2. Basic Equations
We consider the formation of a new phase cluster on a rough rigid surface. For the description of the bulk properties of the ambient and the newly formed phases we use the model of a binary solid or liquid regular solution. The chemical potentials
of each of the two components (
) of a regular solution can be written in the form [
14]
where
is the Boltzmann constant,
T is the absolute temperature,
x and
are the molar fractions of the second and first components, correspondingly (for unambiguity we consider the solvent as the first component and the dissolved substance as the second component),
is the interaction parameter describing specific properties of the considered system, and
is the critical temperature of the system.
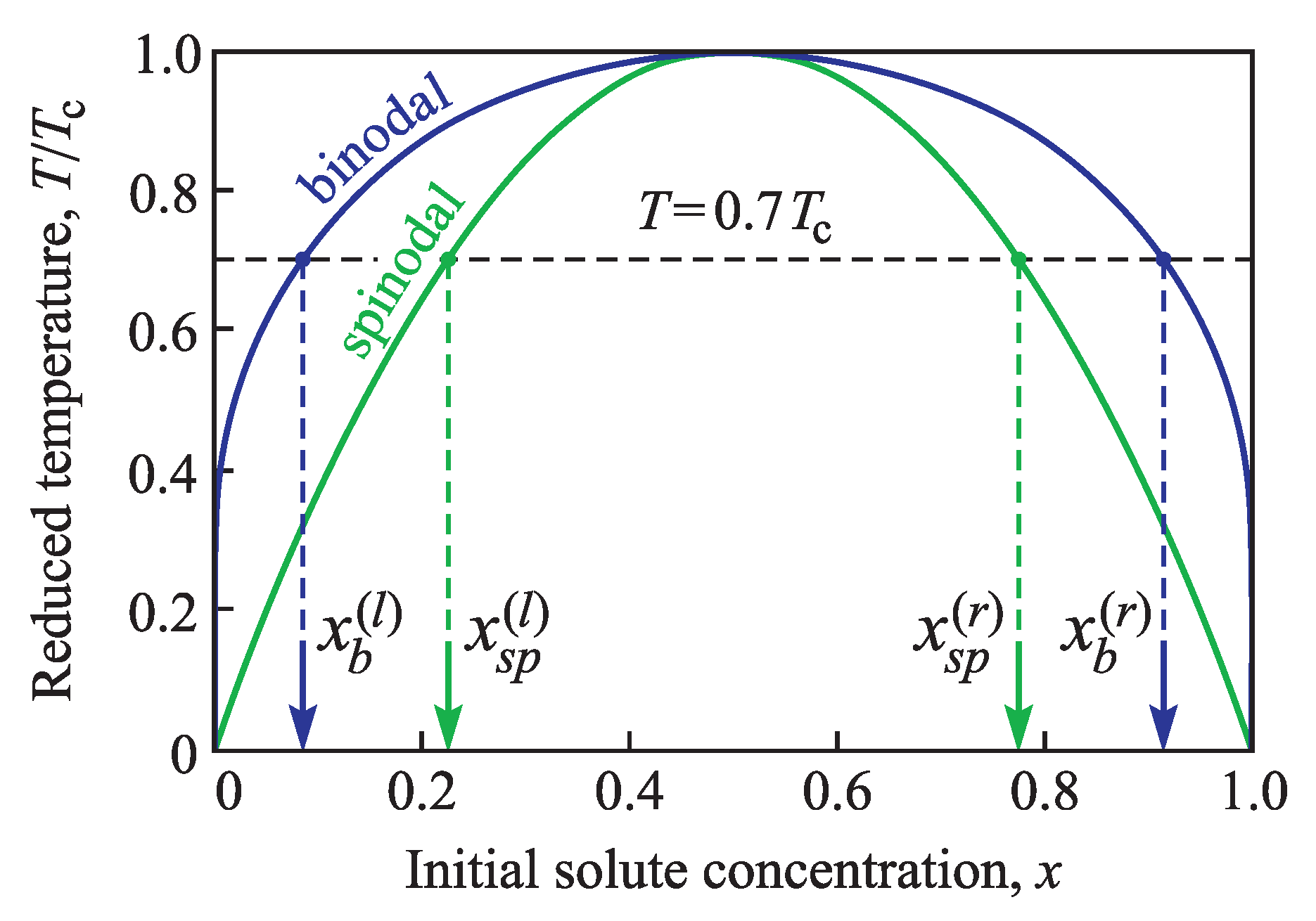
In thermodynamics, the binodal curve is the locus of phase states (in
-diagram) where two distinct phases may coexist in equilibrium. This coexistence curve is defined by the condition at which the chemical potentials of solution components are equal in each phase. The extremum of the binodal curve in temperature is known as a critical point. At this point, the binodal curve coincides with the extremum of the spinodal curve. The spinodal curve in its turn is the locus of the phase states where the system’s local stability with respect to small fluctuations is broken and is defined by the condition that the second derivative of Gibbs free energy (with respect to concentration
x) is zero. Therefore, in our case at constant external pressure the positions of the binodal and spinodal on the phase diagram
are determined by the following equations,
The values of the left binodal (
) and spinodal (
) branches, calculated at temperature
, are, correspondingly
Both curves are symmetric with respect to
; therefore, the corresponding values for the right-hand side branches are
These values are especially distinguished in
Figure 1.
Let us assume that due to the change in temperature or composition, the system is transferred into a metastable state somewhere between left binodal and spinodal (
, see
Figure 1). After this sudden transfer, temperature and composition are maintained unchanged. For this system first we define the parameters of the critical cluster nucleated in a conic pore depending on the created supersaturation (i.e., the molar concentration of the dissolved substance,
x). We remind that as an example of surface imperfections we choose a conic pore. This approach allows us to simplify the analysis and at the same time to receive main results of the defect influence on the nucleation process.
In a binary solution a new phase will be nucleated as a result of the redistribution of molecules (atoms) in space. Following Gibbs model [
6], we consider a new phase cluster as a spatially homogeneous part of the system with a composition, however, different from the ambient phase. The boundary is modeled by a mathematical surface of zero thickness with a corresponding value of the tension surface [
1,
6,
12]. The change of the thermodynamic potential (Gibbs free energy
G) of the binary system owing to the creation of a cluster in form of a spherical cone with the radius
R in a conic pore (
Figure 2) can be given by [
1,
2,
3,
11]
Here specific interphase energies (surface tensions) of the corresponding boundaries are denoted as:
(cluster (
)–pore (
s)),
(outer solution (
)–pore (
s)), and
(cluster (
)–outer solution (
)). Next,
and
are the boundary surface area between the cluster and pore, and the outer solution, correspondingly (
Figure 2),
and
are the chemical potentials of both components (
) in the cluster and outside it (see Equations (
1) and (
2)). The indices
and
denote the parameters of the cluster and the ambient phase, accordingly. For the description of the cluster state the numbers of atoms of a kind 1 and 2 are used as independent variables,
and
(the index
in
and
is omitted to simplify the notations). The total number of atoms in a cluster is
.
For simplification, similarly to [
10,
12], the particle volume
is supposed not to depend on composition (
, where
a is the interatomic distance).
The radius of curvature,
R, of the spherical cone (for simplicity we will name it “cluster radius”) is determined by the number of particles in the cluster,
, via
where
is expressed through the contact angle,
, and the cone angle,
, as
The change in Gibbs free energy due to cluster creation is determined in correspondence with Equation (
7) as [
2,
3,
11]
For a wettable surface (
) at low cone angle of
the cluster outer surface becomes concave, the contribution of the surface component in the work of its formation becomes negative, and the cluster can start to grow in the range
at any initial size. However, this conclusion is correct only for a conic pore which has a sufficiently large depth. Indeed, when the cluster grows up to a flat surface surrounding the pore its surface becomes convex, and, actually, one must consider nucleation on a flat surface [
12]. If a pore is not deep, the cluster does not succeed to grow up to a critical size, and the effect of the pore decreases. This particular case is beyond the scope of the present work, therefore we shall limit ourselves here to the range of angles
(see
Figure 2).
In the derivation of Equation (
11) we have neglected possible modifications of the solution composition caused by the nucleation process. This effect is not essential at an early stage of nucleation for sufficiently large systems. An analysis of the effect of such changes in systems of small sizes is given in [
10,
15]. At such conditions, the function
in Equation (
11) has the meaning of the thermodynamic driving force of cluster formation. It is determined by the relation [
2,
3,
11],
The dependence of function
on cluster composition,
, for different values of the supersaturations,
x, is shown in
Figure 3.
The regions of metastability are in composition ranges
(between the left branches of the binodal and spinodal) and
(between the right branches of the spinodal and binodal). The function
has one maximum and two minima (for
). The first minimum,
, corresponds to the state of the ambient phase. The second minimum,
, corresponds to the final macroscopic state of the precipitating phase, to which a cluster evolves at fixed composition of the surrounding solution,
x. It is determined by the minimum of the bulk contribution to the Gibbs free energy (
Figure 3),
At the spinodal,
, the function
has an inflection point corresponding to
,
. The range
is thermodynamically unstable. The maximum of the function
in this region corresponds to the initial state,
, there are also two local minima of the function
at
and
. Similar to Equation (
14) they are determined by the equation
Figure 4 illustrates the dependence of the concentrations
and
on the initial composition
x of the surrounding solution in the whole possible range of compositions. Taking into account the symmetry with respect to the substitution
, we consider only initial states with a composition
.
At any given pressure and temperature, the thermodynamic driving force for cluster formation should be positive, i.e.,
(the bulk contribution to Gibbs free energy is decreased in this case [
11]). This condition holds for
where
is the solution of the equation
The function
is represented in
Figure 4 by a dashed line, and the composition of the critical cluster for homogeneous nucleation
is shown by a solid line. The evolution of an initially metastable state proceeds along the following path:
. It starts at the initial state
and propagates through a critical cluster (
C) to
. For an initially unstable state two variants of evolution are possible, first
, with a decrease of the cluster concentration to
, and second
, with an increase in the concentration up to
. Generally, the inequality
holds, it goes over to an equality only at
and
. In the latter case, when
, the cluster can evolve without overcoming a potential barrier. This process corresponds to spinodal decomposition. As will be shown below, at heterogeneous nucleation in a conic pore a similar situation can arise even at appreciably smaller supersaturations
.
The Young equation determines the mechanical equilibrium at the contact line of three phases [
1,
2,
3,
4,
5]
Assuming that this condition is fulfilled and taking into account Equation (
9) we obtain from Equation (
12) the interfacial contribution of a new phase cluster to the Gibbs free energy as
Thus, the work of cluster formation at heterogeneous nucleation in a conic pore can be written as
where
and the catalytic factor
is determined by Equation (
9). Equations (
19)–(
21) are fulfilled at all possible values of the cone pore angle
, contact angle
, and radius
R (note, however, that here we do not consider the case of a concave outer surface of the cluster, when
). Equation (
19) is similar to that obtained for heterogeneous nucleation on a smooth planar surface [
11] and differs only by the factor
(which equals unity for a flat surface, when
). The catalytic factor
within the framework of the generalized Gibbs approach becomes dependent not only on the angles
and
, but also on the compositions of the initial phase and the critical cluster. The specific interfacial energy between two phases with compositions
and
x, can be expressed according to Becker [
14] (see also [
16]) as
Here is the respective value, when a new phase cluster and the solution surrounding it are in equilibrium, i.e., and .
For further analysis it is convenient to introduce the dimensionless variables
In these variables Equation (
20) takes the form
As already was noted above,
R is the radius of the cluster surface contacting with the ambient solution, and it can have positive, infinite, and negative values. Therefore, it is more convenient to use as independent variables for the description of the cluster state the numbers of atoms in the cluster
instead of
. Also is convenient to normalize these quantities to
as
To simplify the notations we omit primes, then Equation (
26) takes the form
where
. The parameters of the critical cluster,
, are determined by a solution of the set of equations
The work of formation of the critical cluster is determined by
Above relations are the basic for the subsequent analysis of surface roughness on the properties of critical clusters in heterogeneous nucleation on rough surfaces. This analysis we will start with the discussion of the contact angle.
3. Determination of the Contact Angle
In classical nucleation theory the parameters of a cluster are taken to be widely equal to the properties of the newly evolving macroscopic phase. By this reason, the values of the specific surface energies in Young’s equation, Equation (
18), are constants for some given values of pressure and temperature. Consequently, the contact angle is also constant. In the generalized Gibbs approach, parameters of a new phase cluster are functions of the supersaturation, therefore the contact angle
and, consequently, the catalytic factor
also depend on supersaturation.
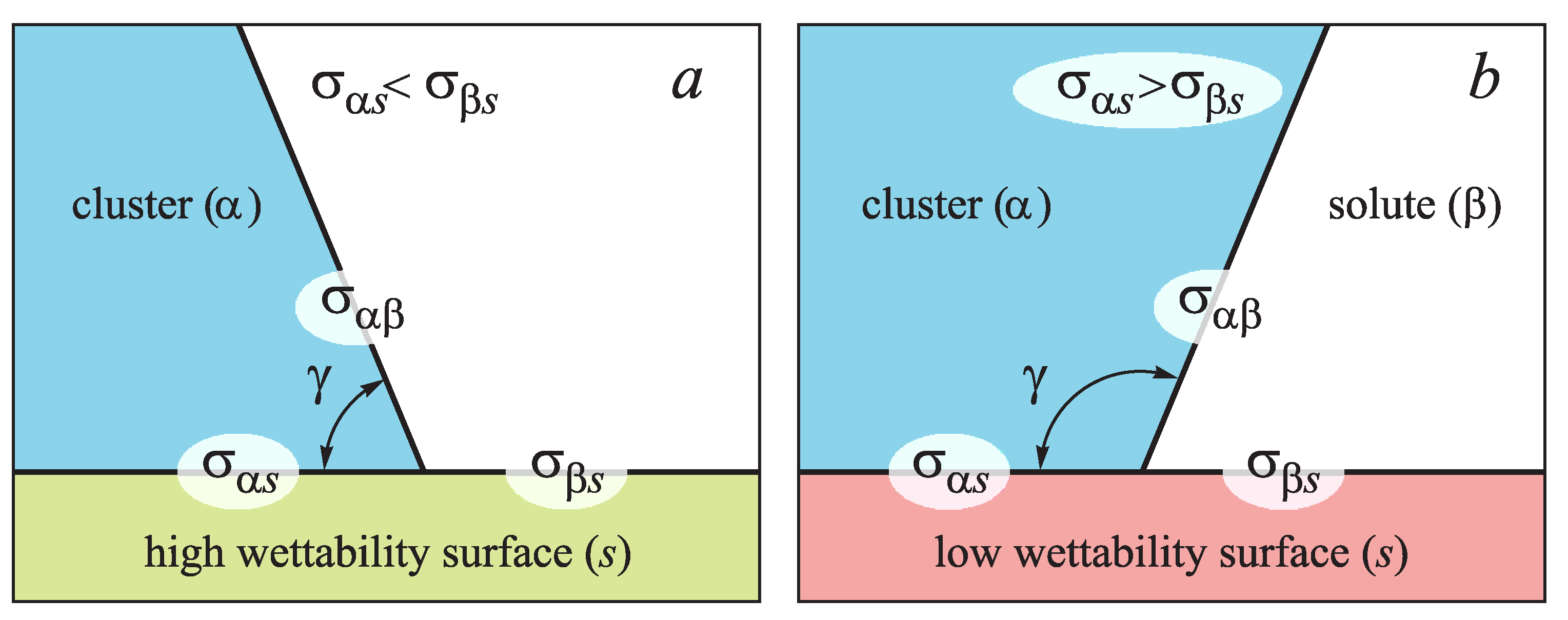
For the case when the surface tension of the cluster boundary with the pore surfaces is less than that between solutions and the same surfaces (
), and the contact angle determined by Young Equation (
18), as
is less than 90
, the surface is well wettable (
Figure 5a). Otherwise,
, the contact angle is larger than 90
, the surface is badly wettable (
Figure 5b). In the present work we consider only the first case, when wettability is good and the influence of surface defects becomes most apparent.
For the determination of the contact angle one must know the specific interfacial energies of all boundaries as functions of the cluster and surrounding solution compositions (for unification of the notations we shall use the term “fluid” both for cluster and solution and denote it with a subindex “f”) in the whole range from the left binodal,
, up to the right binodal,
. It is easy to show that in a simple linear approximation the specific interfacial energy of the fluid-surface interphase can be expressed as (details see [
13])
Here, as above (see Equation (
23)), the index “0” relates to the case, when a new phase cluster and surrounding solution are in equilibrium, i.e.,
and
, and the quantities without index “0” denote parameters for current composition (cluster or solution). From Equation (
32) it follows that
It is evident that the difference is a linear function of . It is positive when (good wetting) and is negative when (bad wetting) in correspondence with above-stated definition. This difference determines the degree of catalytic activity of the solid surface at heterogeneous nucleation.
From Equation (
23) with allowance for Equations (
31) and (
33) we obtain an expression determining the contact angle
as a function of the compositions of the cluster,
, and of the surrounding solution,
x,
where
Thus, for the further analysis there is no need in the knowledge of the specific interfacial energies; it merely required to know the equilibrium contact angle .
In the considered case of cluster nucleation on a well-wettable surface the angle
, defining the catalytic activity factor Equation (
9), has values larger zero only when
where
is determined by the equation
Intersection of the plots
and
(see Equations (
16) and (
17) and
Figure 6) determines the position of the spinodal
for heterogeneous nucleation
At
the catalytic activity factor equals zero,
, i.e., in this case the nucleation of a new phase cluster in a pore proceeds in a mode when the energy barrier is absent, like spinodal decomposition of the unstable homogeneous system.
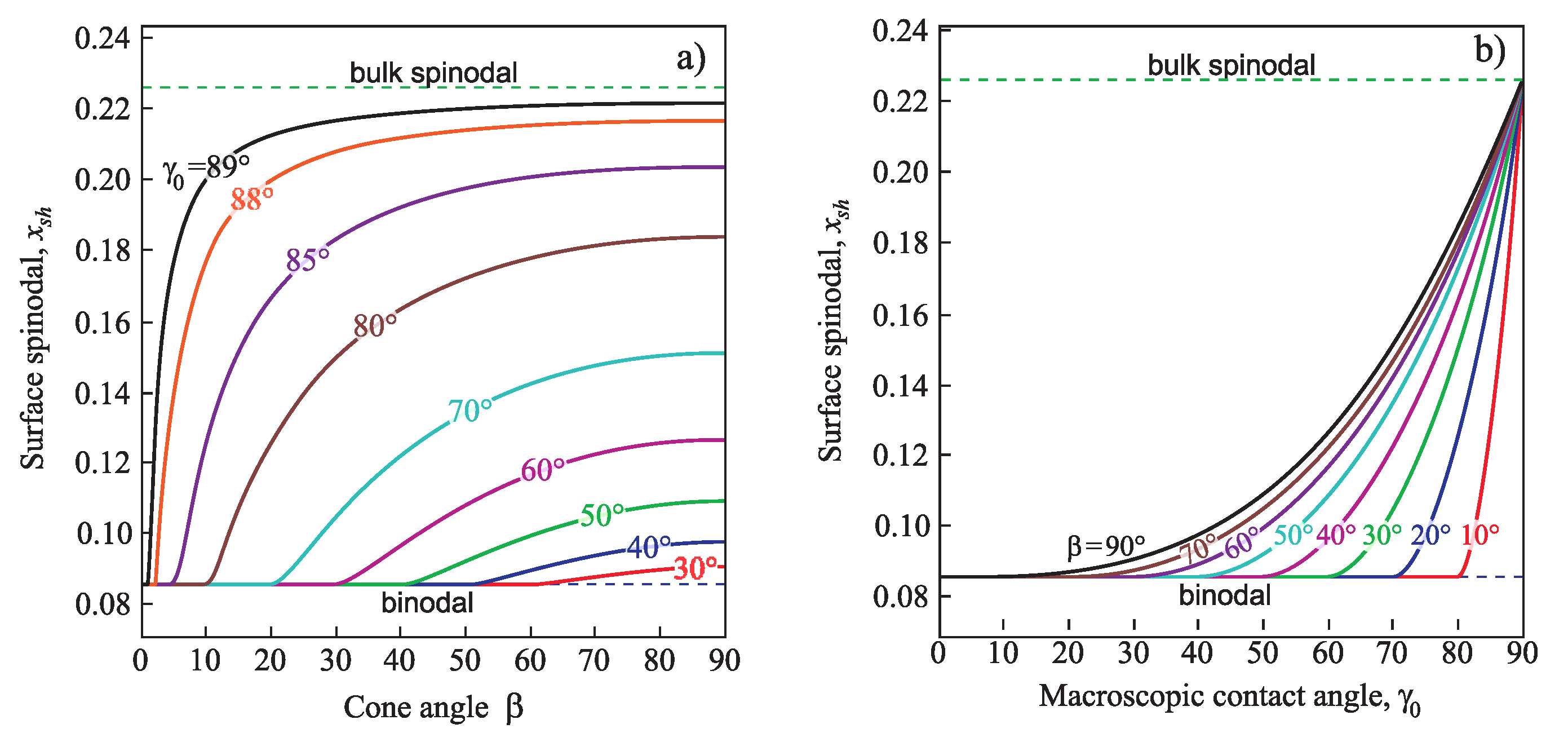
Figure 7 presents the dependence of the heterogeneous spinodal position
on cone angle
and contact angle
.
Is evident that the spinodal for heterogeneous nucleation is located nearer to the binodal as both the pore cone angle
(
Figure 7a) and the macroscopic contact angle
(
Figure 7b) yield its shift to decreasing values of
x. If the equilibrium contact angle is equal to
, the heterogeneous spinodal coincides with the macroscopic one, i.e.,
, like in the case of the homogeneous nucleation.
4. Heterogeneous Nucleation in a Conic Pore: Results
For a metastable state of the initial solution,
, the work of critical cluster formation in the space
has characteristic saddle points properties near to the parameters of the critical cluster,
. The surface is shown in
Figure 8 for the case of nucleation of a new phase cluster in a pore with an angle
and for an equilibrium contact angle
and at the composition of the ambient phase equal to
. The “valley” at
corresponds to the initial state, and the saddle point to the critical cluster. Its parameters are determined by Equations (
29). In the course of its growth, the new phase cluster passes through a saddle point. Finally, its composition tends to an equilibrium value nearly equal to the respective value on the right binodal
.
The composition of a critical cluster,
, is shown in
Figure 9 and
Figure 10 in dependence on the initial supersaturation for the case of nucleation in a conic pore with various angles
and two different values of the equilibrium contact angle
(
Figure 9) and
(
Figure 10). With an increase of the supersaturation from an initial value close to the binodal the concentration of the second component in the critical cluster,
, decreases down to the minimum value
at
(
Figure 9 and
Figure 10). If the supersaturation increases further,
grows linearly (see Equation (
36) and
Figure 6).
According to the classical nucleation theory the size of a critical cluster tends to infinity for initial phase composition approaching the binodal. With an increase of the supersaturation grows the critical cluster size decreases. At
, the critical size in its classical interpretation does not exist anymore because cluster growth can proceed without overcoming a thermodynamic potential barrier starting from
(or, in a more precise formulation, starting with one structural unit). However, in terms of the generalized Gibbs approach, in contrast to the classical theory and in agreement with density functional computations [
7,
8], this decrease in size may be followed by an increase with a further increase of the supersaturation (
Figure 11 and
Figure 12). Consequently, the transition from metastable to thermodynamically unstable states proceeds here in a quite different way. As one consequence it follows that near to the spinodal the formation of critical clusters will, in general, not proceed via the saddle point of the thermodynamic potential surface but via a ridge point (for details see [
17,
18]).
For the equilibrium contact angle
and small values of the cone pore angles
, along with the increase of the supersaturation the critical cluster size decreases monotonically from infinity at the binodal up to values of
x at the spinodal for heterogenous nucleation
(
Figure 11). Then it exhibits a discontinuity and becomes equal to zero at further increase of the supersaturation. For planar solids surfaces
, the decrease of the critical cluster size with increasing supersaturation is followed by its further increase. This increase is then also followed by a similar discontinuity at
.
When the equilibrium contact angle equals
and the cone pore angle has values in the range
the dependence of the critical cluster size on supersaturation is non-monotonic: first
decreases from infinity at the binodal, then the decrease is followed by its growth up to
, and for
the critical cluster size becomes equal to zero (
Figure 12).
Figure 13 and
Figure 14 illustrate the normalized work of formation of a critical cluster,
, in dependence on supersaturation for nucleation in conic pores with various angles
, and
, the equilibrium contact angle is
(
Figure 13) and
(
Figure 14). The work of formation of a cluster decreases from infinity at the binodal, and for
it becomes equal to zero. The less the cone pore angle
and the contact angle
are, the faster the work of a critical cluster formation decreases.
The work of critical cluster formation determines widely the steady-state nucleation rate,
J. It can be expressed generally as (see, for example [
1,
4])
The pre-exponential factor,
, is determined by the diffusion coefficients of the solution and by the number of possible nucleation centers per unit area. The quantity
is determined by Equation (
25).
Figure 15 and
Figure 16 supply us with a comparison of the normalized nucleation rates,
, in conic pores with various angles
, and
determined within the generalized (solid lines) and via the classical Gibbs (dotted line) approaches in the case of good wettability (
and
). The calculations were performed for a temperature
with
K and the parameters
and
with
m. The nucleation rate calculated via the generalized Gibbs approach is much higher than the results obtained via the classical theory. With the increase of the supersaturation it reaches the maximum value,
, at
.