Evidence for Maintained Post-Encoding Memory Consolidation Across the Adult Lifespan Revealed by Network Complexity
Abstract
1. Introduction
Participants
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. fMRI Scans
2.4. fMRI Acquisition and Preprocessing
2.5. Resting-state fMRI Analysis
2.6. MultiScale Entropy (MSE) Analysis
2.7. Multilevel Modeling Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luo, L.; Craik, F.I. Aging and memory: A cognitive approach. Can. J. Psychiat. 2008, 53, 346–353. [Google Scholar] [CrossRef]
- Naveh-Benjamin, M. Adult age differences in memory performance: Tests of an associative deficit hypothesis. J. Exp. Psychol. Learn. Mem. Cogn. 2000, 26, 1170–1187. [Google Scholar] [CrossRef]
- Bastin, C.; Besson, G.; Simon, J.; Delhaye, E.; Geurten, M.; Willems, S.; Salmon, E. An Integrative Memory model of recollection and familiarity to understand memory deficits. Behav. Brain Sci. 2019, 1, 1–66. [Google Scholar] [CrossRef]
- Mitchell, K.J.; Johnson, M.K. Source monitoring 15 years later: What have we learned from fMRI about the neural mechanisms of source memory? Psychol. Bull. 2009, 135, 638–677. [Google Scholar] [CrossRef]
- Maillet, D.; Rajah, M.N. Age-related differences in brain activity in the subsequent memory paradigm: A meta-analysis. Neurosci. Biobehav. Rev. 2014, 45, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G. The hippocampo-neocortical dialogue. Cereb. Cortex 1996, 6, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Diekelmann, S.; Born, J. The memory function of sleep. Nat. Rev. Neurosci. 2010, 11, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Tononi, G.; Cirelli, C. Sleep function and synaptic homeostasis. Sleep Med. Rev. 2006, 10, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Nadel, L.; Hupbach, A.; Gomez, R.; Newman-Smith, K. Memory formation, consolidation and transformation. Neurosci. Biobehav. Rev. 2012, 36, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Bliwise, D.L. Sleep in normal aging and dementia. Sleep 1993, 16, 40–81. [Google Scholar] [CrossRef]
- Ohayon, M.M.; Carskadon, M.A.; Guilleminault, C.; Vitiello, M.V. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep 2004, 27, 1255–1273. [Google Scholar] [CrossRef] [PubMed]
- Scullin, M.K. Sleep, memory, and aging: The link between slow-wave sleep and episodic memory changes from younger to older adults. Psychol. Aging 2013, 28, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.F.; Jadhav, S.P.; Frank, L.M. Hippocampal replay in the awake state: A potential substrate for memory consolidation and retrieval. Nat. Neurosci. 2011, 14, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Tambini, A.; Ketz, N.; Davachi, L. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron 2010, 65, 280–290. [Google Scholar] [CrossRef]
- Schlichting, M.L.; Preston, A.R. Hippocampal-medial prefrontal circuit supports memory updating during learning and post-encoding rest. Neurobiol. Learn. Mem. 2016, 134, 91–106. [Google Scholar] [CrossRef]
- van Kesteren, M.T.R.; Fernández, G.; Norris, D.G.; Hermans, E.J. Persistent schema-dependent hippocampal-neocortical connectivity during memory encoding and postencoding rest in humans. Proc. Natl. Acad. Sci. USA 2010, 107, 7550–7555. [Google Scholar] [CrossRef]
- Jacobs, H.I.; Dillen, K.N.; Risius, O.; Göreci, Y.; Onur, O.A.; Fink, G.R.; Kukolja, J. Consolidation in older adults depends upon competition between resting-state networks. Front. Aging Neurosci. 2015, 6, 344. [Google Scholar] [CrossRef]
- Mary, A.; Wens, V.; Op de Beeck, M.; Leproult, R.; De Tiège, X.; Peigneux, P. Age-related differences in practice-dependent resting-state functional connectivity related to motor sequence learning. Hum. Brain Mapp. 2017, 38, 923–937. [Google Scholar] [CrossRef]
- Oren, N.; Ash, E.; Shapira-Lichter, I.; Elkana, O.; Reichman-Eisikovits, O.; Chomsky, L.; Lerner, Y. Changes in resting-state functional connectivity of the hippocampus following cognitive effort predict memory decline at the older age—A longitudinal fMRI study. Front. Aging Neurosci. 2019, 11, 163. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of complex physiologic time series. Phys. Rev. Lett. 2002, 89, 68–102. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of biological signals. Phys. Rev. E 2005, 71, 021906. [Google Scholar] [CrossRef] [PubMed]
- Tononi, G.; Sporns, O.; Edelman, G.M. A measure for brain complexity: Relating functional segregation and integration in the nervous system. Proc. Natl. Acad. Sci. USA 1994, 91, 5033–5037. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.T.; Jirsa, V.K.; Spiegler, A.; McIntosh, A.R.; Deco, G. Bottom up modeling of the connectome: Linking structure and function in the resting brain and their changes in aging. Neuroimage 2013, 80, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Vakorin, V.A.; Lippé, S.; McIntosh, A.R. Variability of brain signals processed locally transforms into higher connectivity with brain development. J. Neurosci. 2011, 31, 6405–6413. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, A.R.; Vakorin, V.; Kovacevic, N.; Wang, H.; Diaconescu, A.; Protzner, A.B. Spatiotemporal dependency of age-related changes in brain signal variability. Cereb. Cortex 2014, 24, 1806–1817. [Google Scholar] [CrossRef]
- Heisz, J.J.; Vakorin, V.; Ross, B.; Levine, B.; McIntosh, A.R. A trade-off between local and distributed information processing associated with remote episodic versus semantic memory. J. Cogn. Neurosci. 2013, 26, 41–53. [Google Scholar] [CrossRef]
- Song, D.; Chang, D.; Zhang, J.; Peng, W.; Shang, Y.; Gao, X.; Wang, Z. Reduced brain entropy by repetitive transcranial magnetic stimulation on the left dorsolateral prefrontal cortex in healthy young adults. Brain Imaging Behav. 2019, 13, 421–429. [Google Scholar] [CrossRef] [PubMed]
- McDonough, I.M.; Nashiro, K. Network complexity as a measure of information processing across resting-state networks: Evidence from the Human Connectome Project. Front. Hum. Neurosci. 2014, 8, 409. [Google Scholar] [CrossRef]
- Smith, R.X.; Yan, L.; Wang, D.J. Multiple time scale complexity analysis of resting state FMRI. Brain Imaging Behav. 2014, 8, 284–291. [Google Scholar] [CrossRef]
- Kavcic, V.; Ni, H.; Zhu, T.; Zhong, J.; Duffy, C.J. White matter integrity linked to functional impairments in aging and early Alzheimer’s disease. Alzheimers Dement. 2008, 4, 381–389. [Google Scholar] [CrossRef]
- Friston, K.J. The disconnection hypothesis. Schizophr. Res. 1998, 30, 115–125. [Google Scholar] [CrossRef]
- Just, M.A.; Cherkassky, V.L.; Keller, T.A.; Minshew, N.J. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain 2004, 127, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Kana, R.K.; Libero, L.E.; Moore, M.S. Disrupted cortical connectivity theory as an explanatory model for autism spectrum disorders. Phys. Life Rev. 2011, 8, 410–437. [Google Scholar] [CrossRef] [PubMed]
- Murias, M.; Swanson, J.M.; Srinivasan, R. Functional connectivity of frontal cortex in healthy and ADHD children reflected in EEG coherence. Cereb. Cortex 2006, 17, 1788–1799. [Google Scholar] [CrossRef]
- Carballedo, A.; Scheuerecker, J.; Meisenzahl, E.; Schoepf, V.; Bokde, A.; Möller, H.J.; Doyle, M.; Wiesmann, M.; Frodl, T. Functional connectivity of emotional processing in depression. J. Affect. Disorders 2011, 134, 272–279. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Y.; Chen, D.; Li, C.; Qiu, Y.; Wang, J. Altered electroencephalogram complexity in autistic children shown by the multiscale entropy approach. Neuroreport 2017, 28, 169. [Google Scholar] [CrossRef]
- Hadoush, H.; Alafeef, M.; Abdulhay, E. Brain Complexity in Children with Mild and Severe Autism Spectrum Disorders: Analysis of Multiscale Entropy in EEG. Brain Topogr. 2019, 32, 914–921. [Google Scholar] [CrossRef]
- Bosl, W.; Tierney, A.; Tager-Flusberg, H.; Nelson, C. EEG complexity as a biomarker for autism spectrum disorder risk. BMC Med. 2011, 9, 18. [Google Scholar] [CrossRef]
- Hager, B.; Yang, A.C.; Brady, R.; Meda, S.; Clementz, B.; Pearlson, G.D.; Sweeney, J.A.; Tamminga, C.; Keshavan, M. Neural complexity as a potential translational biomarker for psychosis. J. Affect. Disorders 2017, 216, 89–99. [Google Scholar] [CrossRef]
- Yang, A.C.; Hong, C.J.; Liou, Y.J.; Huang, K.L.; Huang, C.C.; Liu, M.E.; Lo, M.T.; Huang, N.E.; Peng, C.K.; Lin, C.P.; et al. Decreased resting-state brain activity complexity in schizophrenia characterized by both increased regularity and randomness. Hum. Brain Mapp. 2015, 36, 2174–2186. [Google Scholar] [CrossRef]
- Lipsitz, L.A. Physiological complexity, aging, and the path to frailty. Sci. Aging Knowl. Environ. 2004, 16, pe16. [Google Scholar] [CrossRef] [PubMed]
- Mcbride, J.C.; Zhao, X.; Munro, N.B.; Smith, C.D.; Jicha, G.A.; Hively, L.; Broster, L.S.; Schmitt, F.A.; Kryscio, R.J.; Jiang, Y. Spectral and complexity analysis of scalp EEG characteristics for mild cognitive impairment and early Alzheimer’s disease. Comput. Meth. Prog. Biomed. 2014, 114, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Gu, H.; Luo, Q. Sample entropy reveals an age-related reduction in the complexity of dynamic brain. Sci. Rep. 2017, 7, 7990. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Krishnan, A.P.; Yan, L.; Smith, R.X.; Kilroy, E.; Alger, J.R.; Ringman, J.M.; Wang, D.J. Complexity and synchronicity of resting state blood oxygenation level-dependent (BOLD) functional MRI in normal aging and cognitive decline. J. Magn. Reson. Imaging 2013, 38, 36–45. [Google Scholar] [CrossRef]
- Sokunbi, M.O. Sample entropy reveals high discriminative power between young and elderly adults in short fMRI data sets. Front. Neuroinform. 2014, 8. [Google Scholar] [CrossRef]
- Yang, A.C.; Huang, C.C.; Yeh, H.L.; Liu, M.E.; Hong, C.J.; Tu, P.C.; Chen, J.F.; Huang, N.E.; Peng, C.K.; Lin, C.P.; et al. Complexity of spontaneous BOLD activity in default mode network is correlated with cognitive function in normal male elderly: A multiscale entropy analysis. Neurobiol. Aging 2013, 34, 428–438. [Google Scholar] [CrossRef]
- Kielar, A.; Deschamps, T.; Chu, R.K.O.; Jokel, R.; Khatamian, Y.B.; Chen, J.J.; Meltzer, J.A. Identifying dysfunctional cortex: Dissociable effects of stroke and aging on resting state dynamics in MEG and fMRI. Front. Aging Neurosci. 2016, 8, 40. [Google Scholar] [CrossRef]
- McDonough, I.M.; Siegel, J.T. The Relation between White Matter Microstructure and Network Complexity: Implications for Processing Efficiency. Front. Int. Neurosci. 2018, 12, 43. [Google Scholar] [CrossRef]
- Greicius, M.D.; Krasnow, B.; Reiss, A.L.; Menon, V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 253–258. [Google Scholar] [CrossRef]
- Laird, A.R.; Fox, P.M.; Eickhoff, S.B.; Turner, J.A.; Ray, K.L.; McKay, D.R.; Glahn, D.C.; Beckmann, C.F.; Smith, S.M.; Fox, P.T. Behavioral interpretations of intrinsic connectivity networks. J. Cogn. Neurosci. 2011, 23, 4022–4037. [Google Scholar] [CrossRef]
- Smith, S.M.; Fox, P.T.; Miller, K.L.; Glahn, D.C.; Fox, P.M.; Mackay, C.E.; Filippini, N.; Watkins, K.E.; Toro, R.; Laird, A.R.; et al. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. USA 2009, 106, 13040–13045. [Google Scholar] [CrossRef] [PubMed]
- McDonough, I.M.; Letang, S.K.; Stinson, E.A. Dementia Risk Elevates Brain Activity during Memory Retrieval: A Functional MRI Analysis of Middle Aged and Older Adults. J. Alzheimers Dis. 2019, 70, 1005–1023. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.H.; Tumosa, N.; Chibnall, J.T.; Perry, M.H., III; Morley, J.E. Comparison of the Saint Louis University mental status examination and the mini-mental state examination for detecting dementia and mild neurocognitive disorder—A pilot study. Am. J. Geriatr. Psychiatry 2006, 14, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, C.F.; Smith, S.M. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging 2004, 23, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Avants, B.; Gee, J.C. Geodesic estimation for large deformation anatomical shape averaging and interpolation. Neuroimage 2004, 23, S139–S150. [Google Scholar] [CrossRef]
- Beckmann, C.F.; Mackay, C.E.; Filippini, N.; Smith, S.M. Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. Neuroimage 2009, 47, S148. [Google Scholar] [CrossRef]
- Filippini, N.; MacIntosh, B.J.; Hough, M.G.; Goodwin, G.M.; Frisoni, G.B.; Smith, S.M.; Matthews, P.M.; Beckmann, C.F.; Mackay, C.E. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proc. Natl. Acad. Sci. USA 2009, 106, 7209–7214. [Google Scholar] [CrossRef]
- Lake, D.E.; Richman, J.S.; Griffin, M.P.; Moorman, J.R. Sample entropy analysis of neonatal heart rate variability. Am. J. Physiol. Reg. 2002, 283, R789–R797. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef]
- Sokunbi, M.O.; Fung, W.; Sawlani, V.; Choppin, S.; Linden, D.E.; Thome, J. Resting state fMRI entropy probes complexity of brain activity in adults with ADHD. Psychiatry Res. Neuroimaging 2013, 214, 341–348. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Murty, V.P.; Tompary, A.; Adcock, R.A.; Davachi, L. Selectivity in postencoding connectivity with high-level visual cortex is associated with reward-motivated memory. J. Neurosci. 2017, 37, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Dupret, D.; O’Neill, J.; Pleydell-Bouverie, B.; Csicsvari, J. The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat. Neurosci. 2010, 13, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, P.; Squire, L.R. Memory consolidation and the medial temporal lobe: A simple network model. Proc. Natl. Acad. Sci. USA 1994, 91, 7041–7045. [Google Scholar] [CrossRef]
- Eichenbaum, H.; Cohen, N.J. From Conditioning to Conscious Recollection; Oxford Univ. Press: New York, NY, USA, 2001. [Google Scholar]
- Rugg, M.D.; Vilberg, K.L. Brain networks underlying episodic memory retrieval. Curr. Opin. Neurobiol. 2013, 23, 255–260. [Google Scholar] [CrossRef]
- He, J.; Carmichael, O.; Fletcher, E.; Singh, B.; Iosif, A.-M.; Martinez, O.; Reed, B.; Yonelinas, A.; DeCarli, C. Influence of functional connectivity and structural MRI measures on episodic memory. Neurobiol. Aging 2012, 33, 2612–2620. [Google Scholar] [CrossRef]
- Wang, L.; LaViolette, P.; O’Keefe, K.; Putcha, D.; Bakkour, A.; Van Dijk, K.R.A.; Pihlajamaki, M.; Dickerson, B.C.; Sperling, R.A. Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. Neuroimage 2010, 51, 910–917. [Google Scholar] [CrossRef]
- Tambini, A.; Davachi, L. Persistence of hippocampal multivoxel patterns into postencoding rest is related to memory. Proc. Natl. Acad. Sci. USA 2013, 110, 19591–19596. [Google Scholar] [CrossRef]
- Park, D.C.; Polk, T.A.; Park, R.; Minear, M.; Savage, A.; Smith, M.R. Aging reduces neural specialization in ventral visual cortex. Proc. Natl. Acad. Sci. USA 2004, 101, 13091–13095. [Google Scholar] [CrossRef]
- Park, J.; Carp, J.; Kennedy, K.M.; Rodrigue, K.M.; Bischof, G.N.; Huang, C.M.; Rieck, J.R.; Polk, T.A.; Park, D.C. Neural broadening or neural attenuation? Investigating age-related dedifferentiation in the face network in a large lifespan sample. J. Neurosci. 2012, 32, 2154–2158. [Google Scholar] [CrossRef] [PubMed]
- Grady, C.L.; Haxby, J.V.; Horwitz, B.; Schapiro, M.B.; Rapoport, S.I.; Ungerleider, L.G.; Mishkin, M.; Carson, R.E.; Herscovitch, P. Dissociation of object and spatial vision in human extrastriate cortex: Age-related changes in activation of regional cerebral blood flow measured with [15 O] water and positron emission tomography. J. Cogn. Neurosci. 1992, 4, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Craik, F.I.M.; Byrd, M. Aging and cognitive deficits: The role of attentional resources. In Aging and Cognitive Processes; Craik, F.I.M., Trehub, S., Eds.; Plenum: New York, NY, USA, 1982; pp. 191–211. [Google Scholar]
- Craik, F.I.M.; Jennings, J.M. Human memory. In The Handbook of Aging and Cognition; Craik, F.I.M., Salthouse, T.A., Eds.; Erlbaum: Hillsdale, MI, USA, 1992; pp. 51–110. [Google Scholar]
- Dywan, J.; Jacoby, L. Effects of aging on source monitoring: Differences in susceptibility to false fame. Psychol. Aging 1990, 5, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Jennings, J.M.; Jacoby, L.L. Automatic versus intentional uses of memory: Aging, attention, and control. Psychol. Aging 1993, 8, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Maillet, D.; Schacter, D.L. Default network and aging: Beyond the task-negative perspective. Trends Cogn. Sci. 2016, 20, 646–648. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baltes, P.B.; Baltes, M.M. Psychological perspectives on successful aging: The model of selective optimization with compensation. In Successful Aging: Perspectives from the Behavioral Sciences, 1st ed.; University of Cambridge: Cambridge, UK, 1990; pp. 1–34. [Google Scholar]
- van Kesteren, M.T.; Rijpkema, M.; Ruiter, D.J.; Morris, R.G.; Fernández, G. Building on prior knowledge: Schema-dependent encoding processes relate to academic performance. J. Cogn. Neurosci. 2014, 26, 2250–2261. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Cho, R.Y.; Murata, T.; Mizuno, T.; Kikuchi, M.; Mizukami, K.; Kosaka, H.; Takahashi, K.; Wada, Y. Age-related variation in EEG complexity to photic stimulation: A multiscale entropy analysis. Clin. Neurophysiol. 2009, 120, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; McIntosh, A.R.; Kovacevic, N.; Karachalios, M.; Protzner, A.B. Age-related multiscale changes in brain signal variability in pre-task versus post-task resting-state EEG. J. Cogn. Neurosci. 2016, 28, 971–984. [Google Scholar] [CrossRef]
- Barnes, A.; Bullmore, E.T.; Suckling, J. Endogenous human brain dynamics recover slowly following cognitive effort. PLoS ONE 2009, 4, e6626. [Google Scholar] [CrossRef]
- Grigg, O.; Grady, C.L. Task-related effects on the temporal and spatial dynamics of resting-state functional connectivity in the default network. PLoS ONE 2006, 5, e13311. [Google Scholar] [CrossRef]
- Pyka, M.; Beckmann, C.F.; Schöning, S.; Hauke, S.; Heider, D.; Kugel, H.; Arolt, V.; Konrad, C. Impact of working memory load on fMRI resting state pattern in subsequent resting phases. PLoS ONE 2009, 4, e7198. [Google Scholar] [CrossRef]
- Sala-Llonch, R.; Pena-Gomez, C.; Arenaza-Urquijo, E.M.; Vidal-Piñeiro, D.; Bargallo, N.; Junque, C.; Bartres-Faz, D. Brain connectivity during resting state and subsequent working memory task predicts behavioural performance. Cortex 2012, 48, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Reuter-Lorenz, P.A.; Park, D.C. How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychol. Rev. 2014, 24, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Schooler, J.W.; Engstler-Schooler, T.Y. Verbal overshadowing of visual memories: Some things are better left unsaid. Cogn. Psychol. 1990, 22, 36–71. [Google Scholar] [CrossRef]
- Meissner, C.A.; Brigham, J.C. A meta-analysis of the verbal overshadowing effect in face identification. Appl. Cogn. Psychol. 2001, 15, 603–616. [Google Scholar] [CrossRef]
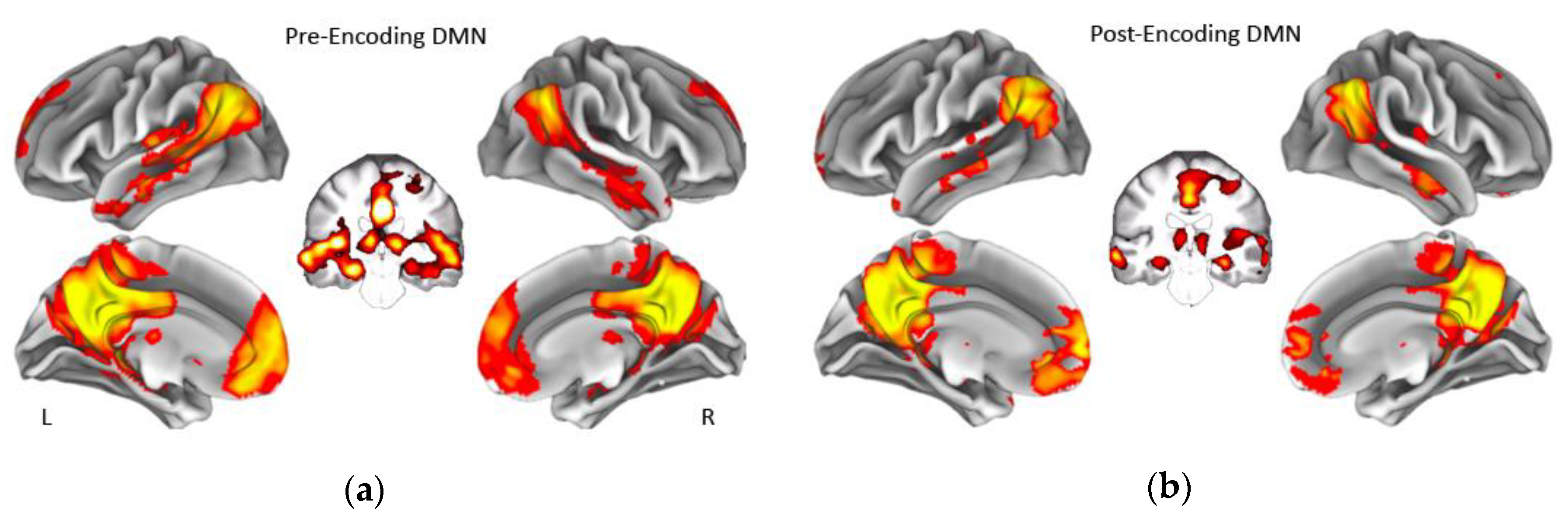

| Young Adults | Middle-Aged Adults | Older Adults | Group Differences | |
|---|---|---|---|---|
| N | 20 | 31 | 35 | - |
| Mean Age | 23.35 (3.25) | 54.29 (2.84) | 66.17 (4.02) | F (2,83) = 987.70, p < 0.001 |
| Age Range | 20–30 | 50–60 | 61–74 | - |
| Sex (F/M) | 11 (55%)/9 (45%) | 17 (54%)/14 (46%) | 23 (66%)/12 (34%) | χ2 (2) = 1.01, p = 0.61 |
| Ethnoracial Category | χ2 (4) = 22.66, p < 0.001 | |||
| Non-Hispanic White | 12 (60%) | 14 (45%) | 28 (80%) | - |
| African American | 1 (5%) | 13 (42%) | 7 (20%) | - |
| Other | 7 (35%) | 4 (13%) | 0 (0%) | - |
| Years of Education | 15.00 (2.20) | 14.26 (2.67) | 13.83 (2.93) | F (2,83) = 1.20, p = 0.30 |
| SLUMS Score | - | 26.48 (2.95) 1 | 26.21 (2.91) | t (62.7) = 0.38, p = 0.70 |
| Associative Memory Performance | 0.57 (0.17) | 0.36 (0.14) | 0.32 (0.09) | F (2,83) = 24.55, p < 0.001 |
| Premorbid IQ | 106.65 (10.69) | 95.16 (16.08) | 107.55 (16.70) | F (2,83) = 6.20, p = 0.003 |
| Dementia Risk | - | 5.32 (1.85) | 5.03 (2.08) | t (62.9) = 0.66, p = 0.51 |
| DMN Post–Pre Encoding (Model 1) | DMN Pre-Encoding (Model 2) | DMN Post-Encoding (Model 3) | DMN Post–Pre Encoding (Model 4) | OT Post–Pre Encoding (Model 5) | CO Post–Pre Encoding (Model 6) | LFP Post–Pre Encoding (Model 7) | RFP Post–Pre Encoding (Model 8) | |
|---|---|---|---|---|---|---|---|---|
| Intercept | −0.0084 (0.013) | 0.9764 (0.012) *** | 0.9683 (0.013) *** | −0.0242 (0.024) | −0.0051 (0.016) | −0.0162 (0.017) | −0.0674 (0.015) *** | −0.0745 (0.017) *** |
| Timescale | 0.0099 (0.013) | 0.0148 (0.012) | 0.0117 (0.013) | 0.0065 (0.015) | −0.0010 (0.006) | 0.0021 (0.007) | 0.0175 (0.006) ** | 0.0156 (0.007) * |
| MSE Pre-Encoding | −0.1789 (0.009) *** | - | - | −0.1813 (0.010) *** | −0.1836 (0.009) *** | −0.1800 (0.009) *** | −0.1913 (0.009) *** | −0.1992 (0.009) *** |
| Age | 0.0376 (0.014) ** | −0.0002 (0.014) | 0.0374 (0.014) * | 0.0750 (0.036) * | 0.0017 (0.016) | −0.0155 (0.015) | −0.0155 (0.014) | −0.0039 (0.015) |
| Sex (Ref. = Male) | −0.0036 (0.010) | 0.0096 (0.010) | −0.0027 (0.011) | −0.0034 (0.012) | 0.0014 (0.012) | 0.0021 (0.011) | 0.0104 (0.011) | 0.0032 (0.011) |
| Premorbid IQ | −0.0090 (0.013) | 0.0015 (0.013) | −0.0084 (0.013) | −0.0222 (0.015) | 0.0123 (0.014) | −0.0055 (0.014) | −0.0020 (0.013) | −0.0074 (0.014) |
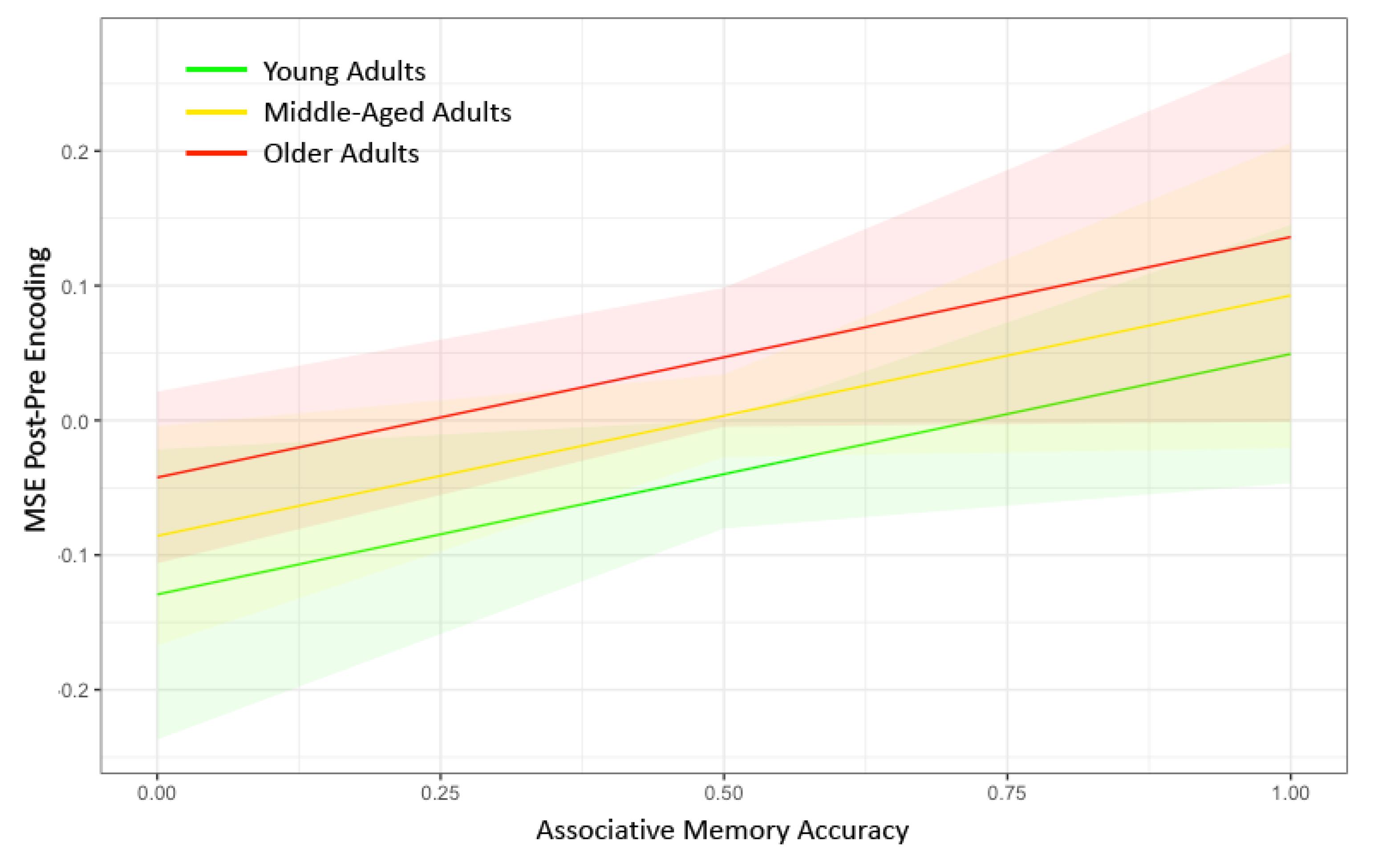
| Memory Accuracy | 0.0321 (0.016) * | 0.0083 (0.016) | 0.0329 (0.016) * | 0.0538 (0.021) ** | 0.0156 (0.018) | −0.0039 (0.017) | −0.0238 (0.016) | 0.0144 (0.017) |
| Dementia Risk | - | - | - | 0.0052 (0.007) | - | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDonough, I.M.; Letang, S.K.; Erwin, H.B.; Kana, R.K. Evidence for Maintained Post-Encoding Memory Consolidation Across the Adult Lifespan Revealed by Network Complexity. Entropy 2019, 21, 1072. https://doi.org/10.3390/e21111072
McDonough IM, Letang SK, Erwin HB, Kana RK. Evidence for Maintained Post-Encoding Memory Consolidation Across the Adult Lifespan Revealed by Network Complexity. Entropy. 2019; 21(11):1072. https://doi.org/10.3390/e21111072
Chicago/Turabian StyleMcDonough, Ian M., Sarah K. Letang, Hillary B. Erwin, and Rajesh K. Kana. 2019. "Evidence for Maintained Post-Encoding Memory Consolidation Across the Adult Lifespan Revealed by Network Complexity" Entropy 21, no. 11: 1072. https://doi.org/10.3390/e21111072
APA StyleMcDonough, I. M., Letang, S. K., Erwin, H. B., & Kana, R. K. (2019). Evidence for Maintained Post-Encoding Memory Consolidation Across the Adult Lifespan Revealed by Network Complexity. Entropy, 21(11), 1072. https://doi.org/10.3390/e21111072





