The Gibbs Paradox
Abstract
1. Introduction
- (i)
- Why is the entropy of the mixing of two gases independent of their degree of similarity—and only zero when the gases are the same? (the discontinuity puzzle).
- (ii)
- How, in classical statistical mechanics, can an extensive entropy function be defined? (the extensivity puzzle).
- (iii)
- How can there not be an entropy of mixing, even for samples of the same gas, in statistical mechanics, classical or quantum?—because surely the particles of the two gases undergo much the same microscopic motions on mixing, be they exactly alike or only approximately similar (the microrealism puzzle).
2. Solutions
2.1. Thermodynamics and the Discontinuity Puzzle
The entropy of mixing has the same value…however alike are the two substances, but suddenly collapses to zero when they are the same. It is the absence of any ‘warning’ of the impending catastrophe, as the substances are made more and more similar, which is the truly paradoxical feature.
Whether such a process is reversible or not depends on how discriminating the observer is. The expression for the entropy depends on whether or not he is able and willing to distinguish between the molecules A and B. This is a paradox only for those who attach more physical reality to the entropy than is implied by its definition.
Now, when we say that two gases are the same, we mean that we cannot separate the one from the other by any known reaction. It is not probable, but it is possible, that two gases derived from different sources but hitherto regarded to be the same, may hereafter be found to be different, and that a method be discovered for separating them by a reversible process. If this should happen, the process of inter-diffusion that we had formerly supposed not to be an instance of dissipation of energy would now be recognised as such an instance.[9] (p. 646)
We might also imagine the case of two gases which should be absolutely identical in all their properties (sensible and molecular) which come into play while they exist as gases either pure or mixed with each other, but which should differ in respect to their attractions between their atoms and the atoms of some other substances, and therefore in their tendency to combine with such substances. In the mixture of such gases by diffusion an increase in entropy would take place, although the process of mixture, dynamically considered, might be absolutely identical in its minutest details (even with respect to the precise path of each atom) with processes which might take place without any increase in entropy. In such respects, entropy stands strongly contrasted with energy.[10] (p. 167)
It follows from this that the idea of dissipation of energy depends on our knowledge. Dissipated energy is energy which we cannot lay hold of and direct at pleasure, such as the energy of the confused agitation of molecules which we call heat. Now, confusion, like the correlative term order, is not a property of material things in themselves, but only in relation to the mind that perceives them.[9] (p. 646)
2.2. Extensivity
In a recent and very important writing, a good part of it is devoted to the definition [of a gaseous mixture according to Gibbs], Mr. Carl Neumann points to a paradoxical consequence of this definition. This paradox, which must have stricken the mind of anyone interested in these questions and which, in particular, was examined by Mr. J. W. Gibbs, is the following:
If we apply the formulas relative to the mixture of two gases to the case when the two gases are identical, we may be driven to absurd consequences.
Suppose a valve is now opened, making a communication between the chambers. We do not regard this as making any change in the entropy, although the masses of gas or liquid diffuse into one another, and although the same process of diffusion would increase the entropy if the masses of fluids were different. It is evident, therefore, that it is equilibrium with respect to generic phases, and not with respect to specific, with which we have to do in the evaluation of entropy, and therefore that we must use the average [over the quotient space] and not [over phase space] as the equivalent of entropy, except in the thermodynamics of bodies in which the number of molecules of the various kinds is constant.[1] (pp. 206–207)
2.3. The Quantum Approach
The paradoxical consequences [of the mixing-entropy formula] start to occur only when we follow Gibbs in imagining gases that are infinitely little different from each other in every respect and thus conceive the case of identical gases as the continuous limit of the general case of different gases. On the contrary, we may well conclude that finite differences of the properties belong to the essence of what we call matter.[19] (p. 697)
It was a famous paradox pointed out for the first time by W. Gibbs, that the same increase of entropy must not be taken into account, when the two molecules are of the same gas, although (according to naive gas-theoretical views) diffusion takes place then too, but unnoticeably to us, because all the particles are alike. The modern view [of quantum mechanics] solves this paradox by declaring that in the second case there is no real diffusion, because exchange between like particles is not a real event—if it were, we should have to take account of it statistically. It has always been believed that Gibbs’ paradox embodied profound thought. That it was intimately linked up with something so important and entirely new [as quantum mechanics] could hardly be foreseen.
3. Reconsidering Indistinguishability
3.1. Indistinguishabilty and Sequence-Position
3.2. Permutations as Active Transformations
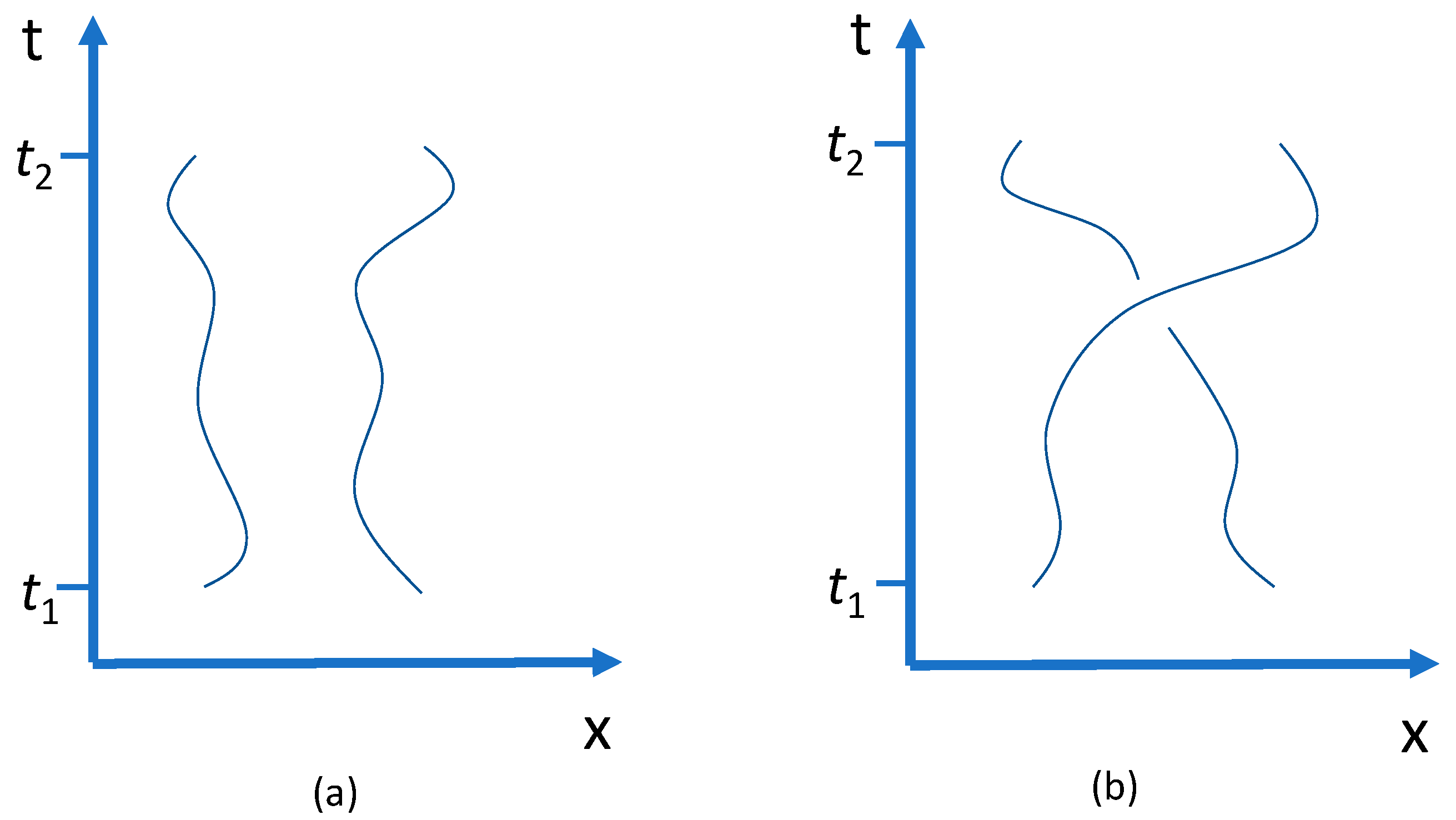
Suppose I show someone two identical balls lying on a table and then ask this person to close his eyes and a few moments later to open them again. I then ask whether or not I have meanwhile switched the two balls around. He cannot tell, since the balls are identical. Yet I know the answer. If I have switched the balls, then I have been able to follow the continuous motion which brought the balls from the initial to the final configuration. This simple example illustrates Boltzmann’s first axiom of classical mechanics, which says, in essence, that identical particles which cannot come infinitely close to each other can be distinguished by their initial conditions and by the continuity of their motion.
3.3. Demarcating Properties
4. The Micro-Realism Puzzle
However, if we admit the mental or even practical possibility to reversibly mix or unmix similar [gas] masses in such a way that every individually determined smallest particle is found in the same ‘state,’ in particular in the same position, after a complete cycle, it cannot be denied that in such a mixing process work can be won even though it does not involve any outward change.
If the two gases are chemically speaking the same, the mixing will not be detectable by looking at the usual thermodynamical quantities. This is so because in thermodynamics we restrict ourselves to the consideration of coarse-grained macroscopic quantities, and this entitles us to describe the mixing of two volumes of gases of the same kind, with equal P and T, as reversible with no increase in entropy. However, if we think of what happens in terms of the motions of individual atoms or molecules, the two processes (irreversible and reversible mixing) are completely similar. In other words, the qualification of the mixing process as irreversible or reversible, and the verdict that the entropy does or does not change, possesses a pragmatic dimension. It depends on what we accept as legitimate methods of discrimination; chemical differences lead to acknowledged thermodynamical entropy differences in a process of mixing, whereas mere differences in where particles come from do not.
4.1. Place of Origin as a Demarcating Property
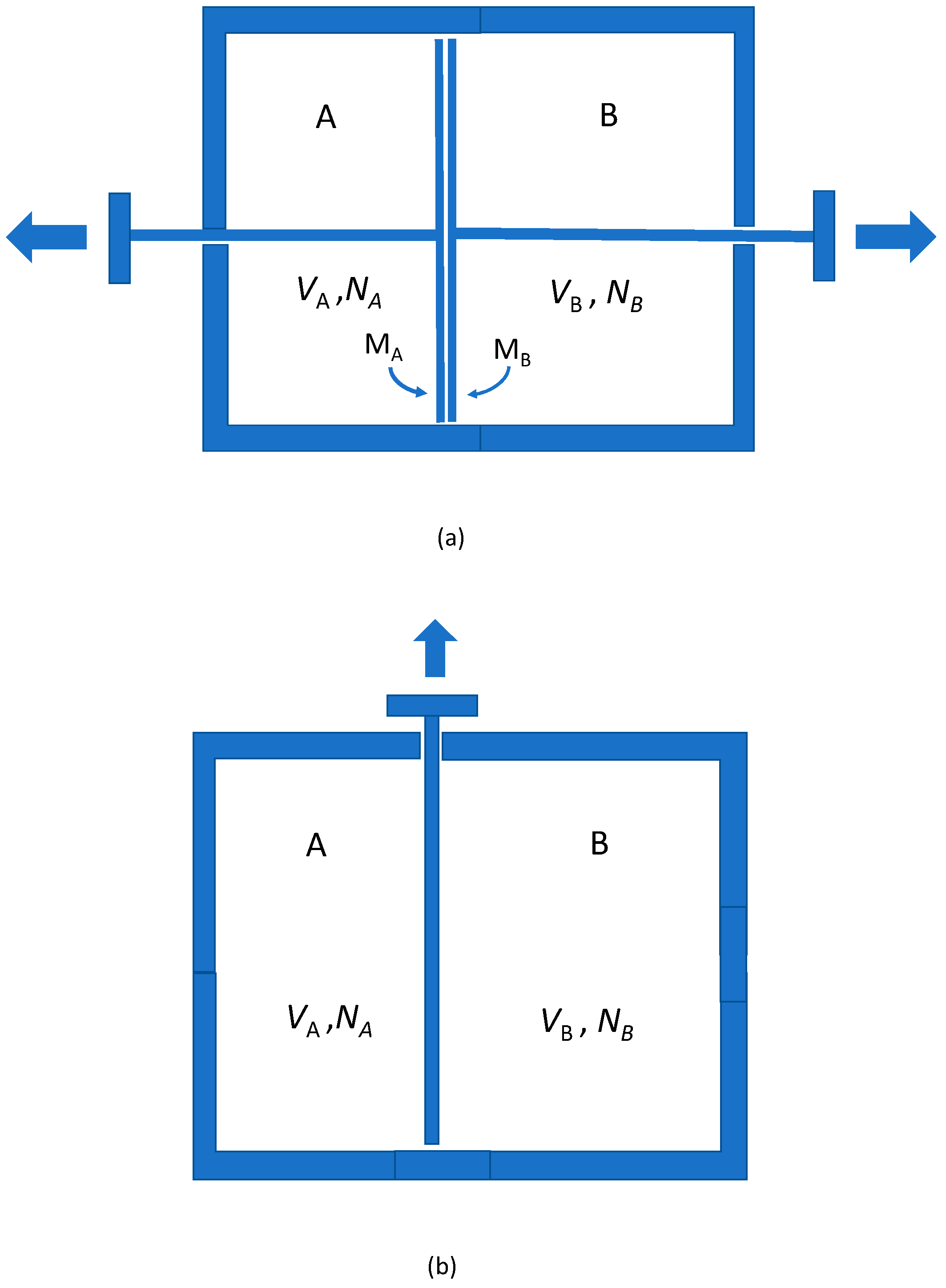
Figuratively speaking, think of submicroscopic computers built into the membrane that perform an ultra-rapid calculation each time a particle hits them, to see where it came from; or the proverbial demon with super-human calculational powers who stops or lets pass particles depending on their origin. In general, of course, allowing expedients of this kind may upset thermodynamical principles, in particular the second law of thermodynamics. However, in the thought experiment we propose here we make a restricted use of these unusual membranes. The idea is merely to employ them for the purpose of demonstrating that if gases are mixed and unmixed by selection on the basis of past particle trajectories and origins, as should be possible according to classical mechanics, this leads to the emergence of an entropy of mixing.
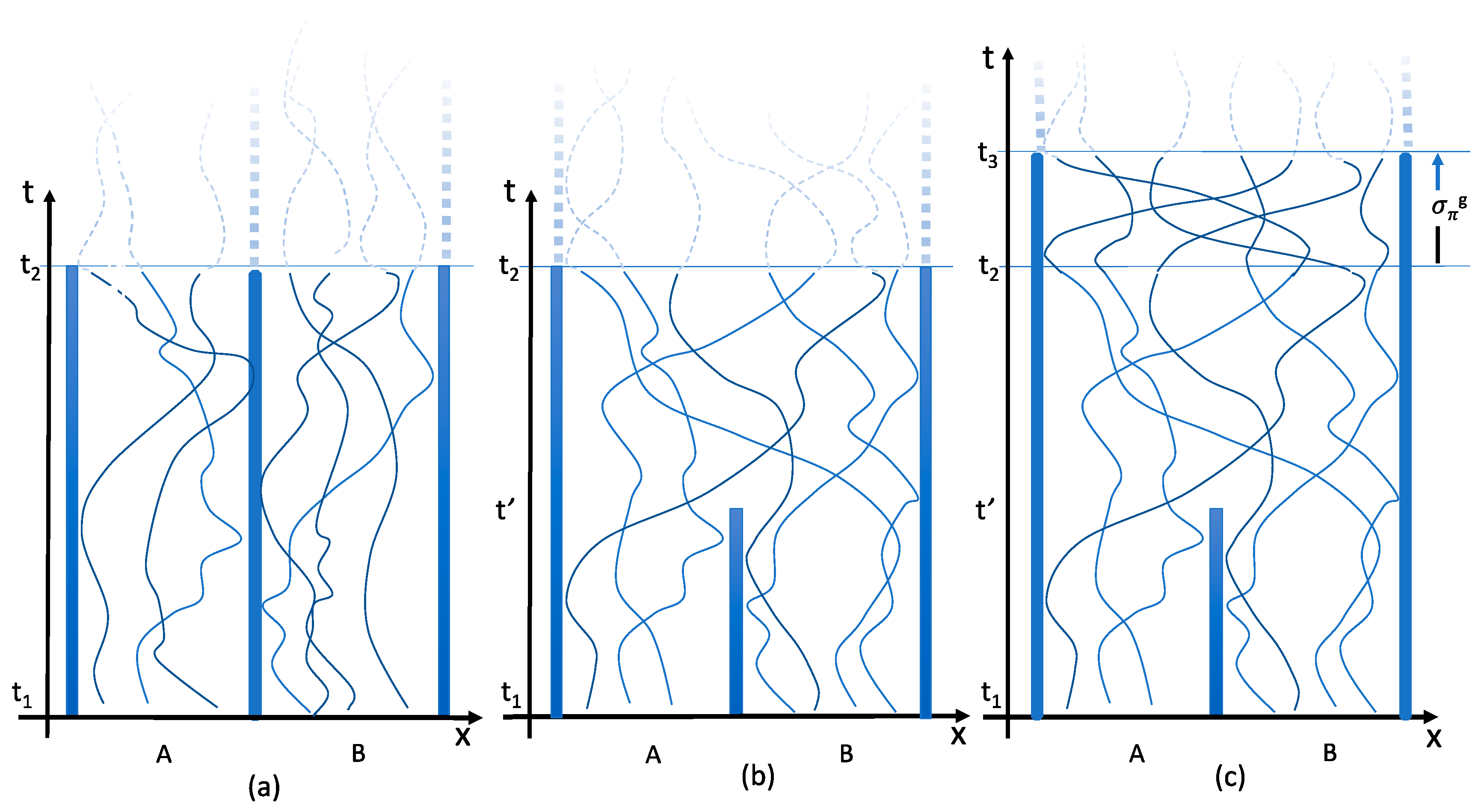
4.2. Equilibration
4.3. Independence
4.4. Particle Trajectories
Consider again two gas-filled chambers, each containing N identical particles. Before the partition is removed the number of available states per particle is V. After the partition has been removed, the number of available states is 2V. The reason is that after the partition’s removal it has become possible for the particles to move to the other chamber. The doubling of the number of available microstates thus expresses a physical freedom that was not present before the partition was taken away. Trajectories in space-time have become possible from the particles’ initial states to states in the other chamber. Particles from the left and right sides can physically exchange their states.
5. Conclusions
Funding
Conflicts of Interest
References
- Gibbs, J. Elementary Principles in Statistical Mechanics; Yale University Press: New Haven, CT, USA, 1902. [Google Scholar]
- Huang, K. Statistical Mechanics, 2nd ed.; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Van Kampen, N. The Gibbs paradox. In Essays in Theoretical Physics in Honour of Dirk ter Haar; Parry, W.E., Ed.; Pergamon Press: Oxford, UK, 1984. [Google Scholar]
- Bach, A. Indistinguishable Classical Particles; Springer: Berlin, Germany, 1997. [Google Scholar]
- Dieks, D. Gibbs paradox revisited. In Explanation, Prediction, and Confirmation, the Philosophy of Science in a European Perspective; Dieks, A., Gonzalez, W., Hartmann, S., Uebel, T., Weber, M., Eds.; Springer: Amsterdam, The Netherlands, 2011; pp. 367–377. [Google Scholar]
- Hestenes, D. Entropy and indistinguishability. Am. J. Phys. 1970, 38, 840–845. [Google Scholar] [CrossRef]
- Fujita, S. On the indistinguishability of classical particles. Found. Phys. 1991, 21, 439–457. [Google Scholar] [CrossRef]
- Denbigh, K.; Redhead, M. Gibbs‘ paradox and non-uniform convergence. Synthese 1989, 81, 283–312. [Google Scholar] [CrossRef]
- Maxwell, J. Diffusion. In Encyclopaedia Britannica, 9th ed.; Baynes, T., Ed.; A. C. Black: London, UK, 1887; Republished in The Scientific Papers of James Clerk Maxwell; Niven, W., Ed.; Cambridge University Press: Cambridge, UK, 1890; Volume 2, pp. 625–646. [Google Scholar]
- Gibbs, J. On the Equilibrium of Heterogeneous Substances; The Connecticut Academy of Arts and Sciences: New Haven, CT, USA, 1878; Volume 3, pp. 343–524. [Google Scholar]
- Jaynes, E. The Gibbs’ paradox. In Maximum-Entropy and Bayesian Methods; Erickson, G., Neudorfer, P., Smith, C., Eds.; Kluwer: Dordrecht, The Netherlands, 1992. [Google Scholar]
- Dieks, D. The logic of identity. Distinguishability and indistinguishability in classical and quantum physics. Found. Phys. 2014, 44, 1302–1316. [Google Scholar] [CrossRef]
- Dieks, D.; Lubberdink, A. How classical particles emerge from the quantum world. Found. Phys. 2011, 41, 1051–1064. [Google Scholar] [CrossRef]
- Dieks, D.; Versteegh, M. The Gibbs paradox and the distinguishability of identical particles. Am. J. Phys. 2011, 79, 741–746. [Google Scholar]
- Maxwell, J. Introductory lectures on experimental physics. In The Scientific Papers of James Clerk Maxwell; Niven, W., Ed.; Cambridge University Press: Cambridge, UK, 1890; Volume 1, pp. 241–255. [Google Scholar]
- Neumann, C. Bemerkungen zur mechanischen Theorie der Wärme. König. Sachs. Gesell. D. Wiss. Z. Leipzig Math-Phys. Klasse Verhand. 1891, 75–156. [Google Scholar]
- Duhem, P. Sur la Dissociation Dans les Systèmes qui Renferment un Mélange de Gaz Parfaits; Travaux et Mémoires des Facultés de Lille: Lille, France, 1892; Volume 2, mémoire 8. [Google Scholar]
- Darrigol, O. The Gibbs paradox: Early history and solutions. Entropy 2018, 20, 443. [Google Scholar] [CrossRef]
- Wiedeburg, O. Das Gibbs’sche Paradoxon. Ann. Phys. 1891, 53, 684–697. [Google Scholar] [CrossRef]
- Ehrenfest, P.; Trkal, V. Deduction of the dissociation equilibrium from the theory of quanta and a calculation of the chemical constant based on this. Proc. Amst. Acad. 1920, 23, 162–183, Reprinted in Paul Ehrenfest, Collected Scientific Papers; Klein, M., Ed.; North-Holland: Amsterdam, The Netherlands, 1959. [Google Scholar]
- Swendsen, R. Statistical mechanics of classical systems with distinguishable particles. J. Stat. Phys. 2002, 107, 1143–1166. [Google Scholar] [CrossRef]
- Swendsen, R. Probability, entropy, and Gibbs’ paradox(es). Entropy 2018, 20, 450. [Google Scholar] [CrossRef]
- Dieks, D. The Gibbs paradox and particle individuality. Entropy 2018, 20, 466. [Google Scholar] [CrossRef]
- Ehrenfest, P.; Kammerlingh Onnes, H. Simplified deduction of the formula from the theory of combinations which Planck uses as the basis of his radiation theory. Proc. Amst. Acad. 1914, 17, 870–872, Reprinted in Paul Ehrenfest, Collected Scientific Papers; Klein, M., Ed.; North-Holland: Amsterdam, The Netherlands, 1959; pp. 353–356. [Google Scholar] [CrossRef]
- Ehrenfest, P. Welche Züge der Lichtenquantenhypothese spielen in der Theorie der Wärmestrahlung eine wesentliche Rolle. Ann. Phys. 1911, 36, 91–118, Reprinted in Paul Ehrenfest, Collected Scientific Papers; Klein, M., Ed.; North-Holland: Amsterdam, The Netherlands, 1959; pp. 185–212. [Google Scholar] [CrossRef]
- Natanson, L. On the statistical theory of radiation. Bull. Acad. Sci. Crac. 1911, 134–148. [Google Scholar]
- Schrödinger, E. Statistical Thermodynamics; Cambridge University Press: Cambridge, UK, 1946. [Google Scholar]
- Saunders, S. On the explanation of quantum statistics. Stud. Hist. Philos. Mod. Phys. 2006, 37, 192–211. [Google Scholar] [CrossRef]
- Saunders, S. Indistinguishables. In The Oxford Handbook of Philosophy of Physics; Batterman, R., Ed.; Oxford University Press: Oxford, UK, 2013; pp. 340–380. [Google Scholar]
- Ghirardi, G.; Marinatto, L.; Weber, Y. Entanglement and properties of composite quantum systems: A conceptual and mathematical analysis. J. Stat. Phys. 2002, 108, 112. [Google Scholar] [CrossRef]
- Ghirardi, G.; Marinatto, L. General criterion for the entanglement of two indistinguishable states’. Phys. Rev. A 2004, 70, 012109. [Google Scholar] [CrossRef]
- Caulton, A. Entanglement by (Anti-)Symmetrisation Does not Violate Bell’s Inequalities. Manuscript in preparation.
- Saunders, S. On the emergence of individuals in physics. In Individuals across the Sciences; Guay, A., Pradeu, T., Eds.; Oxford University Press: New York, NY, USA, 2016. [Google Scholar]
- Pais, A. Subtle is the Lord: The Science and the Life of Albert Einstein; Oxford University Press: Oxford, UK, 1982. [Google Scholar]
- Leinaas, J.; Myrheim, J. On the theory of identical particles. IL Nuovo Cimento 1977, 37B, 1–23. [Google Scholar] [CrossRef]
- Goldstein, S.; Taylor, J.; Tumulka, R.; Zanghi, N. Are all particles identical? J. Phys. 2005, A38, 1567–1576. [Google Scholar] [CrossRef]
- Cates, M.; Manoharan, N. Celebrating Soft Matter’s 10th anniversary: Testing the foundations of classical entropy: Colloid experiments. Soft Matter 2015, 11, 6538–6546. [Google Scholar] [CrossRef] [PubMed]
- Von Neumann, J. Mathematical Foundations of Quantum Mechanics; Beyer, R., Translator; Princeton University Press: Princeton, NJ, USA, 1955. [Google Scholar]
- Landé, A. Solution of the Gibbs entropy paradox. Philos. Sci. 1965, 32, 192–193. [Google Scholar] [CrossRef]
- Leff, H.; Rex, A. (Eds.) Maxwell’s Demon 2: Entropy, Classical and Quantum Information, Computing; Institude of Physics: Bristol, UK, 2003. [Google Scholar]
- Wallace, D. The logic of the past hypothesis. In Time’s Arrows and the Probability Structure of the World; Loewer, B., Weslake, B., Winsberg, E., Eds.; Harvard University Press: Cambridge, MA, USA, 2011; Available online: http://philsci-archive.pitt.edu/id/eprint/8894 (accessed on 24 July 2018).
- Loewer, B.; Weslake, B.; Winsberg, E. (Eds.) Time’s Arrows and the Probability Structure of the World; Harvard University Press: Cambridge, MA, USA, forthcoming.
- Zeh, D. The Physical Basis of the Direction of Time, 5th ed.; Springer: Berlin, Germany, 2007. [Google Scholar]
- Wallace, D. The Emergent Multiverse: Quantum Theory According to the Everett Interpretation; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saunders, S. The Gibbs Paradox. Entropy 2018, 20, 552. https://doi.org/10.3390/e20080552
Saunders S. The Gibbs Paradox. Entropy. 2018; 20(8):552. https://doi.org/10.3390/e20080552
Chicago/Turabian StyleSaunders, Simon. 2018. "The Gibbs Paradox" Entropy 20, no. 8: 552. https://doi.org/10.3390/e20080552
APA StyleSaunders, S. (2018). The Gibbs Paradox. Entropy, 20(8), 552. https://doi.org/10.3390/e20080552




