Study on a Quaternary Working Pair of CaCl2-LiNO3-KNO3/H2O for an Absorption Refrigeration Cycle
Abstract
1. Introduction
2. Experiments
2.1. Materials
2.2. Apparatus and Methods
3. Results and Discussion
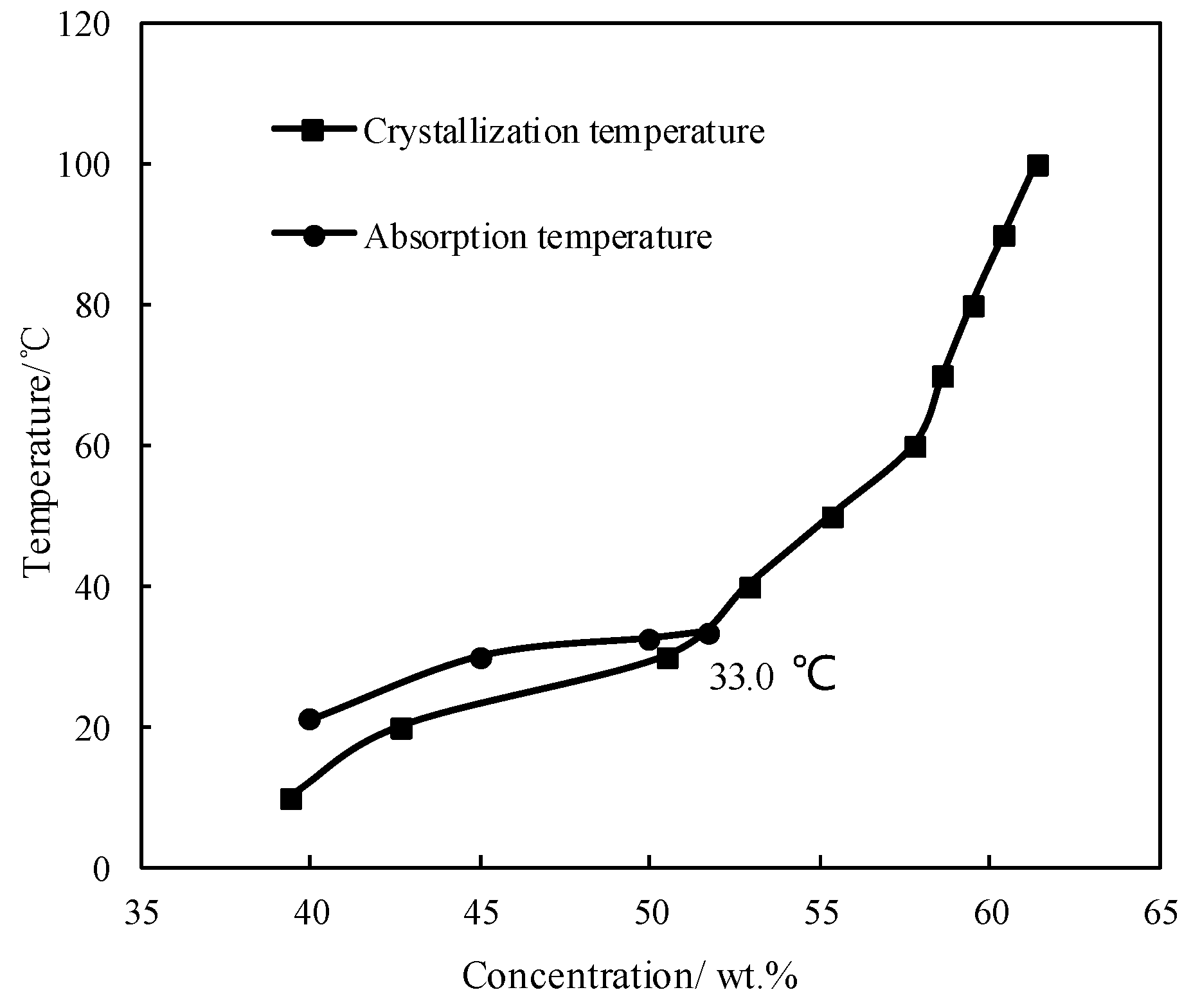
3.1. CaCl2/H2O
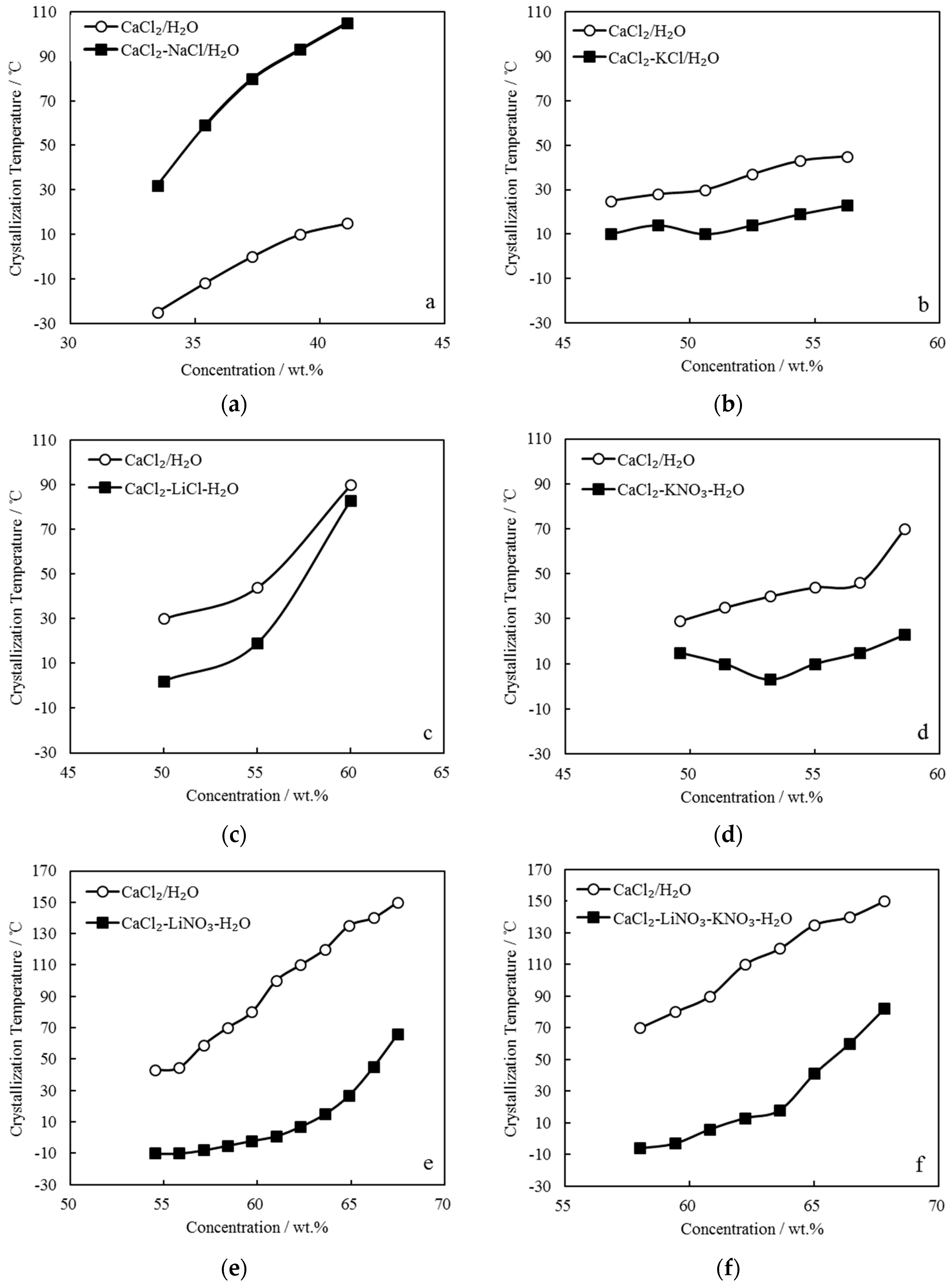
3.2. Measurement of Crystallization Temperature TC
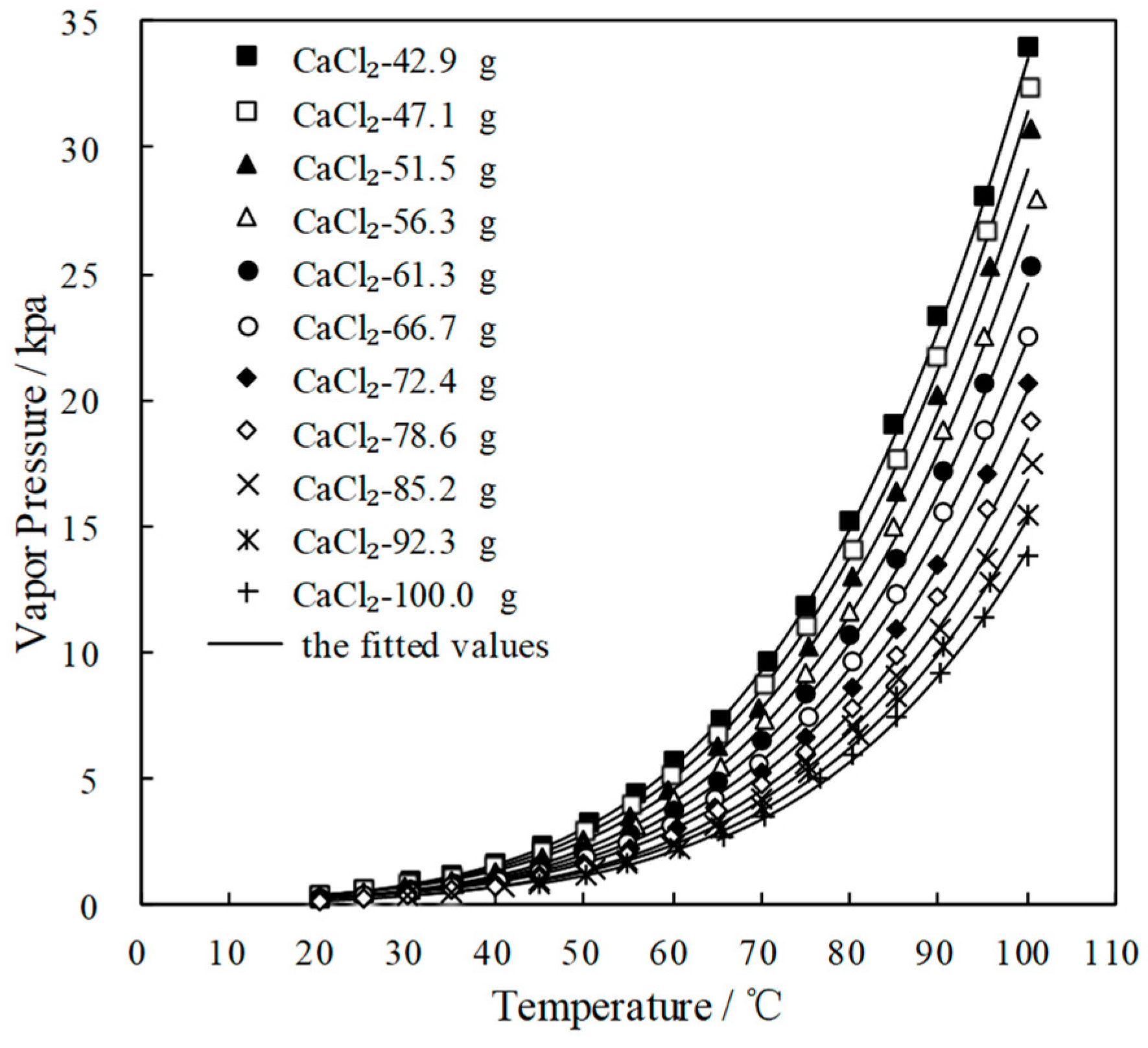
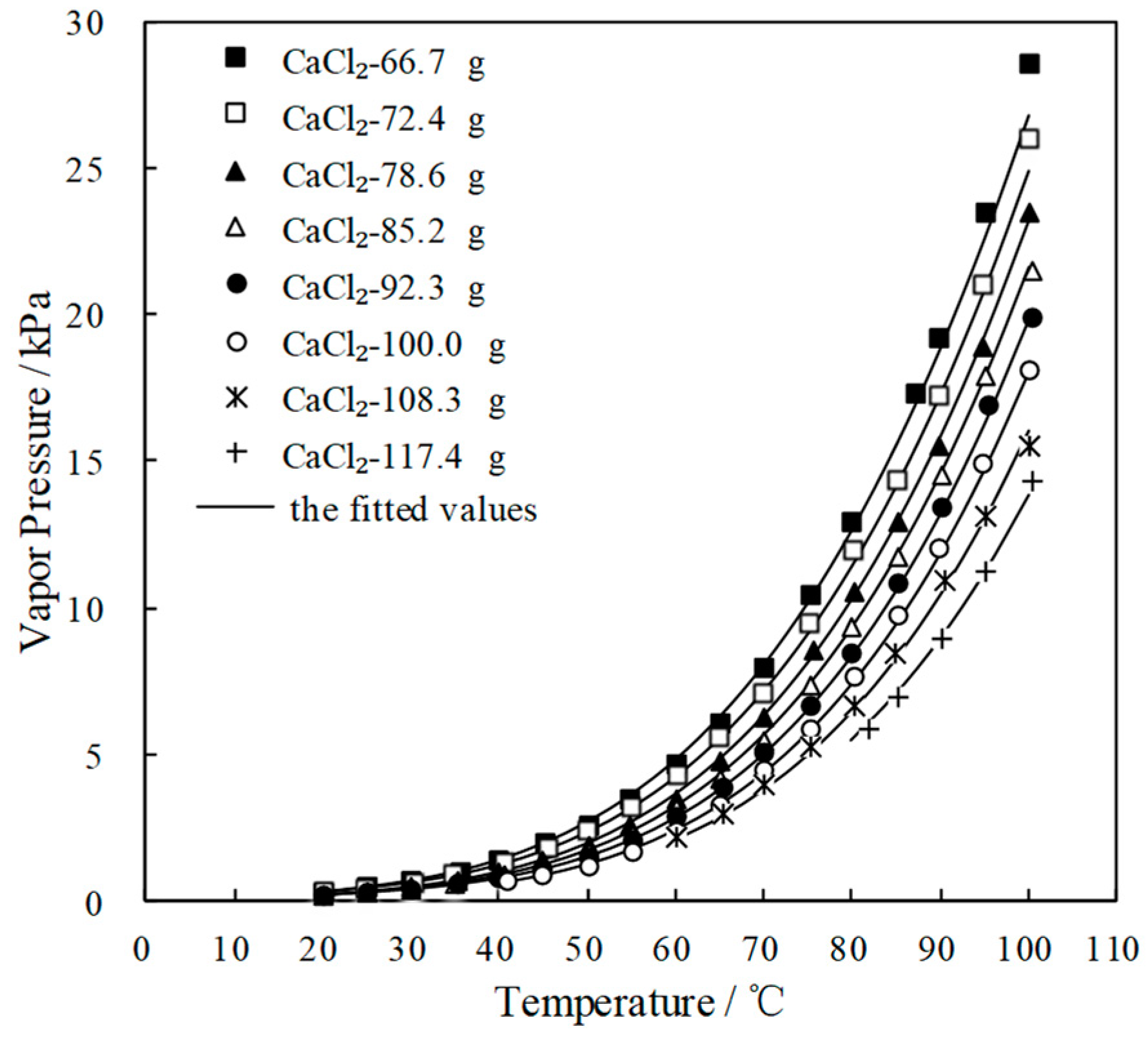
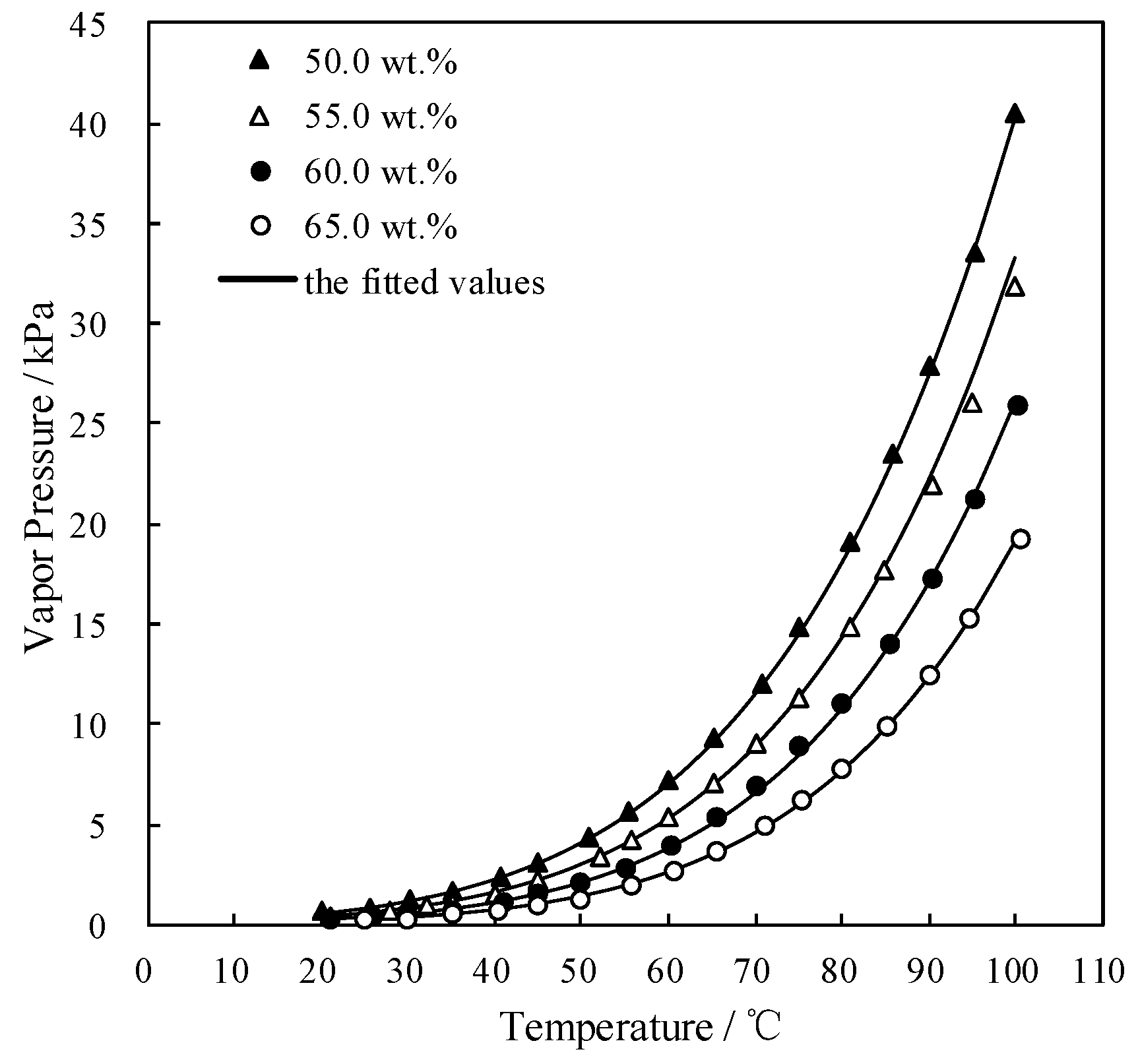
3.3. Measurement of Saturated Vapor Pressure p
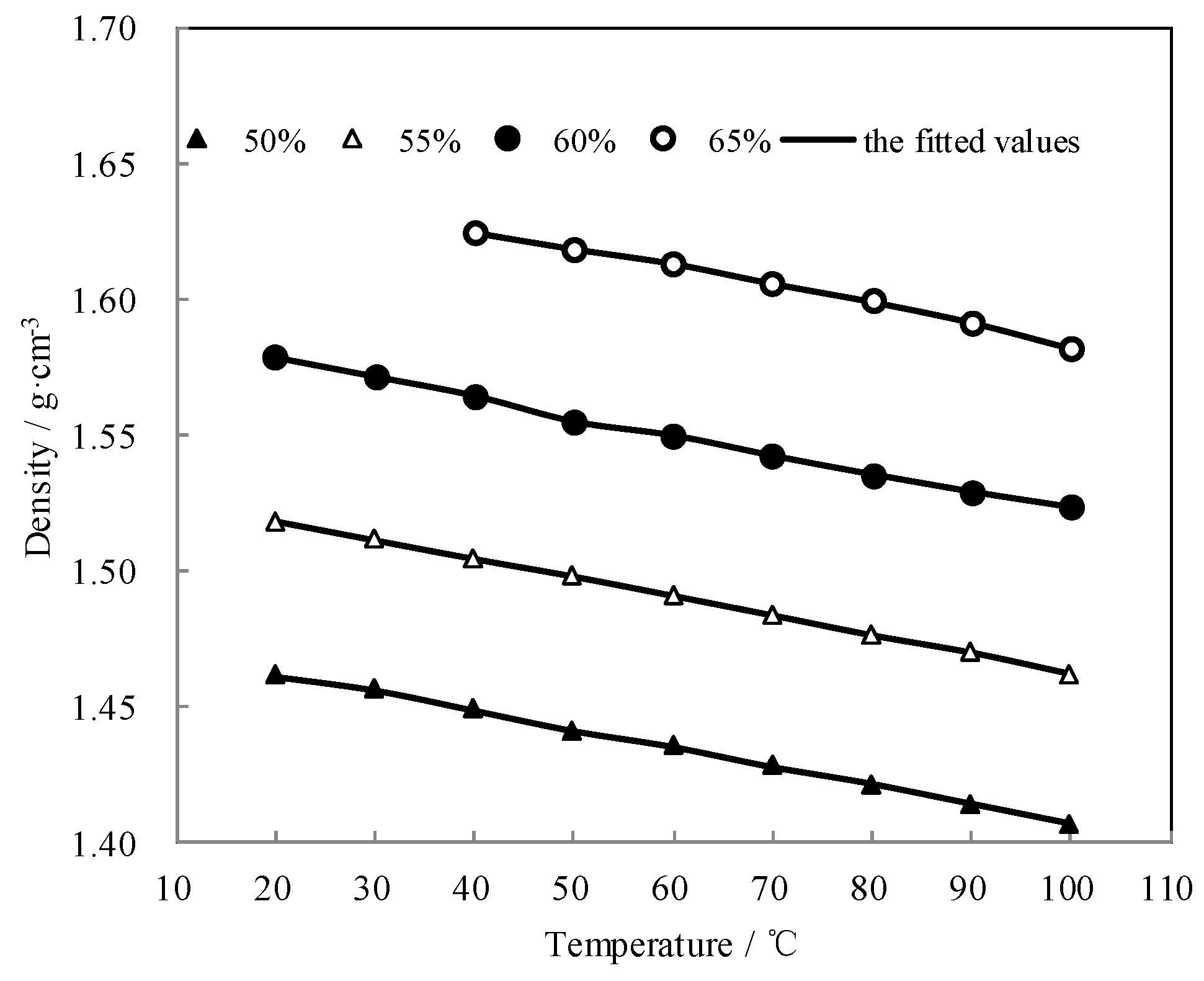
3.4. Measurement of Density ρ
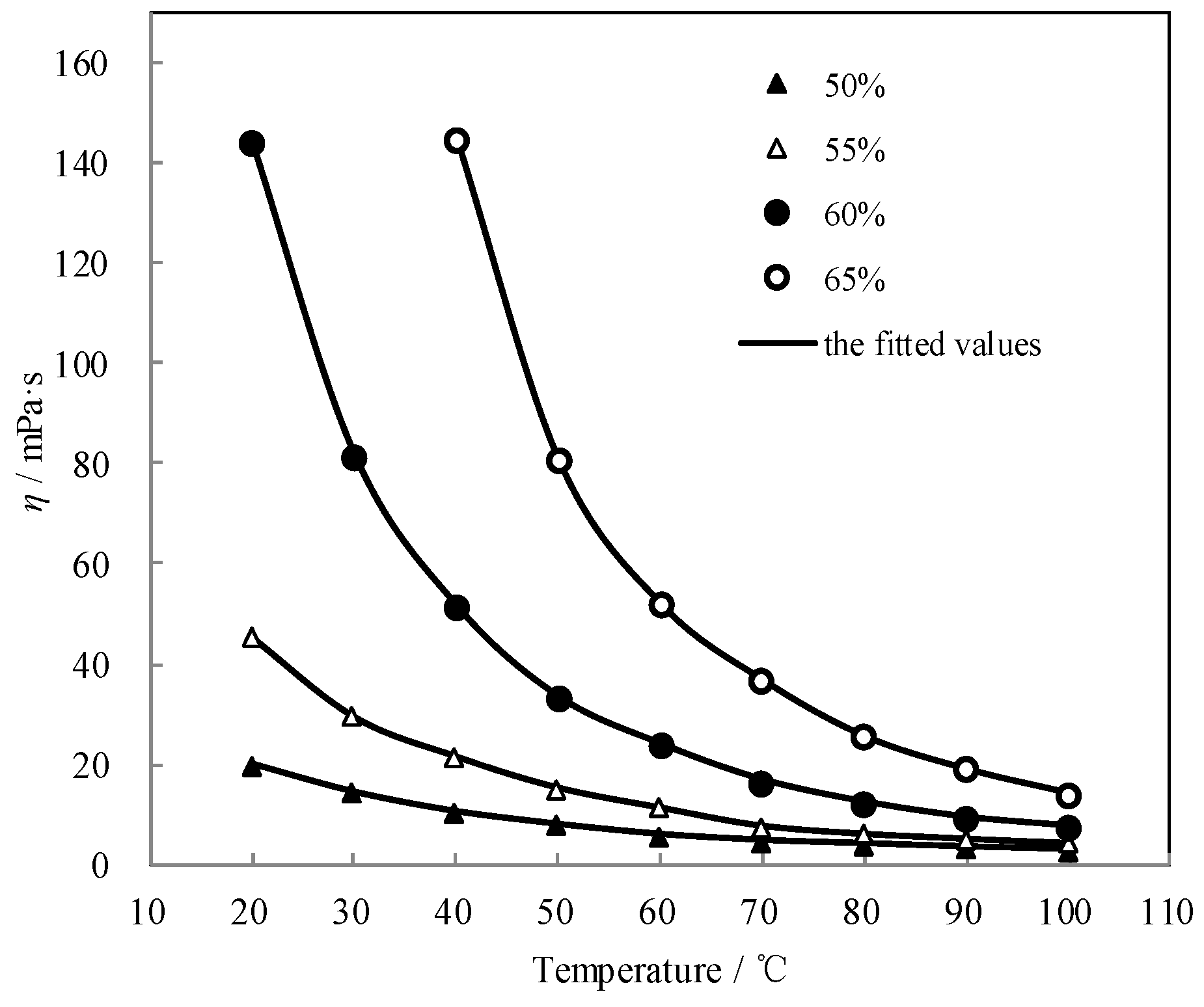
3.5. Measurement of Viscosity η
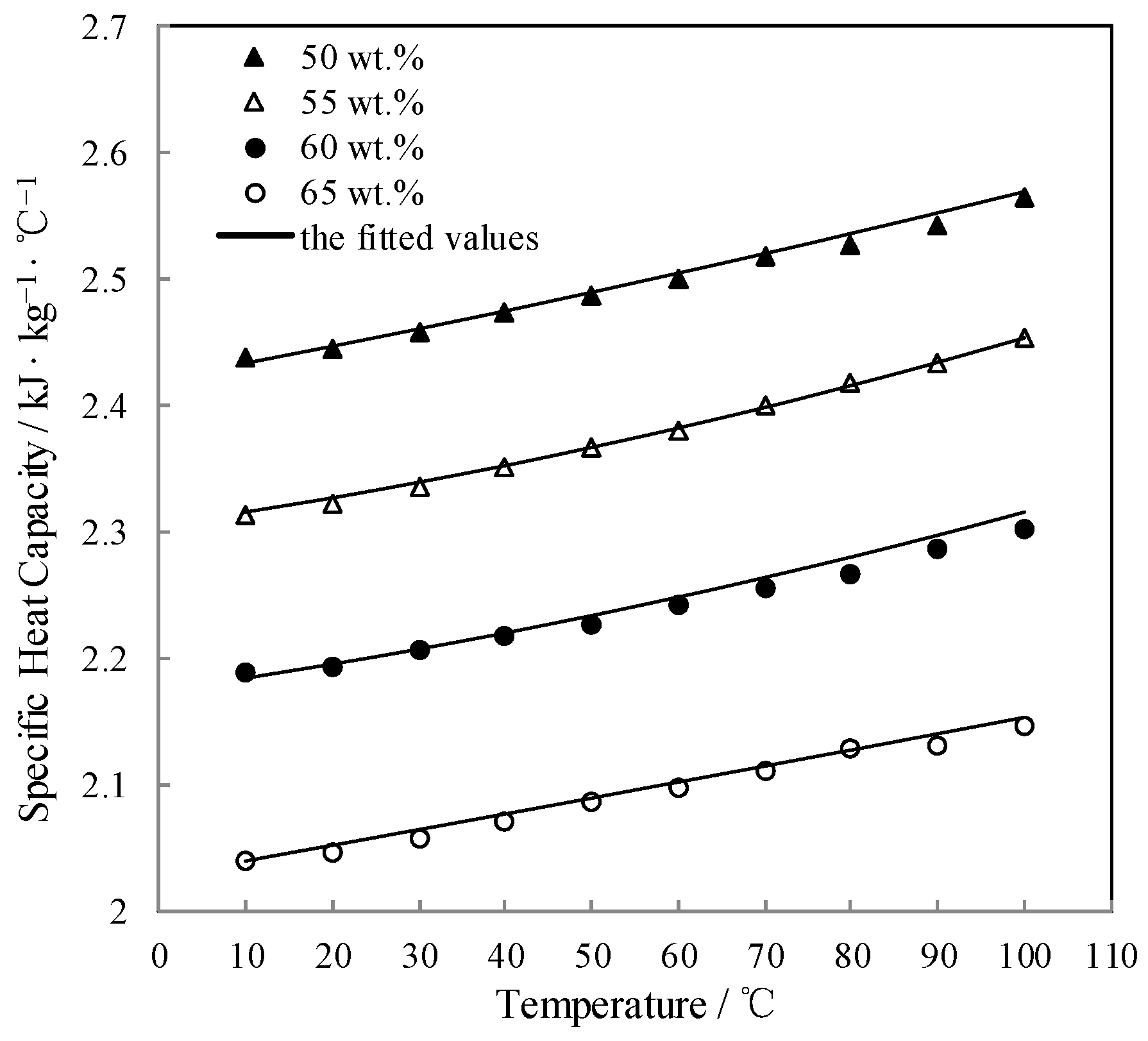
3.6. Measurement of Specific Heat Capacity Cp
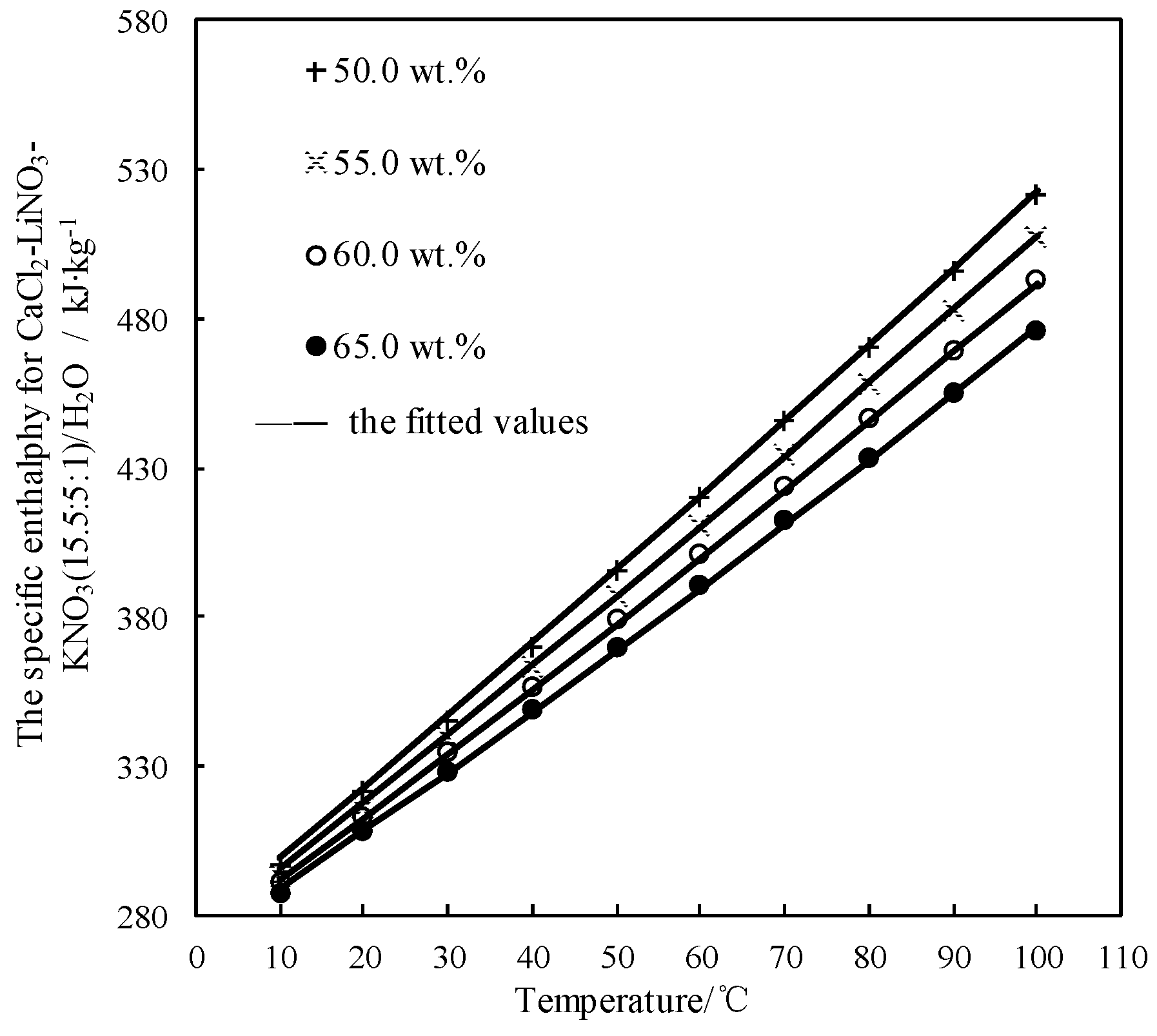
3.7. Calculation of Specific Enthalpy h
3.7.1. Cp of CaCl2, LiNO3, KNO3 and H2O
3.7.2. Measurement of Dissolution Enthalpy ΔHmix
3.7.3. Calculation of Specific Enthalpy h
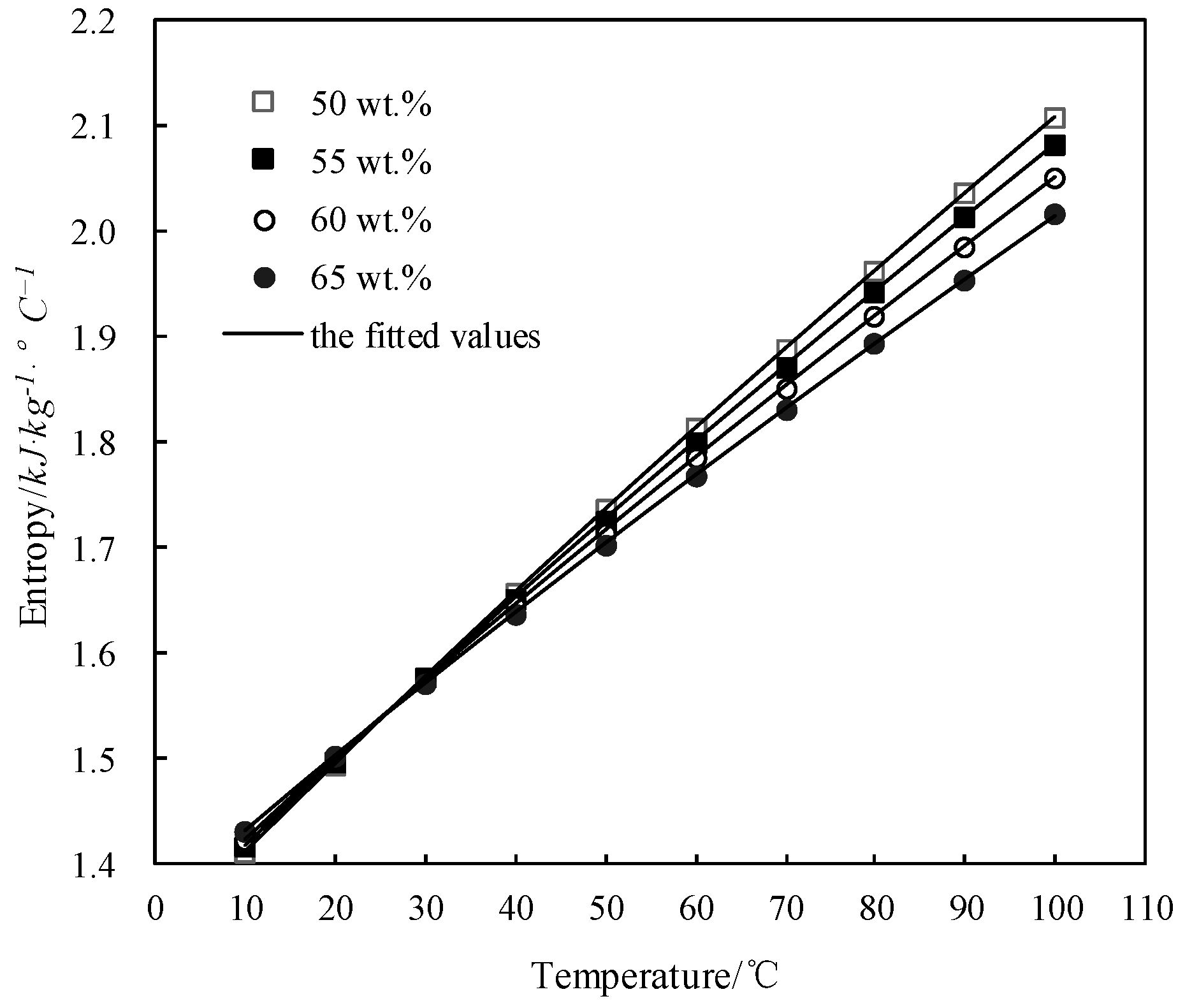
3.8. Calculation of Specific Entropy s
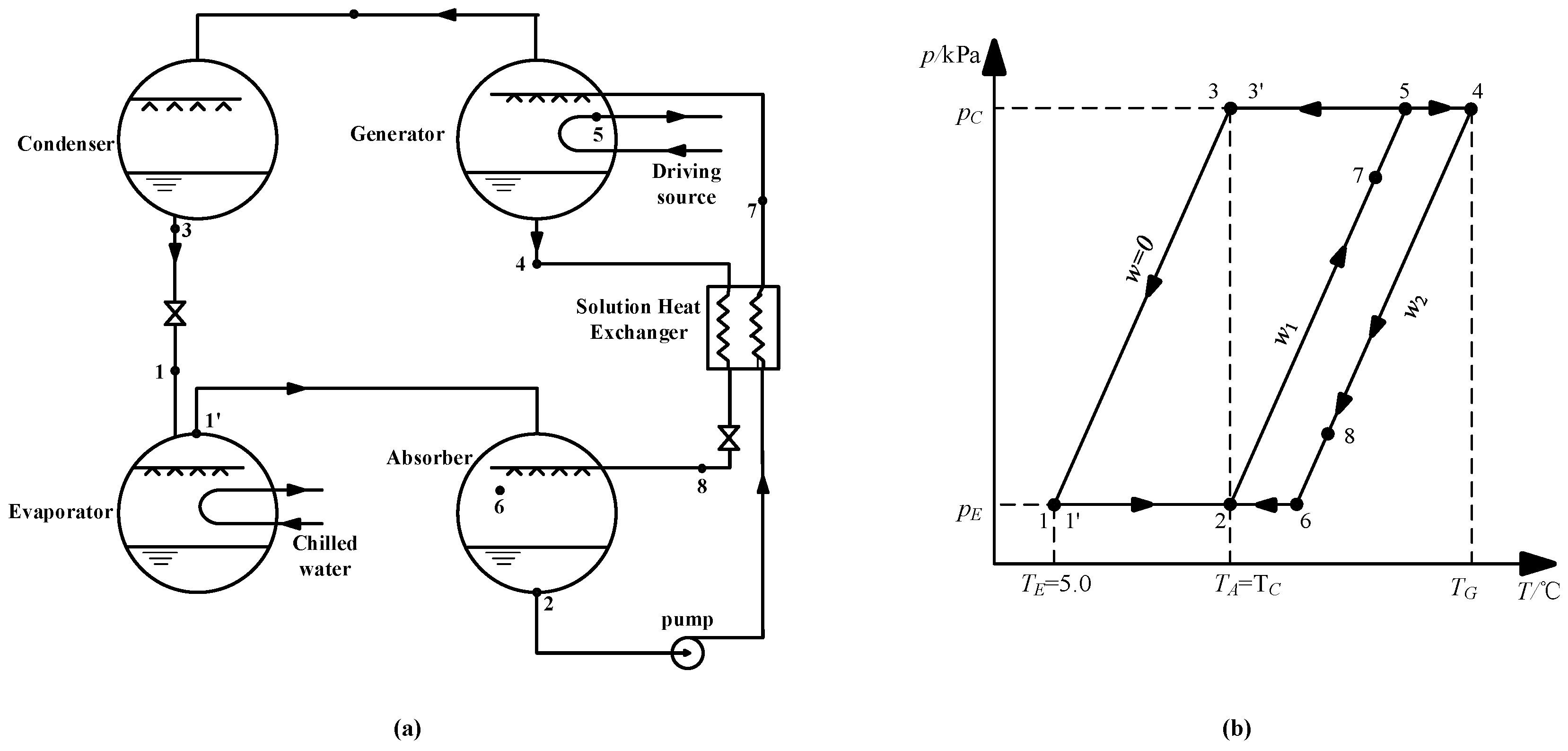
3.9. Application for an Absorption Refrigeration Cycle
3.9.1. Absorption Refrigeration Cycle Using CaCl2-LiNO3-KNO3(15.5:5:1)/H2O
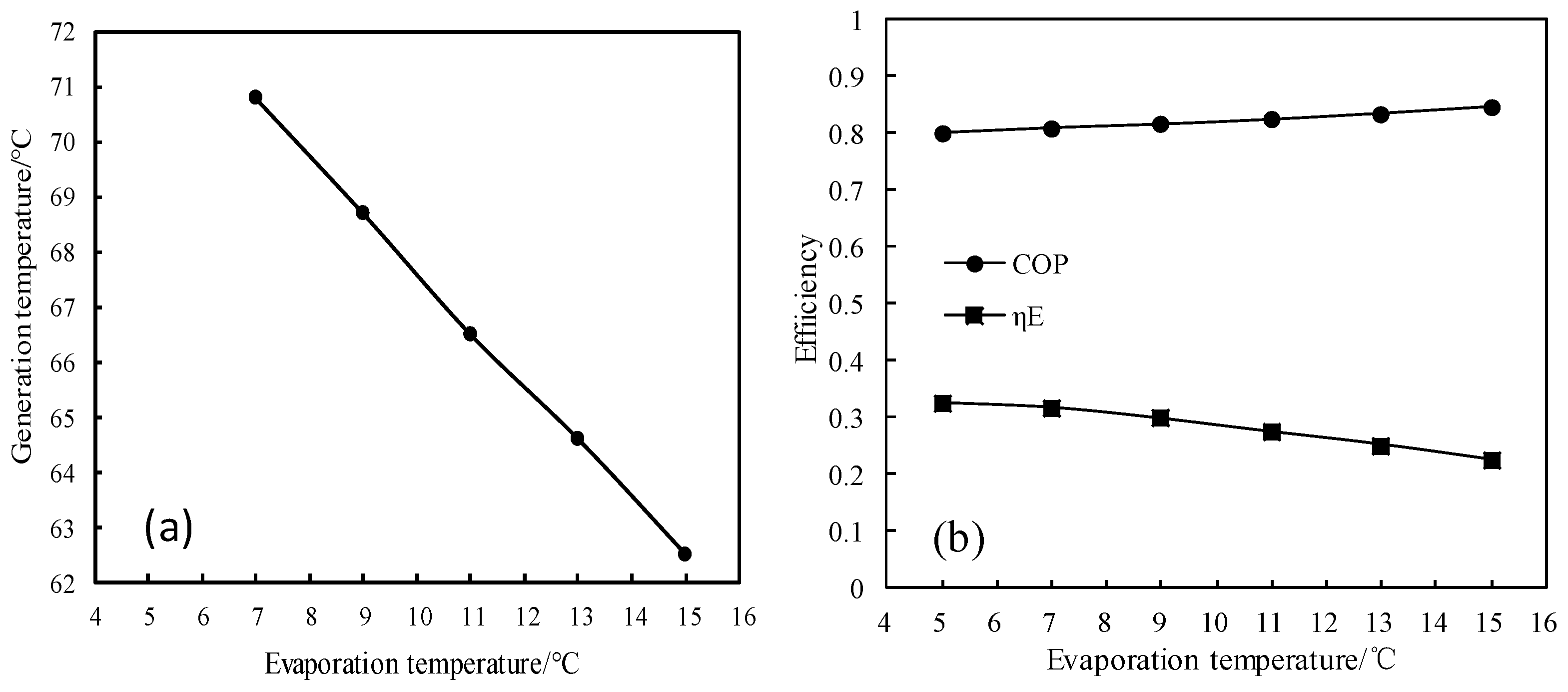
3.9.2. Analysis of COP and Exergy Efficiency
3.10. Measurement of Corrosion Rate RC
4. Conclusions
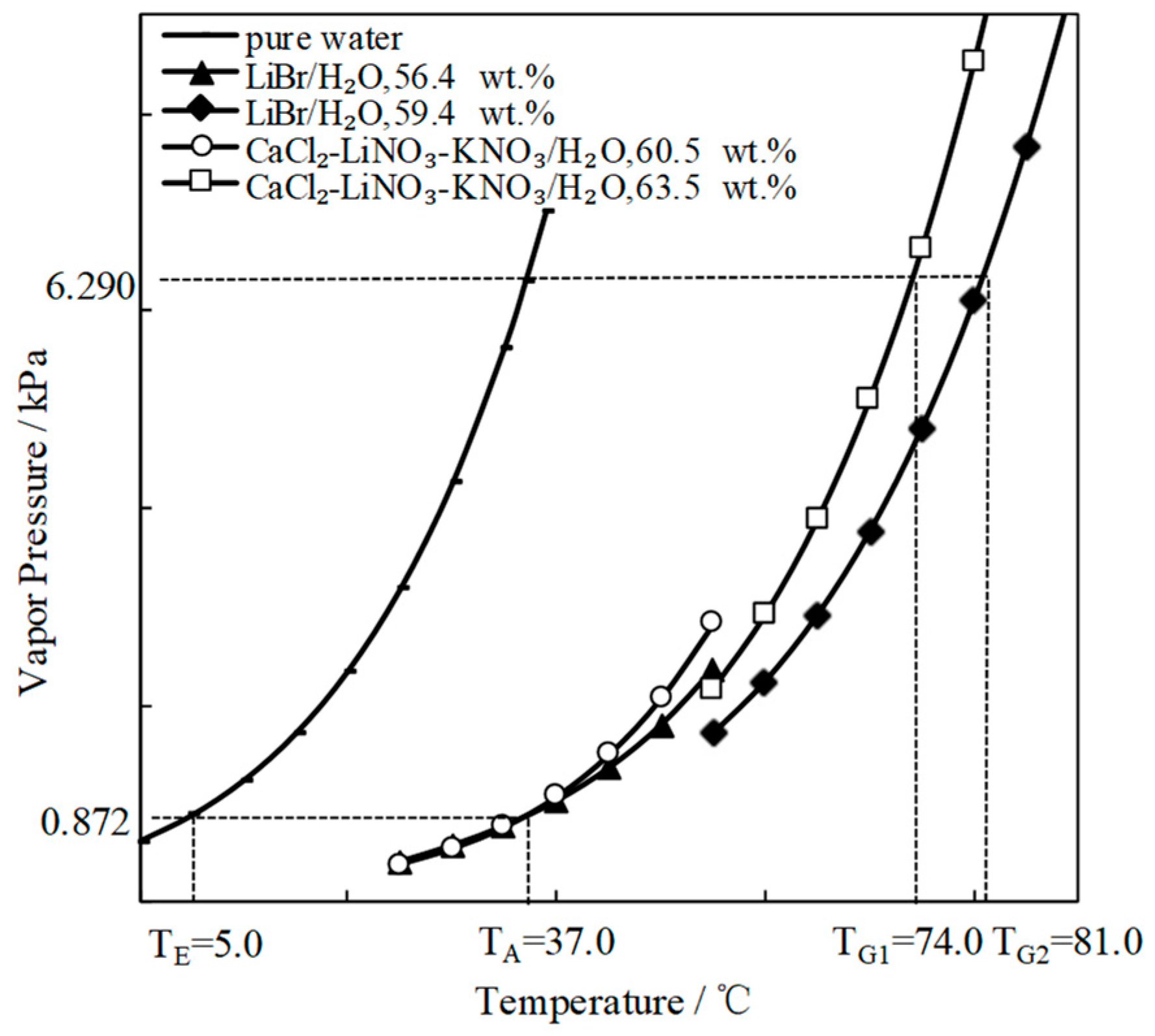
- When compared with LiBr/H2O, for an identical adsorption temperature at 0.872 kPa, which is a typical pressure of absorber, CaCl2/H2O had a lower absorption temperature at 6.290 kPa, which is a typical pressure of generator, meaning that CaCl2/H2O basically had a better refrigeration characteristic for an absorption refrigeration cycle. However, the absorption ability of CaCl2/H2O was not strong enough for achieving an evaporation temperature of 5 °C or lower, because of its high crystallization temperature.
- The crystallization temperature was significantly lowered when combining CaCl2/H2O with LiNO3 or LiNO3+KNO3. As a result, the absorption ability of CaCl2-LiNO3/H2O or CaCl2-LiNO3-KNO3/H2O was essentially improved.
- For an absorption refrigeration cycle using CaCl2-LiNO3-KNO3(15.5:5:1)/H2O as the working pair, the generation temperature that is required for achieving an evaporation temperature of 5 °C was 74.0 °C, which was 7.0 °C lower than that using LiBr/H2O.
- When compared with LiBr/H2O under the same conditions, COP and ηE of the absorption refrigeration cycle with CaCl2-LiNO3-KNO3(15.5:5:1)/H2O were improved by 0.04 and 0.06, respectively.
- RC of carbon steel and copper in 63.5 wt.% solution of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O at 80.0 °C and pH 9.7 were 14.31 and 2.04 μm∙y−1, respectively, which indicated that the corrosivity of the proposed working pair could meet the requirements for practical applications.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| T | temperature, °C |
| w | mass concentration, % |
| TC | crystallization temperature, °C |
| p | saturated vapor pressure, kPa |
| ρ | density, g·cm−3 |
| η | dynamic viscosity, mPa·s |
| Cp | specific heat capacity, kJ·kg−1·K−1 |
| ΔHmix | dissolution enthalpy, kJ·kg−1 |
| h | specific enthalpy, kJ·kg−1 |
| s | specific entropy, kJ·kg−1·K−1 |
| AARD | average absolute relative deviation |
| a | circulation ratio |
| COP | coefficient of performance |
| E | exergy |
| ΔE | exergy destrction |
| ηE | exergy efficiency |
| RC | corrosion rate, μm∙y−1 |
References
- Srikhirin, P.; Aphornratana, S.; Chungpaibulpatana, S. A review of absorption refrigeration technologies. Renew. Sustain. Energy Rev. 2001, 5, 343–372. [Google Scholar] [CrossRef]
- Wang, C. Application and development of absorption refrigeration technology. Energy Technol. 2000, 21, 31–35. [Google Scholar]
- Hong, D.; Tang, L.; He, Y. A novel absorption refrigeration cycle. Appl. Therm. Eng. 2010, 30, 2045–2050. [Google Scholar] [CrossRef]
- Abed, A.M.; Alghoul, M.A.; Sopian, K.; Majdi, H.S.; Al-Shamani, A.N.; Muftah, A.F. Enhancement aspects of single stage absorption cooling cycle: A detailed review. Renew. Sustain. Energy Rev. 2017, 77, 1010–1045. [Google Scholar] [CrossRef]
- Bellos, E.; Tzivanidis, C.; Antonopoulos, K.A. Exergetic, energetic and financial evaluation of a solar driven absorption cooling system with various collector types. Appl. Therm. Eng. 2016, 102, 749–759. [Google Scholar] [CrossRef]
- Leonzio, G. Solar systems integrated with absorption heat pumps and thermal energy storages: State of art. Renew. Sustain. Energy Rev. 2017, 70, 492–505. [Google Scholar] [CrossRef]
- Alobaid, M.; Hughes, B.; Calautit, J.K.; O’Connor, D.; Heyes, A. A review of solar driven absorption cooling with photovoltaic thermal systems. Renew. Sustain. Energy Rev. 2017, 76, 728–742. [Google Scholar] [CrossRef]
- Mehrabian, M.A.; Shahbeik, A.E. Thermodynamic modelling of a single-effect LiBr-H2O absorption refrigeration cycle. Proc. Inst. Mech. Eng. Part E-J. Process Mech. Eng. 2005, 219, 261–273. [Google Scholar] [CrossRef]
- Izquierdo, M.; Lizarte, R.; Marcos, J.D.; Gutiérrez, G. Air conditioning using an air-cooled single effect lithium bromide absorption chiller: Results of a trial conducted in Madrid in August 2005. Appl. Therm. Eng. 2008, 28, 1074–1081. [Google Scholar] [CrossRef][Green Version]
- Kaushik, S.C.; Arora, A. Energy and exergy analysis of single effect and series flow double effect water–lithium bromide absorption refrigeration systems. Int. J. Refrig. 2009, 32, 1247–1258. [Google Scholar] [CrossRef]
- Sumathy, K.; Huang, Z.; Li, Z. Solar absorption cooling with low grade heat source—A strategy of development in South China. Sol. Energy 2002, 72, 155–165. [Google Scholar] [CrossRef]
- Li, Z.; Jing, Y.; Liu, J. Thermodynamic study of a novel solar LiBr/H2O absorption chiller. Energy Build. 2016, 133, 565–576. [Google Scholar] [CrossRef]
- Sun, J.; Fu, L.; Zhang, S. A review of working fluids of absorption cycles. Renew. Sustain. Energy Rev. 2012, 16, 1899–1906. [Google Scholar] [CrossRef]
- N’tsoukpoe, K.E.; Perier-Muzet, M.; Le-ierrès, N.; Luo, L.; Mangin, D. Thermodynamic study of a LiBr–H2O absorption process for solar heat storage with crystallisation of the solution. Sol. Energy 2014, 104, 2–15. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, R.; Wang, H. Experimental evaluation of a variable effect LiBr-water absorption chiller designed for high-efficient solar cooling system. Int. J. Refrig. 2015, 59, 135–143. [Google Scholar] [CrossRef]
- Lin, P.; Wang, R.; Xia, Z. Numerical investigation of a two-stage air-cooled absorption refrigeration system for solar cooling: Cycle analysis and absorption cooling performances. Renew. Energy 2011, 36, 1401–1412. [Google Scholar] [CrossRef]
- Malinina, O.S.; Baranenko, A.V.; Zaitsev, A.V. Influence of the average daily outdoor air parameters on the efficiency of solar lithium bromide-water absorption refrigeration machine. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2018; Volume 2007, p. 030040. [Google Scholar]
- Mortazavi, M.; Schmid, M.; Moghaddam, S. Compact and efficient generator for low grade solar and waste heat driven absorption systems. Appl. Energy 2017, 198, 173–179. [Google Scholar] [CrossRef]
- Bourouis, M.; Vallès, M.; Medrano, M.; Coronas, A. Performance of air-cooled absorption air-conditioning systems working with water-(LiBr+ Lil+ LiNO3+ LiCl). Part E J. Process Mech. Eng. 2005, 219, 205–213. [Google Scholar] [CrossRef]
- Jian, S.; Lin, F.; Shigang, Z. Performance calculation of single effect absorption heat pump using LiBr+ LiNO3+ H2O as working fluid. Appl. Therm. Eng. 2010, 30, 2680–2684. [Google Scholar] [CrossRef]
- Chen, W.; Bai, Y. Thermal performance of an absorption-refrigeration system with [emim] Cu2Cl5/NH3 as working fluid. Energy 2016, 112, 332–341. [Google Scholar] [CrossRef]
- Bellos, E.; Tzivanidis, C.; Antonopoulos, K.A. Exergetic and energetic comparison of LiCl-H2O and LiBr-H2O working pairs in a solar absorption cooling system. Energy Convers. Manag. 2016, 123, 453–461. [Google Scholar] [CrossRef]
- Wang, M.; Ferreira, C.A.I. Absorption heat pump cycles with NH3–ionic liquid working pairs. Appl. Energy 2017, 204, 819–830. [Google Scholar] [CrossRef]
- Luo, C.; Chen, K.; Li, Y.; Su, Q. Crystallization Temperature, Vapor Pressure, Density, Viscosity, and Specific Heat Capacity of the LiNO3/[BMIM]Cl/H2O Ternary System. J. Chem. Eng. Data 2017, 62, 3043–3052. [Google Scholar] [CrossRef]
- Luo, C.; Li, Y.; Chen, K.; Li, N.; Su, Q. Thermodynamic properties and corrosivity of a new absorption heat pump working pair: Lithium nitrate+ 1-butyl-3-methylimidazolium bromide+ water. Fluid Phase Equilib. 2017, 451, 25–39. [Google Scholar] [CrossRef]
- Luo, C.; Li, Y.; Li, N.; Wang, Y.; Su, Q. Thermophysical properties of lithium nitrate+ 1-ethyl-3-methylimidazolium diethylphosphate+ water system. J. Chem. Thermodyn. 2018, 126, 160–170. [Google Scholar] [CrossRef]
- Luo, C.; Wang, Y.; Li, Y.; Wu, Y.; Su, Q.; Hu, T. Thermodynamic properties and application of LiNO3-[MMIM][DMP]/H2O ternary working pair. Renew. Energy 2019, 134, 147–160. [Google Scholar] [CrossRef]
- Luo, C.; Li, Y.; Li, N.; Su, Q. Thermodynamic properties and evaluation of the lithium nitrate–imidazole IL–water ternary systems as new working fluids for a double-effect AHP cycle. Int. J. Refrig. 2018, 90, 58–72. [Google Scholar] [CrossRef]
- Li, N.; Luo, C.; Su, Q. A working pair of CaCl2–LiBr–LiNO3/H2O and its application in a single-stage solar-driven absorption refrigeration cycle. Int. J. Refrig. 2018, 86, 1–13. [Google Scholar] [CrossRef]
- Li, N.; Luo, C.; Su, Q. Thermophysical properties and corrosivity of CaCl2-LiBr-LiNO3-KNO3/H2O working pair. Chin. J. Process Eng. 2018, 18, 764–768. [Google Scholar]
- Li, N.; Luo, C.; Su, Q. Thermophysical properties and applications of CaCl2-LiBr(1.35:1)/H2O as a working pair. Chin. J. Eng. 2018, 40, 167–176. [Google Scholar]
- Li, N.; Li, Y.; Luo, C.; Su, Q. Thermophysical properties and applications of CaCl2-LiNO3/H2O ternary working pair. Chem. Ind. Eng. Prog. 2018, 37, 4625–4637. [Google Scholar]
- Park, Y.; Kim, J.S.; Lee, H.; Yu, S.I. Density, vapor pressure, solubility, and viscosity for water+ lithium bromide+ lithium nitrate+ 1, 3-propanediol. J. Chem. Eng. Data 1997, 42, 145–148. [Google Scholar] [CrossRef]
- Safarov, J.T. Vapor pressure of heat transfer fluids of absorption refrigeration machines and heat pumps: Binary solutions of lithium nitrate with methanol. J. Chem. Thermodyn. 2005, 37, 1261–1267. [Google Scholar] [CrossRef]
- Verevkin, S.; Safarov, J.; Bich, E.; Hassel, E.; Heintz, A. Study of vapour pressure of lithium nitrate solutions in ethanol. J. Chem. Thermodyn. 2006, 38, 611–616. [Google Scholar] [CrossRef]
- Gao, Q.; Zheng, D.; Jiang, C. Thermodynamic study on the working of HEAT–TYPE absorption heat pump. Petrochem. Technol. 1993, 22, 382–392. [Google Scholar]
- Chen, D.; Xie, J. Technology and Application of Heat Pump; Chemical Industry Press: Beijing, China, 2006; pp. 206–220. [Google Scholar]
- Yang, D.; Zhu, Y.; Liu, S.; Lv, H.; Luo, C. Thermodynamic Properties of a Ternary AHP Working Pair: Lithium Bromide+ 1-Ethyl-3-methylimidazolium Chloride+ H2O. J. Chem. Eng. Data 2019, 64, 574–583. [Google Scholar] [CrossRef]
- Aprhornratana, S.; Eames, I.W. Thermodynamic analysis of absorption refrigeration cycles using the second law of thermodynamics method. Int. J. Refrig. 1995, 18, 244–252. [Google Scholar] [CrossRef]



| Reagent | Mass Concentration Purity | Provenance |
|---|---|---|
| CaCl2 | >0.96 | Sinopharm Chemical Reagent Beijing |
| NaCl | >0.995 | Sinopharm Chemical Reagent Beijing |
| KNO3 | >0.99 | Sinopharm Chemical Reagent Beijing |
| KCl | >0.995 | Sinopharm Chemical Reagent Beijing |
| LiCl | >0.95 | Tianjin Jinke Chemical |
| LiNO3 | >0.995 | Tianjin Jinke Chemical |
| Ultrapure water | Home-made |
| Component | C | Mn | Si | P | S | Zn | Pb | Sn | Fe | Cu |
|---|---|---|---|---|---|---|---|---|---|---|
| Carbon steel Q235 | 0.16 | 0.53 | 0.3 | 0.035 | 0.04 | -- | -- | -- | balance | -- |
| Copper T6 | -- | -- | 0.006 | -- | 0.01 | 0.005 | 0.05 | 0.05 | 0.05 | balance |
| Instrument | Parameter | Accuracy |
|---|---|---|
| Analytical balance | 0–2100 g | ±0.1 g |
| Precision thermostat | −30–150 °C | ±0.5 °C |
| Oil bath | 20–300 °C | ±1.0 °C |
| Digital absolute pressure gauge | 0–110 kPa | ±0.01 kPa |
| Precision viscometer oil bath | 0–230 °C | ±0.05 °C |
| Capillary pycnometer | 50 mL | ±0.03% |
| Ubbelohde capillary viscometer | 0.36 mm, 0.46 mm, 0.58 mm, 073 mm | ±0.02% |
| Micro reaction calorimeter | 0–180 °C | ±0.001 °C |
| x/g | Saturated Vapor Pressure p (kPa) at Each Temperature T (°C) CaCl2(x)-LiNO3(35.0 g)/H2O(65.0 g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 42.9 | T | 20.0 | 25.0 | 30.5 | 35.0 | 40.0 | 45.3 | 50.4 | 55.7 | 60.2 |
| p | 0.457 | 0.641 | 0.934 | 1.256 | 1.693 | 2.369 | 3.278 | 4.431 | 5.707 | |
| T | 65.3 | 70.8 | 75.0 | 80.0 | 85.0 | 90.0 | 95.0 | 100.0 | ||
| p | 7.376 | 9.632 | 11.825 | 15.204 | 19.002 | 23.331 | 28.101 | 34.022 | ||
| 47.1 | T | 20.0 | 25.0 | 30.2 | 35.0 | 40.1 | 45.3 | 50.2 | 55.5 | 59.9 |
| p | 0.419 | 0.585 | 0.834 | 1.138 | 1.535 | 2.139 | 2.948 | 3.998 | 5.136 | |
| T | 65.2 | 70.3 | 75.1 | 80.2 | 85.2 | 90.0 | 95.4 | 100.3 | ||
| p | 6.823 | 8.739 | 11.035 | 14.118 | 17.692 | 21.770 | 26.701 | 32.375 | ||
| 51.5 | T | 20.0 | 25.0 | 29.9 | 35.0 | 40.1 | 45.2 | 50.0 | 55.2 | 59.6 |
| p | 0.372 | 0.520 | 0.733 | 1.010 | 1.377 | 1.910 | 2.617 | 3.564 | 4.565 | |
| T | 65.1 | 69.8 | 75.2 | 80.4 | 85.3 | 90.0 | 95.8 | 100.6 | ||
| p | 6.269 | 7.846 | 10.244 | 13.031 | 16.382 | 20.208 | 25.300 | 30.728 | ||
| 56.3 | T | 20.0 | 25.0 | 30.3 | 35.3 | 40.1 | 45.1 | 50.0 | 55.7 | 60.1 |
| p | 0.330 | 0.460 | 0.674 | 0.921 | 1.233 | 1.688 | 2.336 | 3.217 | 4.188 | |
| T | 65.3 | 70.5 | 75.0 | 79.9 | 85.0 | 90.4 | 95.0 | 101.0 | ||
| p | 5.523 | 7.333 | 9.228 | 11.688 | 14.998 | 18.869 | 22.500 | 27.967 | ||
| 61.3 | T | 20.1 | 25.0 | 30.3 | 35.2 | 40.2 | 45.1 | 50.1 | 55.3 | 60.0 |
| p | 0.295 | 0.419 | 0.603 | 0.826 | 1.124 | 1.531 | 2.113 | 2.844 | 3.704 | |
| T | 65.0 | 70.2 | 75.1 | 80.1 | 85.1 | 90.5 | 95.1 | 100.5 | ||
| p | 4.894 | 6.490 | 8.349 | 10.671 | 13.678 | 17.233 | 20.685 | 25.265 | ||
| 66.7 | T | 20.2 | 25.0 | 30.3 | 35.1 | 40.2 | 45.1 | 50.1 | 54.9 | 59.9 |
| 0.269 | 0.378 | 0.552 | 0.752 | 1.024 | 1.375 | 1.860 | 2.471 | 3.220 | ||
| T | 64.6 | 69.8 | 75.2 | 80.2 | 85.2 | 90.5 | 95.2 | 100.0 | ||
| 4.265 | 5.647 | 7.470 | 9.654 | 12.358 | 15.597 | 18.870 | 22.562 | |||
| 72.4 | T | 20.1 | 25.0 | 30.1 | 35.0 | 40.0 | 44.9 | 50.0 | 55.2 | 60.4 |
| p | 0.247 | 0.341 | 0.492 | 0.671 | 0.899 | 1.186 | 1.657 | 2.237 | 3.092 | |
| T | 64.6 | 70.0 | 75.0 | 80.4 | 85.1 | 90.0 | 95.5 | 100.2 | ||
| p | 3.930 | 5.268 | 6.678 | 8.678 | 10.960 | 13.507 | 17.127 | 20.709 | ||
| 78.6 | T | 20.1 | 25.0 | 30.0 | 35.1 | 40.0 | 45.1 | 50.1 | 55.0 | 59.9 |
| p | 0.218 | 0.298 | 0.430 | 0.592 | 0.804 | 1.106 | 1.515 | 2.019 | 2.722 | |
| T | 65.2 | 70.2 | 74.9 | 80.2 | 85.1 | 90.0 | 95.5 | 100.3 | ||
| p | 3.709 | 4.810 | 6.099 | 7.805 | 9.901 | 12.206 | 15.709 | 19.204 | ||
| 85.2 | T | 30.1 | 35.0 | 41.1 | 45.0 | 51.1 | 54.9 | 60.0 | 64.9 | 69.9 |
| p | 0.390 | 0.529 | 0.768 | 0.981 | 1.420 | 1.780 | 2.385 | 3.205 | 4.185 | |
| T | 75.0 | 80.2 | 85.3 | 90.2 | 95.4 | 100.6 | ||||
| p | 5.624 | 7.135 | 9.098 | 10.996 | 13.756 | 17.535 | ||||
| 92.3 | T | 45.1 | 50.1 | 54.9 | 60.8 | 65.4 | 70.2 | 75.5 | 81.1 | 85.1 |
| p | 0.886 | 1.188 | 1.645 | 2.303 | 3.048 | 3.903 | 5.226 | 6.777 | 8.311 | |
| T | 90.5 | 95.8 | 100.0 | |||||||
| p | 10.276 | 12.820 | 15.447 | |||||||
| 100.0 | T | 65.7 | 70.4 | 76.7 | 80.2 | 85.1 | 90.2 | 95.2 | 100.1 | |
| p | 2.711 | 3.552 | 5.050 | 6.000 | 7.432 | 9.260 | 11.383 | 13.858 | ||
| y/g | Saturated Vapor Pressure p (kPa) at Each Temperature T (°C) CaCl2(y)-LiNO3(25.0 g)-KNO3(5.0 g)/H2O(70.0 g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 66.7 | T | 20.0 | 25.0 | 30.1 | 35.5 | 40.0 | 45.3 | 50.0 | 54.9 | 60.0 |
| p | 0.352 | 0.511 | 0.741 | 1.064 | 1.434 | 1.987 | 2.638 | 3.508 | 4.660 | |
| T | 65.1 | 70.1 | 75.2 | 80.0 | 87.2 | 89.9 | 95.0 | 100.0 | ||
| p | 6.119 | 8.024 | 10.490 | 13.001 | 17.346 | 19.223 | 23.450 | 28.590 | ||
| 72.4 | T | 20.0 | 25.0 | 30.2 | 34.9 | 40.6 | 45.6 | 50.0 | 55.2 | 60.4 |
| p | 0.315 | 0.455 | 0.650 | 0.911 | 1.353 | 1.850 | 2.403 | 3.250 | 4.283 | |
| T | 65.1 | 69.9 | 75.2 | 80.3 | 85.0 | 89.7 | 94.9 | 100.0 | ||
| p | 5.560 | 7.106 | 9.469 | 11.911 | 14.327 | 17.200 | 21.021 | 26.025 | ||
| 78.6 | T | 20.0 | 25.0 | 29.9 | 35.1 | 40.0 | 45.0 | 50.0 | 54.9 | 60.0 |
| p | 0.259 | 0.371 | 0.530 | 0.762 | 1.049 | 1.427 | 1.940 | 2.634 | 3.552 | |
| T | 65.1 | 69.8 | 75.5 | 80.1 | 85.0 | 89.9 | 94.9 | 100.1 | ||
| p | 4.825 | 6.306 | 8.546 | 10.555 | 12.913 | 15.540 | 18.898 | 23.535 | ||
| 85.2 | T | 20.0 | 25.0 | 30.0 | 35.0 | 40.5 | 45.0 | 50.0 | 55.0 | 60.2 |
| p | 0.219 | 0.312 | 0.445 | 0.627 | 0.928 | 1.239 | 1.740 | 2.311 | 3.200 | |
| T | 65.0 | 69.8 | 75.1 | 80.0 | 85.1 | 90.0 | 95.2 | 100.2 | ||
| p | 4.205 | 5.499 | 7.402 | 9.378 | 11.789 | 17.909 | 21.505 | |||
| 92.3 | T | 20.0 | 25.0 | 30.0 | 35.4 | 39.9 | 45.0 | 50.0 | 55.1 | 60.1 |
| p | 0.198 | 0.285 | 0.399 | 0.581 | 0.793 | 1.136 | 1.563 | 2.150 | 2.925 | |
| T | 65.2 | 70.0 | 75.3 | 79.9 | 85.2 | 90.0 | 95.5 | 100.2 | ||
| p | 3.875 | 5.048 | 6.683 | 8.448 | 10.859 | 13.429 | 16.906 | 19.867 | ||
| 100.0 | T | 41.0 | 44.9 | 50.0 | 55.0 | 60.2 | 65.0 | 70.1 | 75.1 | 80.3 |
| p | 0.673 | 0.889 | 1.244 | 1.730 | 2.454 | 3.349 | 4.519 | 5.911 | 7.656 | |
| T | 85.2 | 89.9 | 94.9 | 99.9 | ||||||
| p | 9.761 | 12.060 | 14.960 | 18.163 | ||||||
| 108.3 | T | 60.0 | 65.2 | 70.0 | 75.2 | 80.1 | 84.8 | 90.4 | 95.1 | 100.1 |
| p | 2.205 | 3.045 | 4.051 | 5.252 | 6.686 | 8.464 | 10.993 | 13.110 | 15.587 | |
| 117.4 | T | 82.0 | 85.1 | 90.0 | 95.0 | 100.3 | ||||
| p | 5.921 | 6.971 | 9.009 | 11.303 | 14.387 | |||||
| i | Ai | Bi | Ci | AARD |
|---|---|---|---|---|
| 0 | 4.896 × 10−1 | −1.985 × 100 | −3.624 × 100 | 1.55% |
| 1 | −1.172 × 10−1 | 5.756 × 10−1 | −1.189 × 101 | |
| 2 | 1.024 × 10−2 | −2.990 × 10−1 | −3.963 × 101 | |
| 3 | −1.768 × 10−4 | 3.046 × 10−3 | −1.894 × 101 | |
| 4 | 9.452 × 10−7 | −8.957 × 10−6 | −4.335 × 100 |
| i | Ai | Bi | Ci | AARD |
|---|---|---|---|---|
| 0 | −2.262 × 10−2 | −3.844 × 10−1 | 5.668 × 10−1 | 2.34% |
| 1 | 5.616 × 10−1 | 1.184 × 100 | 1.367 × 100 | |
| 2 | −2.523 × 10−2 | −1.498 × 10−2 | 5.033 × 100 | |
| 3 | 4.274 × 10−4 | −3.524 × 10−3 | −3.042 × 101 | |
| 4 | −2.421 × 10−6 | 2.944 × 10−5 | −1.620 × 101 |
| w/wt.% | Saturated Vapor Pressure p (kPa) at Each Temperature T (°C) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 50.0 | T | 20.1 | 25.7 | 30.2 | 35.3 | 40.6 | 45.0 | 50.7 | 55.4 | 60.0 |
| p | 0.634 | 0.901 | 1.225 | 1.705 | 2.371 | 3.064 | 4.325 | 5.626 | 7.206 | |
| T | 65.2 | 70.7 | 75.0 | 80.9 | 85.7 | 90.0 | 95.2 | 100.0 | ||
| p | 9.336 | 12.075 | 14.800 | 19.100 | 23.536 | 27.931 | 33.519 | 40.578 | ||
| 55.0 | T | 21.2 | 28.0 | 32.1 | 35.0 | 39.9 | 45.1 | 52.0 | 55.6 | 60.0 |
| p | 0.443 | 0.712 | 0.939 | 1.148 | 1.569 | 2.210 | 3.407 | 4.249 | 5.387 | |
| T | 65.2 | 70.2 | 75.0 | 80.9 | 85.0 | 90.4 | 95.0 | 100.0 | ||
| p | 7.060 | 9.016 | 11.301 | 14.825 | 17.699 | 21.912 | 26.011 | 31.898 | ||
| 60.0 | T | 21.0 | 26.1 | 30.0 | 35.0 | 41.0 | 45.0 | 50.0 | 55.1 | 60.4 |
| p | 0.280 | 0.412 | 0.541 | 0.769 | 1.166 | 1.492 | 2.071 | 2.855 | 4.026 | |
| T | 65.5 | 70.2 | 75.0 | 80.0 | 85.4 | 90.4 | 95.2 | 100.1 | ||
| p | 5.355 | 6.901 | 8.873 | 11.106 | 14.063 | 17.268 | 21.176 | 25.912 | ||
| 65.0 | T | 25.1 | 30.0 | 35.0 | 40.5 | 45.0 | 50.0 | 55.6 | 60.5 | 65.6 |
| p | 0.226 | 0.341 | 0.490 | 0.744 | 0.985 | 1.339 | 1.958 | 2.675 | 3.665 | |
| T | 71.1 | 75.4 | 80.1 | 85.2 | 90.0 | 94.6 | 100.5 | |||
| p | 4.971 | 6.282 | 7.749 | 9.894 | 12.395 | 15.241 | 19.243 | |||
| w/wt.% | Density ρ (g·cm−3) at Each Temperature T (°C) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 50.0 | T | 20.0 | 30.0 | 40.0 | 50.0 | 60.0 | 70.0 | 80.0 | 90.0 | 100.0 |
| ρ | 1.4614 | 1.4563 | 1.4488 | 1.4412 | 1.4352 | 1.4278 | 1.4215 | 1.4141 | 1.4067 | |
| 55.0 | T | 20.0 | 30.0 | 40.0 | 50.0 | 60.0 | 70.0 | 80.0 | 90.0 | 100.0 |
| ρ | 1.5181 | 1.5111 | 1.5042 | 1.4977 | 1.4906 | 1.4834 | 1.4761 | 1.4696 | 1.4618 | |
| 60.0 | T | 20.0 | 30.0 | 40.0 | 50.0 | 60.0 | 70.0 | 80.0 | 90.0 | 100.0 |
| ρ | 1.5784 | 1.5713 | 1.5642 | 1.5547 | 1.5495 | 1.5420 | 1.5351 | 1.5287 | 1.5229 | |
| 65.0 | T | 40.0 | 50.0 | 60.0 | 70.0 | 80.0 | 90.0 | 100.0 | ||
| ρ | 1.6245 | 1.6185 | 1.6131 | 1.6058 | 1.5991 | 1.5915 | 1.5820 | |||
| w/wt.% | Viscosity η (mPa·s) at Each Temperature T (°C) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 50.0 | T | 20.0 | 30.0 | 40.0 | 50.0 | 60.0 | 70.0 | 80.0 | 90.0 | 100.0 |
| H | 20.13 | 14.51 | 10.56 | 8.00 | 5.98 | 4.77 | 4..11 | 3.47 | 2.95 | |
| 55.0 | T | 20.0 | 30.0 | 40.0 | 50.0 | 60.0 | 70.0 | 80.0 | 90.0 | 100.0 |
| H | 45.51 | 29.57 | 21.63 | 15.40 | 11.42 | 7.79 | 6.19 | 5.18 | 4.35 | |
| 60.0 | T | 20.0 | 30.0 | 40.0 | 50.0 | 60.0 | 70.0 | 80.0 | 90.0 | 100.0 |
| H | 144.05 | 81.88 | 51.66 | 33.48 | 24.07 | 16.91 | 12.51 | 9.47 | 7.71 | |
| 65.0 | T | 40.0 | 50.0 | 60.0 | 70.0 | 80.0 | 90.0 | 100.0 | ||
| H | 144.90 | 80.97 | 52.26 | 37.07 | 25.91 | 19.38 | 14.55 | |||
| w/wt.% | Specific Heat Capacity Cp (kJ·kg−1·K −1) at Each Temperature T (°C) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50.0 | T | 10.0 | 20.0 | 30.0 | 40.0 | 50.0 | 60.0 | 70.0 | 80.0 | 90.0 | 100.0 |
| Cp | 2.438 | 2.444 | 2.458 | 2.473 | 2.487 | 2.501 | 2.518 | 2.527 | 2.542 | 2.563 | |
| 55.0 | T | 10.0 | 20.0 | 30.0 | 40.0 | 50.0 | 60.0 | 70.0 | 80.0 | 90.0 | 100.0 |
| Cp | 2.313 | 2.321 | 2.335 | 2.350 | 2.366 | 2.380 | 2.400 | 2.417 | 2.432 | 2.452 | |
| 60.0 | T | 10.0 | 20.0 | 30.0 | 40.0 | 50.0 | 60.0 | 70.0 | 80.0 | 90.0 | 100.0 |
| Cp | 2.189 | 2.193 | 2.206 | 2.216 | 2.227 | 2.241 | 2.254 | 2.266 | 2.286 | 2.303 | |
| 65.0 | T | 10.0 | 20.0 | 30.0 | 40.0 | 50.0 | 60.0 | 70.0 | 80.0 | 90.0 | 100.0 |
| Cp | 2.039 | 2.045 | 2.057 | 2.071 | 2.085 | 2.098 | 2.110 | 2.127 | 2.130 | 2.146 | |
| Reagent | Specific Heat Capacity Cp (kJ·kg−1·K−1) at Each Temperature T (°C) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| KNO3 | T | 10.0 | 20.0 | 30.0 | 40.0 | 50.0 | 60.0 | 70.0 | 80.0 | 90.0 | 100.0 |
| Cp | 0.930 | 0.952 | 0.968 | 0.974 | 0.988 | 1.040 | 1.053 | 1.072 | 1.090 | 1.119 | |
| w/wt.% | 50.0 | 55.0 | 60.0 | 65.0 |
|---|---|---|---|---|
| ΔHmix/kJ·kg−1 | 149.310 | 150.501 | 150.275 | 151.292 |
| w/wt.% | Specific Enthalpy h (kJ·kg−1) at Each Temperature T (°C) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50.0 | T | 10.0 | 20.0 | 30.0 | 40.0 | 50.0 | 60.0 | 70.0 | 80.0 | 90.0 | 100.0 |
| h | 296.787 | 321.185 | 345.720 | 370.395 | 395.214 | 420.183 | 445.305 | 470.584 | 496.025 | 521.632 | |
| 55.0 | T | 10.0 | 20.0 | 30.0 | 40.0 | 50.0 | 60.0 | 70.0 | 80.0 | 90.0 | 100.0 |
| h | 293.028 | 316.239 | 339.568 | 363.026 | 386.622 | 410.368 | 434.272 | 458.345 | 482.598 | 507.039 | |
| 60.0 | T | 10.0 | 20.0 | 30.0 | 40.0 | 50.0 | 60.0 | 70.0 | 80.0 | 90.0 | 100.0 |
| h | 290.874 | 312.767 | 334.776 | 356.907 | 379.172 | 401.578 | 424.134 | 446.849 | 469.733 | 492.793 | |
| 65.0 | T | 10.0 | 20.0 | 30.0 | 40.0 | 50.0 | 60.0 | 70.0 | 80.0 | 90.0 | 100.0 |
| h | 287.666 | 308.113 | 328.685 | 349.382 | 370.205 | 391.155 | 412.233 | 433.438 | 454.773 | 476.237 | |
| w/wt.% | Entropy s (kJ·kg−1·K−1) at Each Temperature T (°C) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50.0 | T | 10.0 | 20.0 | 30.0 | 40.0 | 50.0 | 60.0 | 70.0 | 80.0 | 90.0 | 100.0 |
| s | 1.412 | 1.496 | 1.578 | 1.658 | 1.736 | 1.813 | 1.889 | 1.963 | 2.036 | 2.108 | |
| 55.0 | T | 10.0 | 20.0 | 30.0 | 40.0 | 50.0 | 60.0 | 70.0 | 80.0 | 90.0 | 100.0 |
| s | 1.417 | 1.498 | 1.576 | 1.652 | 1.727 | 1.800 | 1.872 | 1.943 | 2.014 | 2.083 | |
| 60.0 | T | 10.0 | 20.0 | 30.0 | 40.0 | 50.0 | 60.0 | 70.0 | 80.0 | 90.0 | 100.0 |
| s | 1.422 | 1.500 | 1.574 | 1.645 | 1.716 | 1.785 | 1.852 | 1.919 | 1.985 | 2.050 | |
| 65.0 | T | 10.0 | 20.0 | 30.0 | 40.0 | 50.0 | 60.0 | 70.0 | 80.0 | 90.0 | 100.0 |
| s | 1.431 | 1.502 | 1.571 | 1.638 | 1.704 | 1.769 | 1.832 | 1.895 | 1.955 | 2.016 | |
| Working Pair | CaCl2-LiNO3- KNO3 (15.5:5:1)/H2O | LiBr/ H2O | CaCl2-LiBr-LiNO3- KNO3(16.2:2:2:1) /H2O | CaCl2-LiNO3- LiBr (8.72:1:1)/H2O | CaCl2-LiBr (1.35:1) /H2O |
|---|---|---|---|---|---|
| Dilute solution/wt.% | 60.5 | 56.4 | 58.5 | 57.3 | 55.8 |
| Strong solution/wt.% | 63.5 | 59.4 | 61.5 | 60.3 | 58.8 |
| Generation temperature/°C | 74.0 | 81.0 | 74.8 | 73.3 | 74.8 |
| Point | Stream | p/kPa | T/°C | w/wt.% | h/kJ·kg−1 | D/kg·s−1 | s/kJ·kg−1·K−1 |
|---|---|---|---|---|---|---|---|
| 1 | Water | 0.872 | 5.0 | 0 | 439.6 | 1.0 | 0.07621 |
| 1ʹ | Vapor | 0.872 | 5.0 | 0 | 2927.9 | 1.0 | 9.02690 |
| 2 | Dilute solution | 0.872 | 37.0 | 60.5 | 349.2 | 21.2 | 0.09149 |
| 3 | Water | 6.290 | 37.0 | 0 | 573.5 | 1.0 | 0.53190 |
| 4 | Strong solution | 6.290 | 74.0 | 63.5 | 424.4 | 20.2 | 0.33201 |
| 4ʹ | Vapor | 6.290 | 74.0 | 0 | 3054.2 | 1.0 | 8.5368 |
| 5 | Dilute solution | 6.290 | 69.2 | 60.5 | 420.9 | 21.2 | 0.31351 |
| 6 | Strong solution | 0.872 | 41.0 | 63.5 | 353.7 | 20.2 | 0.11525 |
| 7 | Dilute solution | -- | 63.9 | 60.5 | 409.9 | 21.2 | 0.27782 |
| 8 | Strong solution | -- | 44.3 | 63.5 | 360.7 | 20.2 | 0.13754 |
| Working Pair | CaCl2-LiNO3-KNO3 (15.5:5:1) /H2O | LiBr /H2O | CaCl2-LiBr-LiNO3-KNO3 (16.2:2:2:1) /H2O | CaCl2-LiNO3-LiBr (8.72:1:1) /H2O | CaCl2-LiBr (1.35:1) /H2O |
|---|---|---|---|---|---|
| COP | 0.801 | 0.762 | 0.793 | 0.805 | 0.788 |
| Part | Exergy Destruction/kW | |
|---|---|---|
| CaCl2-LiNO3-KNO3 (15.5:5:1)/H2O | LiBr/H2O | |
| Evaporator | 0.5 | 0.5 |
| Condenser | 0.4 | 0.8 |
| Absorber | 52.1 | 55.3 |
| Generator | 282.6 | 332.7 |
| Heat exchanger | 25.5 | 31.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, N.; Luo, C.; Su, Q. Study on a Quaternary Working Pair of CaCl2-LiNO3-KNO3/H2O for an Absorption Refrigeration Cycle. Entropy 2019, 21, 546. https://doi.org/10.3390/e21060546
Li Y, Li N, Luo C, Su Q. Study on a Quaternary Working Pair of CaCl2-LiNO3-KNO3/H2O for an Absorption Refrigeration Cycle. Entropy. 2019; 21(6):546. https://doi.org/10.3390/e21060546
Chicago/Turabian StyleLi, Yiqun, Na Li, Chunhuan Luo, and Qingquan Su. 2019. "Study on a Quaternary Working Pair of CaCl2-LiNO3-KNO3/H2O for an Absorption Refrigeration Cycle" Entropy 21, no. 6: 546. https://doi.org/10.3390/e21060546
APA StyleLi, Y., Li, N., Luo, C., & Su, Q. (2019). Study on a Quaternary Working Pair of CaCl2-LiNO3-KNO3/H2O for an Absorption Refrigeration Cycle. Entropy, 21(6), 546. https://doi.org/10.3390/e21060546




