Effect of Solution Treatment on the Shape Memory Functions of (TiZrHf)50Ni25Co10Cu15 High Entropy Shape Memory Alloy
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
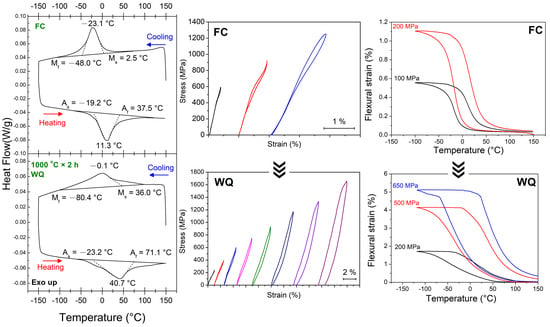
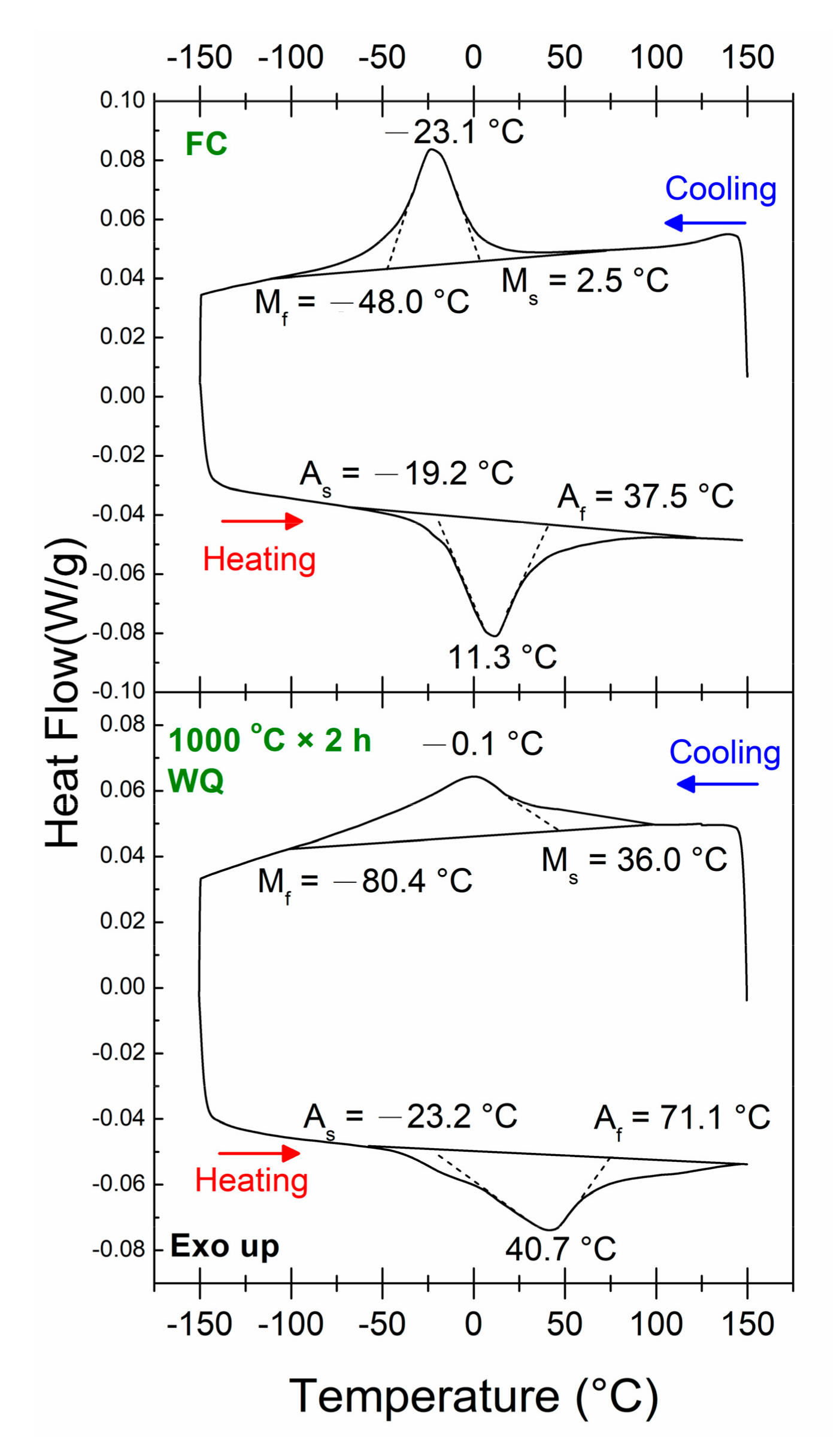
3.1. Transformation Behaviors
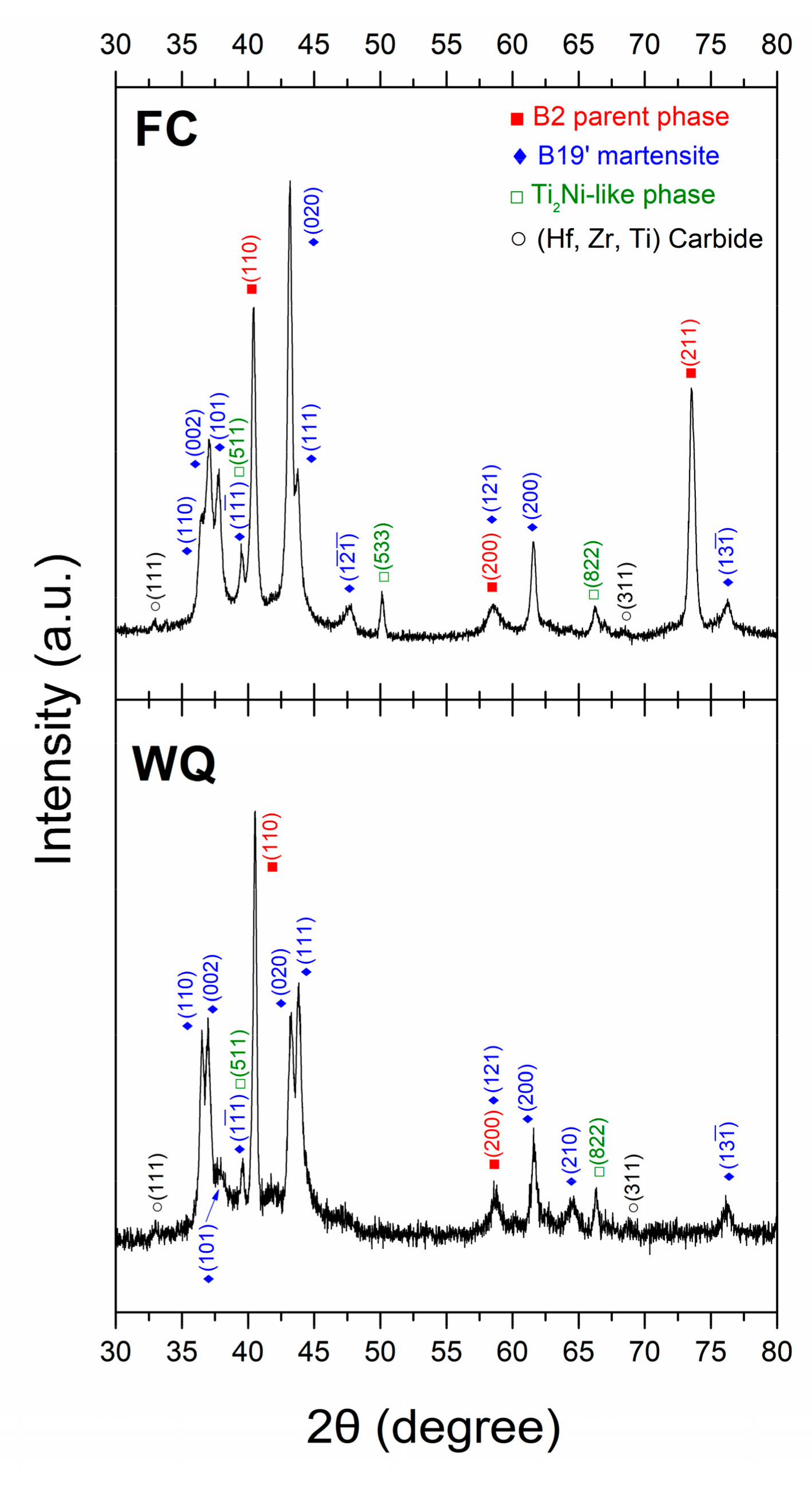
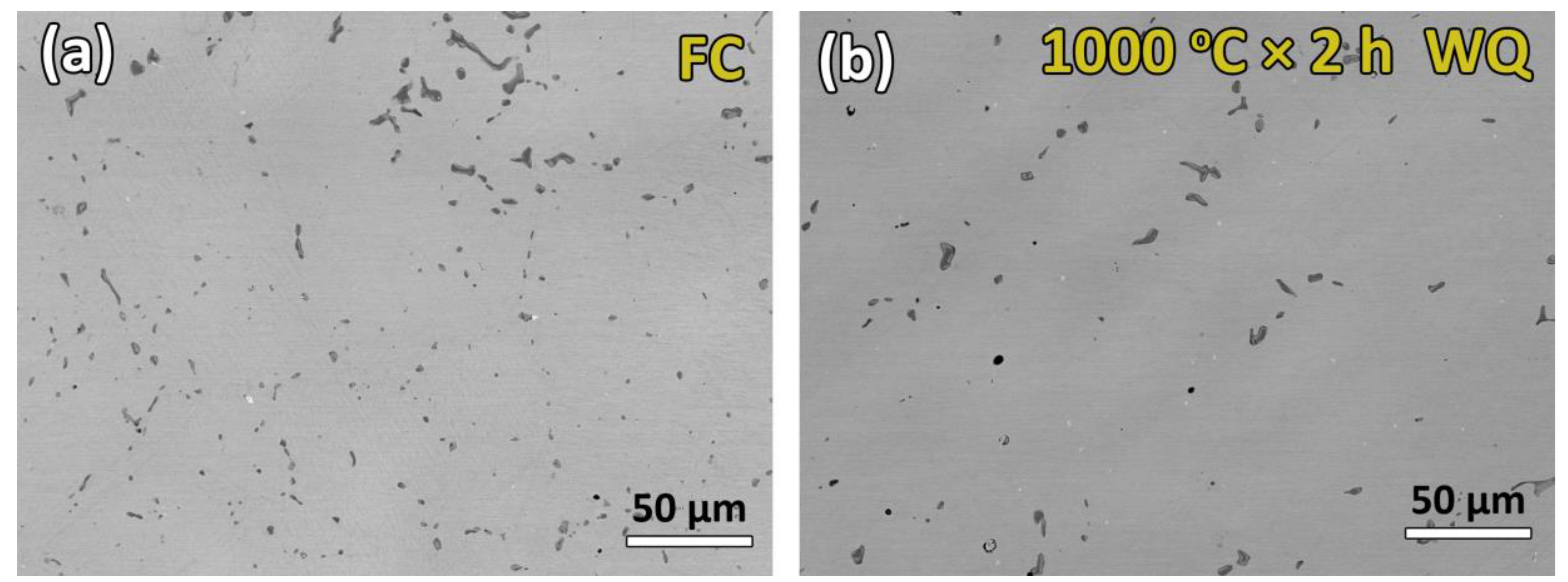
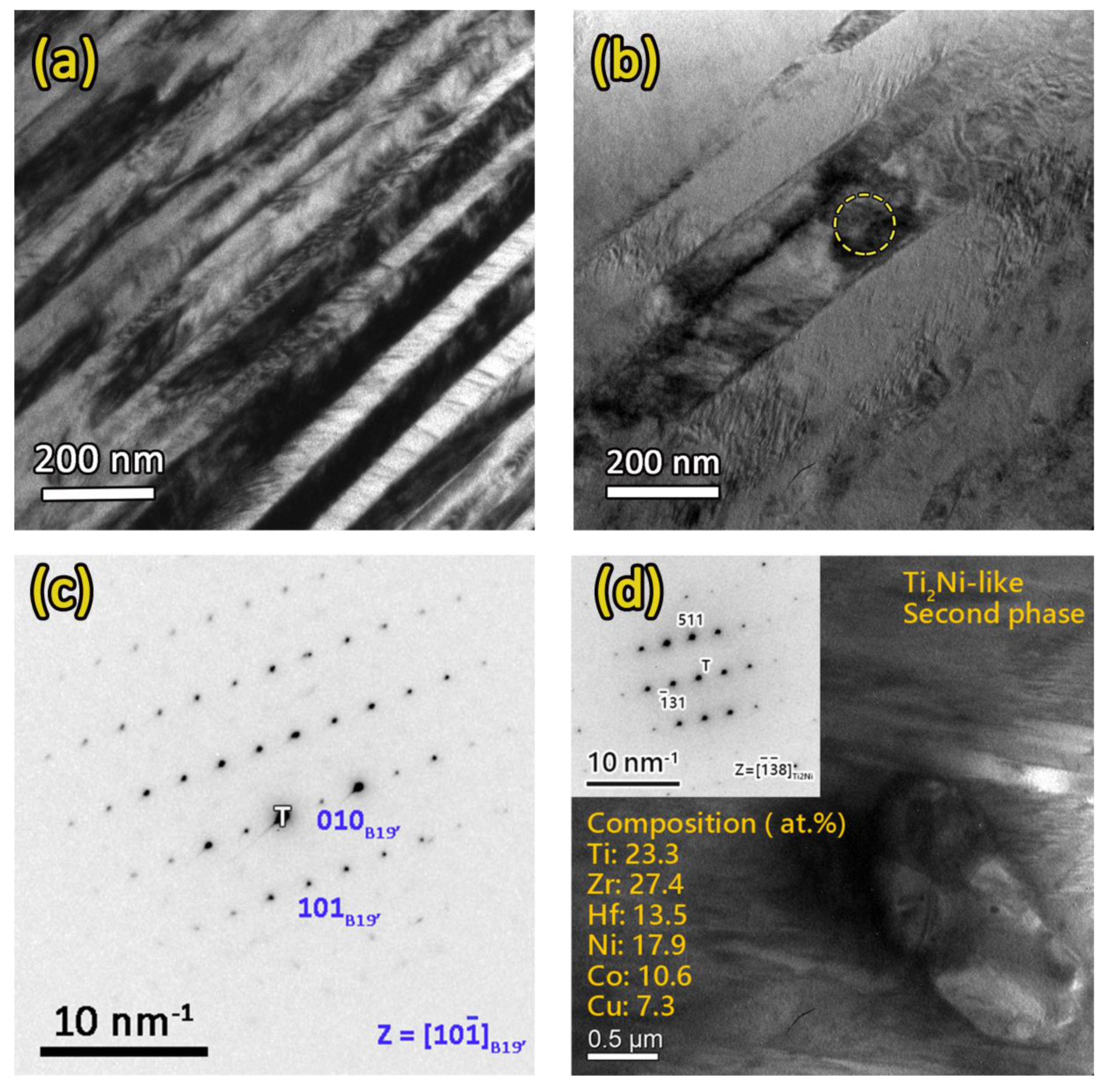
3.2. Microstructure Observations
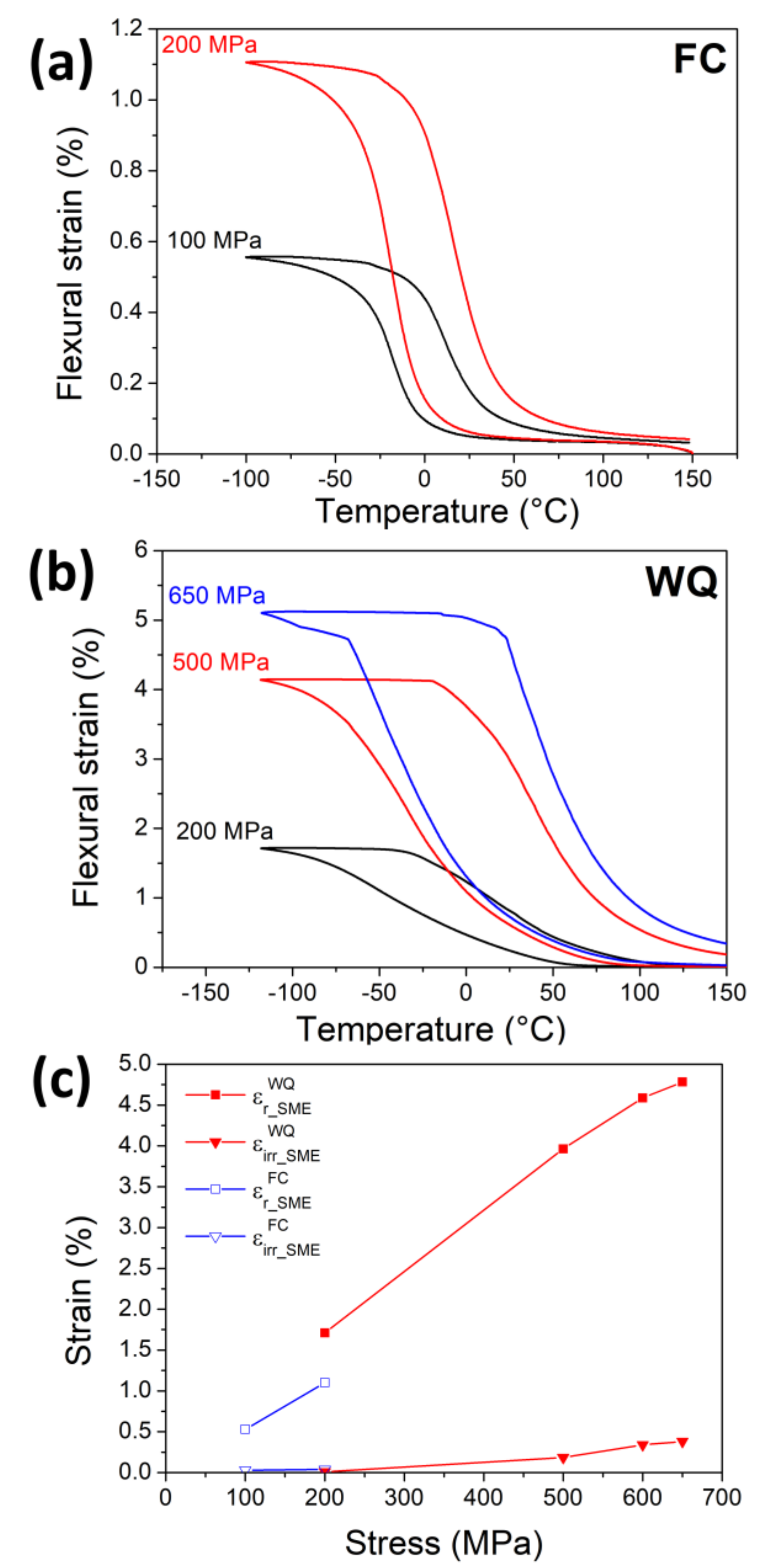
3.3. Changes in Transformation Temperatures
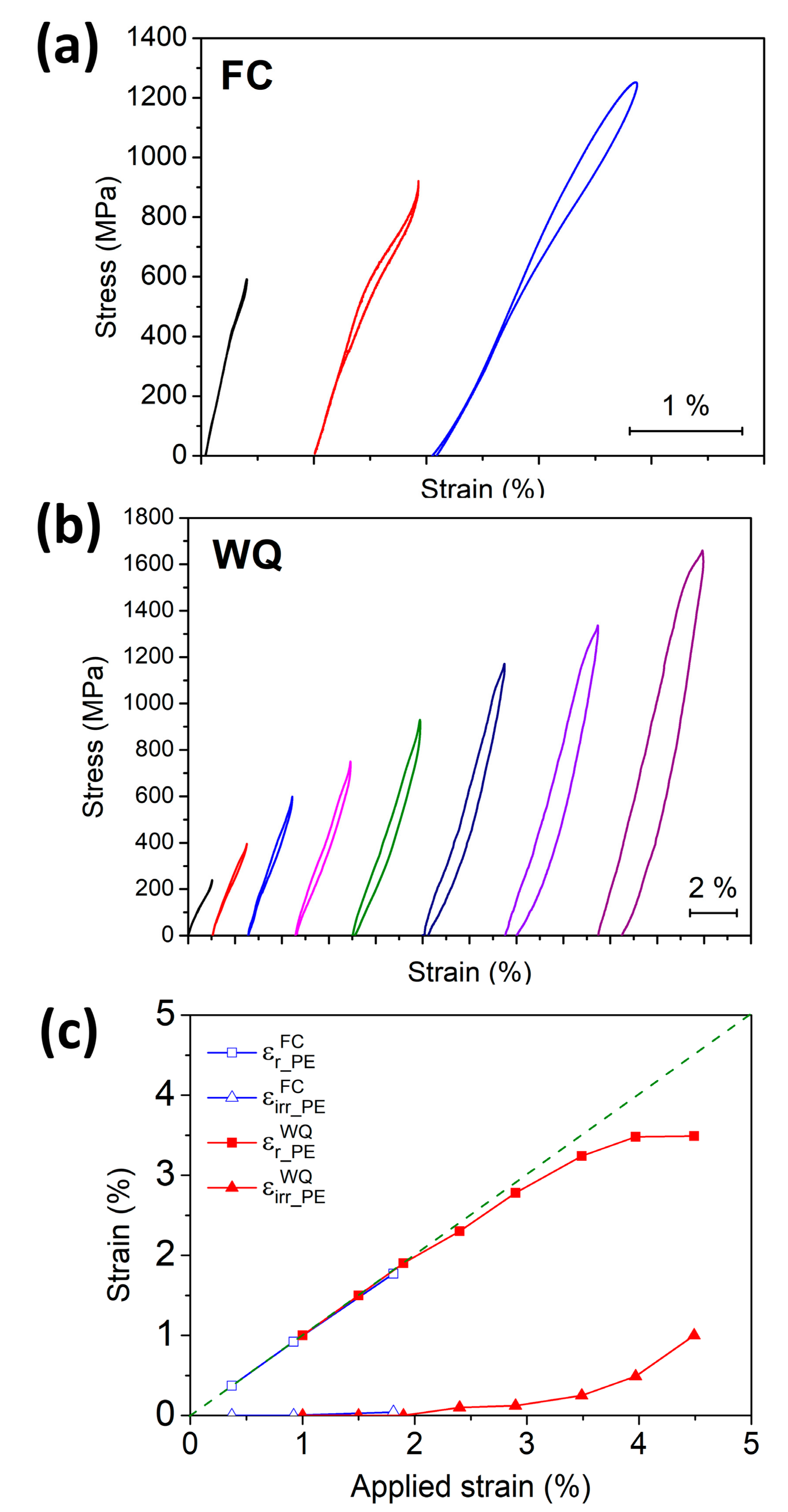
3.4. Shape Memory Effect (SME) and Pseudoelasticity (PE)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Feuerbacher, M.; Lienig, T.; Thomas, C. A single-phase bcc high-entropy alloy in the refractory Zr-Nb-Ti-V-Hf system. Scr. Mater. 2018, 152, 40–43. [Google Scholar] [CrossRef]
- Sohn, S.S.; Kwiatkowski da Silva, A.; Ikeda, Y.; Körmann, F.; Lu, W.; Choi, W.S.; Gault, B.; Ponge, D.; Neugebauer, J.; Raabe, D. Ultrastrong Medium-Entropy Single-Phase Alloys Designed via Severe Lattice Distortion. Adv. Mater. 2019, 31, 1807142. [Google Scholar] [CrossRef] [PubMed]
- Senkov, O.N.; Jensen, J.K.; Pilchak, A.L.; Miracle, D.B.; Fraser, H.L. Compositional variation effects on the microstructure and properties of a refractory high-entropy superalloy AlMo0.5NbTa0.5TiZr. Mater. Des. 2018, 139, 498–511. [Google Scholar] [CrossRef]
- Nagase, T.; Mizuuchi, K.; Nakano, T. Solidification Microstructures of the Ingots Obtained by Arc Melting and Cold Crucible Levitation Melting in TiNbTaZr Medium-Entropy Alloy and TiNbTaZrX (X = V, Mo, W) High-Entropy Alloys. Entropy 2019, 21, 483. [Google Scholar] [CrossRef]
- Lei, Z.; Liu, X.; Wu, Y.; Wang, H.; Jiang, S.; Wang, S.; Hui, X.; Wu, Y.; Gault, B.; Kontis, P.; et al. Enhanced strength and ductility in a high-entropy alloy via ordered oxygen complexes. Nature 2018, 563, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Löbel, M.; Lindner, T.; Pippig, R.; Lampke, T. High-Temperature Wear Behaviour of Spark Plasma Sintered AlCoCrFeNiTi0.5 High-Entropy Alloy. Entropy 2019, 21, 582. [Google Scholar]
- Tseng, K.; Yang, Y.; Juan, C.; Chin, T.; Tsai, C.; Yeh, J. A light-weight high-entropy alloy Al20Be20Fe10Si15Ti35. Sci. China Technol. Sci. 2018, 61, 184–188. [Google Scholar] [CrossRef]
- Singh, P.; Marshal, A.; Smirnov, A.V.; Sharma, A.; Balasubramanian, G.; Pradeep, K.G.; Johnson, D.D. Tuning phase stability and short-range order through Al doping in CoCrFeMn100-xAlx high-entropy alloys. Phys. Rev. Mater. 2019, 3, 075002. [Google Scholar] [CrossRef]
- El-Atwani, O.; Li, N.; Li, M.; Devaraj, A.; Baldwin, J.K.S.; Schneider, M.M.; Sobieraj, D.; Wróbel, J.S.; Nguyen-Manh, D.; Maloy, S.A.; et al. Outstanding radiation resistance of tungsten-based high-entropy alloys. Sci. Adv. 2019, 5, eaav2002. [Google Scholar] [CrossRef] [PubMed]
- Maresca, F.; Curtin, W.A. Mechanistic Origin of High Strength in Refractory BCC High Entropy Alloys up to 1900K. Acta Mater. 2019. In Press. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, Y.; Chen, X.; Fu, X.; Chen, D.; Chen, S.; Gu, L.; Wei, F.; Bei, H.; Gao, Y.; et al. Tuning element distribution, structure and properties by composition in high-entropy alloys. Nature 2019, 574, 223–227. [Google Scholar] [CrossRef]
- Tsai, C.-W.; Lee, C.; Lin, P.-T.; Xie, X.; Chen, S.; Carroll, R.; LeBlanc, M.; Brinkman, B.A.W.; Liaw, P.K.; Dahmen, K.A.; et al. Portevin-Le Chatelier mechanism in face-centered-cubic metallic alloys from low to high entropy. Int. J. Plast. 2019. In Press. [Google Scholar] [CrossRef]
- LaRosa, C.R.; Shih, M.; Varvenne, C.; Ghazisaeidi, M. Solid solution strengthening theories of high-entropy alloys. Mater. Charact. 2019, 151, 310–317. [Google Scholar] [CrossRef]
- Kostiuchenko, T.; Körmann, F.; Neugebauer, J.; Shapeev, A. Impact of lattice relaxations on phase transitions in a high-entropy alloy studied by machine-learning potentials. NPJ Comput. Mater. 2019, 5, 55. [Google Scholar] [CrossRef]
- Lei, Z.; Liu, X.; Wang, H.; Wu, Y.; Jiang, S.; Lu, Z. Development of advanced materials via entropy engineering. Scr. Mater. 2019, 165, 164–169. [Google Scholar] [CrossRef]
- Firstov, G.; Timoshevski, A.; Kosorukova, T.; Koval, Y.; Matviychuk, Y.; Verhovlyuk, P. Electronic and crystal structure of the high entropy TiZrHfCoNiCu intermetallics undergoing martensitic transformation. Matec Web Conf. 2015, 33, 06006. [Google Scholar] [CrossRef]
- Firstov, G.S.; Kosorukova, T.A.; Koval, Y.N.; Odnosum, V.V. High Entropy Shape Memory Alloys. Mater. Today Proc. 2015, 2S, S499–S504. [Google Scholar] [CrossRef]
- Firstov, G.S.; Kosorukova, T.A.; Koval, Y.N.; Verhovlyuk, P.A. Directions for Highe-Temperature Shape Memory Alloy’s Improvement: Straight Way to High-Entropy Materials? Shap. Mem. Superelasticity 2015, 1, 400–407. [Google Scholar] [CrossRef]
- Canadinc, D.; Trehern, W.; Ma, J.; Karaman, I.; Sun, F.; Chaudhry, Z. Ultra-high temperature multi-component shape memory alloys. Scr. Mater. 2019, 158, 83–87. [Google Scholar] [CrossRef]
- Wang, L.; Fu, C.; Wu, Y.; Li, R.; Hui, X.; Wang, Y. Superelastic effect in Ti-rich high entropy alloys via stress-induced martensitic transformation. Scr. Mater. 2019, 162, 112–117. [Google Scholar] [CrossRef]
- Chen, C.H.; Chen, Y.J. Shape memory characteristics of (TiZrHf)50Ni25Co10Cu15 high entropy shape memory alloy. Scr. Mater. 2019, 162, 185–189. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chen, Y.-J.; Shen, J.-J. Microstructure and Mechanical Properties of (TiZrHf)50(NiCoCu)50 High Entropy Alloys. Met. Mater. Int. 2019. [Google Scholar] [CrossRef]
- Frenzel, J.; Wieczorek, A.; Opahle, I.; Maass, B.; Drautz, R.; Eggeler, G. On the effect of alloy composition on martensite start temperatures and latent heats in Ni-Ti-based shape memory alloys. Acta Mater. 2015, 90, 213–231. [Google Scholar] [CrossRef]
- Xue, D.; Zhou, Y.; Ren, X. The effect of aging on the B2-R transformation behaviors in Ti-51at%Ni alloy. Intermetallics 2011, 19, 1752–1758. [Google Scholar] [CrossRef]
- Panchenko, E.Y.; Chumlyakov, Y.I.; Kireeva, I.V.; Ovsyannikov, A.V.; Sehitoglu, H.; Karaman, I.; Maier, Y.H.J. Effect of disperse Ti3N4 particles on the martensitic transformations in titanium nickelide single crystals. Phys. Met. Met. 2008, 106, 577. [Google Scholar] [CrossRef]
- Frenzel, J.; George, E.P.; Dlouhy, A.; Somsen, C.; Wagner, M.F.X.; Eggeler, G. Influence of Ni on martensitic phase transformations in NiTi shape memory alloys. Acta Mater. 2010, 58, 3444–3458. [Google Scholar] [CrossRef]
- Kai, W.-Y.; Chang, K.-C.; Wu, H.-F.; Chen, S.-W.; Yeh, A.-C. Formation mechanism of Ni2Ti4Ox in NITI shape memory alloy. Materialia 2019, 5, 100194. [Google Scholar] [CrossRef]
- DeHoff, R. Thermodynamics in Materials Science; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Hosoda, H.; Hanada, S.; Inoue, K.; Fukui, T.; Mishima, Y.; Suzuki, T. Martensite transformation temperatures and mechanical properties of ternary NiTi alloys with offstoichiometric compositions. Intermetallics 1998, 6, 291–301. [Google Scholar] [CrossRef]
- Otsuka, K.; Ren, X. Recent developments in the research of shape memory alloys. Intermetallics 1999, 7, 511–528. [Google Scholar] [CrossRef]
- Evirgen, A.; Karaman, I.; Santamarta, R.; Pons, J.; Hayrettin, C.; Noebe, R.D. Relationship between crystallographic compatibility and thermal hysteresis in Ni-rich NiTiHf and NiTiZr high temperature shape memory alloys. Acta Mater. 2016, 121, 374–383. [Google Scholar] [CrossRef]
- Carl, M.; Smith, J.D.; Van Doren, B.; Young, M.L. Effect of Ni-Content on the Transformation Temperatures in NiTi-20 at. % Zr High Temperature Shape Memory Alloys. Metals 2017, 7, 511. [Google Scholar] [CrossRef]
- Karaca, H.E.; Acar, E.; Tobe, H.; Saghaian, S.M. NiTiHf-based shape memory alloys. Mater. Sci. Technol. 2014, 30, 1530–1544. [Google Scholar] [CrossRef]
- Evirgen, A.; Karaman, I.; Santamarta, R.; Pons, J.; Noebe, R.D. Microstructural characterization and shape memory characteristics of the Ni50.3Ti34.7Hf15 shape memory alloy. Acta Mater. 2015, 83, 48–60. [Google Scholar] [CrossRef]
- Ishida, A.; Ogawa, K.; Sato, M.; Miyazaki, S. Microstructure of Ti-48.2 at. Pct Ni shape memory thin films. Metall. Mater. Trans. A 1997, 28, 1985–1991. [Google Scholar] [CrossRef]
- Wang, X.; Kustov, S.; Li, K.; Schryvers, D.; Verlinden, B.; Van Humbeeck, J. Effect of nanoprecipitates on the transformation behavior and functional properties of a Ti–50.8at.% Ni alloy with micron-sized grains. Acta Mater. 2015, 82, 224–233. [Google Scholar] [CrossRef]
- Gao, M.C.; Yeh, J.W.; Liaw, P.K.; Zhang, Y. High-Entropy Alloys: Fundamentals and Applications; Springer International Publishing: New York, NY, USA, 2016. [Google Scholar]
- Otsuka, K.; Ren, X. Physical metallurgy of Ti–Ni-based shape memory alloys. Prog. Mater. Sci. 2005, 50, 511–678. [Google Scholar] [CrossRef]
| (at.%) | Ti | Zr | Hf | Ni | Co | Cu | O |
|---|---|---|---|---|---|---|---|
| Furnace-Cooled (FC) | |||||||
| B2 matrix | 16.6 ± 0.26 | 15.3 ± 0.21 | 17.0 ± 0.28 | 25.4 ± 0.21 | 10.5 ± 0.15 | 15.2 ± 0.16 | - |
| (TiZrHf)48.9(NiCoCu)51.1 | |||||||
| Ti2Ni-like | 29.3 ± 1.58 | 24.8 ± 1.47 | 11.7 ± 1.92 | 18.4 ± 0.65 | 7.5 ± 0.42 | 8.0 ± 0.57 | 0.34 ± 0.05 |
| (TiZrHf)65.8(NiCoCu)33.9O0.3 | |||||||
| Water-Quenched (WQ) | |||||||
| B2 Matrix | 18.0 ± 0.17 | 15.3 ± 0.18 | 16.1 ± 0.33 | 25.1 ± 0.15 | 10.4 ± 0.09 | 15.1 ± 0.21 | - |
| (TiZrHf)49.4(NiCoCu)50.6 | |||||||
| Ti2Ni-like | 26.7 ± 0.71 | 22.2 ± 0.50 | 11.6 ± 0.76 | 17.3 ± 0.14 | 7.0 ± 0.31 | 7.1 ± 0.31 | 8.1 ± 0.43 |
| (TiZrHf)60.5(NiCoCu)31.4O8.1 | |||||||
| FC | WQ | |
|---|---|---|
| Area fraction of Ti2Ni-like phase | 2.2 ± 0.18% | 1.5 ± 0.29% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-C.; Chen, Y.-J.; Chen, C.-H. Effect of Solution Treatment on the Shape Memory Functions of (TiZrHf)50Ni25Co10Cu15 High Entropy Shape Memory Alloy. Entropy 2019, 21, 1027. https://doi.org/10.3390/e21101027
Lee H-C, Chen Y-J, Chen C-H. Effect of Solution Treatment on the Shape Memory Functions of (TiZrHf)50Ni25Co10Cu15 High Entropy Shape Memory Alloy. Entropy. 2019; 21(10):1027. https://doi.org/10.3390/e21101027
Chicago/Turabian StyleLee, Hao-Chen, Yue-Jin Chen, and Chih-Hsuan Chen. 2019. "Effect of Solution Treatment on the Shape Memory Functions of (TiZrHf)50Ni25Co10Cu15 High Entropy Shape Memory Alloy" Entropy 21, no. 10: 1027. https://doi.org/10.3390/e21101027
APA StyleLee, H.-C., Chen, Y.-J., & Chen, C.-H. (2019). Effect of Solution Treatment on the Shape Memory Functions of (TiZrHf)50Ni25Co10Cu15 High Entropy Shape Memory Alloy. Entropy, 21(10), 1027. https://doi.org/10.3390/e21101027