Abstract
When compared with LiBr/H2O, an absorption refrigeration cycle using CaCl2/H2O as the working pair needs a lower driving heat source temperature, that is, CaCl2/H2O has a better refrigeration characteristic. However, the crystallization temperature of CaCl2/H2O solution is too high and its absorption ability is not high enough to achieve an evaporation temperature of 5 °C or lower. CaCl2-LiNO3-KNO3(15.5:5:1)/H2O was proposed and its crystallization temperature, saturated vapor pressure, density, viscosity, specific heat capacity, specific entropy, and specific enthalpy were measured to retain the refrigeration characteristic of CaCl2/H2O and solve its problems. Under the same conditions, the generation temperature for an absorption refrigeration cycle with CaCl2-LiNO3-KNO3(15.5:5:1)/H2O was 7.0 °C lower than that with LiBr/H2O. Moreover, the cycle’s COP and exergy efficiency with CaCl2-LiNO3-KNO3(15.5:5:1)/H2O were approximately 0.04 and 0.06 higher than those with LiBr/H2O, respectively. The corrosion rates of carbon steel and copper for the proposed working pair were 14.31 μm∙y−1 and 2.04 μm∙y−1 at 80 °C and pH 9.7, respectively, which were low enough for engineering applications.
1. Introduction
Absorption refrigeration systems can effectively utilize not only industrial waste heat [1,2,3,4], but also low-grade renewable energy, including solar energy and geothermal energy for refrigeration [5,6,7]. As a traditional working pair, LiBr/H2O has been widely used for refrigeration [8,9,10,11,12]. However, studies on new working pairs are still ongoing, because the required temperature of driving heat source for a refrigeration cycle using LiBr/H2O even reaches 88.0 °C [13,14,15], which is too high to use for some low-grade heat sources. Lin et al. [16] studied a double-stage air-cooled NH3/H2O absorption refrigeration system and found that it could effectively lower the temperature of driving heat source for utilizing solar energy. Malinina et al. [17] analyzed the influences of temperature and humidity on a solar energy refrigeration system with LiBr/H2O and calculated the minimum heat-collecting temperatures that are based on solar energy in some cities. Mortazavi et al. [18] designed an absorption refrigeration system with a falling-film generator, which could use lower temperature waste heat or solar energy. Bourouis et al. [19] analyzed the performance of LiBr + LiNO3 + LiCl + LiI + H2O in a vertical tube and found that the crystallization temperature was 35 °C lower than LiBr solution. Sun et al. [20] studied LiBr-LiNO3 (mole ratio: 4:1)/H2O and found the alternative working pair had higher COP and less corrisivity than LiBr/H2O. Chen et al. [21] studied the performance of an absorption refrigeration system using [emim]Cu2Cl5/NH3 as working pair with the UNIFAC model, and results showed that the [emim]Cu2Cl5/NH3 system possessed several advantages, including non-crystallization and non-corrosion. Bellos et al. [22] compared the exergy efficiency between LiCl/H2O and LiBr/H2O, results showed that LiCl/H2O performed better at different ambient temperature levels. Wang et al. [23] measured the properties of different ammonia/ionic liquid working pairs. Luo et al. [24,25,26,27,28] studied various lithium nitrate-ionic liquid/water working pairs. They both found that the working pairs with ionic liquid had excellent characteristics for heating, whereas they were not suitable for refrigeration because of insufficient absorption ability. Li et al. [29,30,31,32] measured the thermophysical properties of several CaCl2-based working pairs, and found that the CaCl2-based working pairs had an excellent refrigeration characteristic. However, their strong corrosivity limited the practical applications.
In this work, to find a new working pair with excellent refrigeration characteristic for absorption refrigeration, various inorganic salts, including NaCl, KCl, LiCl, KNO3, and LiNO3, were added in CaCl2/H2O, and their crystallization temperature and saturated vapor pressure were measured. Furthermore, some other thermophysical properties and corrisivity of the proposed working pair were measured and the performance of an absorption refrigeration cycle with the proposed working pair was analyzed.
2. Experiments
2.1. Materials
Table 1 shows the purities of the reagents used in this work. Table 2 lists the detailed compositions of carbon steel and copper samples used in the corrosion experiments.

Table 1.
Purity of the used regents.

Table 2.
Chemical compositions of carbon steel and copper.
2.2. Apparatus and Methods
To analyze the performance of a working pair, its properties, such as crystallization temperature, saturated vapor pressure, density, viscosity, specific heat capacity, dissolution enthalpy, and corrosion rate, need to be measured.
The crystallization temperature was measured by a dynamic method in a precision thermostat (HX-3010, Bilang, Shanghai). The prepared solution was put in the thermostat at a slightly higher initial temperature. The crystallization temperature was measured by reducing the temperature by 1 °C every 12 hours until crystallization appeared in the solution.
The saturated vapor pressure was measured by a static method. The solution was poured into an autoclave that was assembled with a precision digital absolute pressure gauge (AX-110, Aoxin, Xi’an) and a Pt-100 thermocouple. The autoclave was placed in a precision oil bath (DKU-30, Jinghong, Shanghai) after vacuuming. The data of pressure gauge and thermocouple were obtained, respectively, after stabilization.
The density and viscosity were measured in a precision viscometer oil bath (SYP1003-H, Zhongxi, Beijing). Density measurement was carried out by a capillary pycnometer with a capillary diameter of approximately 1 mm. Ubbelohde capillary viscometers with different fine capillaries was used to carry out the viscosity measurement.
The specific heat capacity and dissolution enthalpy were measured by a micro reaction calorimeter (μRC, THT Co., UK). The measurement of specific heat capacity was conducted by making a 1 °C “step-change” in the measurement temperature. The dissolution enthalpy was measured by an isothermal method, with a solid addition accessory.
The corrosion rate of carbon steel and copper in the solution were measured by a weight loss method. The sample was immerged in the solution for 200 hours. The corrosion rate was calculated according to the mass change of the sample.
References [24,25,26,27,28] give the detailed procedures. All the above experiments were carried out at 101.3 kPa and 25 °C. The properties of water and LiBr/H2O were measured and compared with literature values to validate the above methods. In addition, three parallel experiments were carried out for each measurement to verify the reproducibility. Table 3 lists the accuracy of the instruments.

Table 3.
Measuring range and accuracy of main instruments.
3. Results and Discussion
3.1. CaCl2/H2O
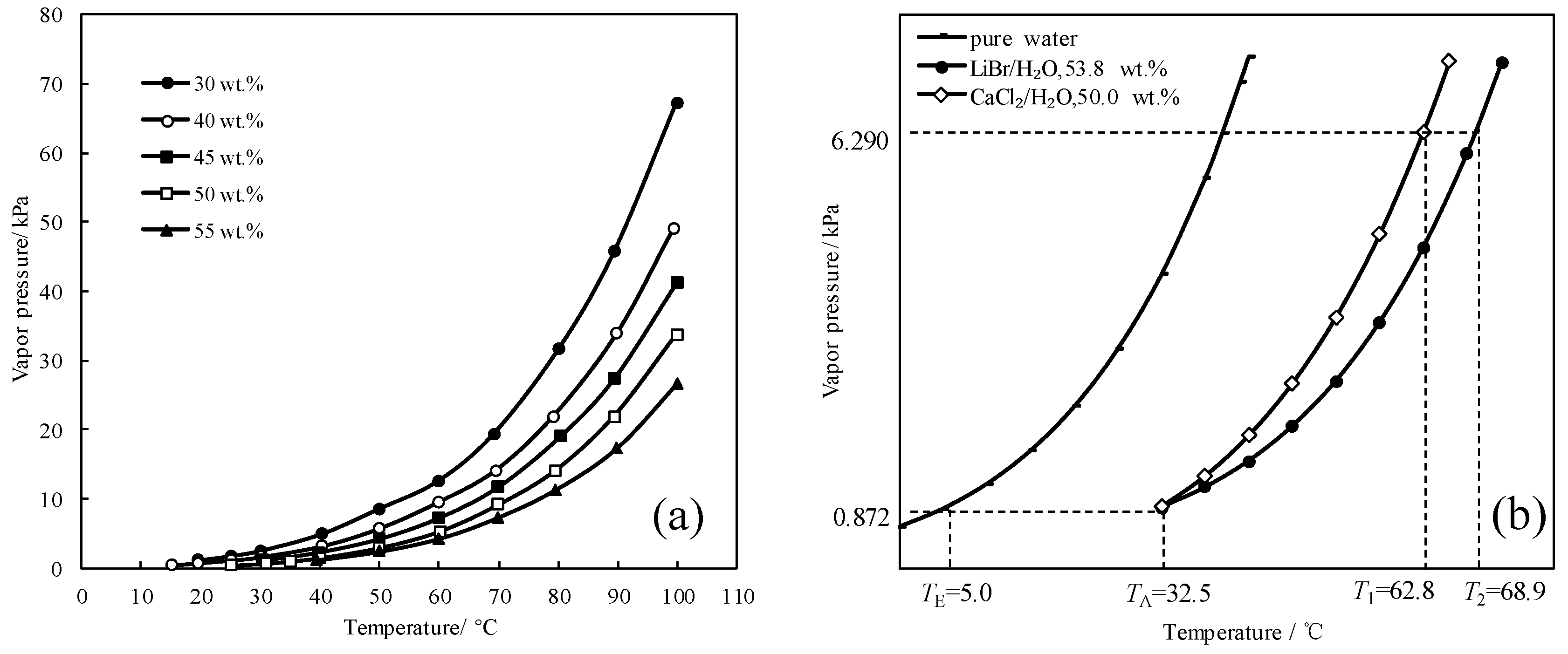
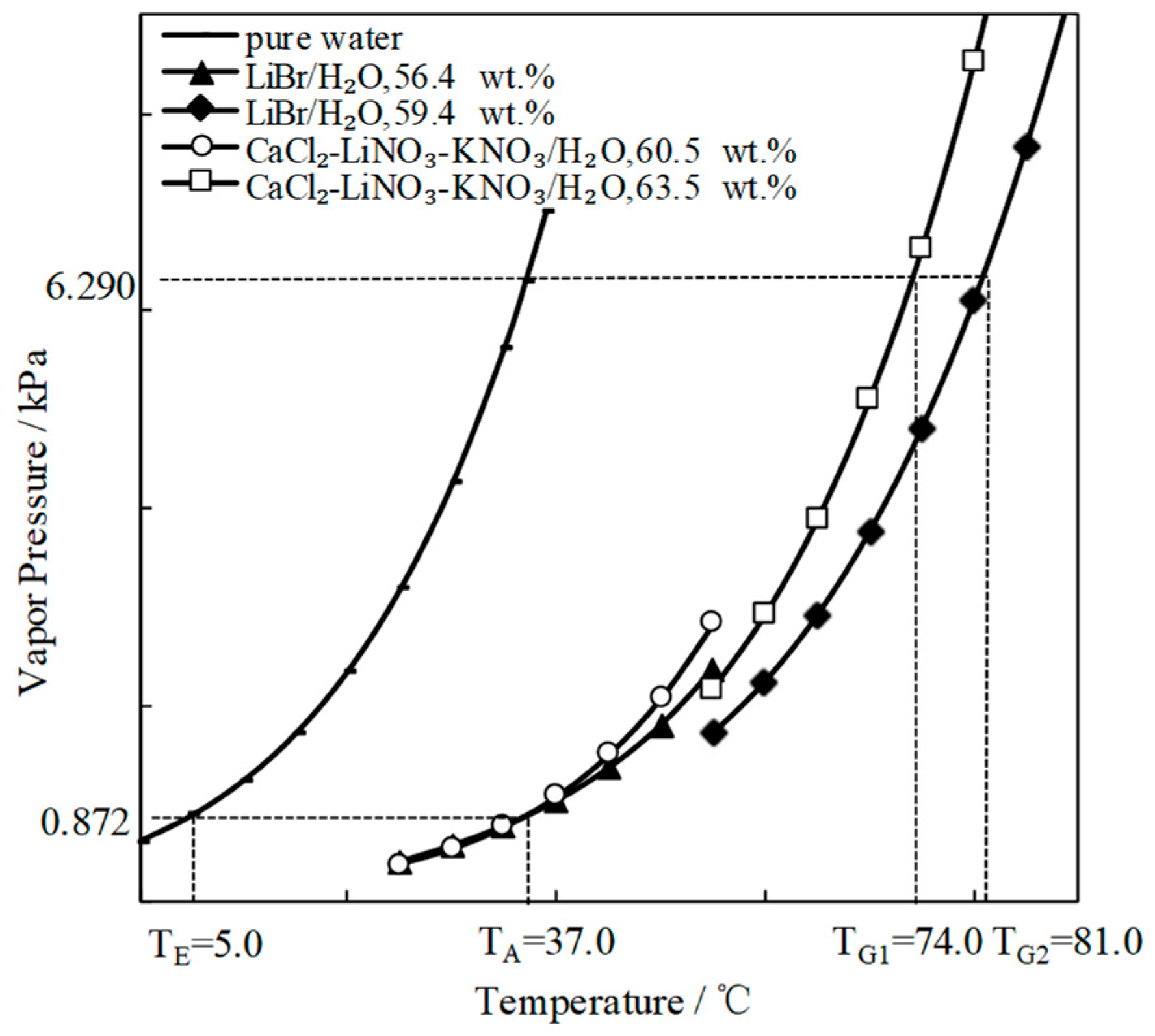
To find a working pair with excellent refrigeration characteristic, the saturated vapor pressures of CaCl2/H2O were measured and are shown in Figure 1a. Figure 1b presents the comparison of the refrigeration characteristic between LiBr/H2O and CaCl2/H2O, it shows that, for an identical pressure of 6.290 kPa, which is a typical pressure in the condenser and generator, CaCl2/H2O had a lower generation temperature than LiBr/H2O, meaning that the refrigeration characteristic of CaCl2/H2O was better than LiBr/H2O.
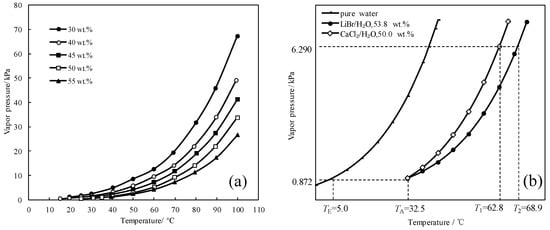
Figure 1.
(a) Saturated vapor pressure of CaCl2/H2O; (b) Comparison of the refrigeration characteristic between LiBr/H2O and CaCl2/H2O.
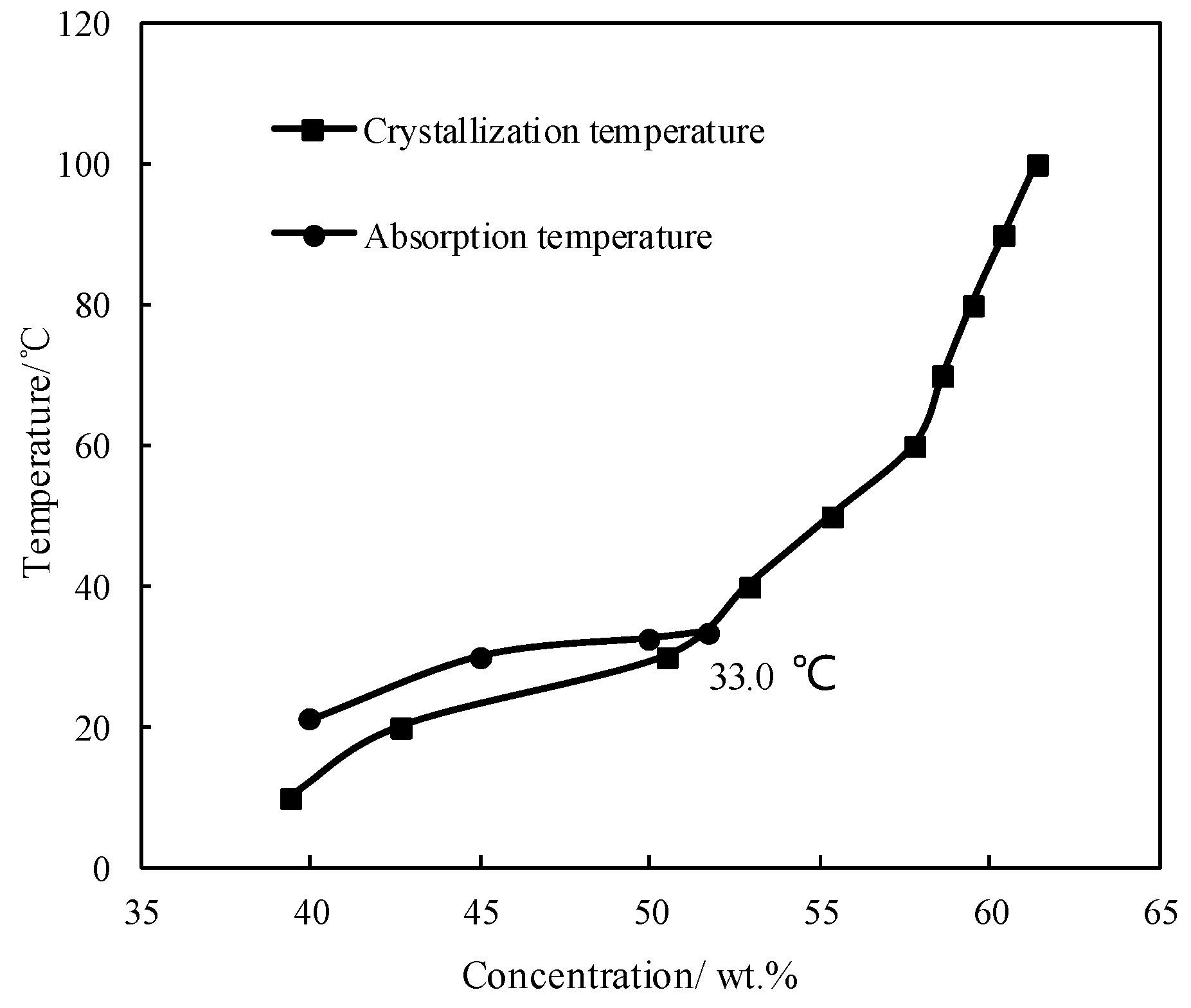
Figure 2 shows the absorption temperature of CaCl2/H2O under an absorption pressure of 0.872 kPa, which corresponds to the typical evaporation temperature of 5 °C. The crystallization temperature of CaCl2/H2O is also plotted in Figure 2 to illustrate the limitation from the crystallization of absorbent. The absorption temperature increased with increasing the concentration, and meet the crystallization temperature at 33.0 °C, which was the maximum absorption temperature under the given conditions. Generally, the absorption temperature in absorber for a refrigeration cycle is 37.0 °C, so the binary working pair of CaCl2/H2O could not be applied for the refrigeration cycle, because of its high crystallization temperature and insufficient absorption ability.
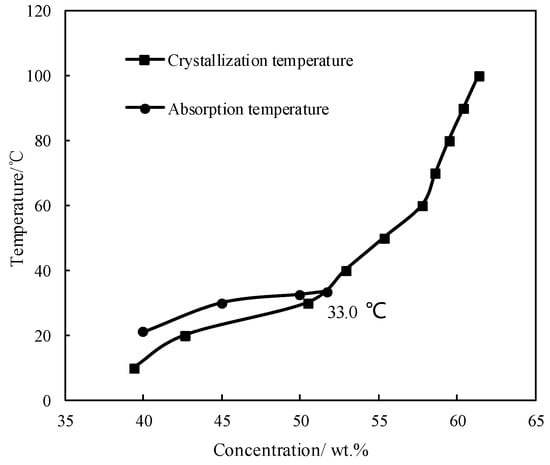
Figure 2.
Crystallization temperature and absorption temperature for CaCl2/H2O.
To improve the absorption ability and reduce the crystallization temperature of CaCl2/H2O, some salts, including NaCl, KCl, LiCl, KNO3, and LiNO3, were combined with CaCl2/H2O, and their saturated vapor pressures and crystallization temperatures were measured.
3.2. Measurement of Crystallization Temperature TC
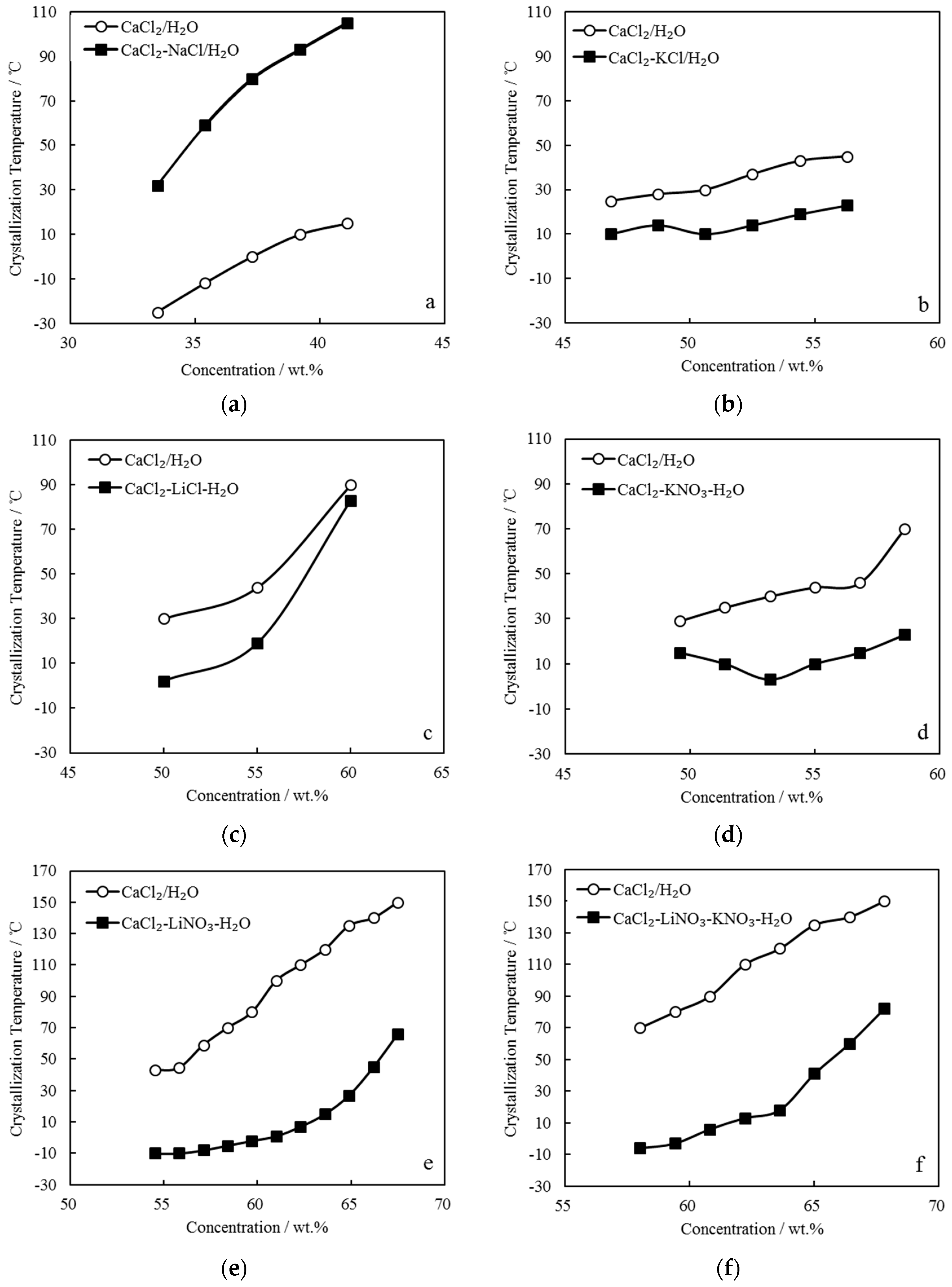
The TC of CaCl2-NaCl/H2O, CaCl2-KCl/H2O, CaCl2-LiCl/H2O, CaCl2-KNO3/H2O, CaCl2-LiNO3/H2O, and CaCl2-LiNO3-KNO3/H2O were measured. Figure 3 gives the comparison of TC between these CaCl2-based working pairs and CaCl2/H2O. Here, the concentration is the solution’s total solute mass concentration.
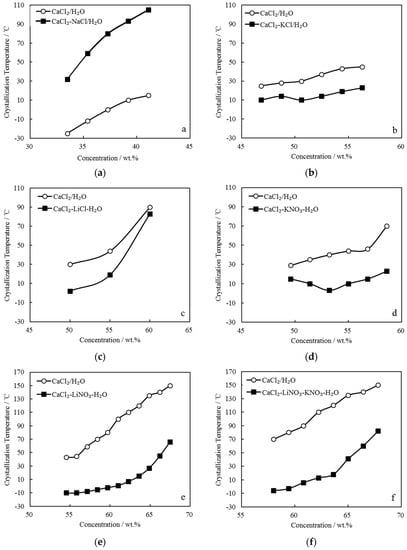
Figure 3.
Comparison of TC between CaCl2/H2O and other CaCl2-based working pairs: (a) CaCl2-NaCl/H2O; (b) CaCl2-KCl/H2O; (c) CaCl2-LiCl/H2O; (d) CaCl2-KNO3/H2O; (e) CaCl2-LiNO3/H2O; and, (f) CaCl2-LiNO3-KNO3/H2O.
Figure 3a shows CaCl2-NaCl/H2O with adding 5.0 g NaCl to CaCl2/H2O, in which CaCl2 were from 42.9 g to 61.3 g and H2O was 95.0 g. The crystallization temperatures of CaCl2-NaCl/H2O were higher than those of CaCl2/H2O under the same concentrations.
Figure 3b shows CaCl2-KCl/H2O with adding 5.0 g KCl to CaCl2/H2O, in which CaCl2 were from 78.6 g to 117.4 g and H2O was 95.0 g. The crystallization temperatures of CaCl2-KCl/H2O were approximately 20.0 °C lower than those of CaCl2/H2O under the same concentrations.
Figure 3c shows CaCl2-LiCl/H2O with adding 10.0 g LiCl to CaCl2/H2O, in which CaCl2 were from 66.7 g to 100.0 g and H2O was 90.0 g. The crystallization temperature of CaCl2-LiCl/H2O was reduced greatly when compared with that of CaCl2/H2O at 50.0 wt.%, whereas with the concentration increasing, the effect of LiCl addition on the crystallization temperature obviously decreased.
Figure 3d shows CaCl2-KNO3/H2O with adding 10.0 g KNO3 to CaCl2/H2O, in which CaCl2 were from 78.6 g to 117.4 g and H2O was 90.0 g. The crystallization temperatures of CaCl2-KNO3/H2O were lower than those of CaCl2/H2O under the same concentrations. Moreover, it decreased with increasing concentration in the range of 49.0 wt.% to 53.5 wt.%, whereas it increased with further increasing concentration.
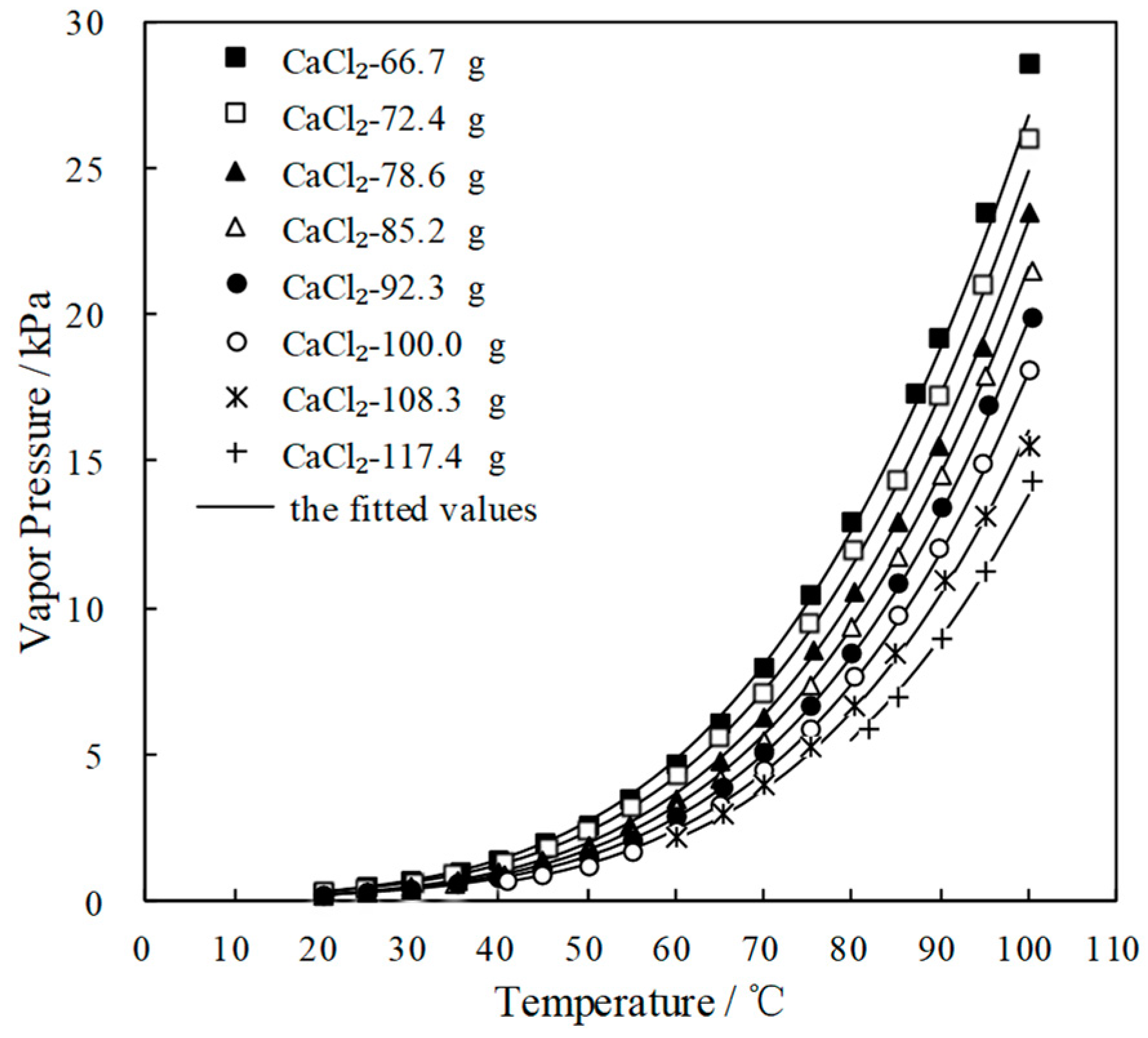
Figure 3e shows CaCl2-LiNO3/H2O with adding 35.0 g LiNO3 to CaCl2/H2O, in which CaCl2 were from 42.9 g to 100.0 g and H2O was 65.0 g. The crystallization temperatures of CaCl2-LiNO3/H2O were significantly reduced when compared with those of CaCl2/H2O under the same concentrations. Corresponding to the concentrations ranging from 55.0 wt.% to 62.0 wt.%, which is a practical concentration range for an absorption refrigeration cycle, the crystallization temperatures of CaCl2-LiNO3/H2O were from -10.0 °C to 7.0 °C, which are sufficiently low to solve the absorbent crystallization problem in summer. However, the addition amount of 35.0 g LiNO3 was relatively large, and it is a disadvantage from the aspect of cost due to LiNO3 being much more expensive than CaCl2.
To depress the cost increase, a part of LiNO3 was replaced with KNO3 for CaCl2-LiNO3/H2O. Figure 3f shows CaCl2-LiNO3-KNO3/H2O with adding 25.0 g LiNO3 and 5.0 g KNO3 to CaCl2/H2O, in which CaCl2 were from 66.7 g to 117.4 g and H2O was 70.0 g. A reduction of crystallization temperature up to 30.0 °C was achieved from 58.0 wt.% to 65.0 wt.%, which indicated that the crystallization problem would not occur in this concentration range.
3.3. Measurement of Saturated Vapor Pressure p
p of CaCl2-LiNO3/H2O and CaCl2-LiNO3-KNO3/H2O with different mass ratios were measured and are shown in Table 4 and Table 5.

Table 4.
p of CaCl2-LiNO3/H2O with different mass ratio.

Table 5.
p of CaCl2-LiNO3-KNO3/H2O with different mass ratio.
The measured p was fitted by Equation (1) [33,34,35].
where Ai, Bi, and Ci are the regression parameters. Equation (2) obtains the average absolute relative deviation (AARD) between the measured values and the fitted values.
where N is total number of data, Pexp is the measured or obtained value, and Pfit is the fitted value.
The regression parameters and AARD were obtained and are shown in Table 6 and Table 7, respectively.

Table 6.
Regression parameters for CaCl2-LiNO3/H2O and average absolute relative deviation (AARD) value.

Table 7.
Regression parameters for CaCl2-LiNO3-KNO3/H2O and AARD value.
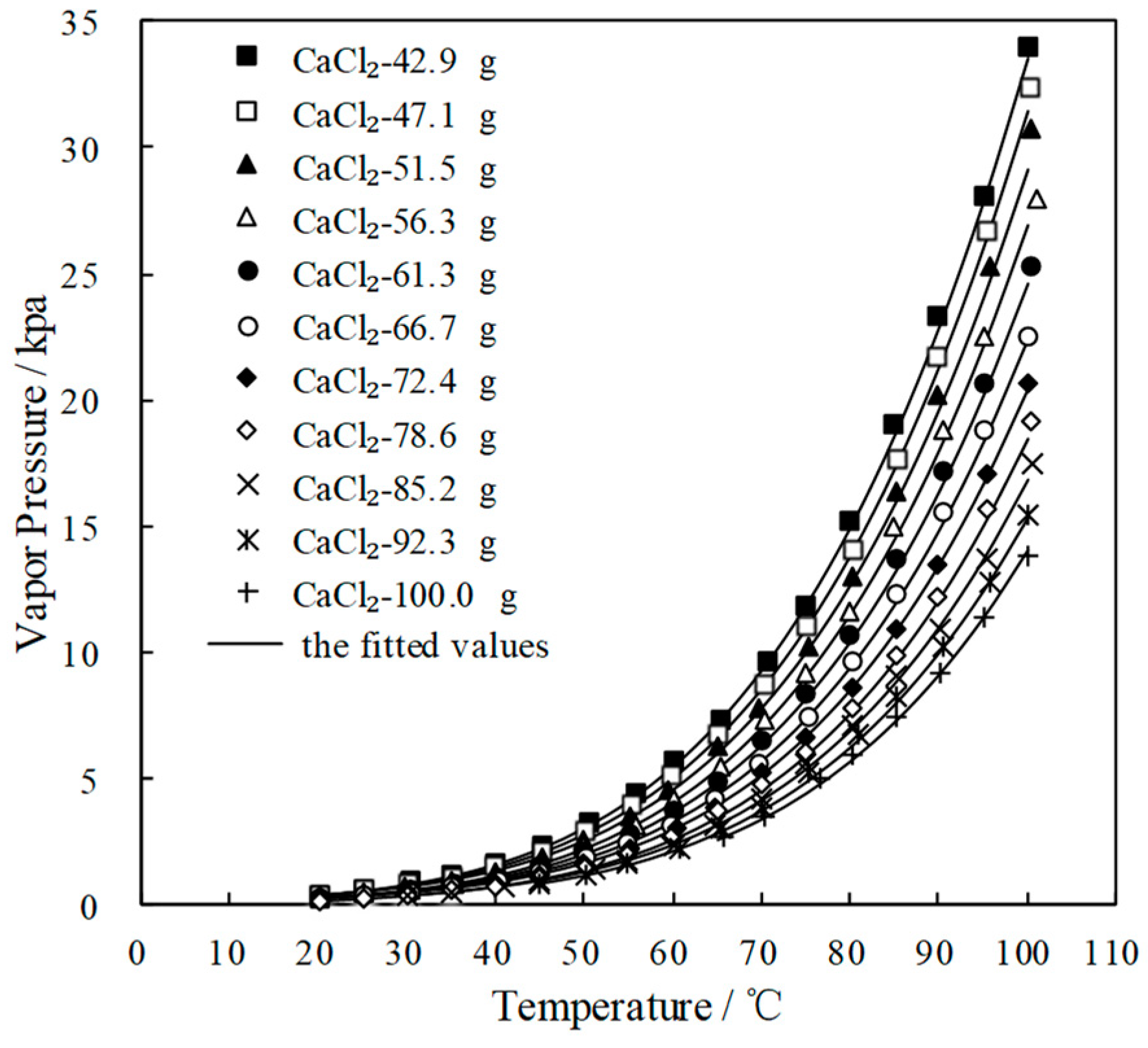
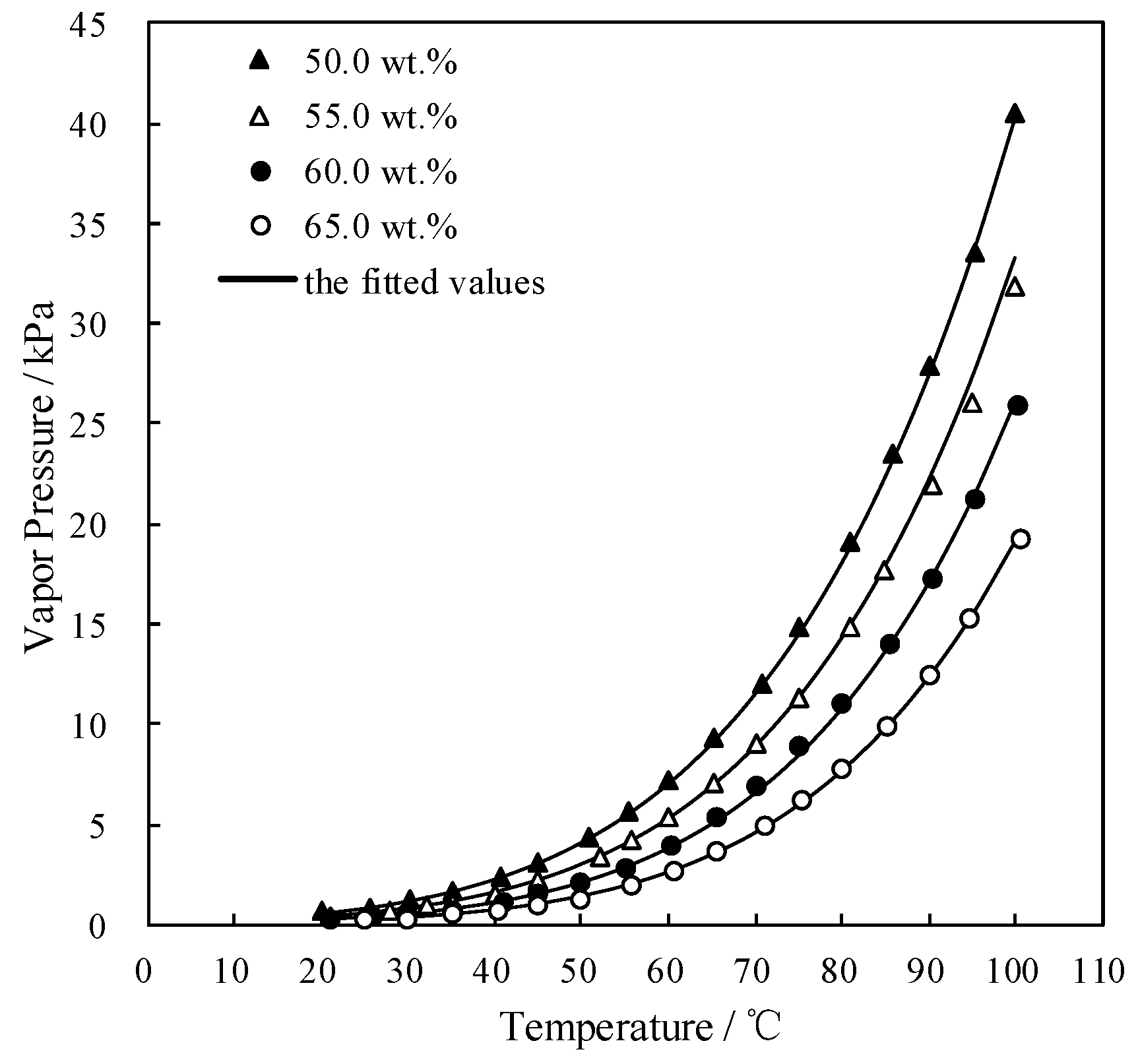
Figure 4 and Figure 5 plot the measured p and fitted value of CaCl2-LiNO3/H2O and CaCl2-LiNO3-KNO3/H2O, respectively. The fitted value agreed well with the measured p. 60.2 wt.% for CaCl2(63.2 g)-LiNO3(35.0 g)/H2O(65.0 g) and 60.5 wt.% for CaCl2(77.3 g)-LiNO3(25.0 g)-KNO3(5.0 g)/H2O(70.0 g) were obtained, respectively, by Equation (1) at 37.0 °C and 0.872 kPa, which are the typical absorption temperature and absorption pressure for a refrigeration cycle. Meanwhile, the absorption temperatures of these two working pairs were 70.5 °C and 69.2 °C, respectively, at 6.290 kPa, which is the typical generation pressure. Therefore, CaCl2(77.3 g)-LiNO3(25.0 g)-KNO3(5.0 g)/H2O(70.0 g), with a solute mass ratio of 15.5:5:1, had been proposed as an alternative working pair for LiBr/H2O. The proposed working pair is expressed as CaCl2-LiNO3-KNO3(15.5:5:1)/H2O in this paper.
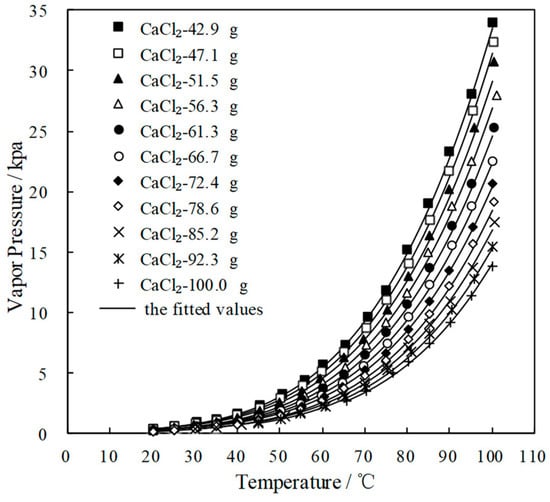
Figure 4.
p of CaCl2-LiNO3/H2O with different mass ratio.
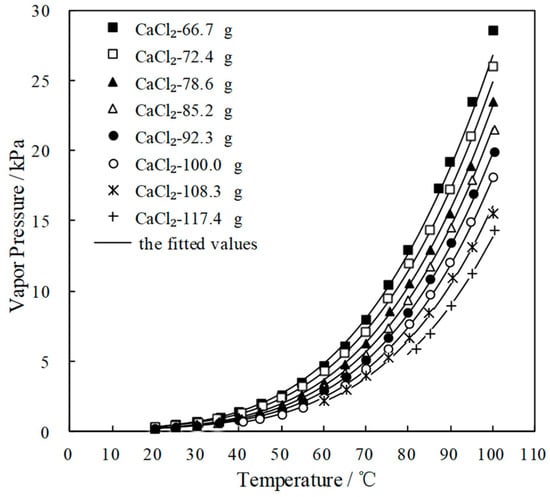
Figure 5.
p of CaCl2-LiNO3-KNO3/H2O with different mass ratio.
The p of this working pair was measured in order to analyze the cycle performance with CaCl2-LiNO3-KNO3(15.5:5:1)/H2O, as shown in Table 8.

Table 8.
p of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O.
The measured p was fitted by Equation (3) and the AARD was obtained to be 1.82% by Equation (2).
Figure 6 shows the measured p and fitted value. The measured p agreed well with the fitted value, which indicated that the p of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O could be obtained with the given corresponding concentration and temperature.
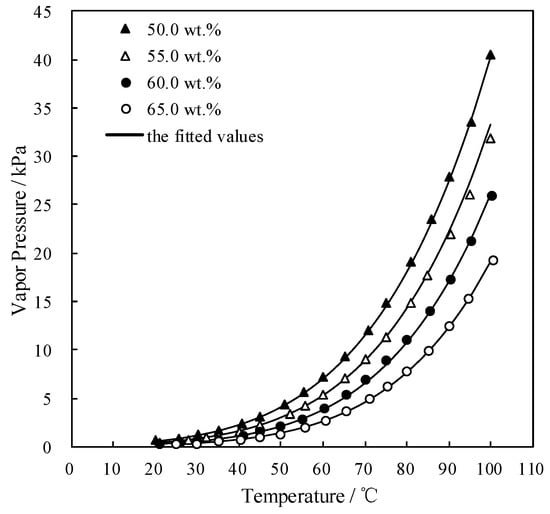
Figure 6.
p of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O.
Figure 7 compares the refrigeration characteristic of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O and LiBr/H2O. The generation temperature of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O was 74.0 °C, which was 7.0 °C lower than that of LiBr/H2O. In other words, the temperature that is required for the driving heat source could be reduced by 7.0 °C through using CaCl2-LiNO3-KNO3(15.5:5:1)/H2O instead of LiBr/H2O.
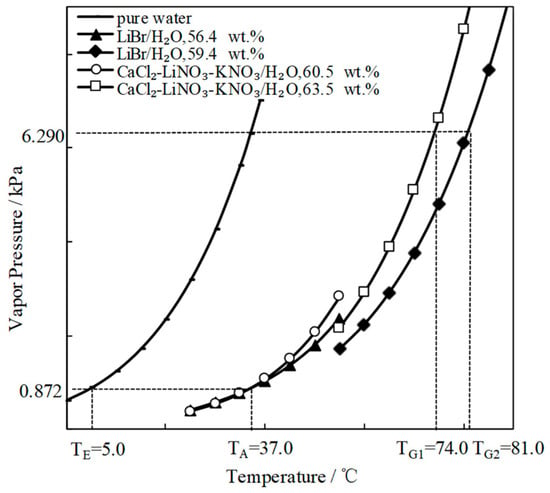
Figure 7.
Comparison of the refrigeration characteristic between LiBr/H2O and CaCl2-LiNO3-KNO3(15.5:5:1)/H2O.
3.4. Measurement of Density ρ
ρ of CaCl2-LiNO3-KNO3(15.5:5:1) /H2O was measured by a capillary pycnometer method. Table 9 lists the results.

Table 9.
ρ of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O.
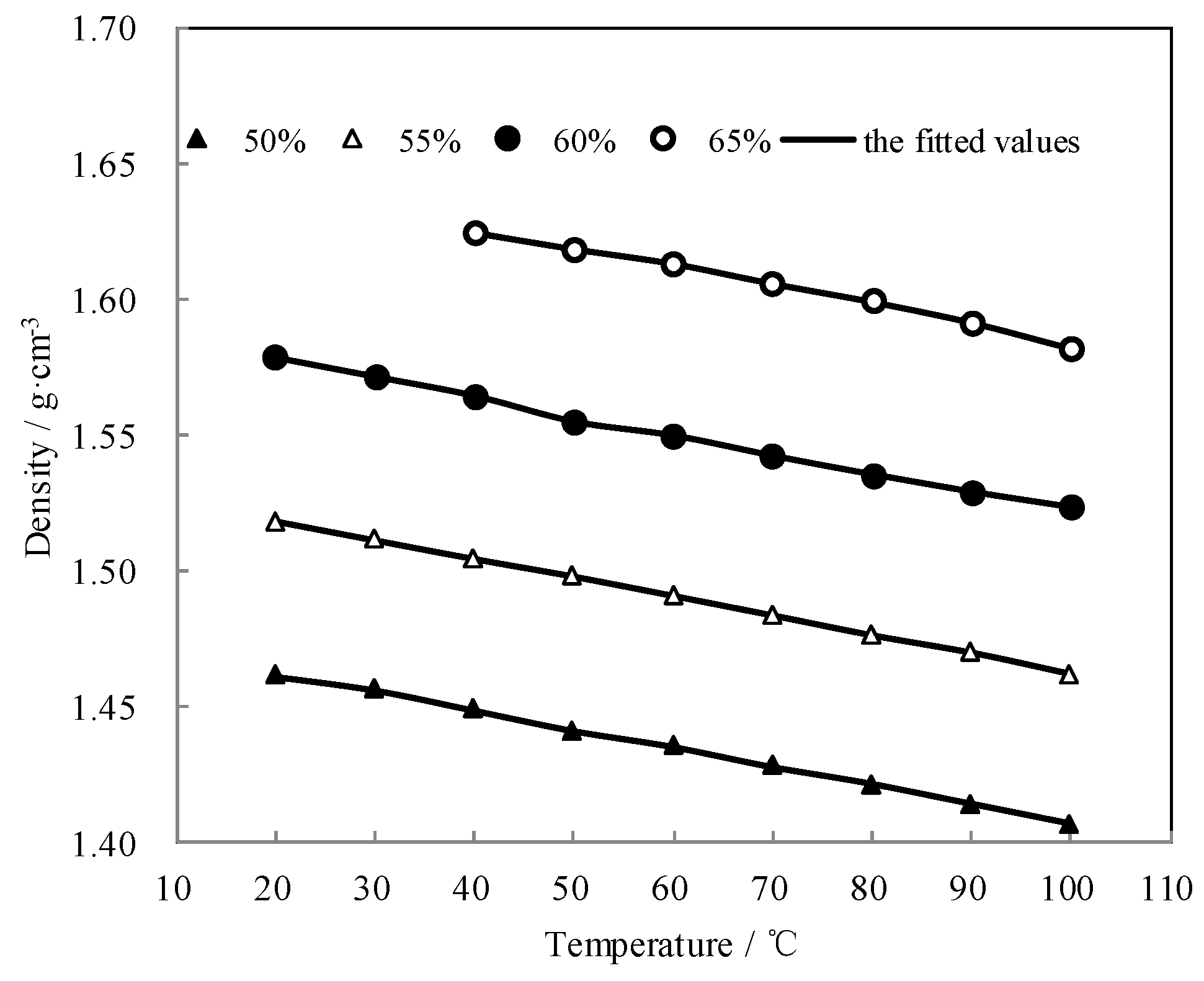
The measured ρ of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O was fitted by Equation (4) and AARD was obtained to be 0.22% by Equation (2).
Figure 8 shows the measured ρ and the fitted value. The measured ρ was highly consistent with the fitted value. The density linearly decreased with the temperature increasing, and it increased with the concentration increasing.
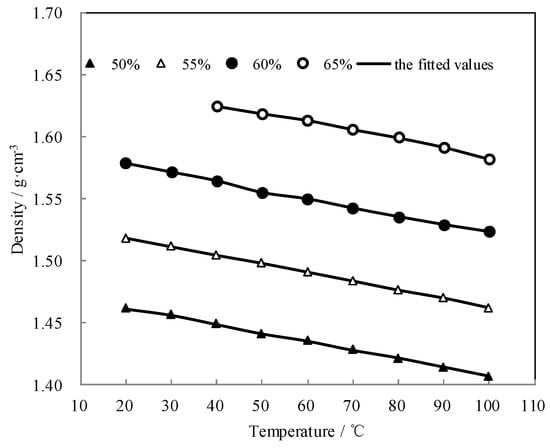
Figure 8.
ρ of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O.
3.5. Measurement of Viscosity η
η of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O was measured by the Ubbelohde capillary viscometer method. Table 10 shows the results.

Table 10.
η of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O.
The measured η of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O was fitted by Equation (5) and the AARD was obtained to be 0.82% by Equation (2).
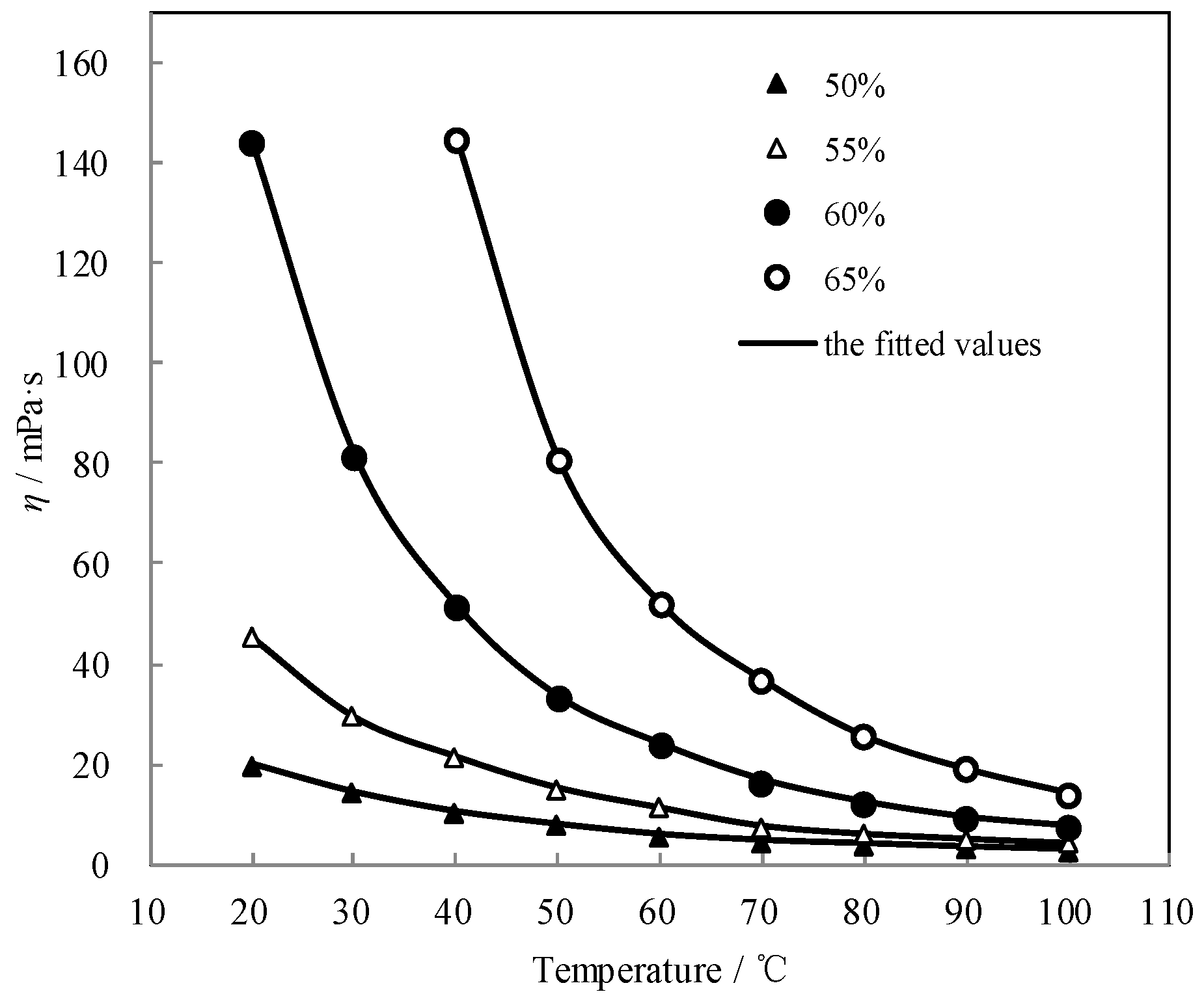
Figure 9 shows the measured η and the fitted value. The measured η agreed well with the fitted value. η exponentially decreased with the temperature increasing, whereas it increased with the concentration increasing.
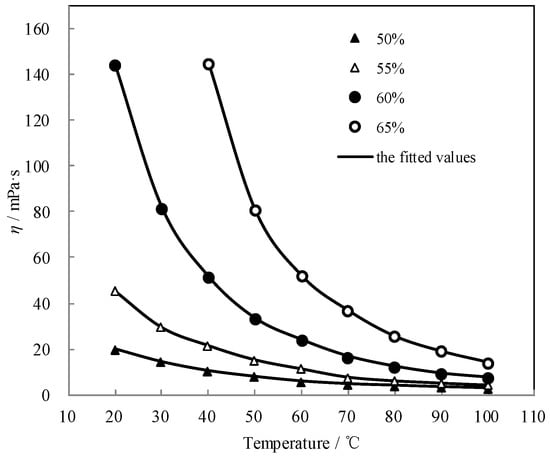
Figure 9.
η of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O.
3.6. Measurement of Specific Heat Capacity Cp
Cp of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O was measured with a micro reaction calorimeter. Table 11 lists the results.

Table 11.
Cp of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O.
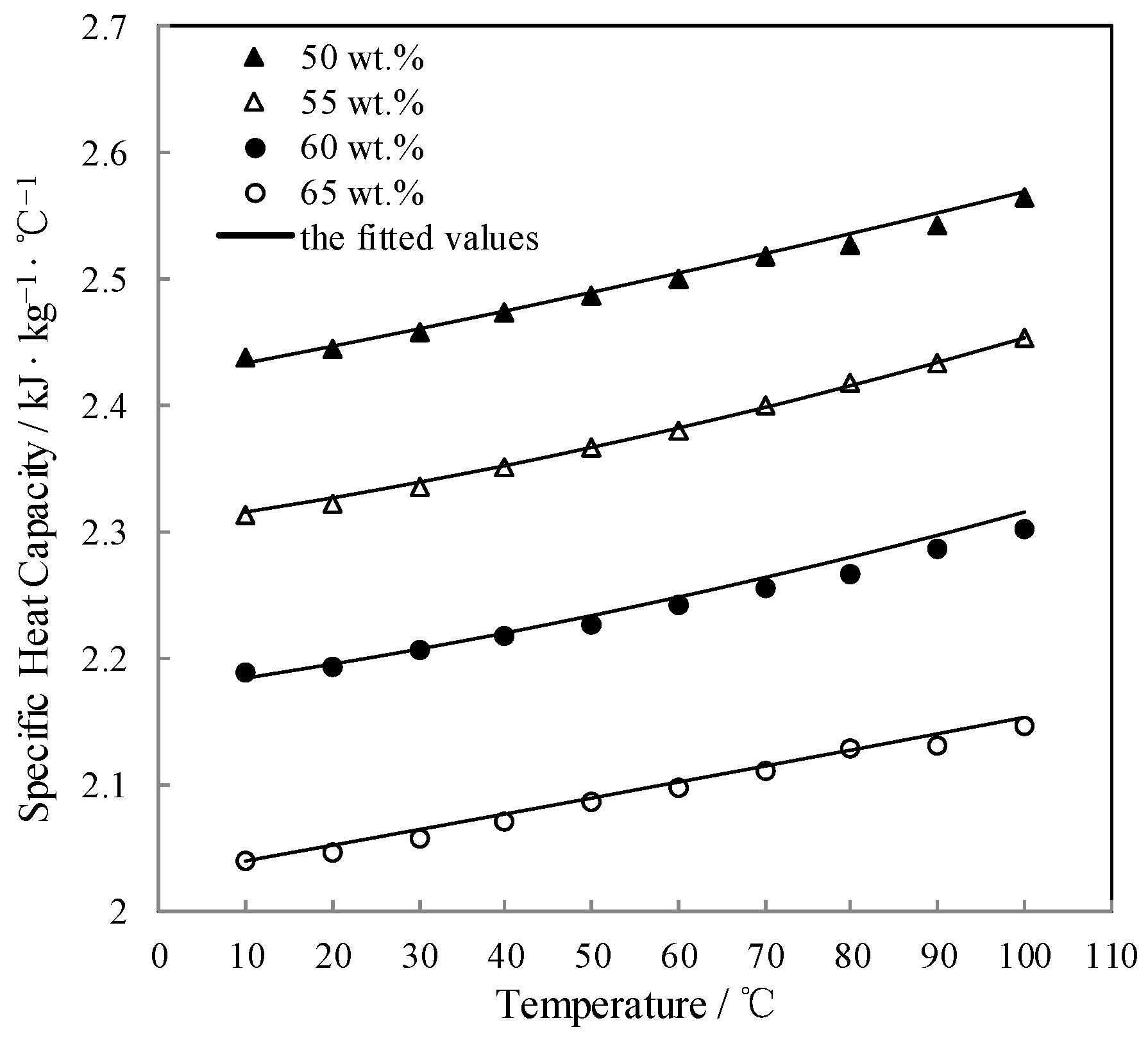
The measured Cp was fitted by Equation (6) and AARD was obtained to be 0.21% by Equation (2).
Figure 10 shows the measured Cp and the fitted value. Cp linearly increased with increasing the temperature.
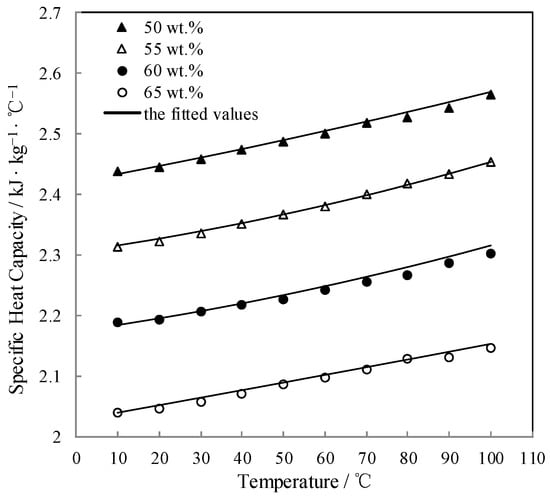
Figure 10.
Cp of CaCl2-LiNO3-KNO3(15.5:5:1) /H2O.
3.7. Calculation of Specific Enthalpy h
3.7.1. Cp of CaCl2, LiNO3, KNO3 and H2O
The Cp of solid KNO3 was measured and is shown in Table 12, Cp of CaCl2, LiNO3, and H2O are given in Reference Literature [29].

Table 12.
Cp of solid KNO3 at atmosphere pressure.
3.7.2. Measurement of Dissolution Enthalpy ΔHmix
ΔHmix of KNO3, LiNO3, and CaCl2 with a mass ratio of 15.5:5:1 were measured at 25.0 °C and are shown in Table 13.

Table 13.
ΔHmix of CaCl2-LiNO3-KNO3(15.5:5:1) /H2O at 25.0 °C.
3.7.3. Calculation of Specific Enthalpy h
h of CaCl2-LiNO3-KNO3(15.5:5:1) /H2O can be obtained from the measured Cp and ΔHmix [25,36,37]. Table 14 lists the obtained results.

Table 14.
h of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O.
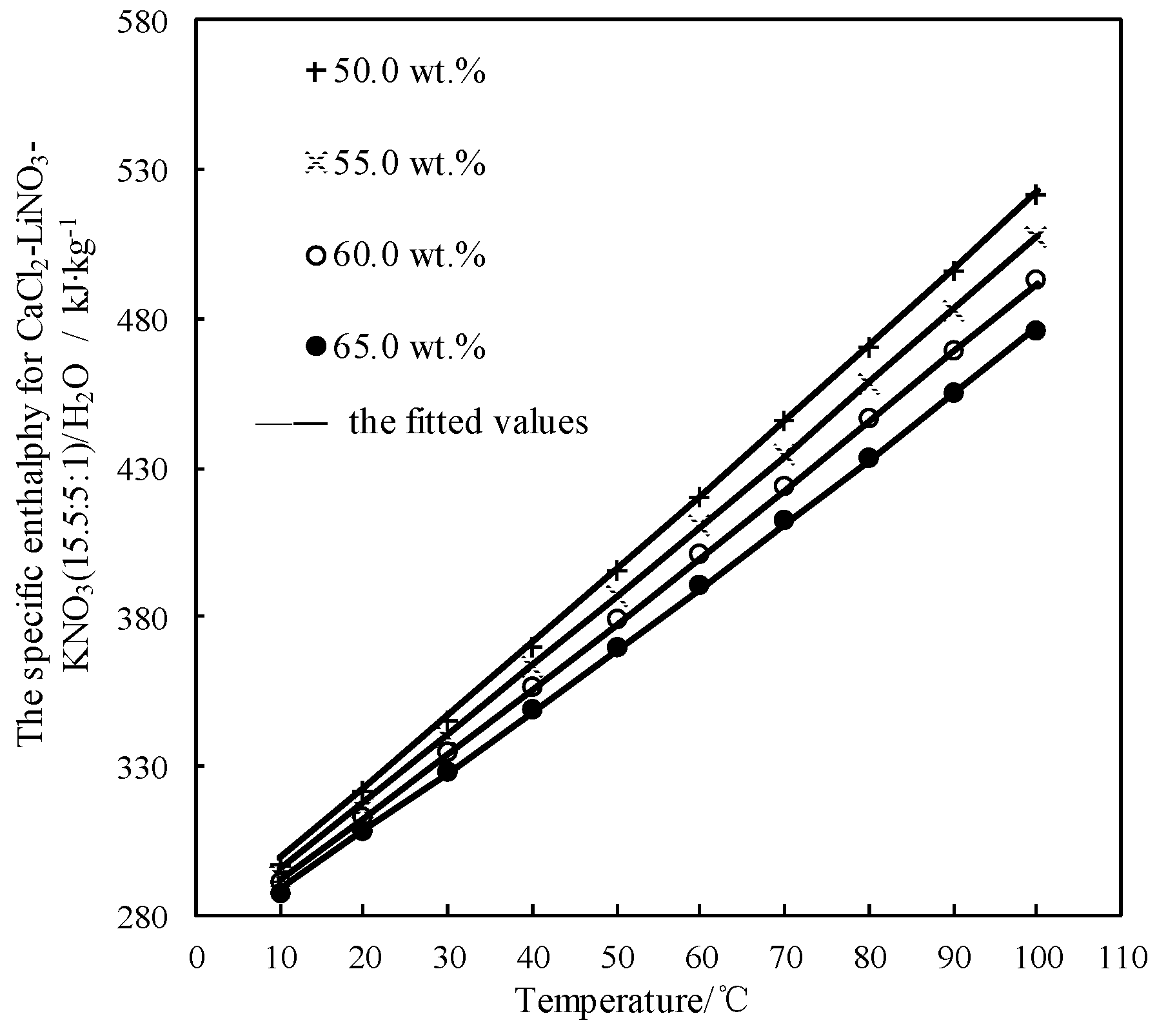
The obtained h was fitted by Equation (7) and AARD was obtained to be 0.07% by Equation (2).
Figure 11 shows the obtained h and the fitted value. h linearly increased with increasing the temperature, and the slope of line slightly increased with reducing the concentration.
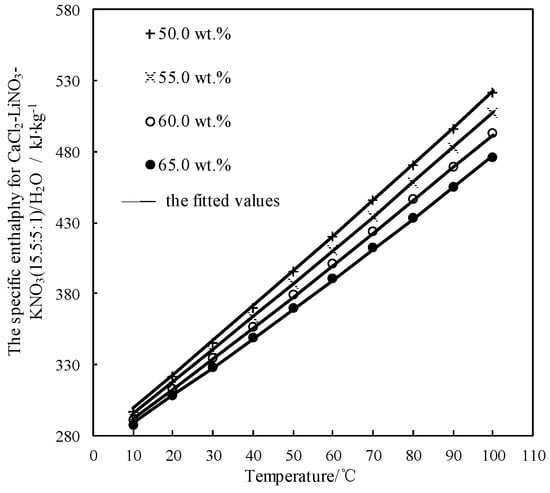
Figure 11.
h of CaCl2-LiNO3-KNO3(15.5:5:1) /H2O.
3.8. Calculation of Specific Entropy s
s of a solution can be also obtained from the measured Cp and ΔHmix [38]. s of CaCl2-LiNO3-KNO3(15.5:5:1) /H2O was obtained and is shown in Table 15.

Table 15.
s of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O.
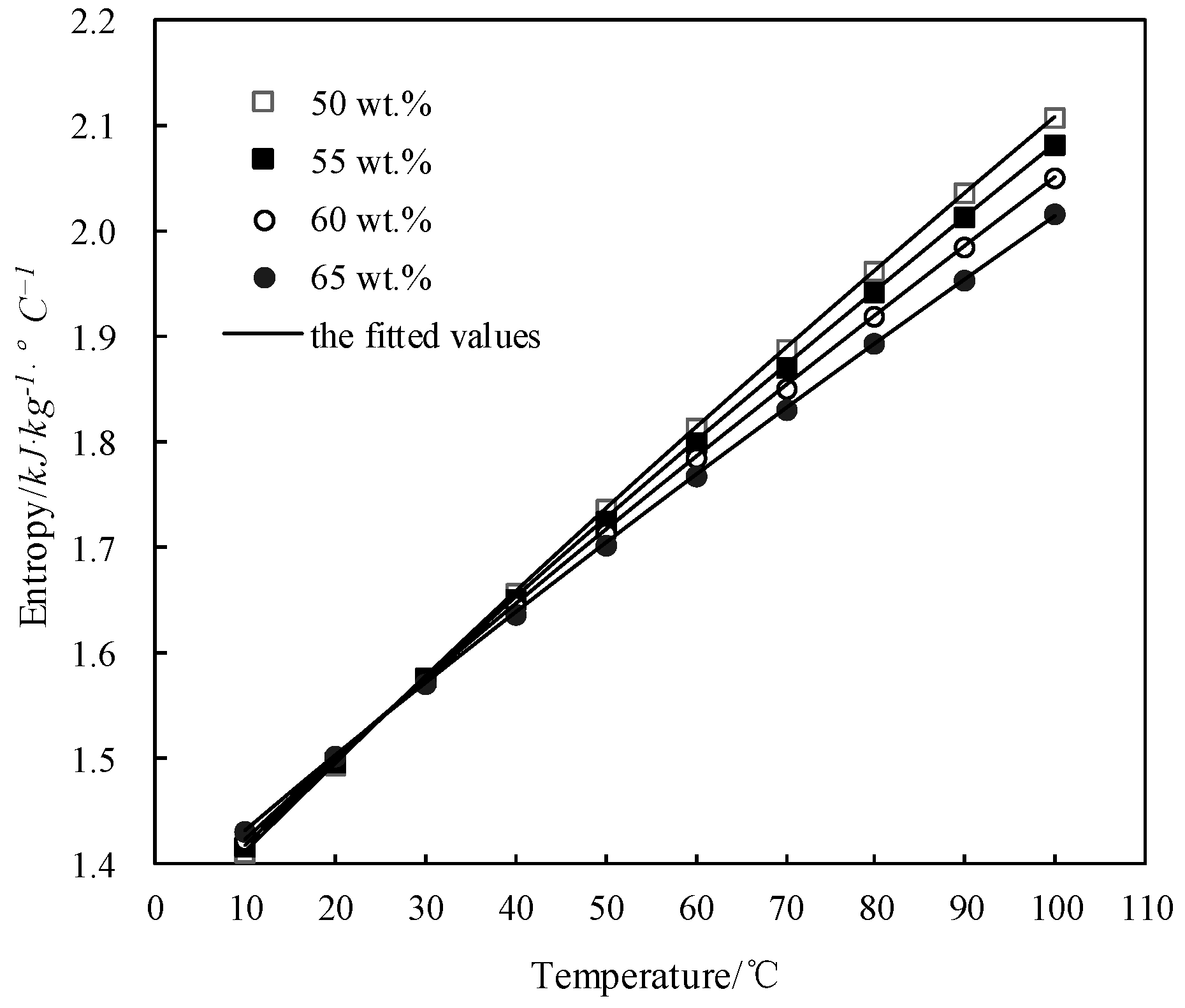
The obtained s of CaCl2-LiNO3-KNO3(15.5:5:1) /H2O was fitted by Equation (8) and the AARD was obtained to be 0.84% by Equation (2).
Figure 12 shows the obtained s and the fitted value. s increased with the temperature increasing and decreased with the concentration increasing when the temperature was above 28 °C, whereas it changed little with the concentration when the temperature was below 28 °C.
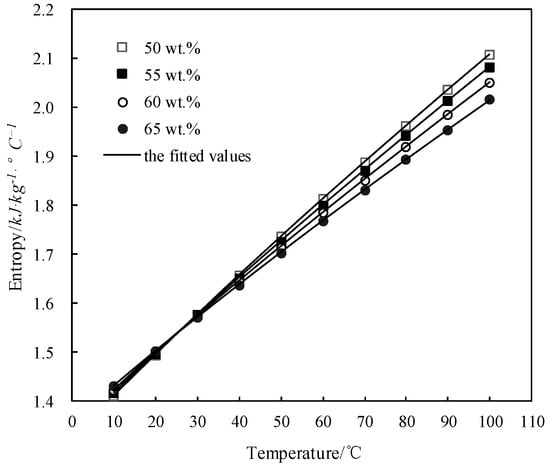
Figure 12.
s of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O.
3.9. Application for an Absorption Refrigeration Cycle
3.9.1. Absorption Refrigeration Cycle Using CaCl2-LiNO3-KNO3(15.5:5:1)/H2O
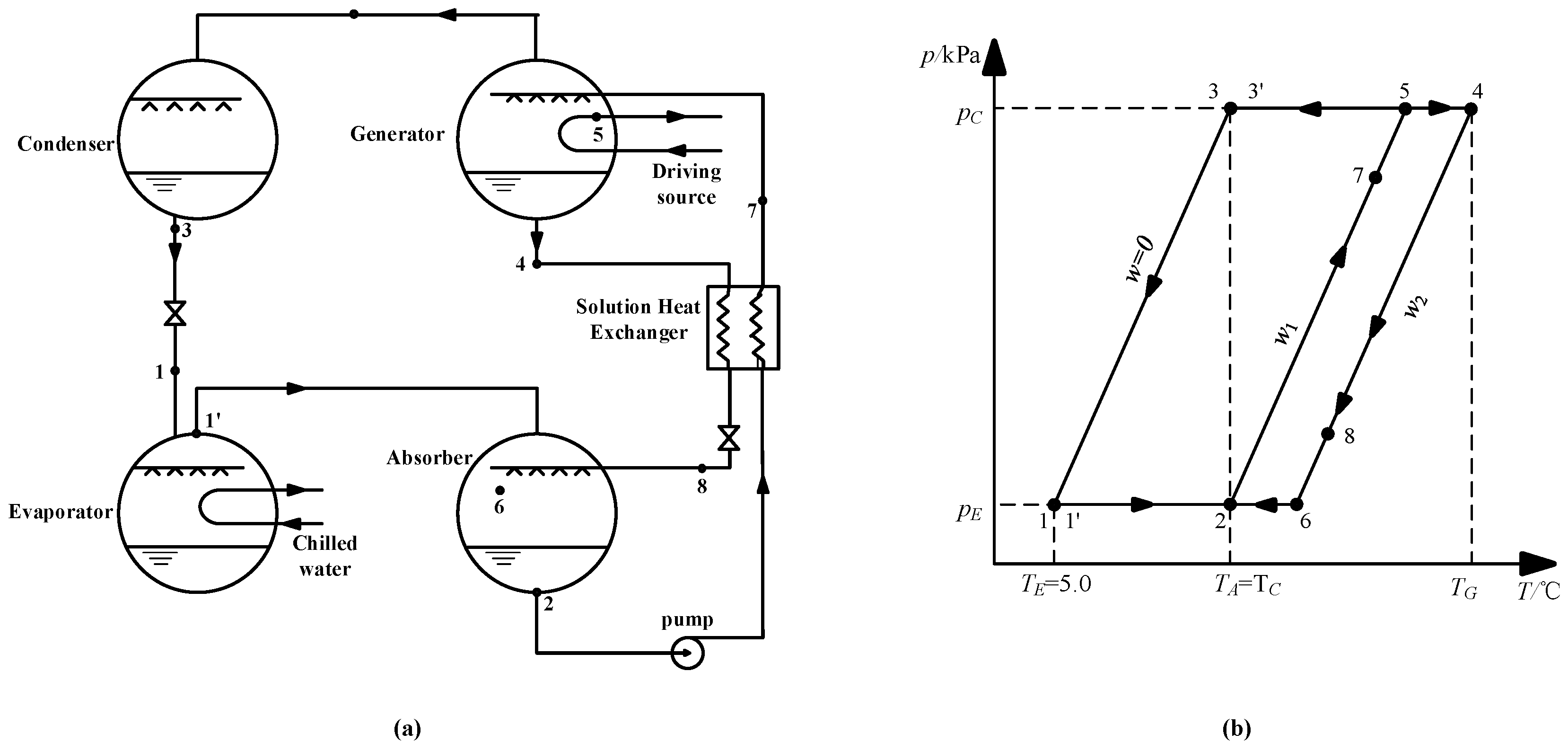
Figure 13a shows the schematic of an absorption refrigeration cycle. Figure 13b is the P-T diagram of the cycle, and the points that are marked in the two diagrams are one-to-one correspondence.
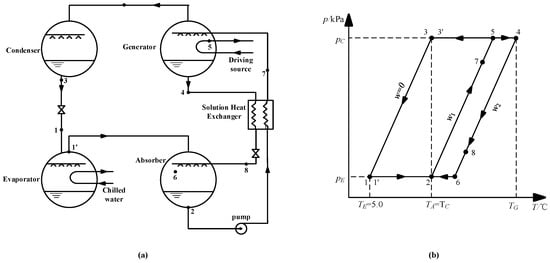
Figure 13.
(a) Schematic of the absorption refrigeration cycle; (b) P-T diagram of the absorption refrigeration cycle.
The working conditions are given, as follows: the evaporation temperature was 5.0 °C; the absorption temperature and condensation temperature were 37.0 °C; and, the evaporation and condensation pressures were 0.872 kPa and 6.290 kPa, respectively. The concentration of dilute solution for CaCl2-LiNO3-KNO3(15.5:5:1)/H2O was figured out to be 60.5 wt.% by Equation (3), and the strong solution was 63.5 wt.%, with a concentration difference of 3.0 wt.%, thus, the generation temperature of the cycle was determined to be 74.0 °C by Equation (3). The same method was applied to calculate the generation temperature while using LiBr/H2O and other CaCl2-based working pairs, including CaCl2-LiBr-LiNO3-KNO3(16.2:2:2:1)/H2O, CaCl2-LiNO3-LiBr(8.72:1:1)/H2O, and CaCl2-LiBr(1.35:1)/H2O. Table 16 lists the results.

Table 16.
Concentration and generation temperature for different working pairs.
As seen in Table 16, the generation temperature was reduced by 7.0 °C through the use of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O instead of LiBr/H2O. The generation temperature differences between the four CaCl2-based working pairs were relatively small.
3.9.2. Analysis of COP and Exergy Efficiency
To analyze the performance of a refrigeration cycle with CaCl2-LiNO3-KNO3(15.5:5:1)/H2O, the state parameters of typical points in Figure 13 were obtained by Equations (3), (7) and (8). Table 17 lists the results.

Table 17.
State parameters of streams in the cycle with CaCl2-LiNO3-KNO3(15.5:5:1)/H2O.
The coefficient of performance (COP) for the absorption refrigeration cycle can be defined as:
where α represents circulating ratio.
COP was obtained to be 0.801 when using CaCl2-LiNO3-KNO3(15.5:5:1)/H2O as the working pair. The COPs for other working pairs were obtained with the same method, and the results are listed in Table 18.

Table 18.
Coefficient of performance (COP) of the cycle with different working pairs.
Table 18 shows that, through using CaCl2-LiNO3-KNO3(15.5:5:1)/H2O instead of LiBr/H2O, the COP was improved by 0.04. Moreover, the exergy destruction in each part of the cycle were analyzed to further compare the performance between CaCl2-LiNO3-KNO3(15.5:5:1)/H2O and LiBr/H2O. Exergy is defined as the maximum possible reversible work that can be obtained from a stream:
where T0 represents the environment temperature that was taken as 25 °C in this paper.
The exergy destructions for each part of the absorption refrigeration cycle were obtained as follows [39].
Evaporator:
Condenser:
Absorber:
Generator:
Heat exchanger:
Table 19 compares the exergy destructions of the absorption cycle with CaCl2-LiNO3-KNO3(15.5:5:1)/H2O and LiBr/H2O. Except the exergy destruction of the evaporator was equal because of the same evaporation condition, the exergy destructions of other parts for CaCl2-LiNO3-KNO3(15.5:5:1)/H2O were lower than those for LiBr/H2O. For the absorption refrigeration cycle, the exergy efficiency (ηE) can be defined as:

Table 19.
The exergy destruction in each part of absorption refrigeration cycle using CaCl2-LiNO3-KNO3(15.5:5:1)/H2O and LiBr/H2O.
The ηE of the absorption refrigeration cycle with CaCl2-LiNO3-KNO3(15.5:5:1)/H2O and LiBr/H2O were obtained to be 0.327 and 0.272, respectively. When compared with COP, the difference in exergy efficiency between the two working pairs was more distinct, which further showed the advantage of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O as an alternative working pair.
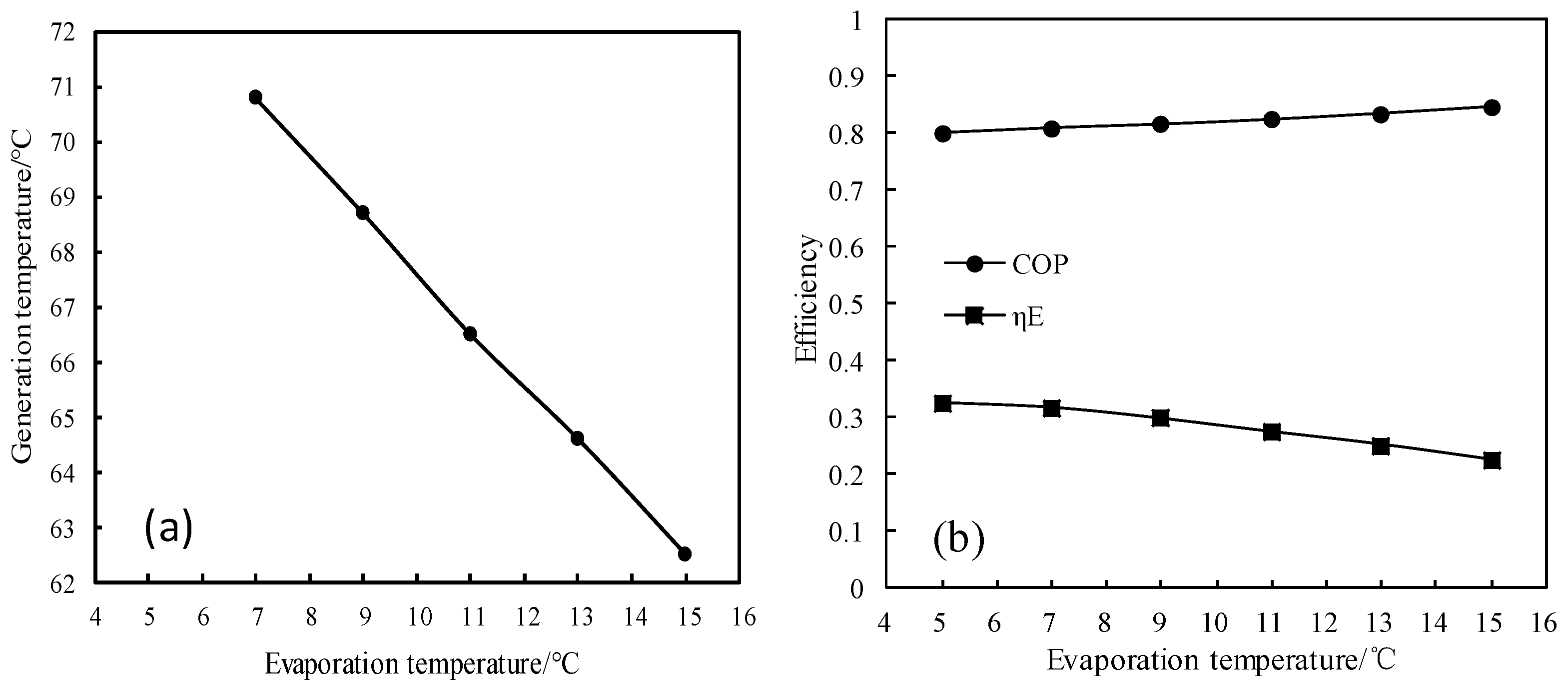
Figure 14 and Figure 15 show the changes of generation temperature and efficiencies (COP and ηE) for CaCl2-LiNO3-KNO3(15.5:5:1)/H2O, with the evaporation temperature varying from 5 °C to 15 °C. As shown in Figure 14a, the generation temperature decreased almost linearly with increasing the evaporation temperature. As shown in Figure 14b, the COP of the absorption refrigeration cycle increased with the evaporation temperature increasing, whereas the exergy efficiency decreased with the evaporation temperature increasing.
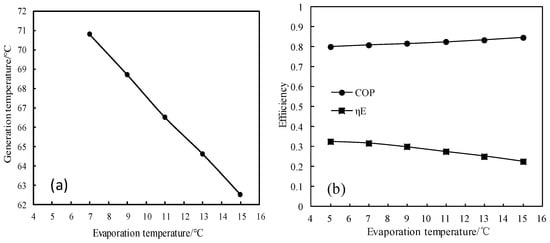
Figure 14.
(a) Variation of the generation temperature with the evaporation temperature; and, (b) Variations of COP and ηE with the evaporation temperature.
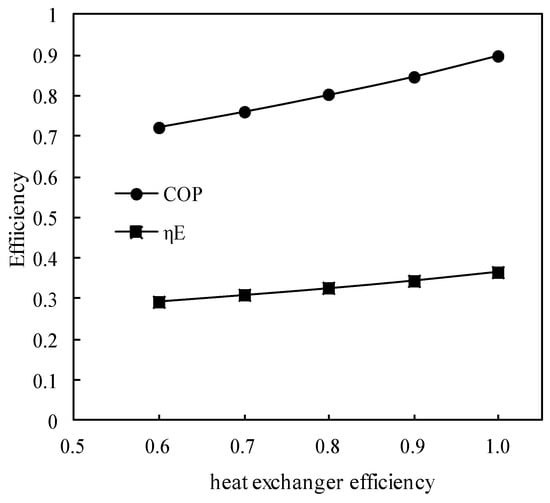
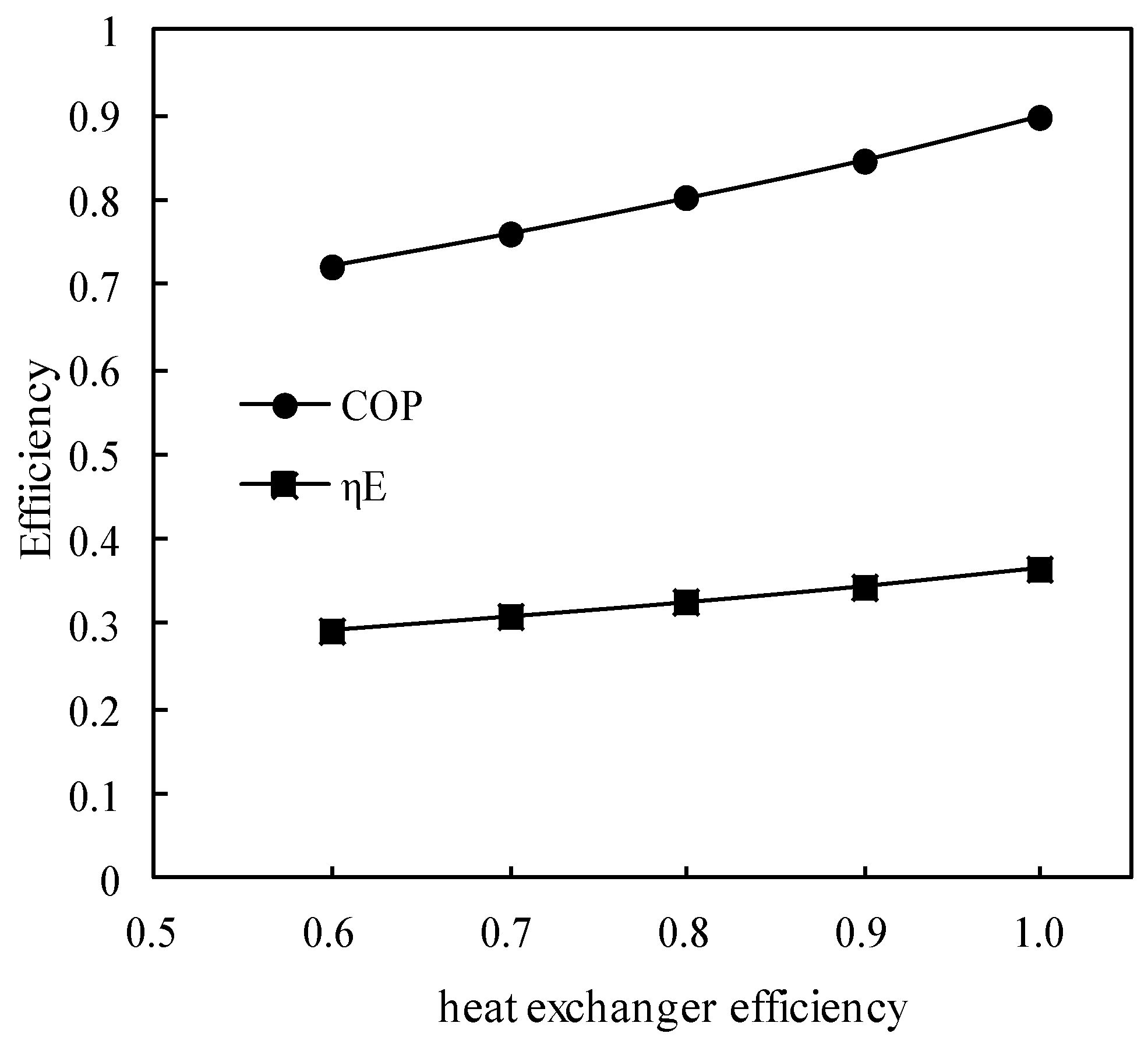
Figure 15.
Variations of COP and ηE with the heat exchanger efficiency.
Figure 15 shows the variations of COP and ηE with the solution heat exchanger efficiency. COP and ηE increased almost linearly with the heat exchanger efficiency increasing, and the increasing slope of COP was greater than that of ηE.
3.10. Measurement of Corrosion Rate RC
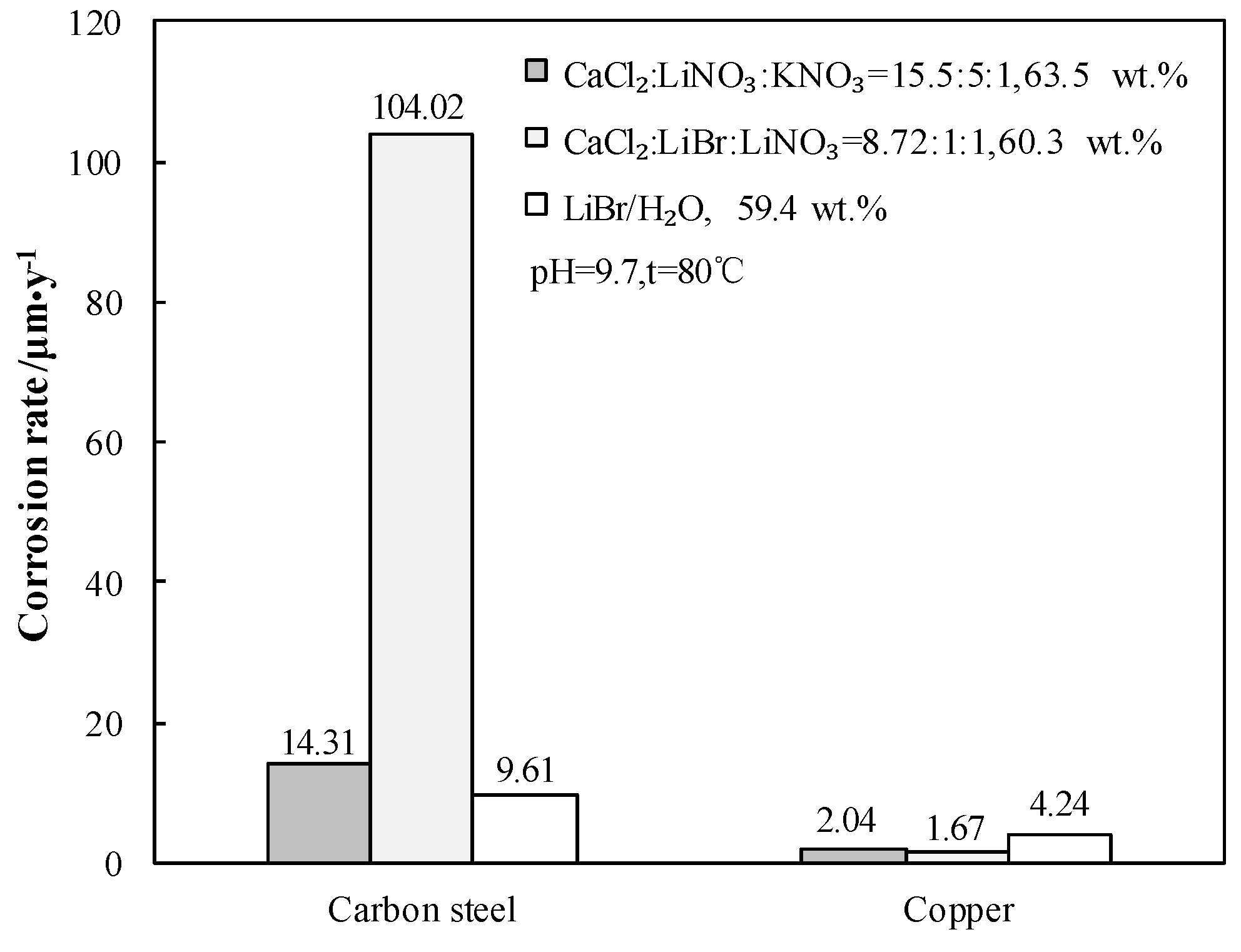
Generally, carbon steel is used as the structural material and copper is used as the heat exchange material for absorption heat pump. The RC of carbon steel and copper in 63.5 wt.% solution of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O were measured at 80.0 °C and pH 9.7. Figure 16 gives the comparison of RC in 63.5 wt.% solution of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O, 59.4 wt.% solution of LiBr/H2O, and 60.3 wt.% solution of CaCl2-LiNO3-LiBr(8.72:1:1)/H2O.

Figure 16.
RC of carbon steel and copper for CaCl2-LiNO3-KNO3(15.5:5:1)/H2O, CaCl2-LiNO3-LiBr(8.72:1:1)/H2O and LiBr/H2O.
Figure 16 shows that the Rc of carbon steel in 63.5 wt.% solution of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O was 14.31 μm∙y−1. Although the corrosivity of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O to carbon steel was stronger than that of LiBr/H2O, it was still acceptable for practical applications. On the other hand, the corrosivity of CaCl2-LiNO3-LiBr(8.72:1:1)/H2O to carbon steel was too strong to be applied, even though it had the lowest generation temperature among the CaCl2-based working pairs. The Rc of copper in 63.5 wt.% solution of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O was 2.04 μm∙y−1, which was smaller than that in 59.4 wt.% solution of LiBr/H2O and it could meet the requirements for engineering applications.
4. Conclusions
- When compared with LiBr/H2O, for an identical adsorption temperature at 0.872 kPa, which is a typical pressure of absorber, CaCl2/H2O had a lower absorption temperature at 6.290 kPa, which is a typical pressure of generator, meaning that CaCl2/H2O basically had a better refrigeration characteristic for an absorption refrigeration cycle. However, the absorption ability of CaCl2/H2O was not strong enough for achieving an evaporation temperature of 5 °C or lower, because of its high crystallization temperature.
- The crystallization temperature was significantly lowered when combining CaCl2/H2O with LiNO3 or LiNO3+KNO3. As a result, the absorption ability of CaCl2-LiNO3/H2O or CaCl2-LiNO3-KNO3/H2O was essentially improved.
- For an absorption refrigeration cycle using CaCl2-LiNO3-KNO3(15.5:5:1)/H2O as the working pair, the generation temperature that is required for achieving an evaporation temperature of 5 °C was 74.0 °C, which was 7.0 °C lower than that using LiBr/H2O.
- When compared with LiBr/H2O under the same conditions, COP and ηE of the absorption refrigeration cycle with CaCl2-LiNO3-KNO3(15.5:5:1)/H2O were improved by 0.04 and 0.06, respectively.
- RC of carbon steel and copper in 63.5 wt.% solution of CaCl2-LiNO3-KNO3(15.5:5:1)/H2O at 80.0 °C and pH 9.7 were 14.31 and 2.04 μm∙y−1, respectively, which indicated that the corrosivity of the proposed working pair could meet the requirements for practical applications.
Author Contributions
Writing—Original Draft preparation, Y.L.; data curation, N.L.; methodology, C.L.; Writing—Review and Editing, Q.S.
Funding
This work was supported by The National Key Research and Development Program of China (2016YFC0400408).
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| T | temperature, °C |
| w | mass concentration, % |
| TC | crystallization temperature, °C |
| p | saturated vapor pressure, kPa |
| ρ | density, g·cm−3 |
| η | dynamic viscosity, mPa·s |
| Cp | specific heat capacity, kJ·kg−1·K−1 |
| ΔHmix | dissolution enthalpy, kJ·kg−1 |
| h | specific enthalpy, kJ·kg−1 |
| s | specific entropy, kJ·kg−1·K−1 |
| AARD | average absolute relative deviation |
| a | circulation ratio |
| COP | coefficient of performance |
| E | exergy |
| ΔE | exergy destrction |
| ηE | exergy efficiency |
| RC | corrosion rate, μm∙y−1 |
References
- Srikhirin, P.; Aphornratana, S.; Chungpaibulpatana, S. A review of absorption refrigeration technologies. Renew. Sustain. Energy Rev. 2001, 5, 343–372. [Google Scholar] [CrossRef]
- Wang, C. Application and development of absorption refrigeration technology. Energy Technol. 2000, 21, 31–35. [Google Scholar]
- Hong, D.; Tang, L.; He, Y. A novel absorption refrigeration cycle. Appl. Therm. Eng. 2010, 30, 2045–2050. [Google Scholar] [CrossRef]
- Abed, A.M.; Alghoul, M.A.; Sopian, K.; Majdi, H.S.; Al-Shamani, A.N.; Muftah, A.F. Enhancement aspects of single stage absorption cooling cycle: A detailed review. Renew. Sustain. Energy Rev. 2017, 77, 1010–1045. [Google Scholar] [CrossRef]
- Bellos, E.; Tzivanidis, C.; Antonopoulos, K.A. Exergetic, energetic and financial evaluation of a solar driven absorption cooling system with various collector types. Appl. Therm. Eng. 2016, 102, 749–759. [Google Scholar] [CrossRef]
- Leonzio, G. Solar systems integrated with absorption heat pumps and thermal energy storages: State of art. Renew. Sustain. Energy Rev. 2017, 70, 492–505. [Google Scholar] [CrossRef]
- Alobaid, M.; Hughes, B.; Calautit, J.K.; O’Connor, D.; Heyes, A. A review of solar driven absorption cooling with photovoltaic thermal systems. Renew. Sustain. Energy Rev. 2017, 76, 728–742. [Google Scholar] [CrossRef]
- Mehrabian, M.A.; Shahbeik, A.E. Thermodynamic modelling of a single-effect LiBr-H2O absorption refrigeration cycle. Proc. Inst. Mech. Eng. Part E-J. Process Mech. Eng. 2005, 219, 261–273. [Google Scholar] [CrossRef]
- Izquierdo, M.; Lizarte, R.; Marcos, J.D.; Gutiérrez, G. Air conditioning using an air-cooled single effect lithium bromide absorption chiller: Results of a trial conducted in Madrid in August 2005. Appl. Therm. Eng. 2008, 28, 1074–1081. [Google Scholar] [CrossRef][Green Version]
- Kaushik, S.C.; Arora, A. Energy and exergy analysis of single effect and series flow double effect water–lithium bromide absorption refrigeration systems. Int. J. Refrig. 2009, 32, 1247–1258. [Google Scholar] [CrossRef]
- Sumathy, K.; Huang, Z.; Li, Z. Solar absorption cooling with low grade heat source—A strategy of development in South China. Sol. Energy 2002, 72, 155–165. [Google Scholar] [CrossRef]
- Li, Z.; Jing, Y.; Liu, J. Thermodynamic study of a novel solar LiBr/H2O absorption chiller. Energy Build. 2016, 133, 565–576. [Google Scholar] [CrossRef]
- Sun, J.; Fu, L.; Zhang, S. A review of working fluids of absorption cycles. Renew. Sustain. Energy Rev. 2012, 16, 1899–1906. [Google Scholar] [CrossRef]
- N’tsoukpoe, K.E.; Perier-Muzet, M.; Le-ierrès, N.; Luo, L.; Mangin, D. Thermodynamic study of a LiBr–H2O absorption process for solar heat storage with crystallisation of the solution. Sol. Energy 2014, 104, 2–15. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, R.; Wang, H. Experimental evaluation of a variable effect LiBr-water absorption chiller designed for high-efficient solar cooling system. Int. J. Refrig. 2015, 59, 135–143. [Google Scholar] [CrossRef]
- Lin, P.; Wang, R.; Xia, Z. Numerical investigation of a two-stage air-cooled absorption refrigeration system for solar cooling: Cycle analysis and absorption cooling performances. Renew. Energy 2011, 36, 1401–1412. [Google Scholar] [CrossRef]
- Malinina, O.S.; Baranenko, A.V.; Zaitsev, A.V. Influence of the average daily outdoor air parameters on the efficiency of solar lithium bromide-water absorption refrigeration machine. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2018; Volume 2007, p. 030040. [Google Scholar]
- Mortazavi, M.; Schmid, M.; Moghaddam, S. Compact and efficient generator for low grade solar and waste heat driven absorption systems. Appl. Energy 2017, 198, 173–179. [Google Scholar] [CrossRef]
- Bourouis, M.; Vallès, M.; Medrano, M.; Coronas, A. Performance of air-cooled absorption air-conditioning systems working with water-(LiBr+ Lil+ LiNO3+ LiCl). Part E J. Process Mech. Eng. 2005, 219, 205–213. [Google Scholar] [CrossRef]
- Jian, S.; Lin, F.; Shigang, Z. Performance calculation of single effect absorption heat pump using LiBr+ LiNO3+ H2O as working fluid. Appl. Therm. Eng. 2010, 30, 2680–2684. [Google Scholar] [CrossRef]
- Chen, W.; Bai, Y. Thermal performance of an absorption-refrigeration system with [emim] Cu2Cl5/NH3 as working fluid. Energy 2016, 112, 332–341. [Google Scholar] [CrossRef]
- Bellos, E.; Tzivanidis, C.; Antonopoulos, K.A. Exergetic and energetic comparison of LiCl-H2O and LiBr-H2O working pairs in a solar absorption cooling system. Energy Convers. Manag. 2016, 123, 453–461. [Google Scholar] [CrossRef]
- Wang, M.; Ferreira, C.A.I. Absorption heat pump cycles with NH3–ionic liquid working pairs. Appl. Energy 2017, 204, 819–830. [Google Scholar] [CrossRef]
- Luo, C.; Chen, K.; Li, Y.; Su, Q. Crystallization Temperature, Vapor Pressure, Density, Viscosity, and Specific Heat Capacity of the LiNO3/[BMIM]Cl/H2O Ternary System. J. Chem. Eng. Data 2017, 62, 3043–3052. [Google Scholar] [CrossRef]
- Luo, C.; Li, Y.; Chen, K.; Li, N.; Su, Q. Thermodynamic properties and corrosivity of a new absorption heat pump working pair: Lithium nitrate+ 1-butyl-3-methylimidazolium bromide+ water. Fluid Phase Equilib. 2017, 451, 25–39. [Google Scholar] [CrossRef]
- Luo, C.; Li, Y.; Li, N.; Wang, Y.; Su, Q. Thermophysical properties of lithium nitrate+ 1-ethyl-3-methylimidazolium diethylphosphate+ water system. J. Chem. Thermodyn. 2018, 126, 160–170. [Google Scholar] [CrossRef]
- Luo, C.; Wang, Y.; Li, Y.; Wu, Y.; Su, Q.; Hu, T. Thermodynamic properties and application of LiNO3-[MMIM][DMP]/H2O ternary working pair. Renew. Energy 2019, 134, 147–160. [Google Scholar] [CrossRef]
- Luo, C.; Li, Y.; Li, N.; Su, Q. Thermodynamic properties and evaluation of the lithium nitrate–imidazole IL–water ternary systems as new working fluids for a double-effect AHP cycle. Int. J. Refrig. 2018, 90, 58–72. [Google Scholar] [CrossRef]
- Li, N.; Luo, C.; Su, Q. A working pair of CaCl2–LiBr–LiNO3/H2O and its application in a single-stage solar-driven absorption refrigeration cycle. Int. J. Refrig. 2018, 86, 1–13. [Google Scholar] [CrossRef]
- Li, N.; Luo, C.; Su, Q. Thermophysical properties and corrosivity of CaCl2-LiBr-LiNO3-KNO3/H2O working pair. Chin. J. Process Eng. 2018, 18, 764–768. [Google Scholar]
- Li, N.; Luo, C.; Su, Q. Thermophysical properties and applications of CaCl2-LiBr(1.35:1)/H2O as a working pair. Chin. J. Eng. 2018, 40, 167–176. [Google Scholar]
- Li, N.; Li, Y.; Luo, C.; Su, Q. Thermophysical properties and applications of CaCl2-LiNO3/H2O ternary working pair. Chem. Ind. Eng. Prog. 2018, 37, 4625–4637. [Google Scholar]
- Park, Y.; Kim, J.S.; Lee, H.; Yu, S.I. Density, vapor pressure, solubility, and viscosity for water+ lithium bromide+ lithium nitrate+ 1, 3-propanediol. J. Chem. Eng. Data 1997, 42, 145–148. [Google Scholar] [CrossRef]
- Safarov, J.T. Vapor pressure of heat transfer fluids of absorption refrigeration machines and heat pumps: Binary solutions of lithium nitrate with methanol. J. Chem. Thermodyn. 2005, 37, 1261–1267. [Google Scholar] [CrossRef]
- Verevkin, S.; Safarov, J.; Bich, E.; Hassel, E.; Heintz, A. Study of vapour pressure of lithium nitrate solutions in ethanol. J. Chem. Thermodyn. 2006, 38, 611–616. [Google Scholar] [CrossRef]
- Gao, Q.; Zheng, D.; Jiang, C. Thermodynamic study on the working of HEAT–TYPE absorption heat pump. Petrochem. Technol. 1993, 22, 382–392. [Google Scholar]
- Chen, D.; Xie, J. Technology and Application of Heat Pump; Chemical Industry Press: Beijing, China, 2006; pp. 206–220. [Google Scholar]
- Yang, D.; Zhu, Y.; Liu, S.; Lv, H.; Luo, C. Thermodynamic Properties of a Ternary AHP Working Pair: Lithium Bromide+ 1-Ethyl-3-methylimidazolium Chloride+ H2O. J. Chem. Eng. Data 2019, 64, 574–583. [Google Scholar] [CrossRef]
- Aprhornratana, S.; Eames, I.W. Thermodynamic analysis of absorption refrigeration cycles using the second law of thermodynamics method. Int. J. Refrig. 1995, 18, 244–252. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).