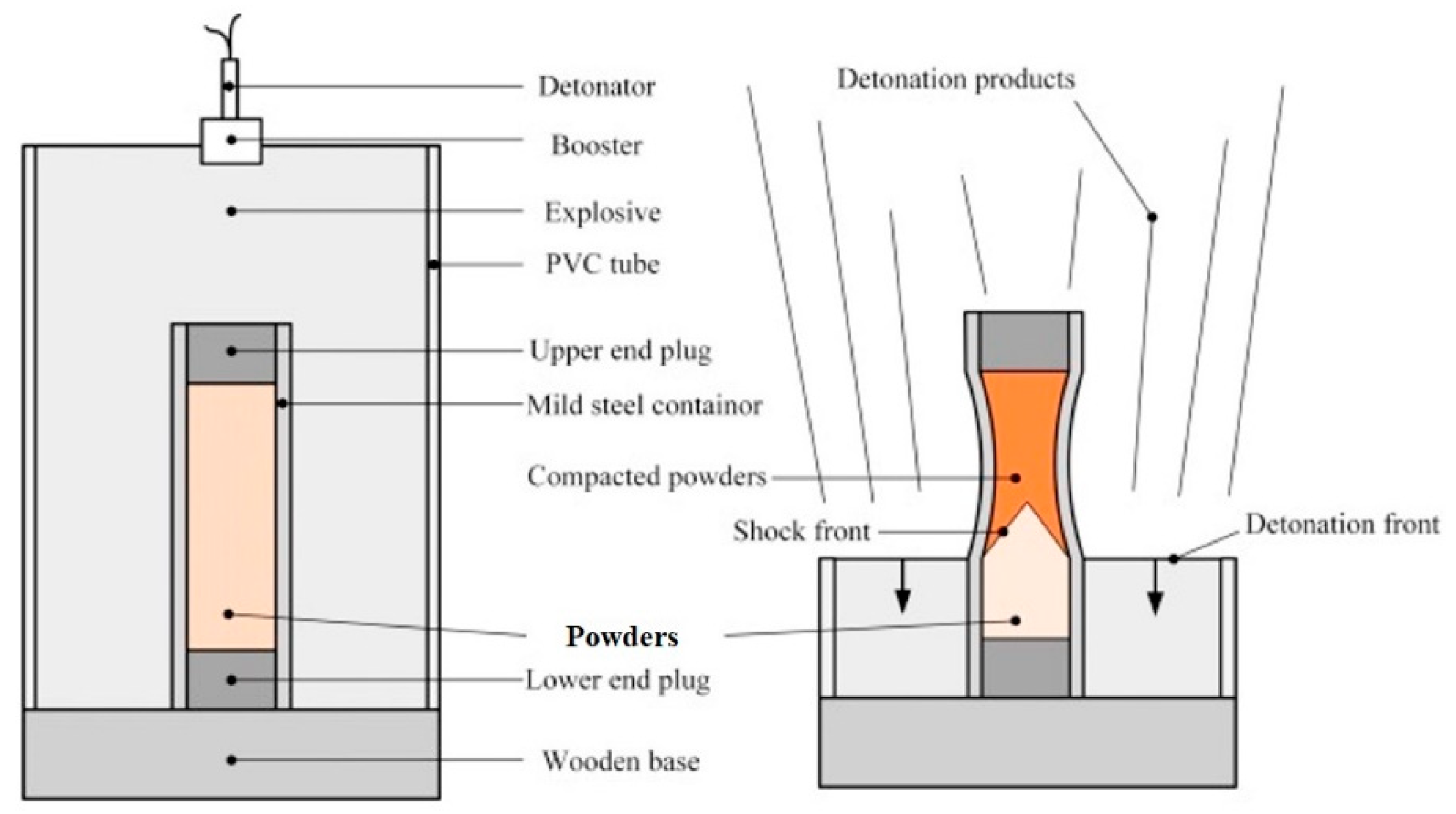
2. Materials and Methods
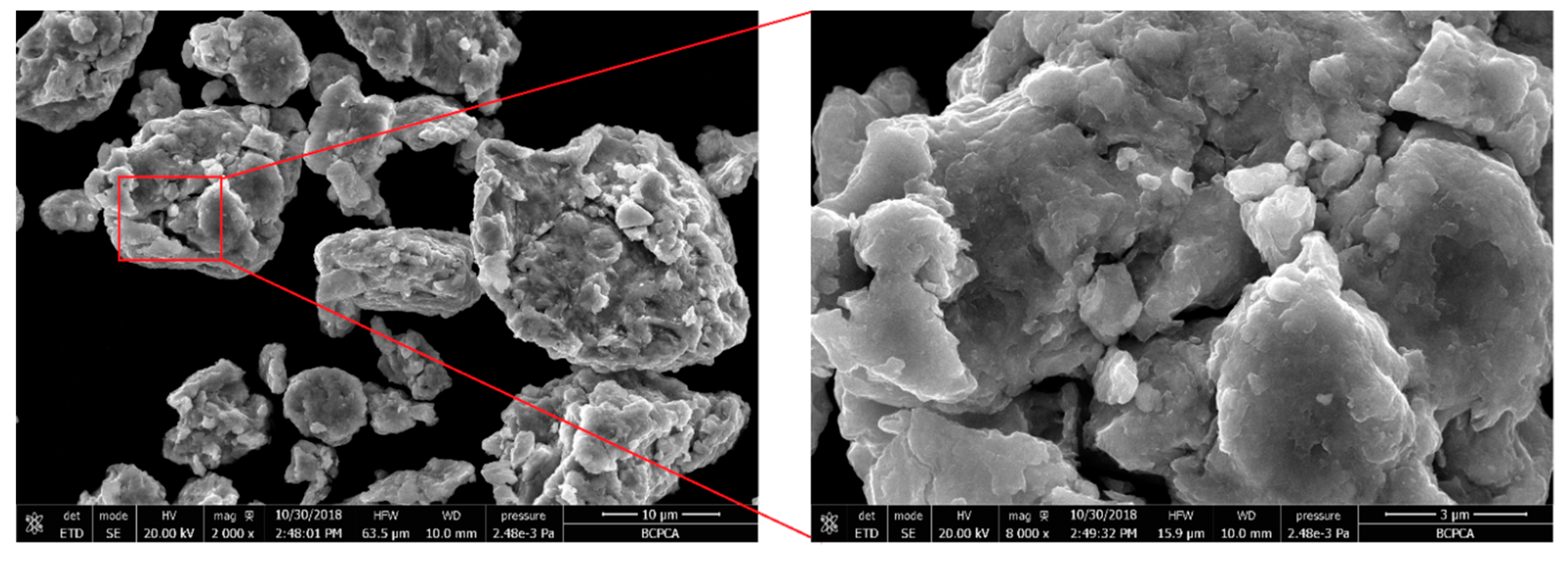
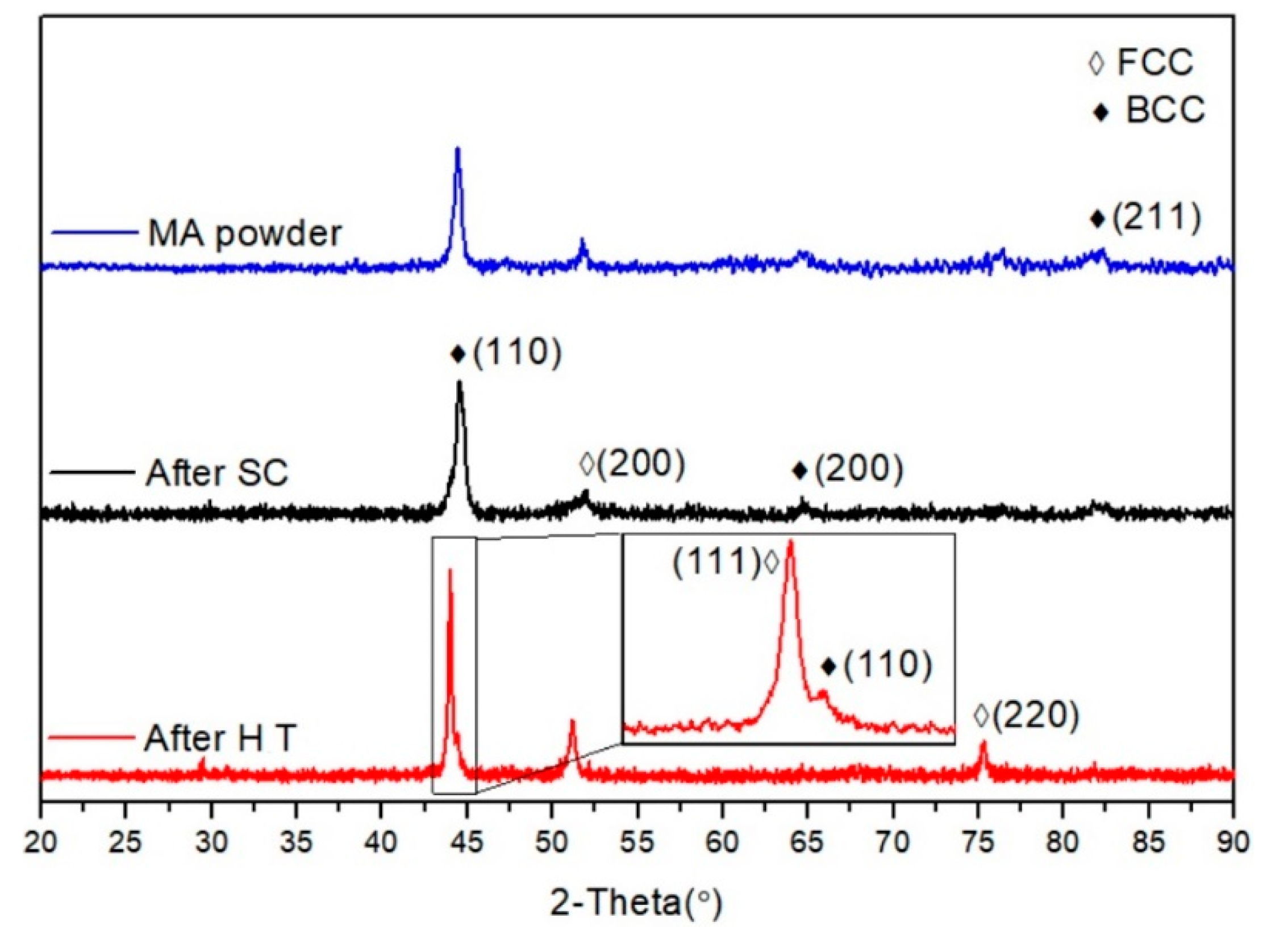
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, K.; Davies, C.; Wu, X. Evolution of microstructure, mechanical and corrosion properties of AlCoCrFeNi high-entropy alloy prepared by direct laser fabrication. J. Alloys Compd. 2017, 694, 971–981. [Google Scholar] [CrossRef]
- Zhang, K.B.; Fu, Z.Y.; Zhang, J.Y.; Shi, J.; Wang, W.M.; Wang, H.; Wang, Y.C.; Zhang, Q.J. Nanocrystalline CoCrFeNiCuAl high-entropy solid solution synthesized by mechanical alloying. J. Alloys Compd. 2009, 485, L31–L34. [Google Scholar] [CrossRef]
- Lee, D.-H.; Park, J.-M.; Yang, G.; He, J.; Lu, Z.; Suh, J.-Y.; Kawasaki, M.; Ramamurty, U.; Jang, J. Nano-graining a particle-strengthened high-entropy alloy. Scr. Mater. 2019, 163, 24–28. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, W.; Wen, H.; Zhang, D.; Chen, Z.; Zheng, B.; Zhou, Y.; Lavernia, E.J. Microstructure and strengthening mechanisms in an FCC structured single-phase nanocrystalline Co25Ni25Fe25Al7.5Cu17.5 high-entropy alloy. Acta Mater. 2016, 107, 59–71. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, J.A.; Zhao, Y.; Lu, Z.; Suh, J.Y.; Kim, J.Y.; Ramamurty, U.; Kawasaki, M.; Langdon, T.G.; Jang, J.-I. Annealing effect on plastic flow in nanocrystalline CoCrFeMnNi high-entropy alloy: A nanomechanical analysis. Acta Mater. 2017, 140, 443–451. [Google Scholar] [CrossRef]
- Lee, D.H.; Seok, M.Y.; Zhao, Y.; Choi, I.C.; He, J.; Lu, Z.; Suh, J.Y.; Ramamurty, U.; Kawasaki, M.; Langdon, T.G.; et al. Spherical nanoindentation creep behavior of nanocrystalline and coarse-grained CoCrFeMnNi high-entropy alloys. Acta Mater. 2016, 109, 314–322. [Google Scholar] [CrossRef]
- Lee, D.H.; Choi, I.C.; Seok, M.Y.; He, J.; Lu, Z.; Suh, J.Y.; Kawasaki, M.; Langdon, T.G.; Jang, J.-I. Nanomechanical behavior and structural stability of a nanocrystalline CoCrFeNiMn high-entropy alloy processed by high-pressure torsion. J. Mater. Res. 2015, 30, 2804–2815. [Google Scholar] [CrossRef]
- Varalakshmi, S.; Kamaraj, M.; Murty, B.S. Processing and properties of nanocrystalline CuNiCoZnAlTi high entropy alloys by mechanical alloying. Mater. Sci. Eng. A 2010, 527, 1027–1030. [Google Scholar] [CrossRef]
- Wang, C.; Ji, W.; Fu, Z. Mechanical alloying and spark plasma sintering of CoCrFeNiMnAl high-entropy alloy. Adv. Powder Technol. 2014, 25, 1334–1338. [Google Scholar] [CrossRef]
- Mohanty, S.; Maity, T.N.; Mukhopadhyay, S.; Sarkar, S.; Gurao, N.P.; Bhowmick, S.; Biswas, K. Powder metallurgical processing of equiatomic AlCoCrFeNi high entropy alloy: Microstructure and mechanical properties. Mater. Sci. Eng. A 2017, 679, 299–313. [Google Scholar] [CrossRef]
- Youssef, K.M.; Zaddach, A.J.; Niu, C.; Irving, D.L.; Koch, C.C. A novel low-density, high-hardness, high-entropy alloy with close-packed single-phase nanocrystalline structures. Mater. Res. Lett. 2014, 3, 95–99. [Google Scholar] [CrossRef]
- Yim, D.; Kim, W.; Praveen, S.; Jang, M.J.; Bae, J.W.; Moon, J.; Kim, E.; Hong, S.J.; Kim, H.S. Shock wave compaction and sintering of mechanically alloyed CoCrFeMnNi high-entropy alloy powders. Mater. Sci. Eng. A 2017, 708, 291–300. [Google Scholar] [CrossRef]
- Wang, B.; Xie, F.; Li, Z.; Zhang, H. Explosive compaction of Al2O3nanopowders. Ceram. Int. 2016, 42, 8460–8466. [Google Scholar] [CrossRef]
- Szewczak, E.; Paszula, J.; Leonov, A.V.; Matyja, H. Explosive consolidation of mechanically alloyed Ti-Al alloys. Mater. Sci. Eng. A 1997, 226–228, 115–118. [Google Scholar] [CrossRef]
- Buzyurkin, A.E.; Kraus, E.I.; Lukyanov, Y.L. The fabrication of boron carbide compacts by explosive consolidation. J. Phys. Conf. Ser. 2016, 774, 012067. [Google Scholar] [CrossRef]
- Farinha, A.R.; Vieira, M.T.; Mendes, R. Explosive consolidation of 316L stainless steel powder—Effect of phase composition. Adv. Powder Technol. 2014, 25, 1469–1473. [Google Scholar] [CrossRef]
- Wang, S.; Sun, C.; Guo, W.; Yan, Q.; Zhou, Z.; Zhang, Y.; Shen, W.; Ge, C. Review on the explosive consolidation methods to fabricate tungsten based PFMs. J. Nucl. Mater. 2014, 455, 174–179. [Google Scholar] [CrossRef]
- Emelchenko, G.A.; Naumenko, I.G.; Veretennikov, V.A.; Gordopolov, Y.A. Shock consolidation of nanopowdered Ni. Mater. Sci. Eng. A 2009, 503, 55–57. [Google Scholar] [CrossRef]
- Chen, T.; Hampikian, J.M.; Thadhani, N.N. Synthesis and characterization of mechanically alloyed and shock-consolidated nanocrystalline NiAl intermetallic. Acta Mater. 1999, 47, 2567–2579. [Google Scholar] [CrossRef]
- Du, S.W.; Aydelotte, B.; Fondse, D.; Wei, C.T.; Jiang, F.; Herbold, E.; Vecchio, K.; Meyers, M.A.; Thadhani, N.N. Explosive compations of intermetallic-forming powder mixtures for fabricating structural energetic materials. AIP Conf. Proc. 2009, 1195, 498–501. [Google Scholar]
- Fredenburg, D.A.; Vogler, T.J.; Saldana, C.J.; Thadhani, N.N. Shock consolidation of nanocrystalline aluminum for bulk component formation. AIP Conf. Proc. 2007, 955, 1029–1032. [Google Scholar]
- Anthony Fredenburg, D.; Thadhani, N.N.; Vogler, T.J. Shock consolidation of nanocrystalline 6061-T6 aluminum powders. Mater. Sci. Eng. A 2010, 527, 3349–3357. [Google Scholar] [CrossRef]
- Ma, S.G.; Liaw, P.K.; Gao, M.C.; Qiao, J.W.; Wang, Z.H.; Zhang, Y. Damping behavior of AlxCoCrFeNi high-entropy alloys by a dynamic mechanical analyzer. J. Alloys Compd. 2014, 604, 331–339. [Google Scholar] [CrossRef]
- Zhang, A.; Han, J.; Meng, J.; Su, B.; Li, P. Rapid preparation of AlCoCrFeNi high entropy alloy by spark plasma sintering from elemental powder mixture. Mater. Lett. 2016, 181, 82–85. [Google Scholar] [CrossRef]
- Ghassemali, E.; Sonkusare, R.; Biswas, K.; Gurao, N.P. In-situ study of crack initiation and propagation in a dual phase AlCoCrFeNi high entropy alloy. J. Alloys Compd. 2017, 710, 539–546. [Google Scholar] [CrossRef]
- Shivam, V.; Basu, J.; Pandey, V.K.; Shadangi, Y.; Mukhopadhyay, N.K. Alloying behaviour, thermal stability and phase evolution in quinary AlCoCrFeNi high entropy alloy. Adv. Powder Technol. 2018, 29, 2221–2230. [Google Scholar] [CrossRef]
- Uporov, S.; Bykov, V.; Pryanichnikov, S.; Shubin, A.; Uporova, N. Effect of synthesis route on structure and properties of AlCoCrFeNi high-entropy alloy. Intermetallics 2017, 83, 1–8. [Google Scholar] [CrossRef]
- Pickering, E.J.; Muñoz-Moreno, R.; Stone, H.J.; Jones, N.G. Precipitation in the equiatomic high-entropy alloy CrMnFeCoNi. Scr. Mater. 2016, 113, 106–109. [Google Scholar] [CrossRef]
- Pickering, E.J.; Stone, H.J.; Jones, N.G. Fine-scale precipitation in the high-entropy alloy Al0.5CrFeCoNiCu. Mater. Sci. Eng. A 2015, 645, 65–71. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, B.S.; Ren, M.X.; Yang, C.; Fu, H.Z. Microstructure and compressive properties of AlCrFeCoNi high entropy alloy. Mater. Sci. Eng. A 2008, 491, 154–158. [Google Scholar] [CrossRef]
- Wang, W.-R.; Wang, W.-L.; Wang, S.-C.; Tsai, Y.-C.; Lai, C.-H.; Yeh, J.-W. Effects of Al addition on the microstructure and mechanical property of AlxCoCrFeNi high-entropy alloys. Intermetallics 2012, 26, 44–51. [Google Scholar] [CrossRef]
- Ji, W.; Fu, Z.; Wang, W.; Wang, H.; Zhang, J.; Wang, Y.; Zhang, F. Mechanical alloying synthesis and spark plasma sintering consolidation of CoCrFeNiAl high-entropy alloy. J. Alloys Compd. 2014, 589, 61–66. [Google Scholar] [CrossRef]
- Munitz, A.; Salhov, S.; Hayun, S.; Frage, N. Heat treatment impacts the micro-structure and mechanical properties of AlCoCrFeNi high entropy alloy. J. Alloys Compd. 2016, 683, 221–230. [Google Scholar] [CrossRef]
- Ma, S.G.; Jiao, Z.M.; Qiao, J.W.; Yang, H.J.; Zhang, Y.; Wang, Z.H. Strain rate effects on the dynamic mechanical properties of the AlCrCuFeNi2 high-entropy alloy. Mater. Sci. Eng. A 2018, 727, 15–19. [Google Scholar] [CrossRef]
- Tang, Q.; Huang, Y.; Cheng, H.; Liao, X.; Langdon, T.G.; Dai, P. The effect of grain size on the annealing-induced phase transformation in an Al0·3CoCrFeNi high entropy alloy. Mater. Des. 2016, 105, 381–385. [Google Scholar] [CrossRef]
- Cui, W.; Karnati, S.; Zhang, X.; Burns, E.; Liou, F. Fabrication of AlCoCrFeNi high-entropy alloy coating on an AISI 304 substrate via a CoFe2Ni intermediate layer. Entropy 2019, 21, 2. [Google Scholar] [CrossRef]
- Ng, C.; Guo, S.; Luan, J.; Wang, Q.; Lu, J.; Shi, S.; Liu, C.T. Phase stability and tensile properties of Co-free Al0.5CrCuFeNi2high-entropy alloys. J. Alloys Compd. 2014, 584, 530–537. [Google Scholar] [CrossRef]
- Kao, Y.F.; Chen, T.J.; Chen, S.K.; Yeh, J.W. Microstructure and mechanical property of as-cast, -homogenized, and -deformed AlxCoCrFeNi (0 ≤ x ≤ 2) high-entropy alloys. J. Alloys Compd. 2009, 488, 57–64. [Google Scholar] [CrossRef]
- Tang, Z.; Senkov, O.N.; Parish, C.M.; Zhang, C.; Zhang, F.; Santodonato, L.J.; Wang, G.; Zhao, G.; Yang, F.; Liaw, P.K. Tensile ductility of an AlCoCrFeNi multi-phase high-entropy alloy through hot isostatic pressing (HIP) and homogenization. Mater. Sci. Eng. A 2015, 647, 229–240. [Google Scholar] [CrossRef]
- Li, W.; Liaw, P.K.; Gao, Y. Fracture resistance of high entropy alloys: A review. Intermetallics 2018, 99, 69–83. [Google Scholar] [CrossRef]
- Joseph, J.; Stanford, N.; Hodgson, P.; Fabijanic, D.M. Understanding the mechanical behaviour and the large strength/ductility differences between FCC and BCC AlxCoCrFeNi high entropy alloys. J. Alloys Compd. 2017, 726, 885–895. [Google Scholar] [CrossRef]
- Niu, S.; Kou, H.; Guo, T.; Zhang, Y.; Wang, J.; Li, J. Strengthening of nanoprecipitations in an annealed Al0.5CoCrFeNi high entropy alloy. Mater. Sci. Eng. A 2016, 671, 82–86. [Google Scholar] [CrossRef]
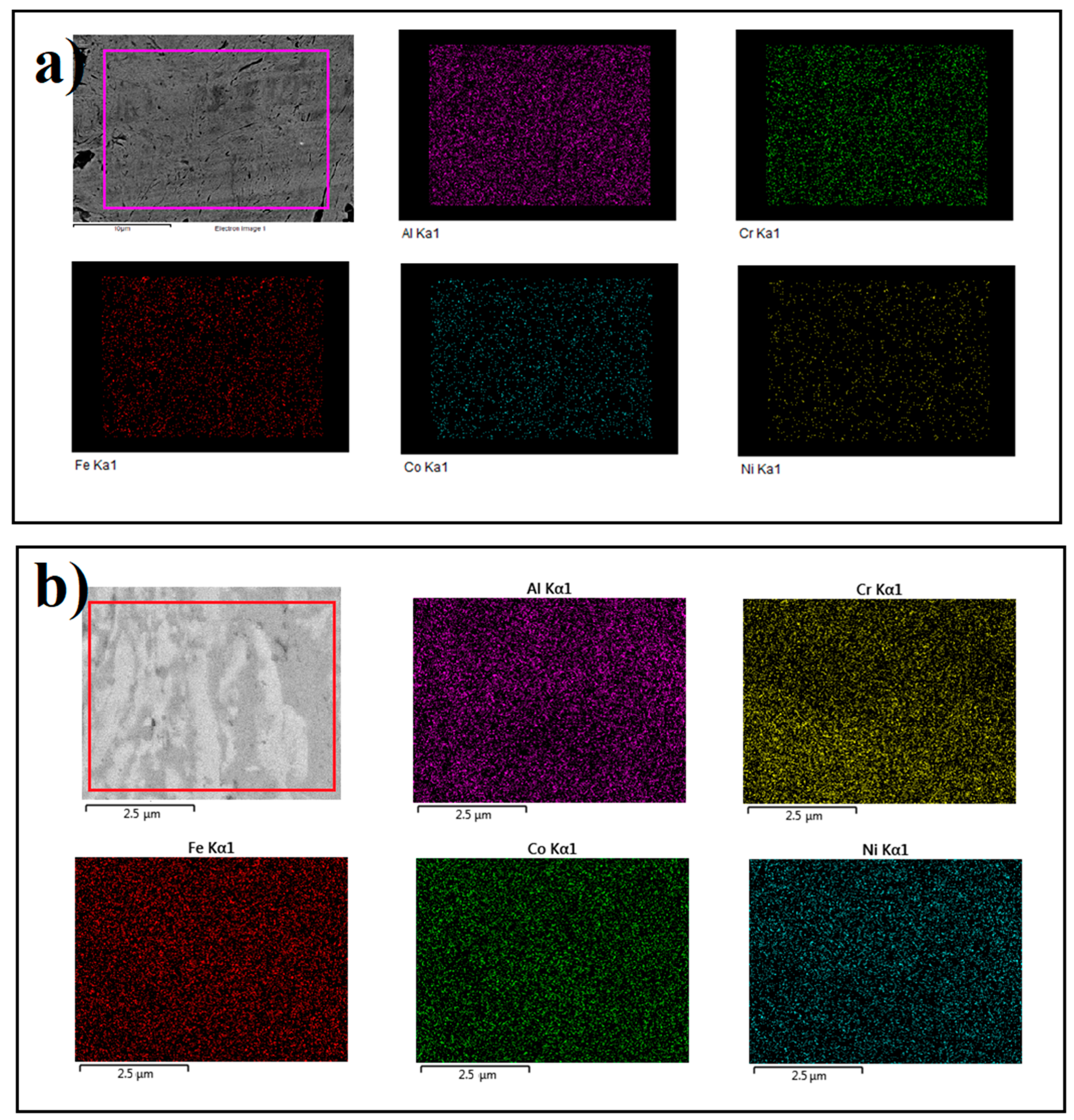

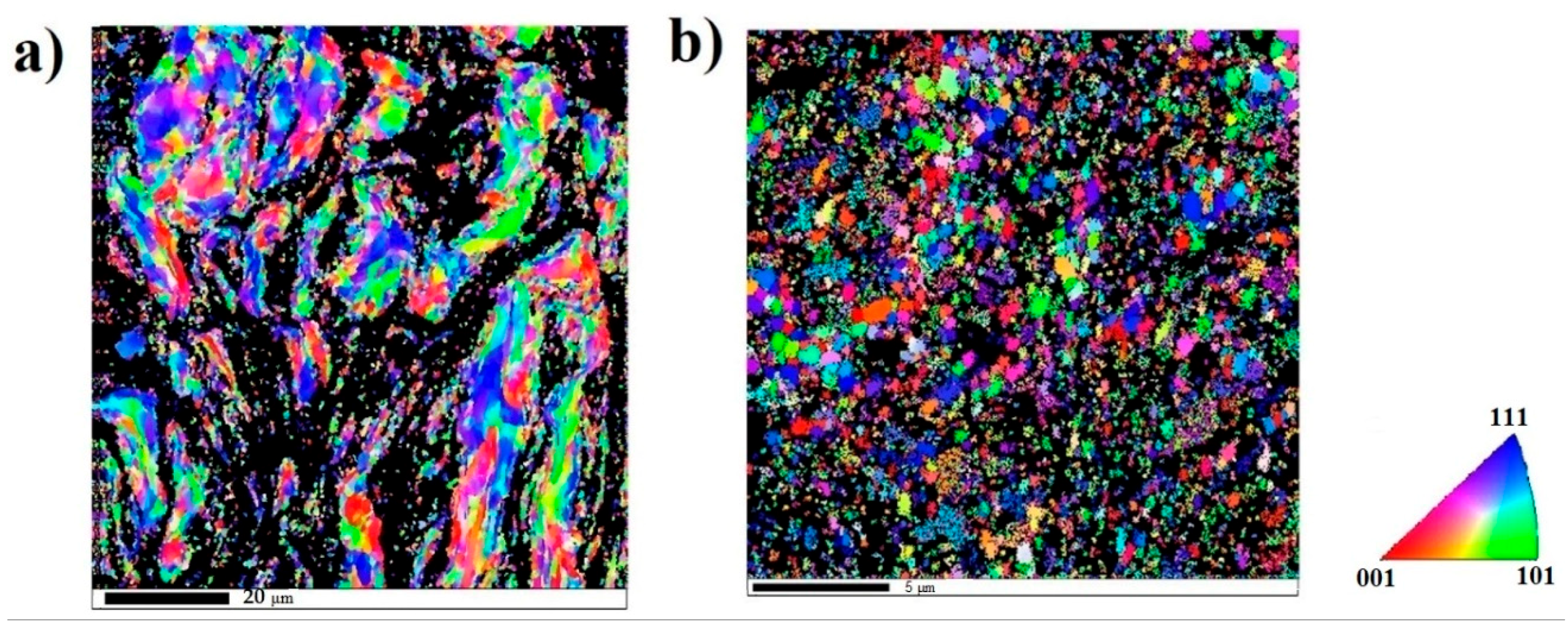

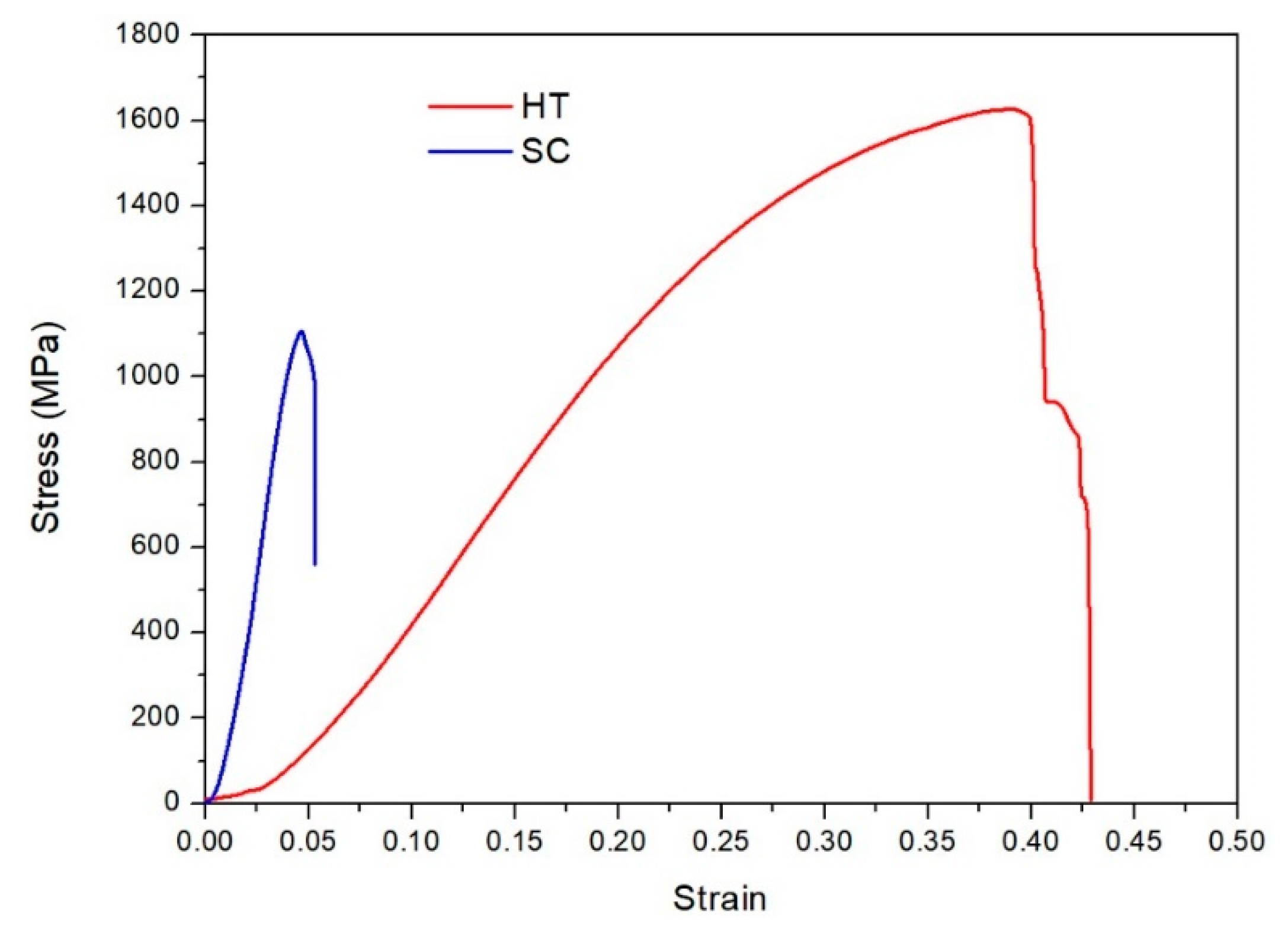

| Zone | Fe (at%) | Co (at%) | Ni (at%) | Al (at%) | Cr (at%) |
|---|---|---|---|---|---|
| 1 | 20.31 ± 0.2 | 20.61 ±0.18 | 20.87 ± 0.1 | 19.35 ± 0.06 | 19.33 ± 0.04 |
| 2 | 24.16 ± 0.1 | 20.99 ± 0.35 | 21.06 ± 0.07 | 16.62 ± 0.03 | 17.17 ± 0.06 |
| 3 | 24.045 ± 0.15 | 20.99 ± 0.07 | 16.68 ± 0.04 | 14.79 ± 0.06 | 23.47 ± 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arab, A.; Guo, Y.; Zhou, Q.; Chen, P. Fabrication of Nanocrystalline AlCoCrFeNi High Entropy Alloy through Shock Consolidation and Mechanical Alloying. Entropy 2019, 21, 880. https://doi.org/10.3390/e21090880
Arab A, Guo Y, Zhou Q, Chen P. Fabrication of Nanocrystalline AlCoCrFeNi High Entropy Alloy through Shock Consolidation and Mechanical Alloying. Entropy. 2019; 21(9):880. https://doi.org/10.3390/e21090880
Chicago/Turabian StyleArab, Ali, Yansong Guo, Qiang Zhou, and Pengwan Chen. 2019. "Fabrication of Nanocrystalline AlCoCrFeNi High Entropy Alloy through Shock Consolidation and Mechanical Alloying" Entropy 21, no. 9: 880. https://doi.org/10.3390/e21090880
APA StyleArab, A., Guo, Y., Zhou, Q., & Chen, P. (2019). Fabrication of Nanocrystalline AlCoCrFeNi High Entropy Alloy through Shock Consolidation and Mechanical Alloying. Entropy, 21(9), 880. https://doi.org/10.3390/e21090880