On the Path to Optimizing the Al-Co-Cr-Cu-Fe-Ni-Ti High Entropy Alloy Family for High Temperature Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
- -
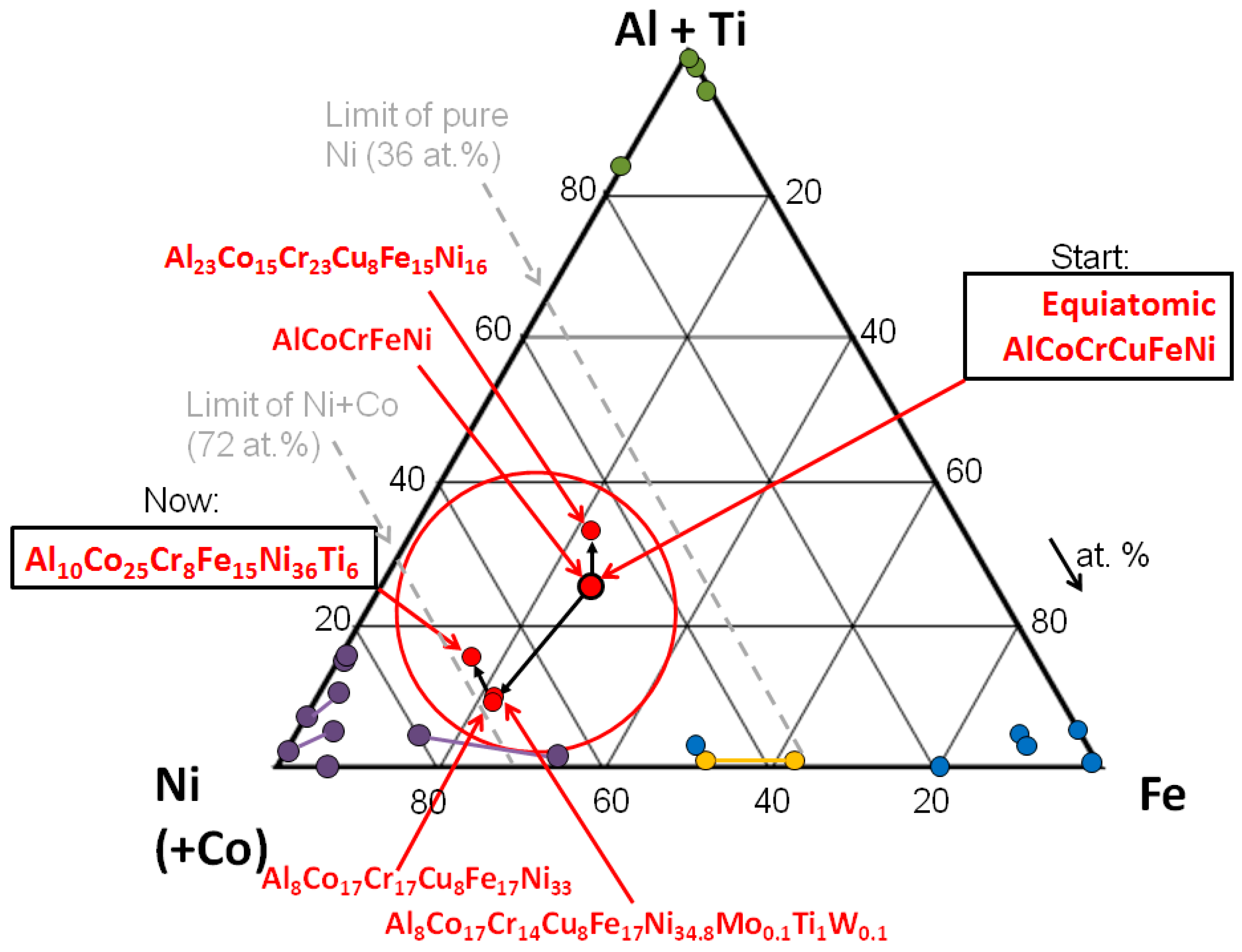
- A reduction of segregated phases is essential. Cu-based phases segregate easily and can thus create disturbances inside the grains as well as at the grain boundaries: the amount of Cu will be decreased (Alloy 3, 4 and 5) or annulled completely (Alloys 2 and 6).
- -
- An optimization of the oxidation properties of the alloy is advantageous: the content of Al and Cr will be increased (Alloy 3).
- -
- A preferential formation of a two phase microstructure in the alloy is desirable. The microstructure of Alloy 1 shows two main phases (except for the Cu-rich phases) with a tendency towards the well-known cubic microstructure in Ni-based alloys: these features will be increased by first increasing the amount of Ni and then fine-tuning by adjusting or adding other elements (Alloys 4, 5 and 6).
- -
- In the bcc alloys, in the Al-Ni rich phase, the amount of Ni and Al is almost the same, in order to form NiAl. In the fcc alloys, there is almost three times as much Ni than Al, which leads to the formation of the γ′ phase.
- -
- In the (Co-)Cr-Fe rich phase, the bcc alloys show a very high amount of Cr, at least 1,2 times higher than the amount of Fe. This is particularly true when observing the relative composition, compared to the nominal one. In the fcc alloys, the relative amounts of Cr, Fe and also Co is much closer. The Co-Cr-Fe matrix composition in the fcc alloys is quite close to the nominal composition of the whole alloy (except for segregating element Cu).
- -
- Ti partitions strongly to the γ′ phase, especially in Alloy 5. Even though the total amount of Ti in Alloy 5 is only 1 at.%, there are 4.6 at.% in the γ′ phase. This strong segregation is only possible because of the low volume fraction of the γ′ phase (about 7%).
4. Summary
- -
- Reduction of segregated phases → The Cu content was reduced or removed completely.
- -
- Optimization of the oxidation properties → the content of Al and Cr was increased
- -
- Preferential formation of a two phase microstructure in the alloy → addition of Ti and/or variation of the composition of the other alloying elements. The final microstructure shows the well-known γ-γ′ microstructure.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yeh, J.W. Nano-structured high-entropy alloys. Knowl. Bridge 2003, 40, 1–2. [Google Scholar]
- Ranganathan, S. Alloyed pleasures: Multimetallic cocktails. Curr. Sci. 2003, 85, 1404–1406. [Google Scholar]
- Tong, C.J.; Chen, M.R.; Chen, S.K.; Yeh, J.W.; Shun, T.T.; Lin, S.J.; Chang, S.Y. Mechanical performance of the AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A 2005, 36A, 1263–1271. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, Y.P.; Dong, Y.; Wang, T.M.; Cao, Z.Q.; Li, T.J. Annealing effects on the microstructure and properties of bulk high-entropy CoCrFeNiTi0.5 alloy casting ingot. Intermetallics 2014, 44, 37–43. [Google Scholar] [CrossRef]
- Ng, C.; Guo, S.; Luan, J.; Shi, S.; Liu, C.T. Entropy-driven phase stability and slow diffusion kinetics in an Al0.5CoCrCuFeNi high entropy alloy. Intermetallics 2012, 31, 165–172. [Google Scholar] [CrossRef]
- Chen, M.R.; Lin, S.J.; Yeh, J.W.; Chen, S.K.; Huang, Y.S.; Tu, C.P. Microstructure and properties of Al0.5CoCrCuFeNiTix (x=0–2.0) high-entropy alloys. Mater. Trans. 2006, 47, 1395–1401. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Shun, T.T.; Hung, C.H.; Lee, C.F. Formation of ordered/disordered nanoparticles in FCC high entropy alloys. J. Alloys Compd. 2010, 493, 105–109. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Singh, S.; Wanderka, N.; Murty, B.S.; Glatzel, U.; Banhart, J. Decomposition in multi-component AlCoCrCuFeNi high-entropy alloy. Acta. Mater. 2011, 59, 182–190. [Google Scholar] [CrossRef]
- Chen, H.Y.; Tsai, C.W.; Tung, C.C.; Yeh, J.W.; Shun, T.T.; Yang, C.C.; Chen, S.K. Effect of the substitution of Co by Mn in Al-Cr-Cu-Fe-Co-Ni high-entropy alloys. Ann. Chim.-Sci. Des Materiaux 2006, 31, 685–698. [Google Scholar] [CrossRef]
- Zhang, K.B.; Fu, Z.Y.; Zhang, J.Y.; Shi, J.; Wang, W.M.; Wang, H.; Wang, Y.C.; Zhang, Q.J. Annealing on the structure and properties evolution of the CoCrFeNiCuAl high-entropy alloy. J. Alloys Compd. 2010, 502, 295–299. [Google Scholar] [CrossRef]
- Tung, C.C.; Yeh, J.W.; Shun, T.T.; Chen, S.K.; Huang, Y.S.; Chen, H.C. On the elemental effect of AlCoCrCuFeNi high-entropy alloy system. Mater. Lett. 2007, 61, 1–5. [Google Scholar] [CrossRef]
- Manzoni, A.; Daoud, H.; Völkl, R.; Glatzel, U.; Wanderka, N. Phase separation in equiatomic AlCoCrFeNi high-entropy alloy. Ultramicroscopy 2013, 132, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, S.G.; Qiao, J.W. Morphology Transition from Dendrites to Equiaxed Grains for AlCoCrFeNi High-Entropy Alloys by Copper Mold Casting and Bridgman Solidification. Metall. Mater. Trans. A-Phys. Metall. Mater. Sci. 2012, 43A, 2625–2630. [Google Scholar] [CrossRef]
- Manzoni, A.; Daoud, H.; Mondal, S.; van Smaalen, S.; Völkl, R.; Glatzel, U.; Wanderka, N. Investigation of phases in Al23Co15Cr23Cu8Fe15Ni16 and Al8Co17Cr17Cu8Fe17Ni33 high entropy alloys and comparison with equilibrium phases predicted by Thermo-Calc. J. Alloys Compd. 2013, 552, 430–436. [Google Scholar] [CrossRef]
- Daoud, H.M.; Manzoni, A.; Volkl, R.; Wanderka, N.; Glatzel, U. Microstructure and Tensile Behavior of Al8Co17Cr17Cu8Fe17Ni33 (at.%) High-Entropy Alloy. JOM 2013, 65, 1805–1814. [Google Scholar] [CrossRef]
- Manzoni, A.M.; Daoud, H.M.; Voelkl, R.; Glatzel, U.; Wanderka, N. Influence of W, Mo and Ti trace elements on the phase separation in Al8Co17Cr17Cu8Fe17Ni33 based high entropy alloy. Ultramicroscopy 2015, 159, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, A.; Inoue, A. Evaluation of glass-forming ability for metallic glasses from time-reduced temperature-transformation diagram. Mater. Trans. 2001, 42, 2374–2381. [Google Scholar] [CrossRef]
- The Version TCCR, ThermoCalc Software AB. Available online: http://www.thermocalc.com (accessed on 17 March 2016).
- Thermotech Ni-Based Superalloys Database, TTNI7, Version 7.0; ThermoCalc Software AB: Stockholm, Sweden, 2006.
- Kuznetsov, A.V.; Shaysultanov, D.G.; Stepanov, N.D.; Salishchev, G.A.; Senkov, O.N. Tensile properties of an AlCrCuNiFeCo high-entropy alloy in as-cast and wrought conditions. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2012, 533, 107–118. [Google Scholar] [CrossRef]
- Daoud, H.M.; Manzoni, A.M.; Wanderka, N.; Glatzel, U. High-Temperature Tensile Strength of Al10Co25Cr8Fe15Ni36Ti6 Compositionally Complex Alloy (High-Entropy Alloy). JOM 2015, 67, 2271–2277. [Google Scholar] [CrossRef]
- Specification Sheet: Alloy 800, 800H, and 800AT. Available online: http://www.sandmeyersteel.com/images/Alloy-800-Spec-Sheet.pdf (accessed on 17 March 2016).
- Special Metals. Available online: http://www.specialmetals.com/documents/Inconel%20alloy%20617.pdf (accessed on 17 March 2016).

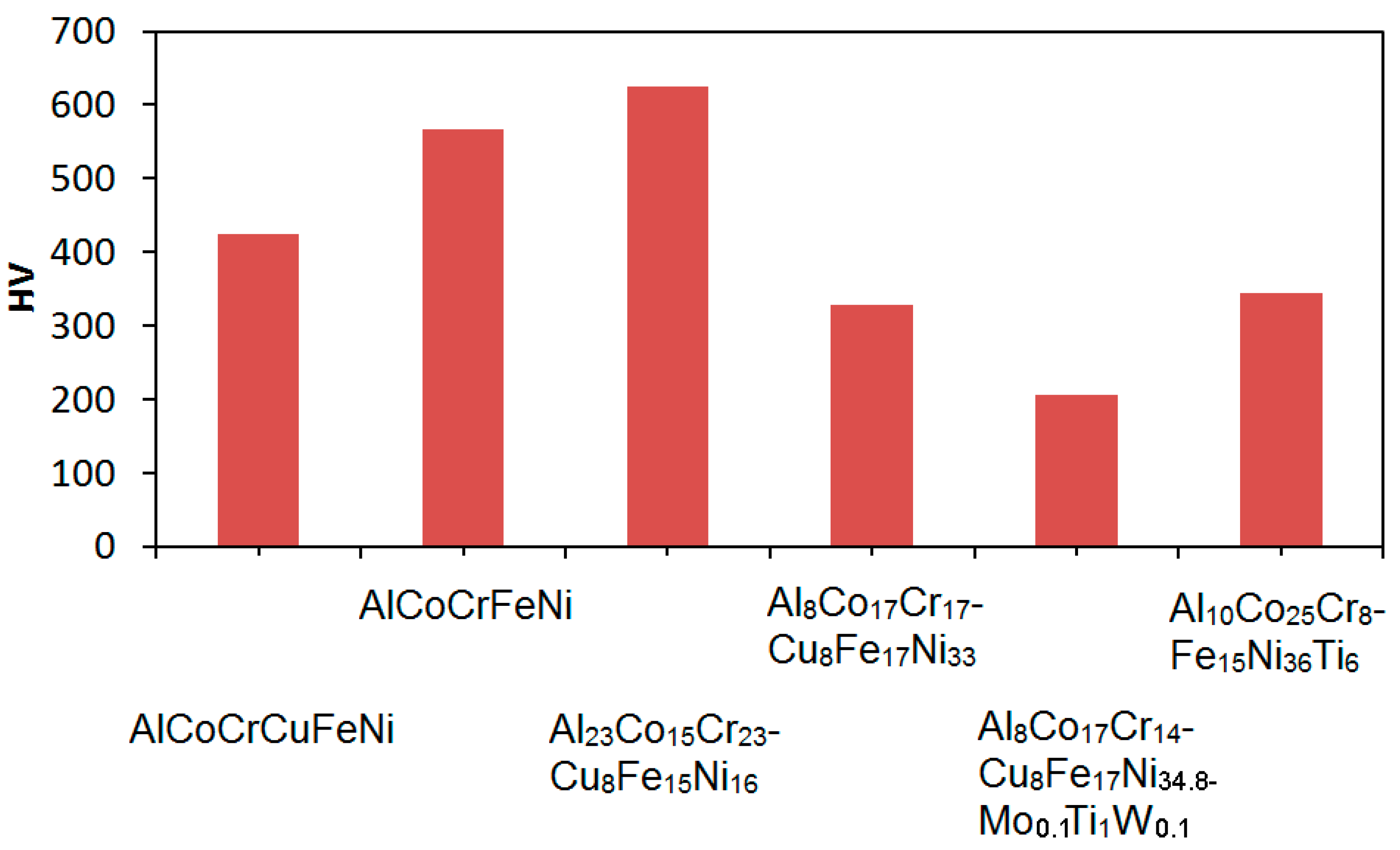
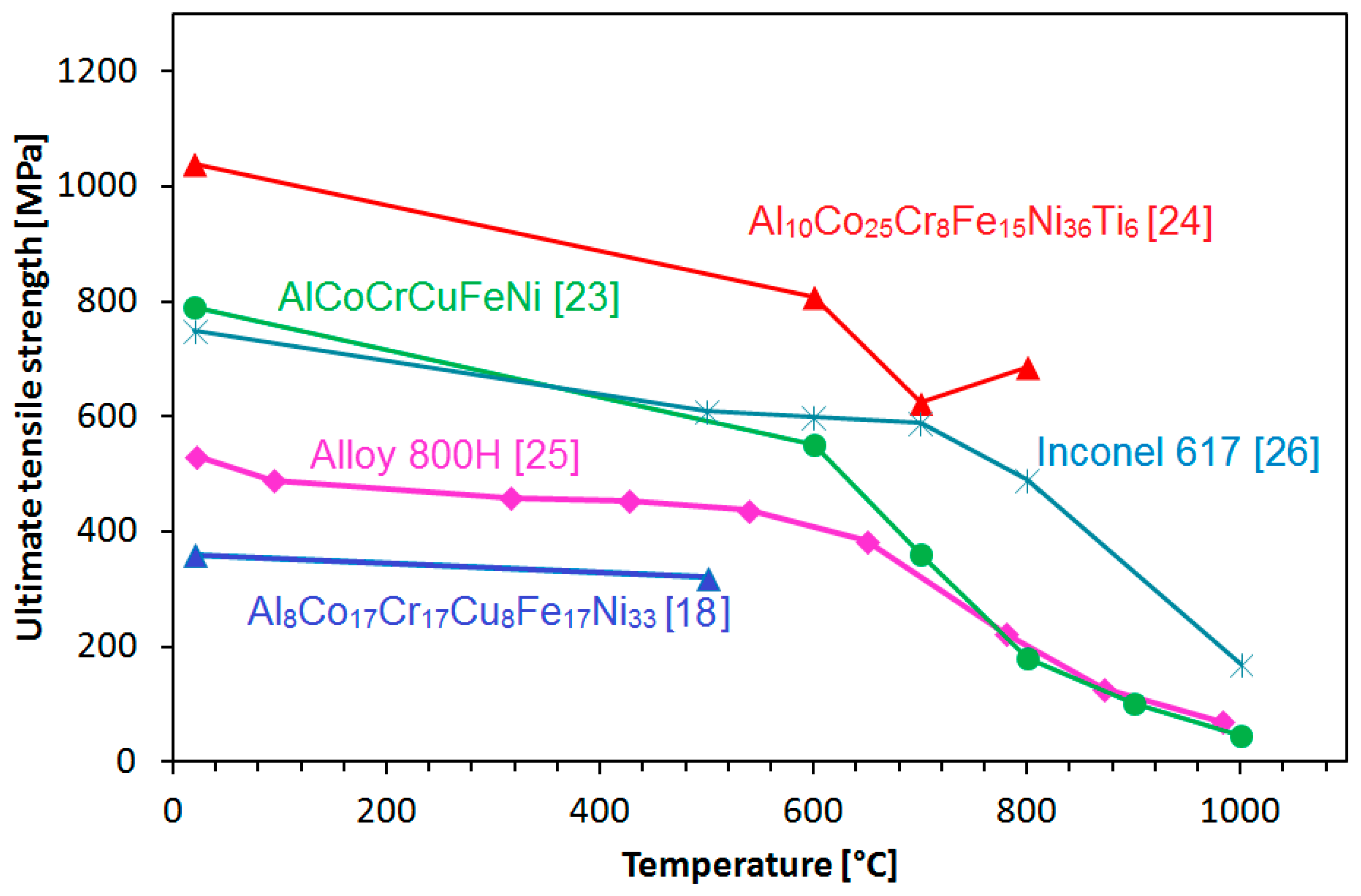
| Alloy | Denomination | Optimum Heat Treatment |
|---|---|---|
| AlCoCrCuFeNi | Alloy 1 | As-cast |
| AlCoCrFeNi | Alloy 2 | As-cast |
| Al23Co15Cr23Cu8Fe15Ni16 | Alloy 3 | As-cast |
| Al8Co17Cr17Cu8Fe17Ni33 | Alloy 4 | 1250 °C/1 h—quenching in water 700 °C/24 h—quenching in water |
| Al8Co17Cr14Cu8Fe17Ni34.8Mo0.1Ti1W0.1 | Alloy 5 | 1250 °C/80 min—quenching in water 875 °C/100 h—quenching in water |
| Al10Co25Cr8Fe15Ni36Ti6 | Alloy 6 | 1220 °C/20 h—cooling in furnace under Ar 900 °C/50 h—cooling in furnace under Ar |
| Phase | Concentration [at.%] | Measuring method | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Co | Cr | Cu | Fe | Ni | W | Mo | Ti | |||
| Alloy 1 (AlCoCrCuFeNi) | |||||||||||
| Al-Ni rich matrix | 29.7 ± 1.5 | 18.4 ± 1.3 | 2.5 ± 0.5 | 8.6 ± 0.9 | 12.7 ± 1.1 | 28.1 ± 1.5 | 3D-AP | [11] | |||
| Cr-Fe rich platelets | 2.8 ± 0.6 | 18.8 ± 1.2 | 39.6 ± 1.4 | 1.3 ± 0.4 | 31.3 ± 1.4 | 6.2 ± 0.5 | 3D-AP | [11] | |||
| Cu rich platelet | 7.8 ± 0.7 | 1.9 ± 0.4 | 0.5 ± 0.1 | 83.5 ± 1.0 | 1.1 ± 0.2 | 5.2 ± 0.6 | 3D-AP | [11] | |||
| Alloy 2 (AlCoCrFeNi) | |||||||||||
| Al-Ni rich matrix | 31.5 ± 3.5 | 19.6 ± 1.9 | 3.7 ± 0.7 | 13.1 ± 1.6 | 32.0 ± 2.1 | 3D-AP | [15] | ||||
| Cr-Fe rich precipitates | 8.5 ± 1.4 | 19.2 ± 1.5 | 35.7 ± 5.3 | 27.0 ± 0.4 | 9.4 ± 1.9 | 3D-AP | [15] | ||||
| Alloy 3 (Al23Co15Cr23Cu8Fe15Ni16) | |||||||||||
| Al-Ni rich matrix | 34.9 ± 3.6 | 19.3 ± 0.8 | 2.5 ± 1.5 | 5.7 ± 2.4 | 9.9 ± 1.1 | 27.5 ± 2.3 | 3D-AP | [17] | |||
| Cr-Fe rich platelets | 10.1 ± 3.0 | 7.1 ± 2.2 | 53.5 ± 6.1 | 1.6 ± 1.2 | 21.7 ± 2.0 | 5.2 ± 1.8 | 3D-AP | [17] | |||
| Cu rich phase | 13.2 ± 3.3 | 1.8 ± 0.1 | 0.3 ± 0.1 | 79.6 ± 6.4 | 1.1 ± 0.3 | 4.0 ± 3.0 | 3D-AP | [17] | |||
| Alloy 4 (Al8Co17Cr17Cu8Fe17Ni33) (heat treatment 700°C 5 h) | |||||||||||
| Al-Ni rich precipitate | 22.4 ± 1.7 | 9.5 ± 0.9 | 5.5 ± 1.1 | 11.2 ± 1.4 | 6.9 ± 71.4 | 44.5 ± 1.6 | 3D-AP | [19] | |||
| Matrix | 5.1 ± 0.3 | 19.3 ± 0.5 | 19.2 ± 0.2 | 4.0 ± 0.8 | 19.9 ± 0.8 | 32.5 ± 0.3 | 3D-AP | [19] | |||
| Alloy 5 (Al8Co17Cr14Cu8Fe17Ni34.8Mo0.1Ti1W0.1) | |||||||||||
| Al-Ni rich precipitate | 20.8 ± 0.0 | 8.8 ± 0.1 | 2.6 ± 0.4 | 7.4 ± 0.3 | 9.5 ± 0.2 | 45.3 ± 0.9 | 0.3 ± 0.0 | 0.8 ± 0.9 | 4.6 ± 0.1 | 3D-AP | [19] |
| Matrix | 7.8 ± 0.4 | 19.5 ± 0.3 | 14.7 ± 0.6 | 4.9 ± 0.2 | 17.9 ± 0.2 | 33.9 ± 0.4 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.9 ± 0.0 | 3D-AP | [19] |
| Alloy 6 (Al10Co25Cr8Fe15Ni36Ti6) | |||||||||||
| Al-Ni rich precipitate | 11.4 ± 0.6 | 22.5 ± 0.6 | 3.5 ± 0.4 | 8.8 ± 0.7 | 45.0 ± 1.5 | 8.7 ± 0.5 | TEM/EDX | This work | |||
| Matrix | 6.9 ± 0.6 | 29.5 ± 0.5 | 9.3 ± 0.4 | 20.4 ± 0.6 | 30.4 ± 1.0 | 3.5 ± 0.4 | TEM/EDX | This work | |||
| Needle precipitate | 24.4 ± 1.3 | 21.9 ± 1.7 | 3.6 ± 0.2 | 10.7 ± 0.4 | 33.9 ± 0.6 | 5.6 ± 0.2 | TEM/EDX | This work | |||
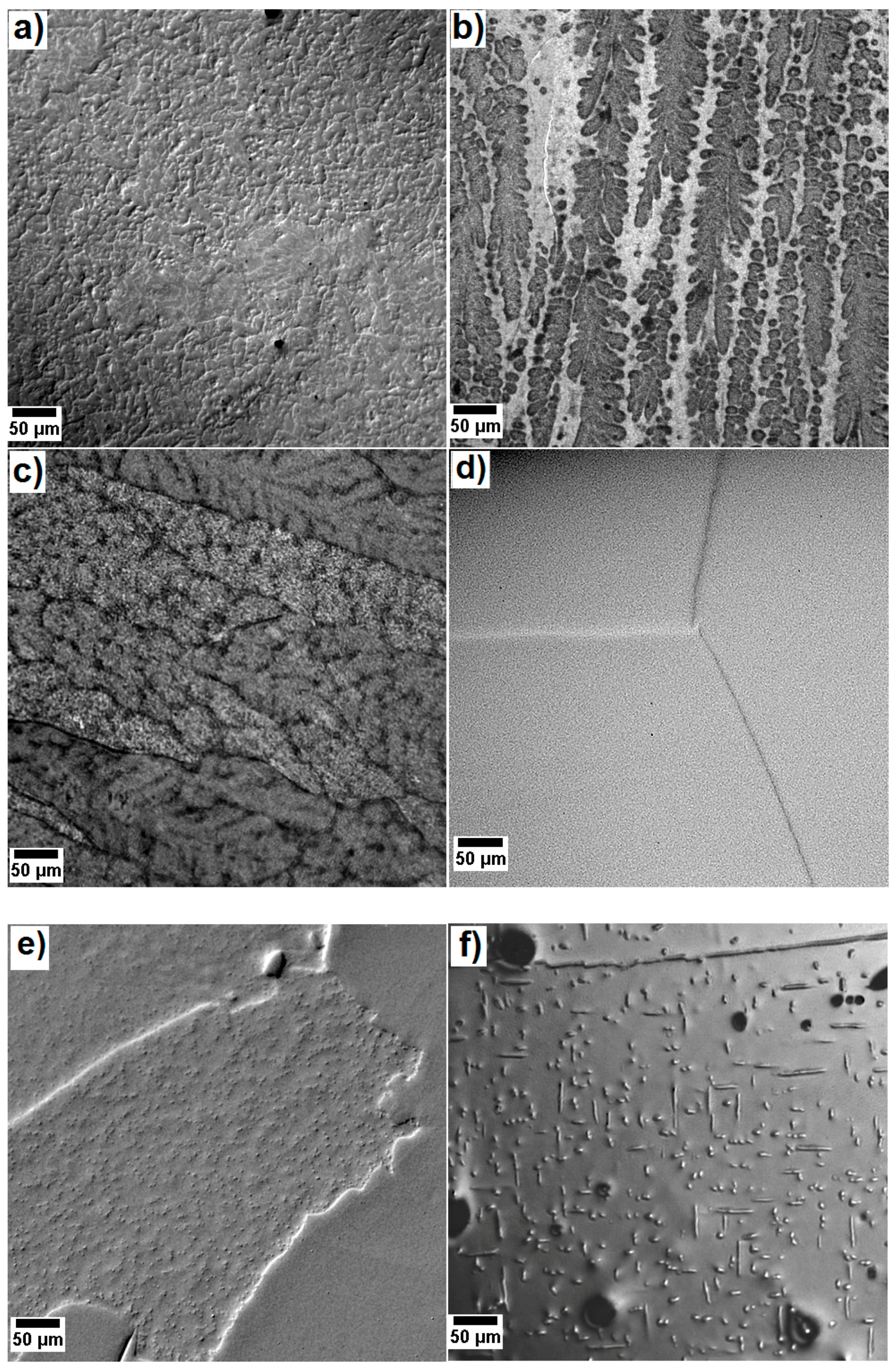
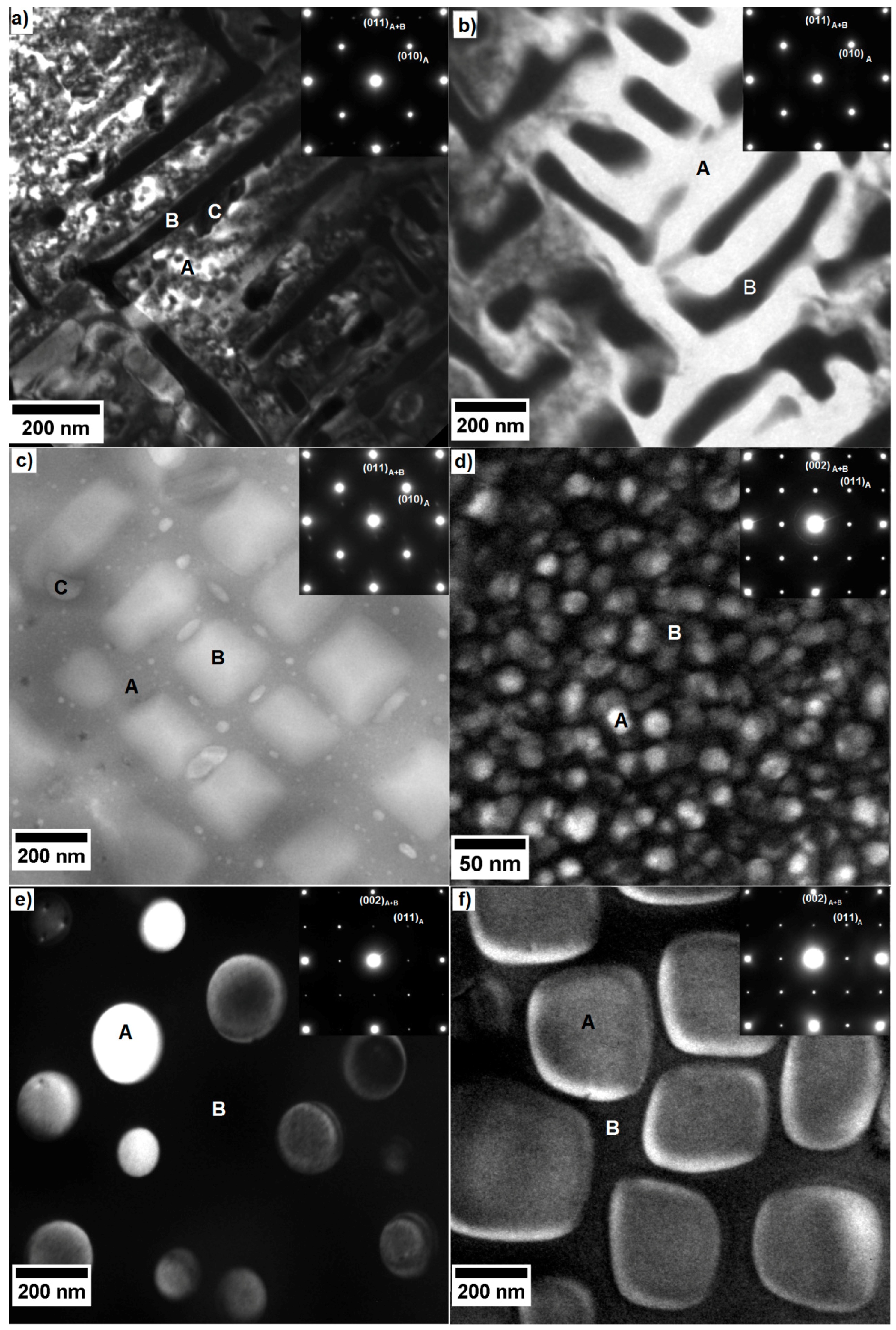
| Alloy | Matrix | Main Precipitating Phase | Average Size of the Main Precipitates | Vol.% of the Precipitating Phase | Additional Phase (s) | Comments | Ref. |
|---|---|---|---|---|---|---|---|
| Alloy 1 AlCoCrCuFeNi | Al-Ni rich, B2 structure | Cr-Fe rich, A2 structure | Platelets, 100–400 nm | ~40 | Cu rich phases B2; L12 | Too brittle for mechanical application | [11] |
| Alloy 2 AlCoCrFeNi | Al-Ni rich, B2 structure | Cr-Fe rich, A2 structure | Cuboids, 50–100 nm | ~30 | - | Too brittle for mechanical application | [15] |
| Alloy 3 Al23Co15Cr23Cu8Fe15Ni16 | Al-Ni rich, B2 structure | Cr-Fe rich, A2 structure | Cuboids, 100–300 nm | ~46 | Cu rich platelets | Too brittle for mechanical application | [17] |
| Alloy 4 Al8Co17Cr17Cu8Fe17Ni33 | Cr-Co-Fe rich, A1 structure | Al-Cu-Ni rich, L12 structure | Spheres, 10–20 nm | 20 | - | Too soft for mechanical application | [17,18,19] |
| Alloy 5 Al8Co17Cr14Cu8Fe17Ni34.8Mo0.1Ti1W0.1 | Cr-Co-Fe rich, A1 structure | Al-Cu-Ni rich, L12 structure | Spheres, 80–200 nm | ~7 | - | Too soft for mechanical application | [19] |
| Alloy 6 Al10Co25Cr8Fe15Ni36Ti6 | Cr-Co-Fe rich, A1 structure | Al-Ni-Ti rich, L12 structure | Cuboids, 160–400 nm | ~40 | Needle precipitate, CaF2 structure | To be investigated | This work |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzoni, A.M.; Singh, S.; Daoud, H.M.; Popp, R.; Völkl, R.; Glatzel, U.; Wanderka, N. On the Path to Optimizing the Al-Co-Cr-Cu-Fe-Ni-Ti High Entropy Alloy Family for High Temperature Applications. Entropy 2016, 18, 104. https://doi.org/10.3390/e18040104
Manzoni AM, Singh S, Daoud HM, Popp R, Völkl R, Glatzel U, Wanderka N. On the Path to Optimizing the Al-Co-Cr-Cu-Fe-Ni-Ti High Entropy Alloy Family for High Temperature Applications. Entropy. 2016; 18(4):104. https://doi.org/10.3390/e18040104
Chicago/Turabian StyleManzoni, Anna M., Sheela Singh, Haneen M. Daoud, Robert Popp, Rainer Völkl, Uwe Glatzel, and Nelia Wanderka. 2016. "On the Path to Optimizing the Al-Co-Cr-Cu-Fe-Ni-Ti High Entropy Alloy Family for High Temperature Applications" Entropy 18, no. 4: 104. https://doi.org/10.3390/e18040104
APA StyleManzoni, A. M., Singh, S., Daoud, H. M., Popp, R., Völkl, R., Glatzel, U., & Wanderka, N. (2016). On the Path to Optimizing the Al-Co-Cr-Cu-Fe-Ni-Ti High Entropy Alloy Family for High Temperature Applications. Entropy, 18(4), 104. https://doi.org/10.3390/e18040104






