Emergence of Animals from Heat Engines – Part 1. Before the Snowball Earths
Abstract
:1. Introduction
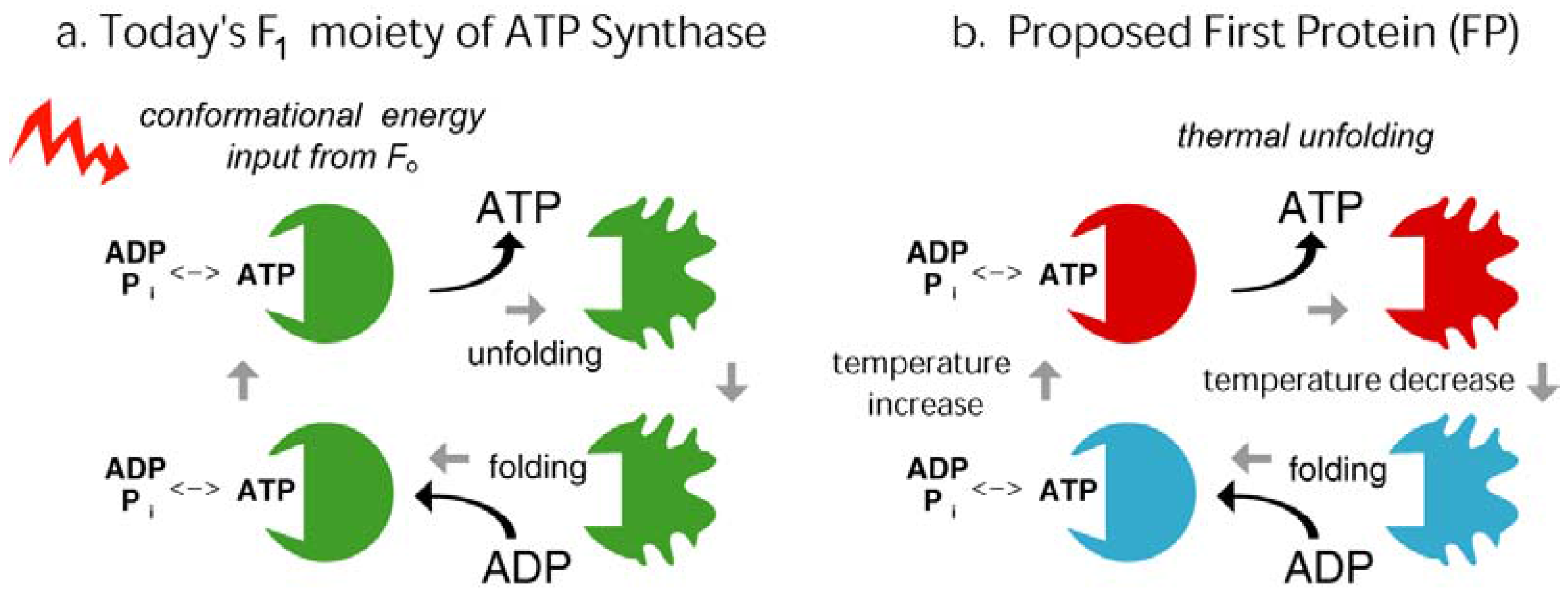
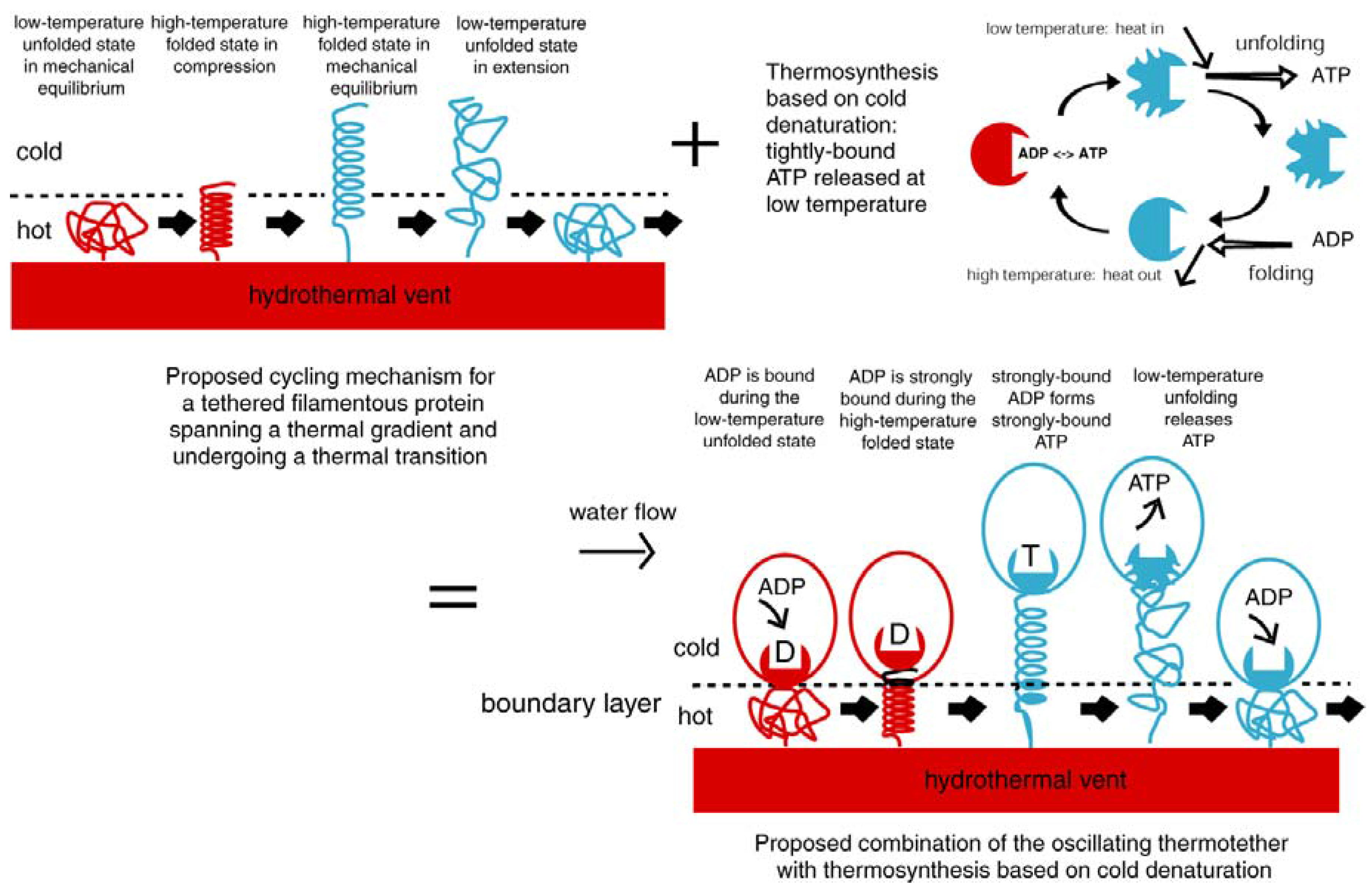
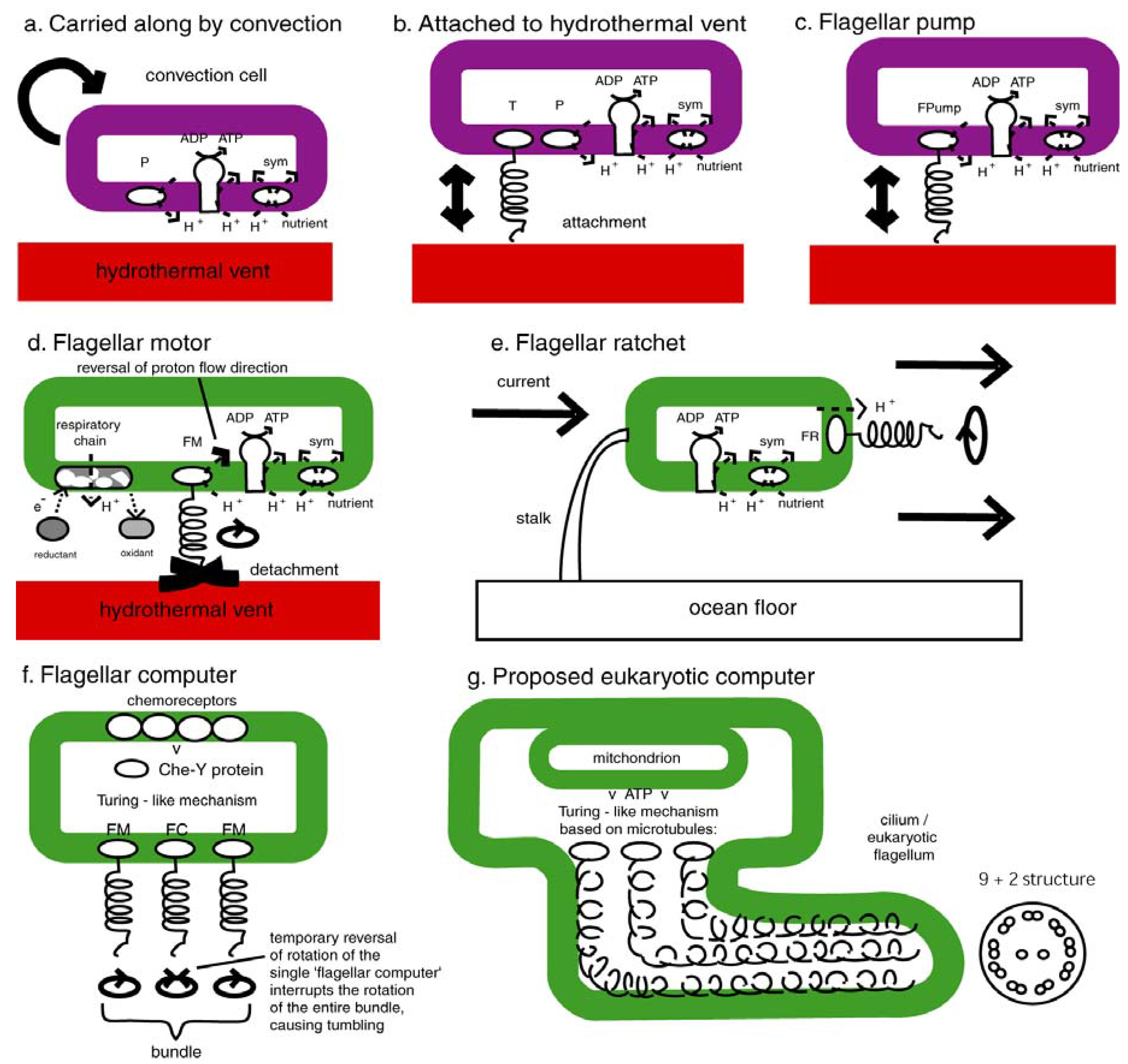
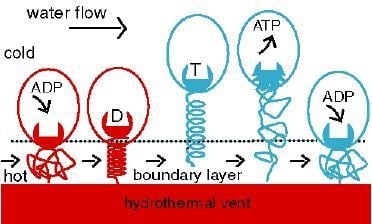
2. Background
2.1. Simple Heat Engines
and convection indeed sustains the weather in the atmosphere, the ocean currents in the hydrosphere, the plate tectonics in the lithosphere, and may sustain the geodynamo in the Earth’s core that generates the Earth’s magnetic field [53,54,55].To heat . . . are due the vast movements which take place on the Earth,
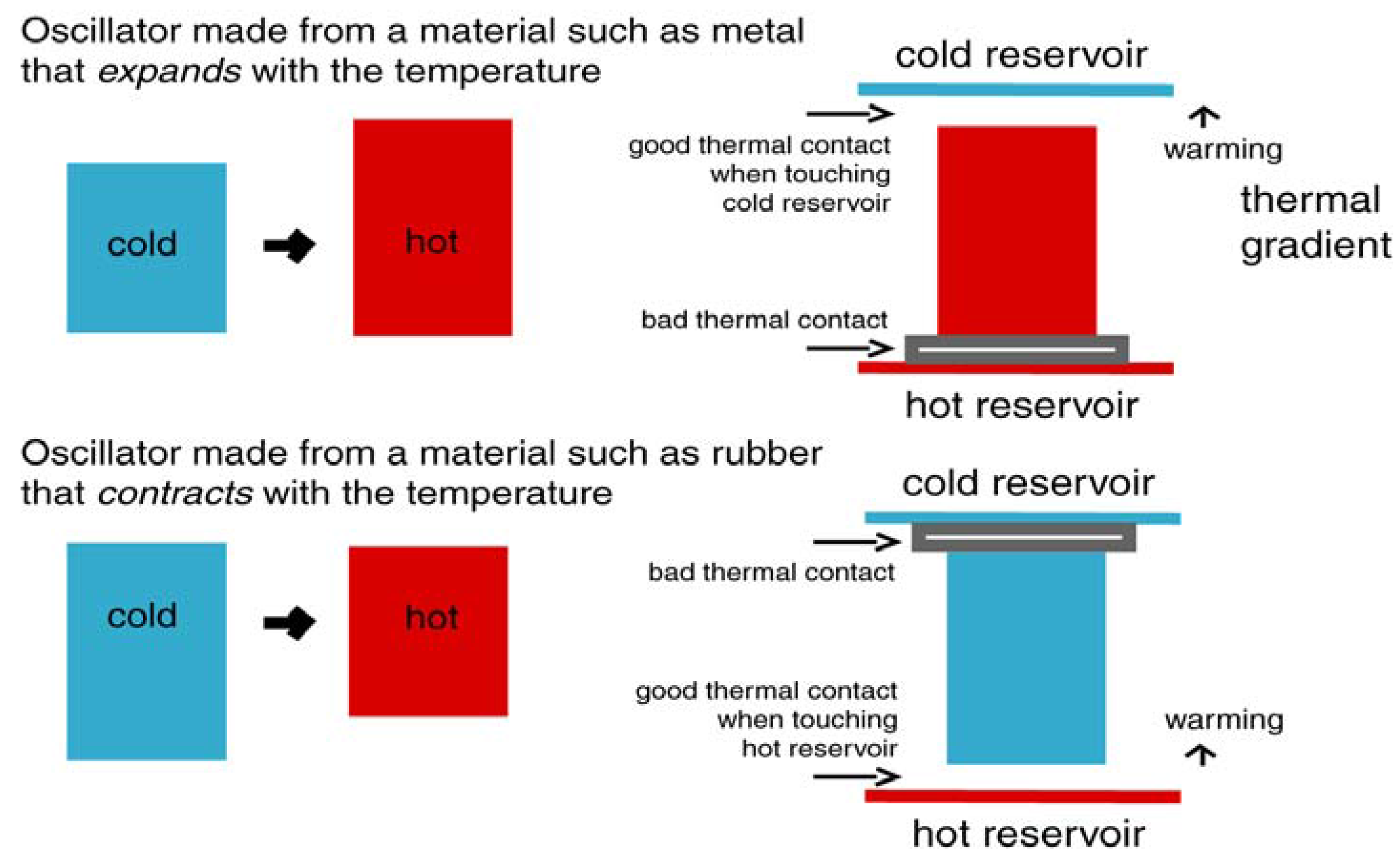
2.2. The Chemiosmotic Mechanism
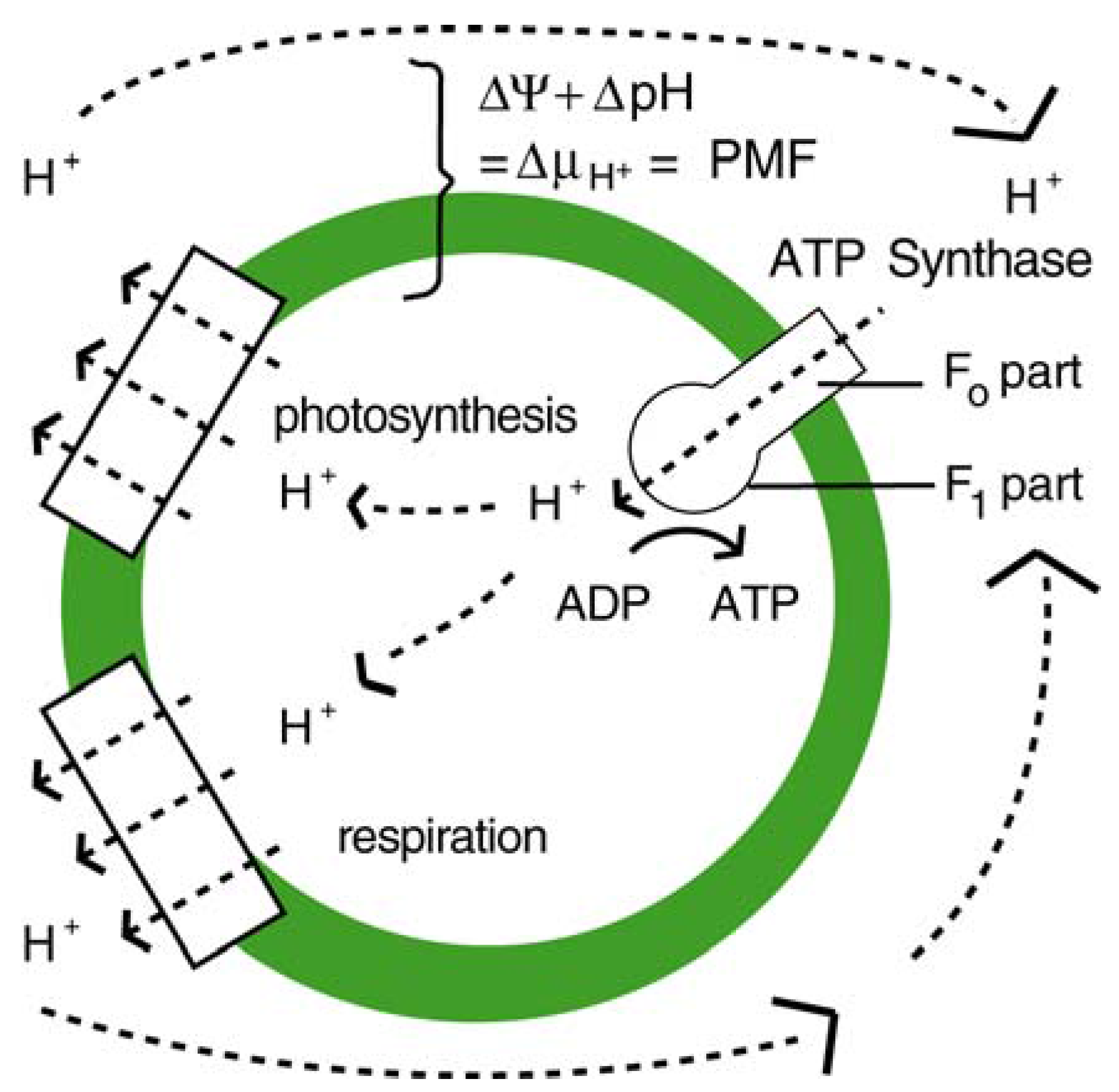
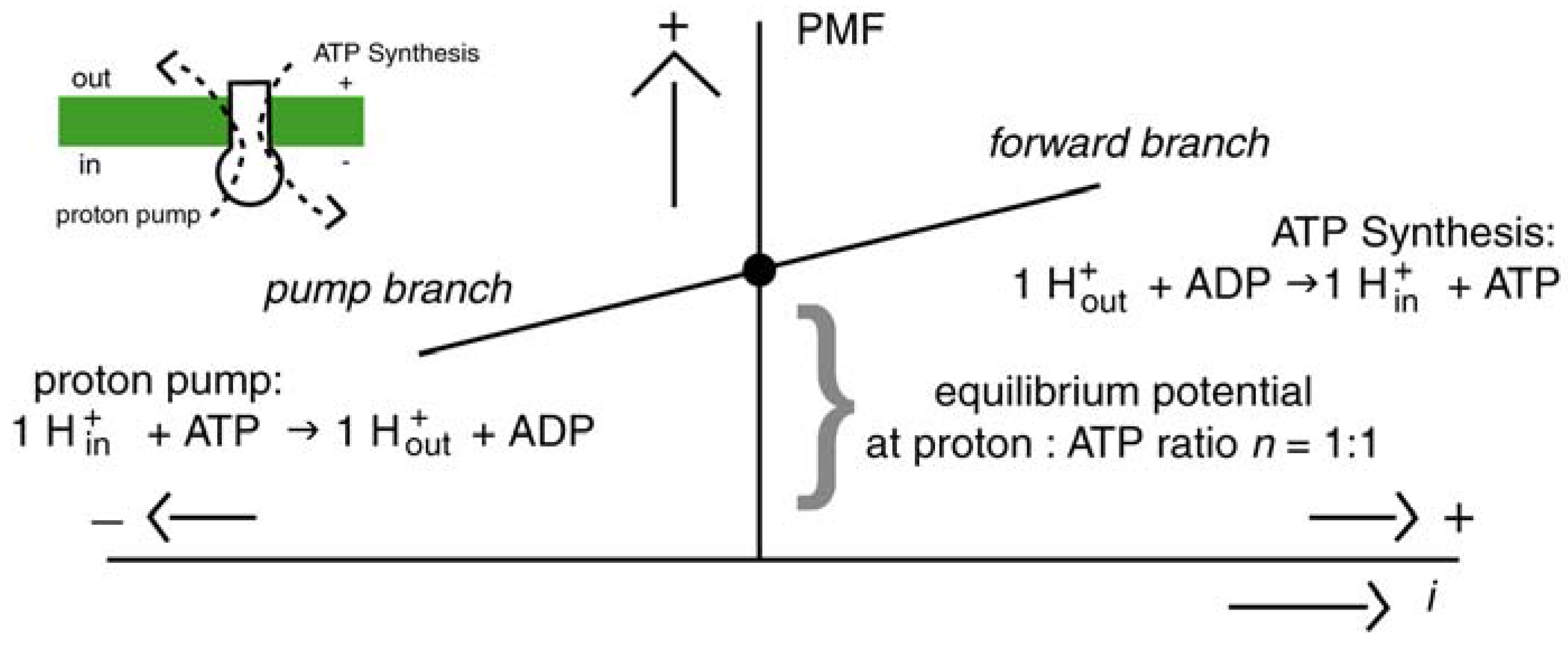

2.3. Thermosynthesis Based on Convection (TSC)
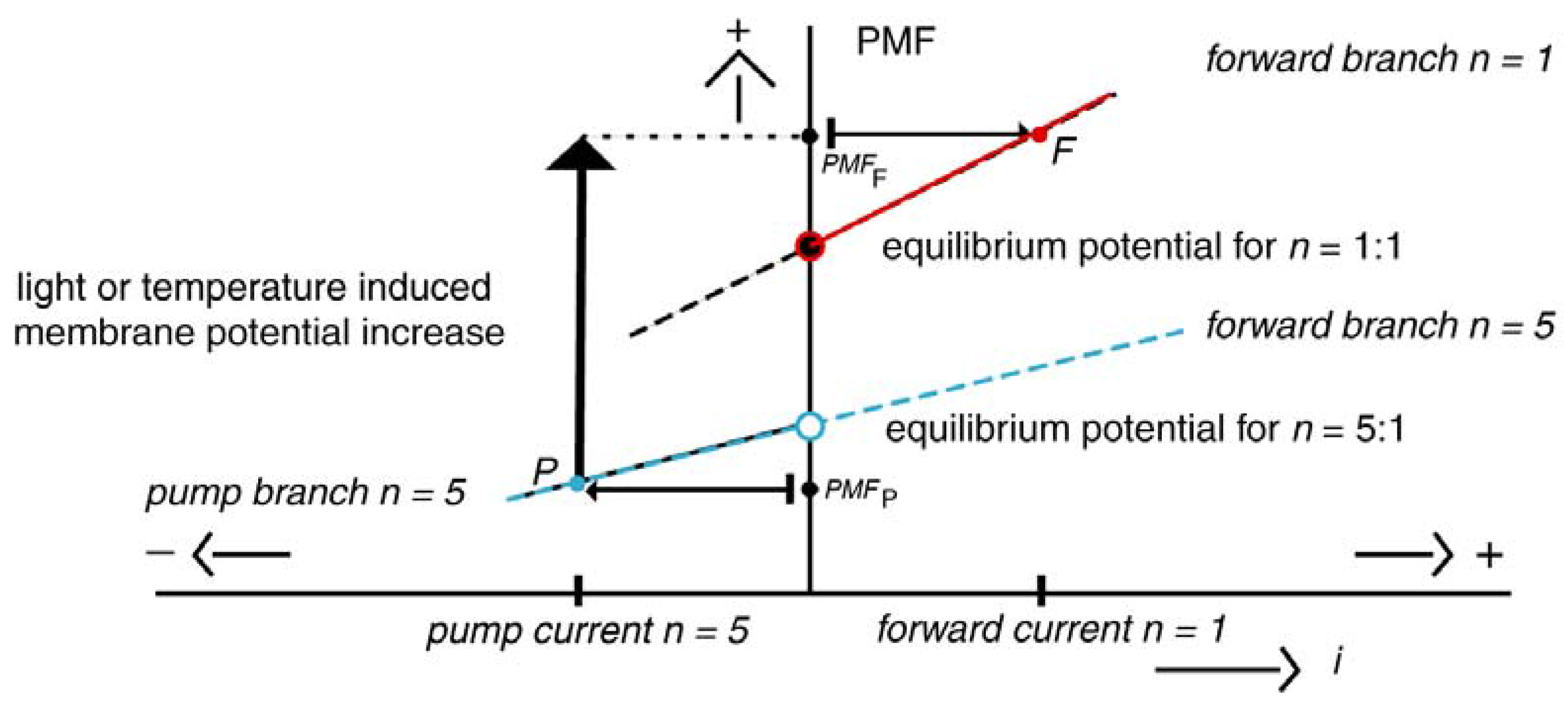
2.4. The Fire: Hydrothermal Vents
RNA and DNA are clearly too unstable to exist in a hot prebiotic environment.
2.5. The Ice: Glaciations on the Early Earth
. . . heat derived from the hydrothermal cooling of newly-emplaced oceanic crust provides the energy source for converting inorganic precursors into organic compounds.
3. Between Fire and Ice: Thermosynthesis in a Thermal Gradient (TSG)
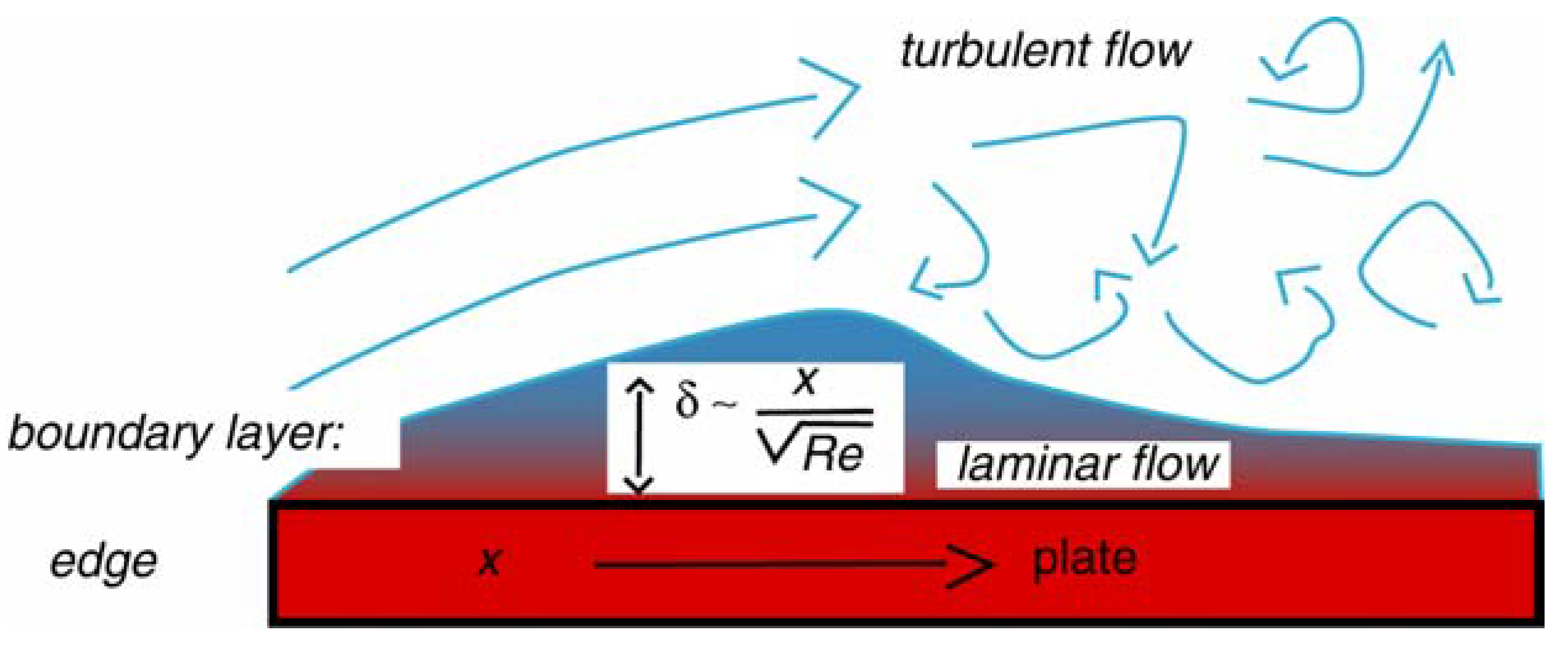
3.1. Thermal Fluctuations on the Surface of a Hydrothermal Vent
3.2. Emergence of the Thermotether: a simple Relaxation Oscillator
. . . all of the microfossils associated with ancient hydrothermal deposits reported to date are filamentous.
Perhaps most significantly, the changes in preferred orientation of filaments in different areas . . . may indicate behavioural variations in different micro-environments, a distinctly biological feature. Similar patterns are evident in modern microbial mats . . . and hot-spring communities . . . indicating a biotic tropism or taxis in response to a gradient or fluctuation in a salient ecological parameter.
4. The Flagellar Motor, the Flagellar Pump, the Flagellar Ratchet and the Flagellar Computer
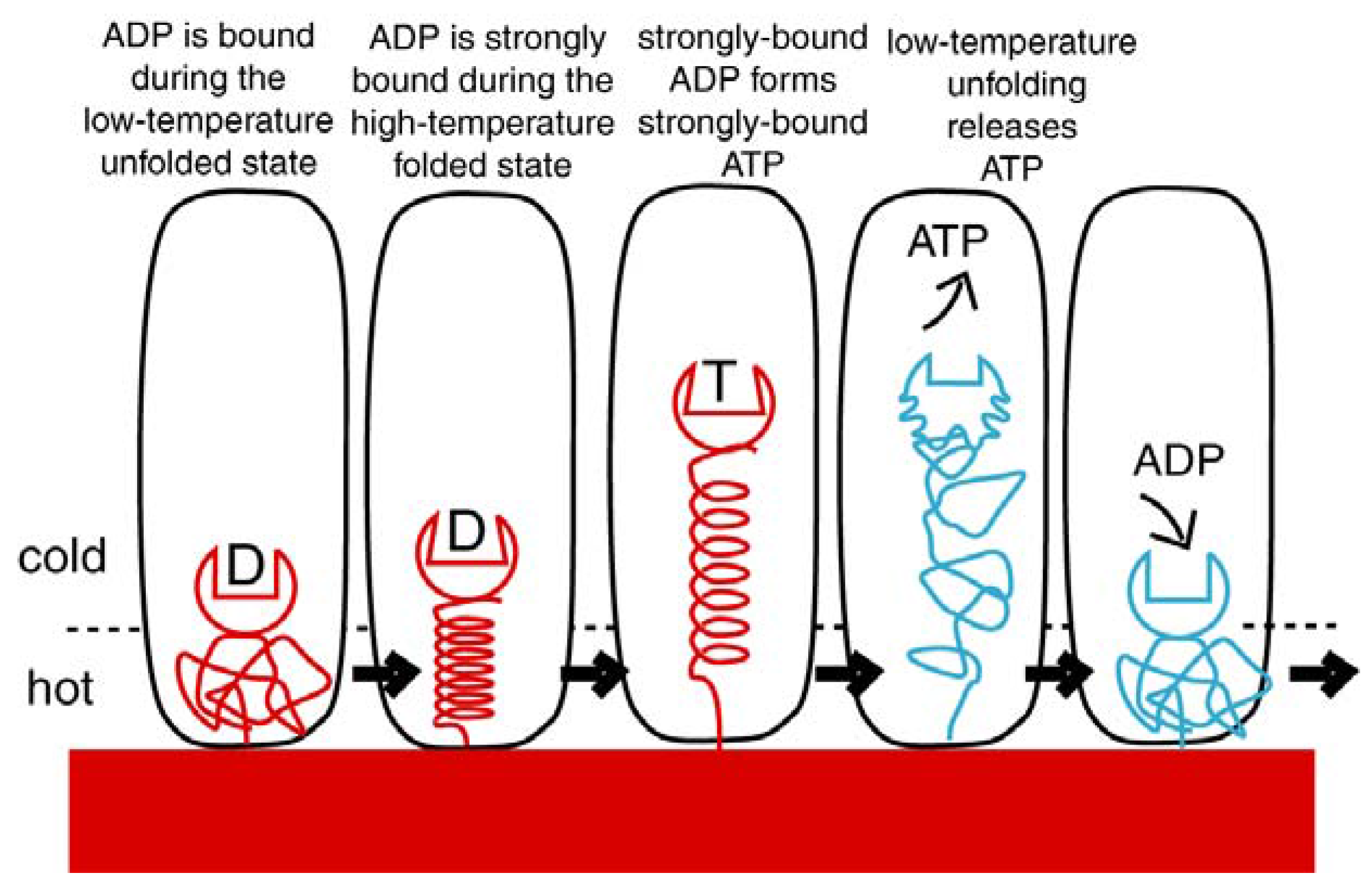
4.1. Emergence of ATP Synthase in a Thermal Gradient
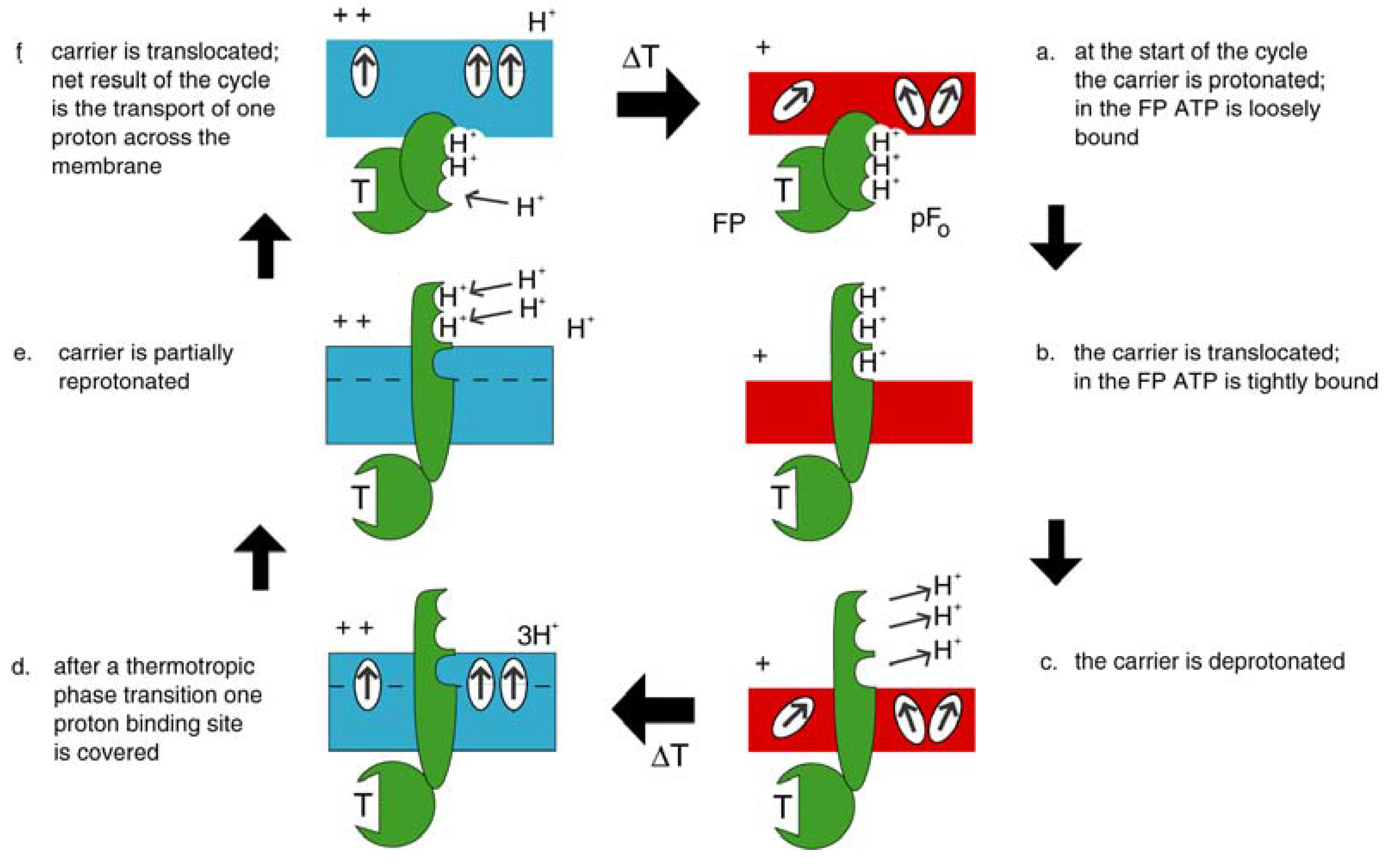
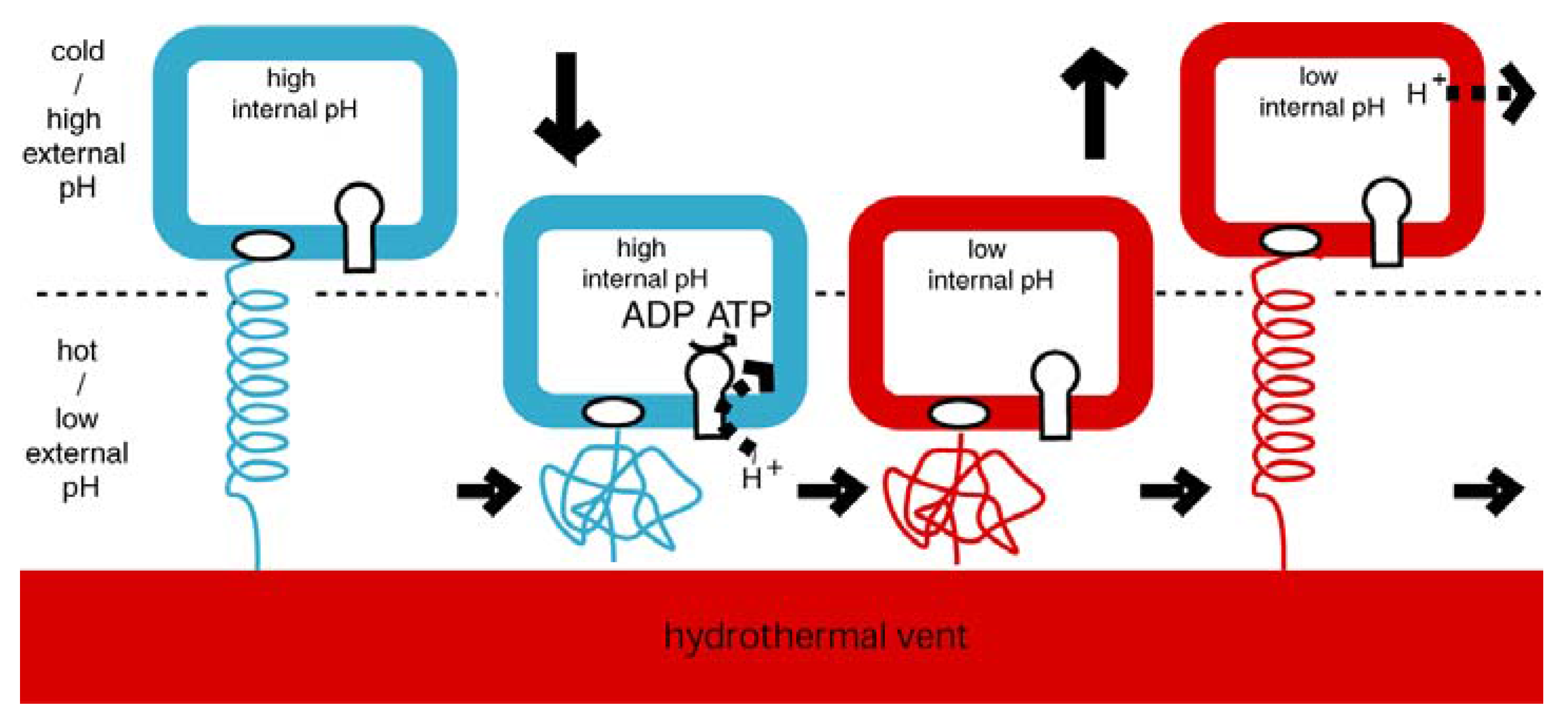
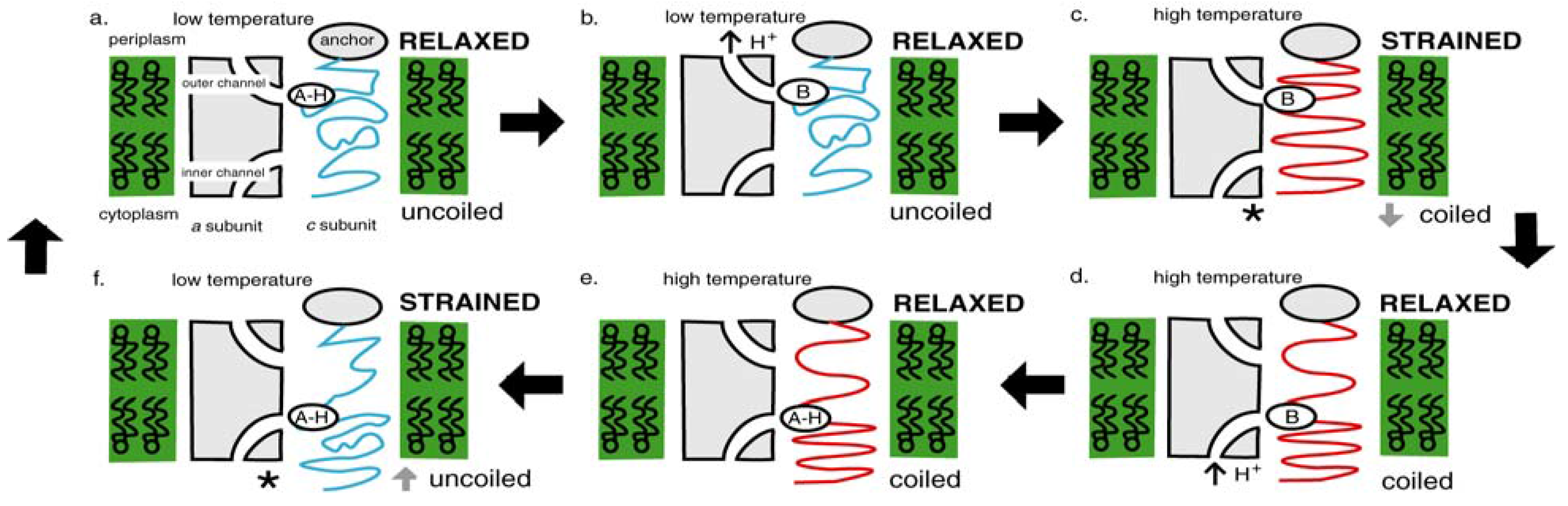
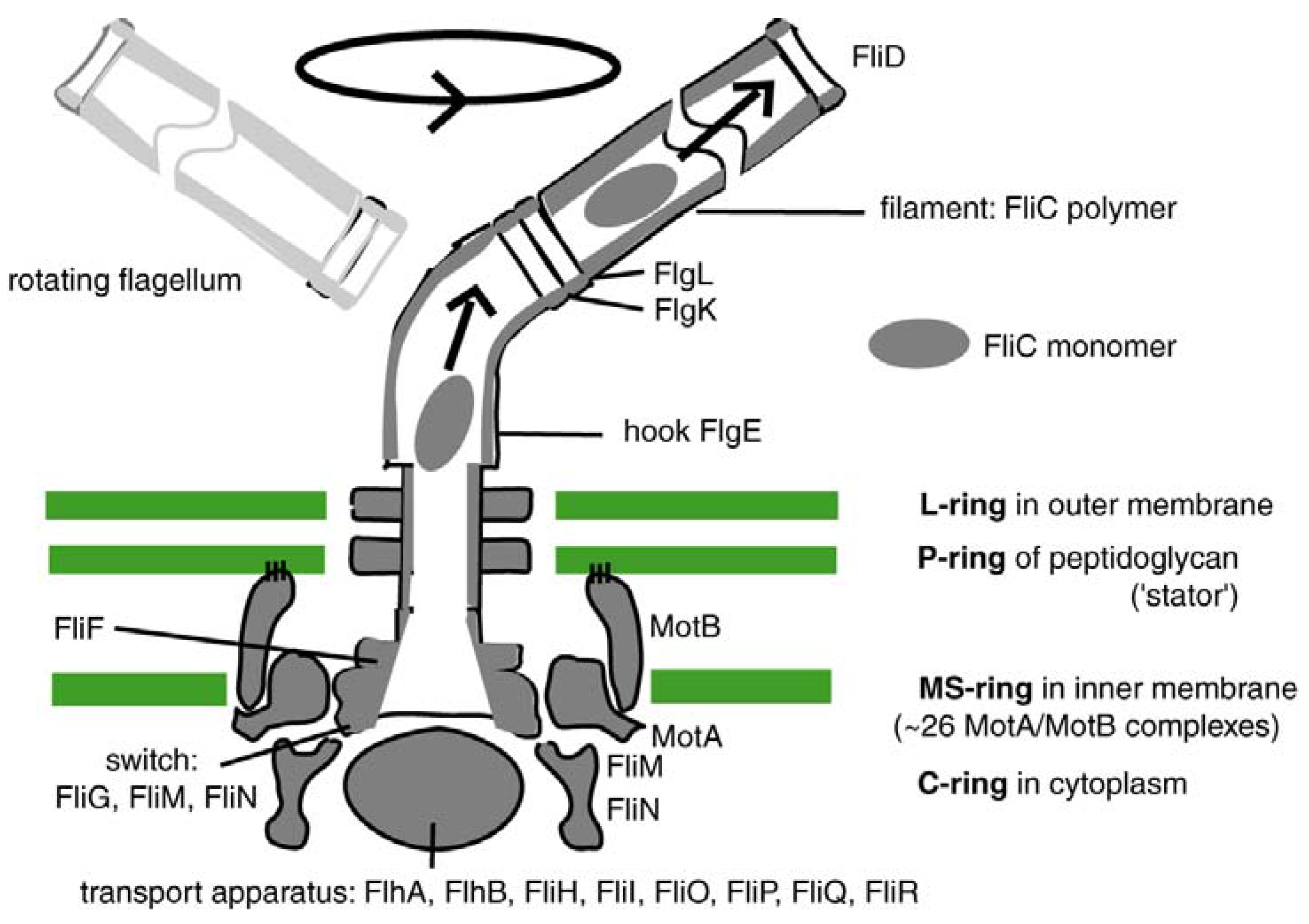
4.2. Today’s Flagellar Motor
The interesting question is not how they swim. Turn anything – if it isn’t perfectly symmetrical, you’ll swim.
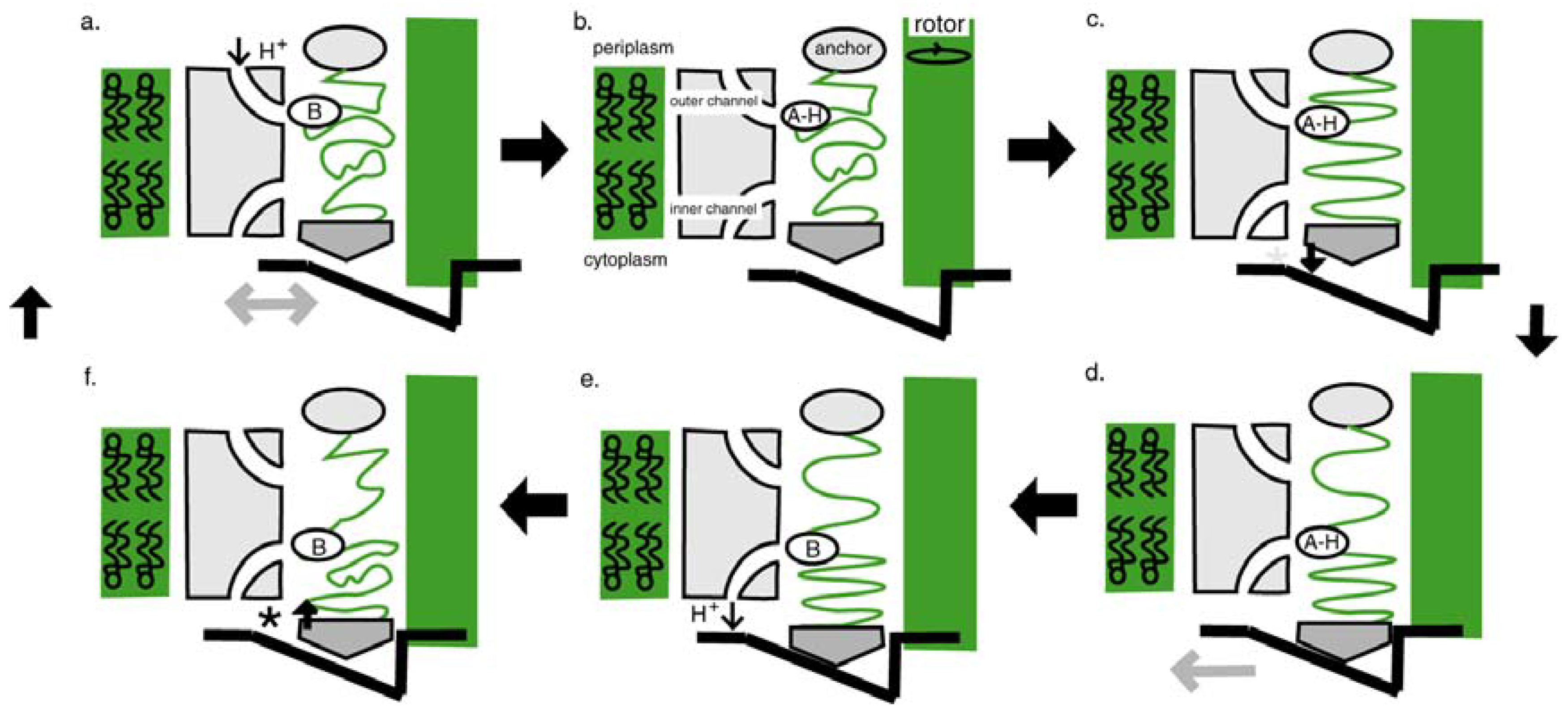
4.3. The Flagellar Pump
4.4. Switch to Isothermy: Emergence of the Flagellar Motor
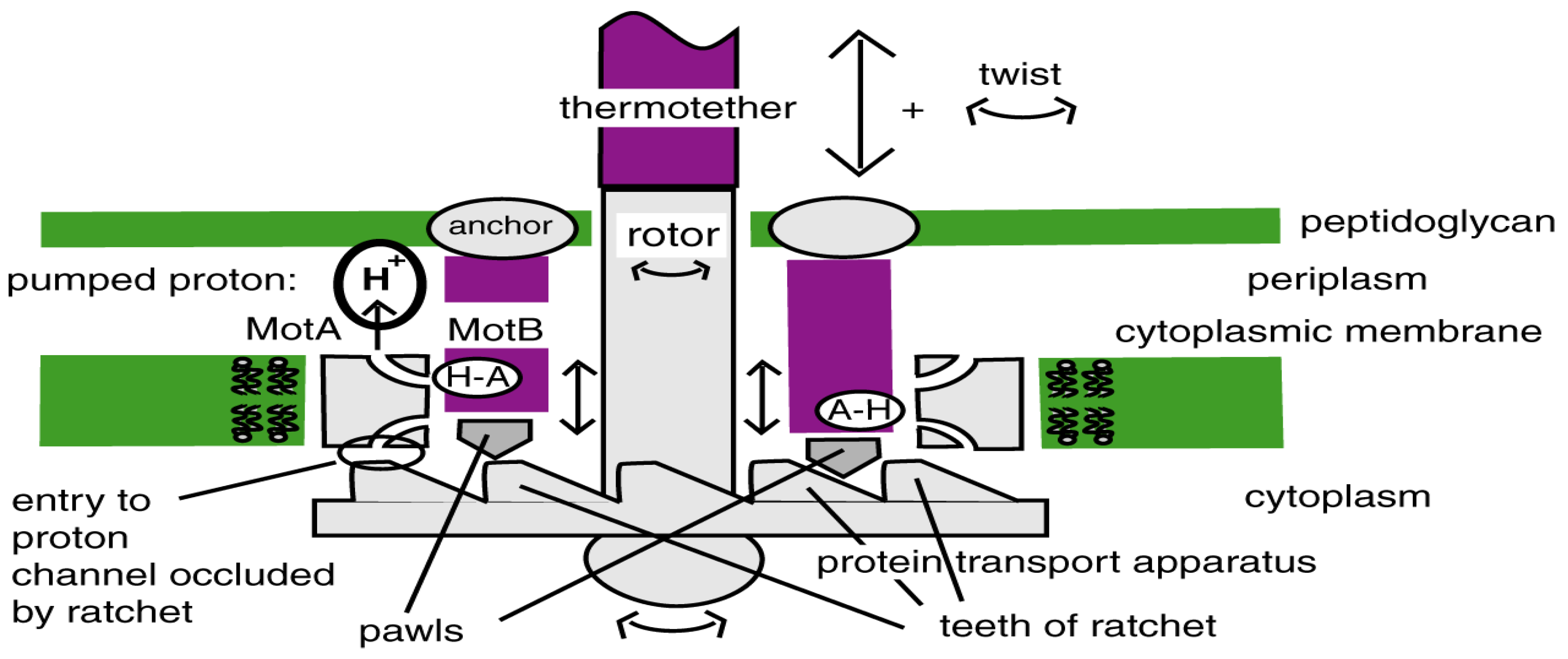
andcore components of the bacterial flagellum originated through the successive duplication and modification of a few, or perhaps, even a single, precursor gene.
The earliest proteins are proximate to the cytoplasmic membrane with later proteins situated distally, first spanning the outer membrane and then giving rise to structures (i.e., the hook, junction, filament, and capping proteins) that extend outside of the bacterial cell. Thus, the flagellum represents a case whereby its order of assembly recapitulates its evolutionary history.

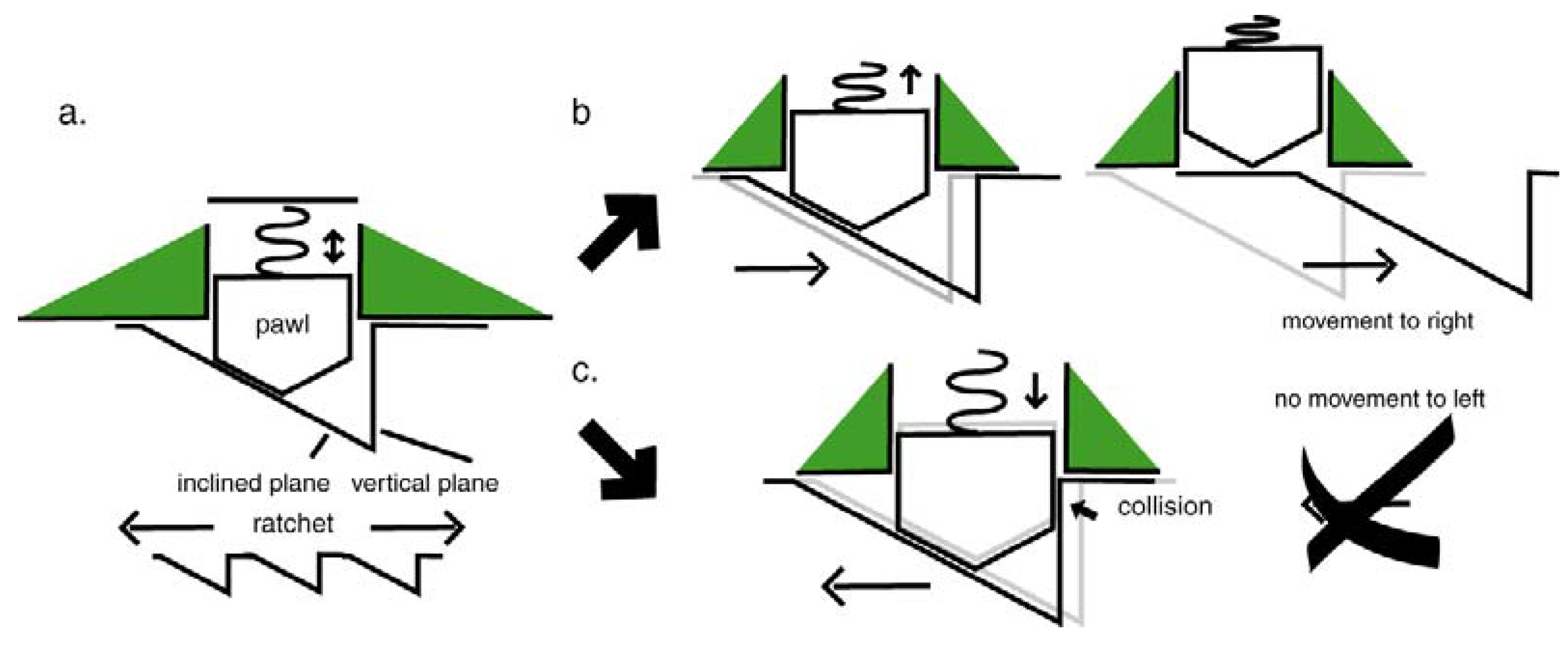
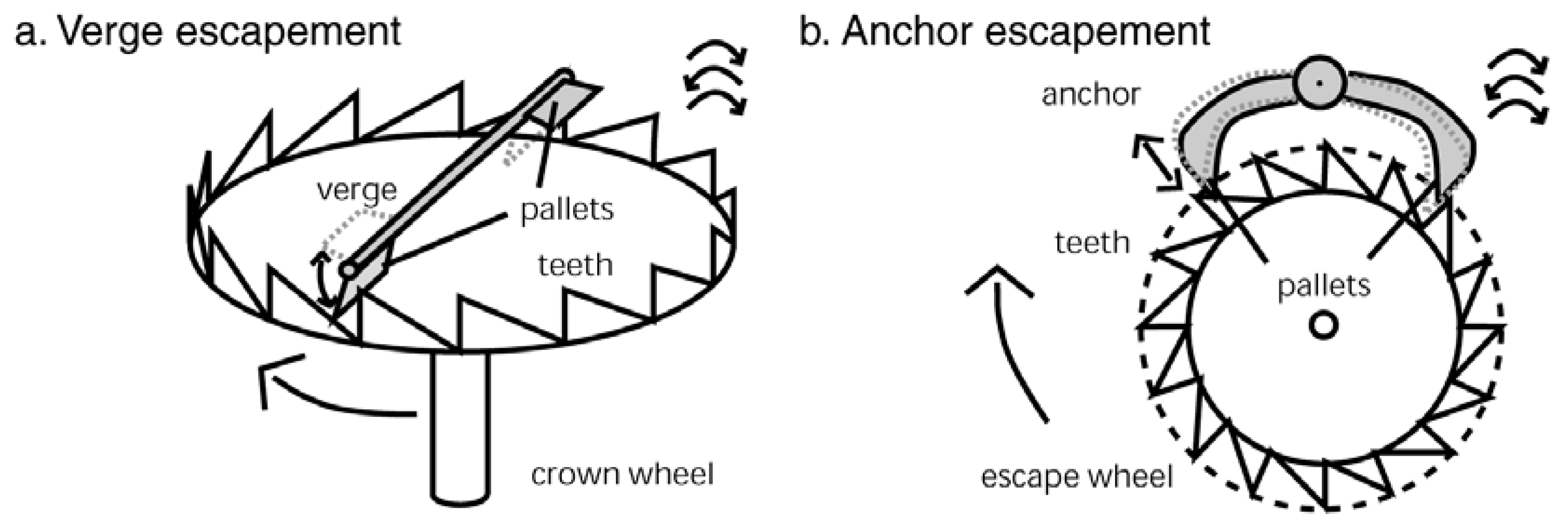
4.5. The Flagellar Ratchet and Feynman’s Ratchet
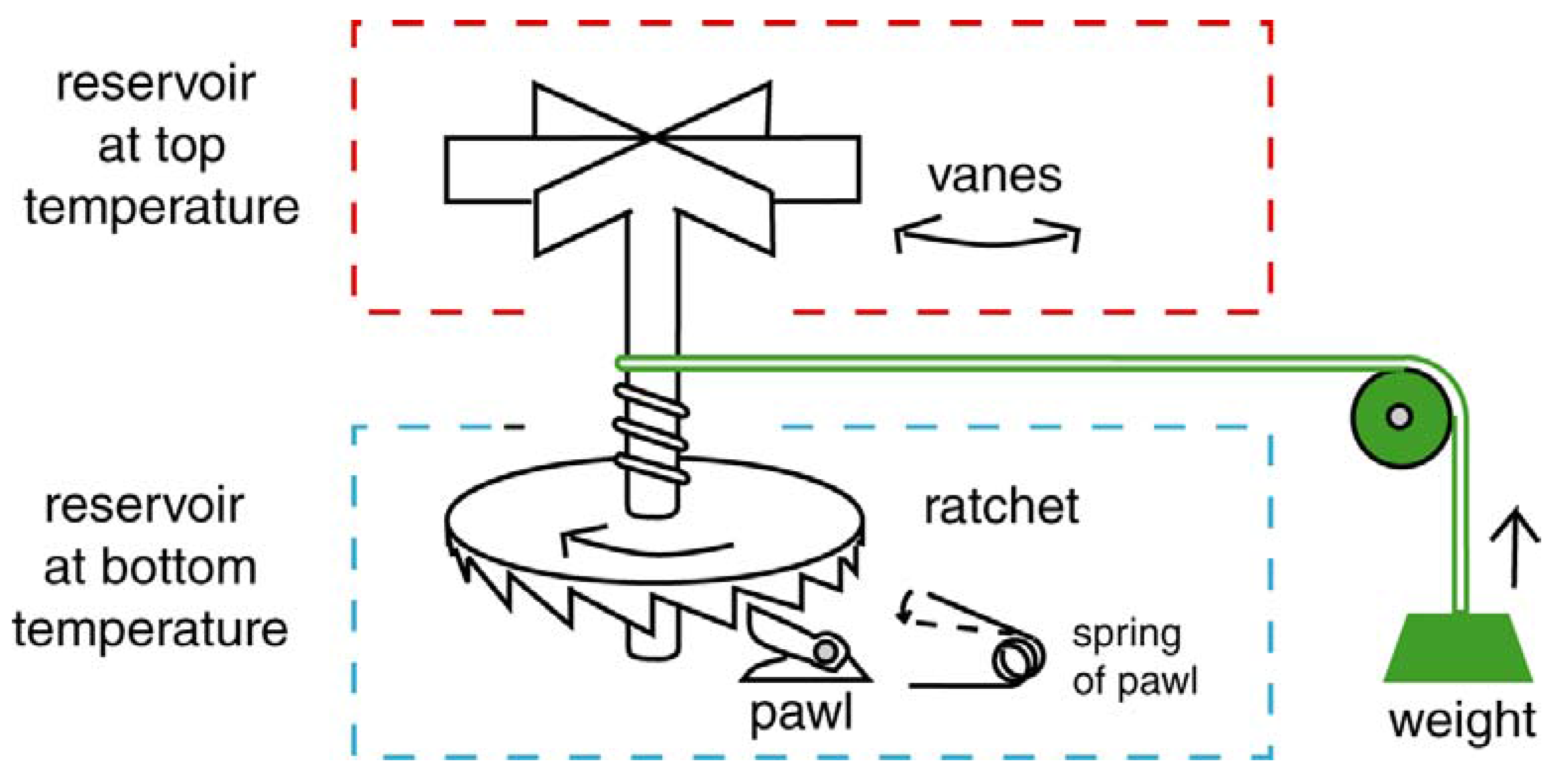
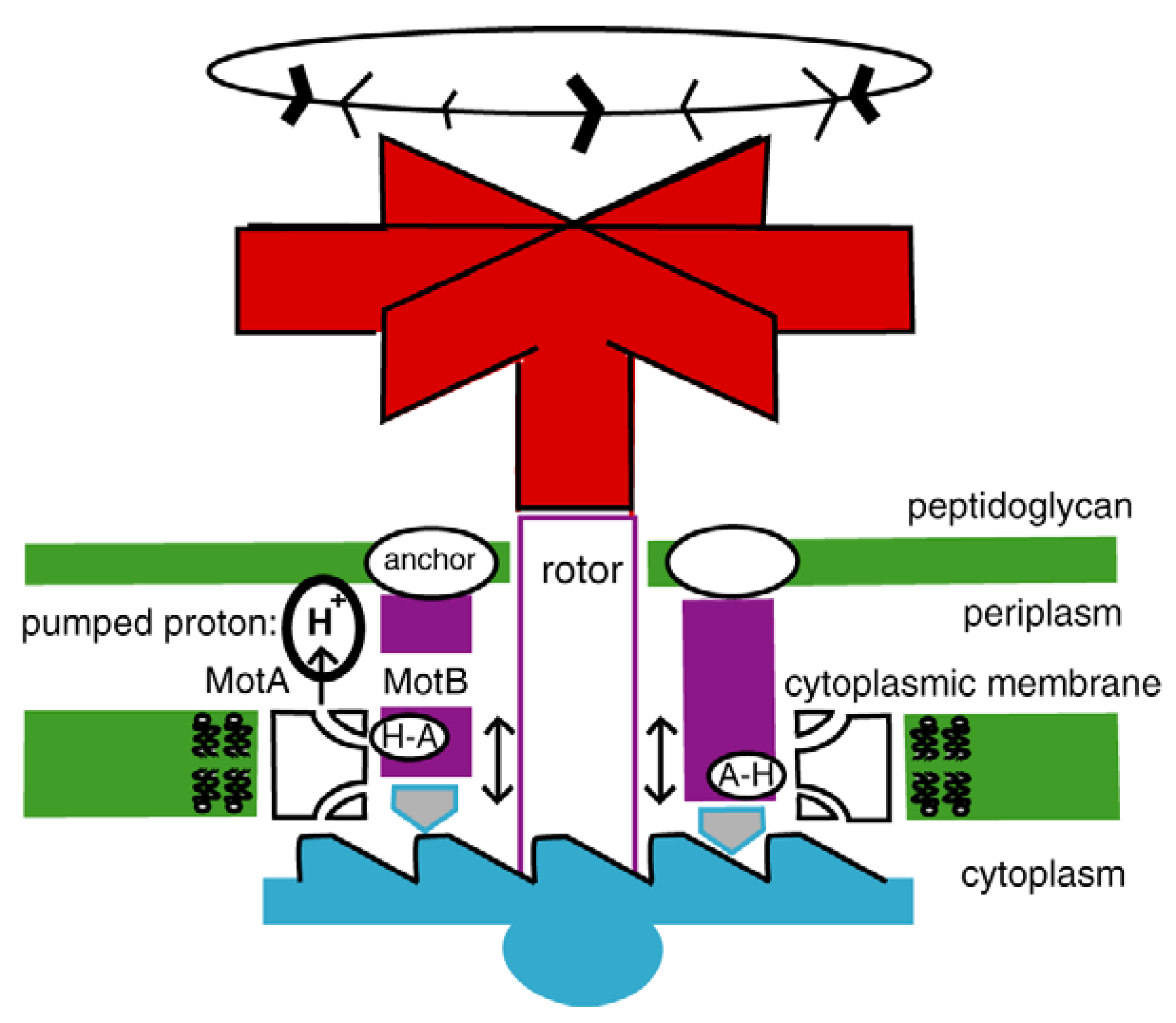
4.6. The Flagellar Computer and the Turing Machine
Beware of the Turing tar-pit in which everything is possible but nothing of interest is easy.
Error is viewed, therefore, not as an extraneous and misdirected or misdirecting accident, but as an essential part of the process under consideration—its importance in the synthesis of automata being fully comparable to that of the factor which is normally considered, the intended and correct logical structure.
Our present treatment of errors is unsatisfactory and ad hoc. It is the author’s conviction, voiced over many years, that error should be treated by thermodynamic methods, and be the subject of thermodynamical theory, as information has been, by the work of L. Szilard and C.E. Shannon.
Turing computation, designed computation, is about halting, computability and universality; it is symbolic, discrete and closed (pre-defined); it is deterministic and sequential (in the sense that probabilistic or parallel variants provide no additional computation power); . . . its calculations are exceedingly fragile to small changes or errors. On the other hand, biological computation, found computation, is about not halting (halting equates system death); it is (mostly) nonsymbolic, continuous and open (constantly adapting and evolving due to the continual flow of matter, energy and information through the system); it is essentially stochastic and massively parallel; . . . and is robust to many classes of errors.
4.7. Emergence of the Eukaryotic Cell
The evolution of the motile cilium was a major innovation during eukaryogenesis that, in synergy with endomembranes, phagocytosis, mitochondria and novel genome organisation, triggered the rapid diversification of the eukaryotes.
Both autogenous and symbiotic scenarios have been put forward to explain the evolutionary origin of the cilium. Autogenous scenarios propose that the cilium evolved by the duplication and divergence of pre-existing components of the eukaryotic cell whereas in symbiotic scenarios, the merger of a motile Spirochete and a host cell is thought to have given rise to the organelle.
5. Discussion
andBiology’s subject matter thus consists of a class of naturally occurring machines, machines that are assumed to be descended from primitive protomachines, the original progenitor of which was self-assembled.
Whether or not abiogenesis, the spontaneous and natural formation of living systems from inanimate materials alone, is explicable in terms of physical theory, it clearly cannot be explained in terms of biology.
It is essential to almost all man-made machines that they introduce nonholonomic constraints, such as ratches, relays, switches and escapements.
A fundamental problem that is common to all ideas regarding the origin of the nucleus is that the underlying mechanism has to be plausible enough to have actually occurred, but at the same time so unlikely that it has only occurred once in four billion years . . .This problem is severe. . . . It is the main reason that they are all coupled to a rare event in evolution, for example the origin of phagotrophy, a karyogenic symbiosis that occurred only in the eukaryotic lineage, or the origin of mitochondria. . . .
6. Conclusions
Acknowledgements
References
- Muller, A.W.J. Thermoelectric energy conversion could be an energy source of living organisms. Phys. Lett. A 1983, 96, 319–321. [Google Scholar] [CrossRef]
- Muller, A.W.J. Thermosynthesis by biomembranes: energy gain from cyclic temperature changes. J. Theor. Biol. 1985, 115, 429–453. [Google Scholar] [CrossRef]
- Muller, A.W.J. A mechanism for thermosynthesis based on a thermotropic phase transition in an asymmetric biomembrane. Physiol. Chem. Phys. Med. NMR 1993, 25, 95–111. [Google Scholar]
- Muller, A.W.J. Were the first organisms heat engines? A new model for biogenesis and the early evolution of biological energy conversion. Prog. Biophys. Mol. Biol. 1995, 63, 193–231. [Google Scholar] [CrossRef]
- Muller, A.W.J. Thermosynthesis as energy source for the RNA world: A model for the bioenergetics of the origin of life. BioSystems 2005, 82, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.W.J. Photosystem 0, a proposed ancestral photosystem without reducing power that that synthesized ATP during light-dark cycling. Available online at http: //www.arXiv.net/physics/0501050 (accessed September 16, 2009).
- Muller, A.W.J.; Schulze-Makuch, D. Sorption heat engines: simple inanimate negative entropy generators. Physica A 2006, 362, 369–381. [Google Scholar] [CrossRef]
- Muller, A.W.J.; Schulze-Makuch, D. Thermal energy and the origin of life. Orig. Life Evol. Biosph. 2006, 36, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Conway Morris, S. Darwin’s dilemma: the realities of the Cambrian explosion. Proc. Trans. R. Soc. B 2006, 361, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Darwin, C. On the Origin of Species by means of Natural Selection; J. Murray: London, UK, 1859. [Google Scholar]
- Glaessner, M.F. The Dawn of Animal Life: a Biohistorical Study; Cambridge University Press: Cambridge, UK, 1984. [Google Scholar]
- McCall, G.J.H. The Vendian (Ediacaran) in the geological record: enigmas in geology’s prelude to the Cambrian explosion. Earth-Sci. Rev. 2006, 77, 1–229. [Google Scholar] [CrossRef]
- Narbonne, G.M. The Ediacara biota: a terminal Neoproterozoic experiment in the evolution of life. GSA Today 1998, 8, 1–6. [Google Scholar]
- Chen, J.-W.; Oliveri, P.; Gao, F.; Dornbos, S.Q.; Li, C.-W.; Bottjer, D.J.; Davidson, E.H. Precambrian animal life: probable developmental and adult cnidarian forms from Southwest China. Dev. Biol. 2002, 248, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Narbonne, G.M. Modular construction of early Ediacaran complex life forms. Science 2004, 305, 1141–1144. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Shen, B.; Zhou, C.; Xie, G.; Yuan, X. A uniquely preserved Ediacaran fossil with direct evidence for a quilted bodyplan. Proc. Nat. Acad. Sci. USA 2005, 102, 10227–10232. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, P.J.; Kaufman, A.J.; Halverson, G.P.; Schrag, D.P. A neoproterozoic snowball Earth. Science 1998, 281, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
- Kirschvink, J.L. Late Proterozoic, low-latitude global glaciation, The Snowball Earth. In The Proterozoic Biosphere: A Multidisciplinary Study; Schopf, J.W., Klein, C., Eds.; Cambridge University Press: Cambridge, UK, 1992; pp. 51–52. [Google Scholar]
- Mawson, D. The late Precambrian ice-age and glacial record of the Bibliando dome. J. Proc. Roy. Soc. New South Wales 1949, 82, 150–174. [Google Scholar]
- Muller, A.W.J. Animal emergence during Snowball Earths by thermosynthesis in submarine hydrothermal vents. Available online at http://precedings.nature.com/documents/3333/version/2 (accessed September 16, 2009).
- Muller, A.W.J. Emergence of animals during Snowball Earths from biological heat engines in the thermal gradient above submarine hydrothermal vents. Orig. Life Evol. Biosph. 2009, 39, 320–321. [Google Scholar]
- Rasmussen, B. Filamentous microfossils in a 3,235-million-year-old volcanogenic massive sulphide deposit. Nature 2000, 405, 676–679. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, B.; van der Mark, J. The heartbeat considered as a relaxation oscillation, and an electrical model of the heart. Philos. Mag. Suppl. 1928, 6, 763–775. [Google Scholar] [CrossRef]
- Brandts, J.F. Heat effects on proteins and enzymes. In Thermobiology; Rose, A.H., Ed.; Academic Press: London, UK, 1967; pp. 25–72. [Google Scholar]
- Todgham, A.E.; Hoaglund, E.A.; Hoffman, G.E. Is cold the new hot? Elevated ubiquitin-conjugated protein levels in tissues of Antarctic fish as evidence for cold-denaturation of proteins in vivo. J. Comp. Physiol. B 2007, 177, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Hueck, C.J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microb. Mol. Biol. Rev. 1998, 62, 379–433. [Google Scholar]
- Feynman, R.; Leighton, R.; Sands, M. The Feynman Lectures on Physics; Addison-Wesley: Reading, MA, USA, 1963. [Google Scholar]
- Machura, L. Performance of Brownian Motors . In Ph.D. Thesis; University of Augsburg: Augsburg, Germany, 2006. [Google Scholar]
- von Smoluchowski, M. Experimentell nachweisbare, der üblichen Thermodynamik widersprechende Molekularphänome. Physik. Zeitschr. 1912, 13, 1069–1080. [Google Scholar]
- Berg, H.C. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 2003, 72, 19–54. [Google Scholar] [CrossRef] [PubMed]
- Turing, A. On computable numbers, with an application to the Entscheidungsproblem. Proc. London Math. Soc. 2 1937, 42, 230–265. [Google Scholar] [CrossRef]
- Cramer, W.A.; Knaff, D.B. Energy Transduction in Biological Membranes. A Textbook of Bioenergetics; Springer-Verlag: New York, NY, USA, 1991. [Google Scholar]
- Dimroth, P.; Von Ballmoos, C.; Meier, T. Catalytic and mechanical cycles in F1-ATP synthases. EMBO Rep. 2006, 7, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P. Coupling of phosphorylation to electron and proton transfer by a chemi-osmotic type of physiology. Nature 1961, 191, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P. Keilin’s respiratory chain concept and its chemiosmotic consequences. Science 1979, 206, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.P. Possible functions of chains of catalysts. J. Theor. Biol. 1961, 1, 1–17. [Google Scholar] [CrossRef]
- Harold, F.M. Gleanings of a chemiosmotic eye. BioEssays 2001, 23, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Carnot, S. Réflexions sur la puissance motrice du feu et sur les machines propres a développer cette puissance; Bachelier: Paris, France, 1824. [Google Scholar]
- Gonzalo, J.A. Ferroelectric materials as energy converters. Ferroelectrics 1976, 11, 423–430. [Google Scholar] [CrossRef]
- Hoh, S.R. Energy converter. U.S. Patent 1963. [Google Scholar]
- Pulvari, C.F.; Garcia, F.J. Conversion of solar to electrical energy utilizing the thermodielectric effect. Ferroelectrics 1978, 22, 769–771. [Google Scholar] [CrossRef]
- Sklar, A.A. A Numerical Investigation of a Thermodielectric Power Generation System . In Ph.D. Thesis; Georgia Institute of Technology: Atlanta, GE, USA, 2005. [Google Scholar]
- Tesla, N. Thermo magnetic motor. U.S. Patent 1889. [Google Scholar]
- Wekjira, J.F. The VT1 Shape Memory Alloy Heat Engine Design . In Ph.D. Thesis; Virginia Polytechnic Institute: Blacksburg, VA, USA, 2001. [Google Scholar]
- Wiegand, W.B.; Snyder, J.W. The rubber pendulum, the Joule effect, and the dynamic stress strain curve. Inst. Rubber Ind. Trans. Proc. 1934, 10, 234–262. [Google Scholar] [CrossRef]
- Mullen, J.G.; Look, G.W.; Konkel, J. Thermodynamics of a simple rubber-band heat engine. Am. J. Phys. 1975, 43, 349–353. [Google Scholar] [CrossRef]
- Savarino, G.; Fisch, M.R. A general physics laboratory investigation of the thermodynamics of a rubber band. Am. J. Phys. 1991, 59, 141–145. [Google Scholar] [CrossRef]
- Singh, R. A rubber heat engine for ground water irrigation in India. Agric. Ecosyst. Environ. 1989, 25, 271–278. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Flory, P.J. Theory of elastic mechanisms in fibrous proteins. J. Am. Chem. Soc. 1956, 78, 5222–5235. [Google Scholar] [CrossRef]
- Wöhlisch, E. Muskelphysiologie vom Standpunkt der kinetische Theorie der Hochelastizität und der Entspannungshypothese des Kontraktionsmechanismus. Naturwissenschaften 1940, 28, 305–312. [Google Scholar] [CrossRef]
- Reed, R.C. The Superalloys. Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Hollerbach, R. On the theory of the geodynamo. Phys. Earth Planet. Interiors 1996, 98, 163–185. [Google Scholar] [CrossRef]
- Roberts, P.H.; Soward, A.M. Dynamo theory. Annu. Rev. Fluid Mech. 1992, 24, 459–512. [Google Scholar] [CrossRef]
- Zhang, K.; Schubert, G. Magnetohydrodynamics in rapidly rotating spherical shells. Annu. Rev. Fluid Mech. 2000, 32, 411–445. [Google Scholar] [CrossRef]
- Schubert, G.; Turcotte, D.L.; Olson, P. Mantle Convection in the Earth and Planets; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Warren, B.A. Deep circulation of the world ocean. In Evolution of Physical Oceanography. Scientific Surveys in Honor of Henry Stommel; Warren, B.A., Ed.; MIT Press: Cambridge, MA, USA, 1981; pp. 6–41. [Google Scholar]
- Wunsch, C.; Ferrari, R. Vertical mixing, energy, and the general circulation of the oceans. Annu. Rev. Fluid Mech. 2004, 36, 281–314. [Google Scholar] [CrossRef]
- Broda, E. The Evolution of the Bioenergetic Processes; Pergamon: Oxford, UK, 1975. [Google Scholar]
- Florkin, M. A history of biochemistry. In Comprehensive Biochemistry; Florkin, M., Stotz, E.H., Eds.; Elsevier: Amsterdam, Netherlands, 1972; Vol. 30, p. 244. [Google Scholar]
- Komissarov, G.G. Photosynthesis as a thermal process. In Current Research in Photosynthesis; Baltscheffsky, M., Ed.; Kluwer: Deventer, 1990; Vol. 4, pp. 107–110. [Google Scholar]
- Van Holde, K.E. The origins of life: a thermodynamic critique. In The Origins of Life and Evolution; Halverson, H.O., Van Holde, K.E., Eds.; Alan Liss: New York, 1980; pp. 31–46. [Google Scholar]
- Engelmann, T.W. Über den Ursprung der Muskelkraft; Juvenile Nonfiction: Leipzig, Germany, 1893. [Google Scholar]
- Fick, A. Einige Bemerkungen zu Engelmann’s Abhandlung über den Ursprung der Muskelkraft. Arch. Ges. Physiol. 1893, 53, 606–615. [Google Scholar] [CrossRef]
- Fick, A. Noch einige Bemerkungen zu Engelmann’s Schrift über den Ursprung der Muskelkraft. Arch. Ges. Physiol. 1893, 54, 313–318. [Google Scholar] [CrossRef]
- Guillaume, C.E. Recherche sur les aciers au nickel. Dilatations aux temperature elevees; resistance electrique. Comptes Rendus Acad. Sci. 1897, 125, 235–238. [Google Scholar]
- Shock, E.L. Geochemical constraints of the origin of organic compounds in hydrothermal systems. Orig. Life. Evol. Biosph. 1990, 20, 331–367. [Google Scholar] [CrossRef]
- Rosing, J.; Slater, E.C. The value of ΔG0 for the hydrolysis of ATP. Biochim. Biophys. Acta 1972, 267, 275–290. [Google Scholar] [CrossRef]
- Giersch, C.; Heber, U.; Kobayashi, Y.; Inoue, Y.; Shibata, K.; Heldt, H.W. Energy charge, phosphorylation potential and proton motive force in chloroplasts. Biochim. Biophys. Acta 1980, 590, 59–73. [Google Scholar] [CrossRef]
- Schlodder, E.; Gräber, P.; Witt, H.T. Mechanism of phosphorylation in chloroplasts. Top. Photosynth. 1982, 4, 105–175. [Google Scholar]
- Hardt, S.L. A measurable consequence of the Mitchell theory of phosphorylation. J. Theor. Biol. 1980, 87, 1–7. [Google Scholar] [CrossRef]
- Kettner, C.; Bertl, A.; Obermyer, G.; Slayman, C.; Bihler, H. Electrophysiological analysis of the yeast V-type proton pump, variable coupling ratio and proton shunt. Biophys. J. 2003, 85, 3730–3738. [Google Scholar] [CrossRef]
- Toei, M.; Gerle, C.; Nakano, M.; Tani, K.; Gyobu, N.; Tamakoshi, M.; Sone, N.; Yoshida, M.; Fujiyoshi, Y.; Mitsuoka, K.; Yokoyama, K. Dodecamer rotor ring defines H+/ATP ratio for ATP synthesis of prokaryotic V-ATPase from Thermus thermophilus. Proc. Nat. Acad. Sci. USA 2007, 104, 20256–20261. [Google Scholar] [CrossRef] [PubMed]
- Tomasek, J.J.; Brusilow, W.S.A. Stoichiometry of energy coupling by proton-translocating ATPases: a history of variability. J. Bioenerg. Biomemb. 2000, 32, 493–500. [Google Scholar] [CrossRef]
- Steigmiller, S.; Turina, P.; Gräber, P. The thermodynamic H+/ATP ratios of the H+-ATP synthases from chloroplasts and Escherichia coli. Proc. Nat. Acad. Sci. USA 2008, 105, 3745–3750. [Google Scholar] [CrossRef] [PubMed]
- Gafni, A.; Boyer, P.D. Modulation of stochiometry of the sarcoplasmic reticulum calcium pump may enhance thermodynamic efficiency. Proc. Nat. Acad. Sci. USA 1985, 82, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Konings, W.N.; Booth, I.R. Do the stoichiometries of ion-linked transport systems vary? Trends Biochem. Sci. 1981, 6, 257–262. [Google Scholar] [CrossRef]
- Feniouk, B.A.; Mulkidjanian, A.Y.; Junge, W. Proton slip in the ATP synthase of Rhodobacter capsulatus: induction, proton conduction, and nucleotide dependence. Biochim. Biophys. Acta 2005, 1706, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Azzone, G.F.; Pozzan, T.; Massari, S.; Bragadin, M. Proton electrochemical gradient and rate of controlled respiration in mitochondria. Biochim. Biophys. Acta 1978, 501, 296–306. [Google Scholar] [CrossRef]
- Azzone, G.F.; Pozzan, T.; Massari, S. Proton electrochemical gradient and phosphate potential in mitochondria. Biochim. Biophys. Acta 1978, 501, 307–316. [Google Scholar] [CrossRef]
- Azzone, G.F.; Pozzan, T.; Massari, S. Proton electrochemical gradient and phosphate potential in submitochondria particles. Biochim. Biophys. Acta 1978, 501, 317–329. [Google Scholar] [CrossRef]
- Boyer, P.D. The ATP synthase—a splendid molecular machine. Annu. Rev. Biochem. 1997, 66, 717–749. [Google Scholar] [CrossRef] [PubMed]
- Boyer, P.D. The binding change mechanism of ATP synthesis. In Membrane Bioenergetics; Lee, C.P., Schatz, G., Enster, L., Eds.; Addison-Wesley: London, UK, 1979; pp. 461–479. [Google Scholar]
- DeMeis, L. Role of water in energy of hydrolysis of phosphate compounds—energy transduction in biological membranes. Biochim. Biophys. Acta 1989, 973, 333–349. [Google Scholar] [CrossRef]
- Muller, A.W.J. The thermosynthesis model for the origin of life and the emergence of regulation by Ca2+. Essays Biochem. 1996, 31, 103–119. [Google Scholar] [PubMed]
- Muller, A.W.J. Finding extraterrestrial organisms living on thermosynthesis. Astrobiology 2003, 3, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Black, S. Pre-cell evolution and the origin of enzymes. Nature 1970, 226, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Copley, S.D. Enzymes with extra talents; moonlighting functions and catalytic promiscuity. Curr. Opin. Chem. Biol. 2003, 7, 265–272. [Google Scholar] [CrossRef]
- Kazlauskas, R.J. Enhancing catalytic promiscuity for biocatalysis. Curr. Opin. Chem. Biol. 2005, 9, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Khersonsky, O.; Roodveldt, C.; Tawfik, D.S. Enzyme promiscuity: evolutionary and mechanistic aspects. Curr. Opinion Chem. Biology 2006, 10, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Hult, K.; Berglund, P. Enzyme promiscuity: mechanism and applications. Trends Biotechnol. 2007, 25, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Jao, S.C.; Huang, L.F.; Tao, Y.S.; Li, W.S. Hydrolysis of organophosphate triesters by Escherichia coli aminopeptidase P. J. Mol. Catal. B-Enzym. 2004, 27, 7–12. [Google Scholar] [CrossRef]
- Villaverde, J.; Cladera, J.; Hartog, A.; Berden, J.; Padros, E.; Dunach, M. Nucleotide and Mg2+ dependency of the thermal denaturation of mitochondrial F1-ATPase. Biophys. J. 1998, 75, 1980–1988. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Freire, E.; McCarthy, R.E. Influence of nucleotide binding site occupancy on the thermal stability of the F1 portion of the chloroplast ATP Synthase. J. Biol. Chem. 1993, 268, 20785–20790. [Google Scholar] [PubMed]
- Hightower, K.E.; McCarty, R.E. Influence of nucleotides on the cold stability of chloroplast coupling factor 1. Biochemistry 1996, 35, 10051–10057. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, A.D. Mitochondrial ATP Synthase: fifteen years later. Biochemistry (Moscow) 1999, 64, 1219–1229. [Google Scholar] [PubMed]
- Sun, F.-J.; Caetano-Anolles, G. The origin and evolution of tRNA inferred from phylogenetic analysis of structure. J. Mol. Evol. 2008, 66, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Cuezva, J.M.; Sánchez-Aragó, M.; Sala, S.; Blanco-Rivero, A.; Ortega, A.D. A message emerging from development: the repression of mitochondrial β-F1-ATPase expression in cancer. J. Bioenerg. Bioembr. 2007, 39, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Corliss, S.B.; Dymond, J.; Gordon, L.I.; Edmond, J.M.; von Herzen, R.P.; Ballard, R.D.; Green, K.; Williams, D.; Bainbridge, A.; Crane, K.; van Andel, T.H. Submarine thermal springs on the Galápagos Rift. Science 1979, 203, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Baross, J.A.; Hoffman, S.E. Submarine hydrothermal vents and associated gradient environments as sites for the origin and evolution of life. Orig. Life 1985, 15, 327–345. [Google Scholar] [CrossRef]
- Corliss, S.B.; Baross, J.A.; Hoffman, S.E. An hypothesis concerning the relationship between submarine hot springs and the origin of life on Earth. Oceanologica Acta 1981, 4 (suppl), 59–69. [Google Scholar]
- Reed, C. Boiling points. Nature 2006, 439, 905–907. [Google Scholar] [CrossRef] [PubMed]
- Bisschoff, J.L.; Rosenbauer, R.J. The critical point of and two-phase boundary of seawater. 200-500 °C. Earth Planet. Sc. Lett. 1984, 68, 172–180. [Google Scholar] [CrossRef]
- Bisschoff, J.L.; Rosenbauer, R.J. Liquid-vapor relations in the critical region of the system NaCl-H2O from 380 to 415 °C: A refined determination of the critical point and the two-phase boundary of seawater. Geochim. Cosmochim. Acta 1988, 52, 2121–2126. [Google Scholar] [CrossRef]
- Miller, S.L.; Bada, J.L. Submarine hot springs and the origin of life. Nature 1988, 334, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.S.; Karson, J.A.; Blackman, D.K.; Früh-green, G.L.; Butterfield, D.A.; Lilley, M.D.; Olson, E.J.; Schrenk, M.O.; Roe, K.K.; Lebon, G.T.; Rivizzigno, P. The AT3-60 ship board party. An off-axis hydrothermal vent field near the Mid-Atlantic Ridge at 30°N. Nature 2001, 412, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Pirajno, F.; van Kranendonk, M.J. Review of hydrothermal processes and systems on Earth and implications for Martian analogues. Aust. J. Earth Sci. 2005, 52, 329–351. [Google Scholar] [CrossRef]
- Bada, J.L.; Lazcano, A. Origin of life, some like it hot—but not the first biomolecules. Science 2002, 296, 1982–1983. [Google Scholar] [CrossRef] [PubMed]
- Woese, C. Bacterial evolution. Microbiol. Rev. 1987, 51, 221–271. [Google Scholar] [PubMed]
- Miller, S.L.; Lazcano, A. The origin of life—did it occur at high temperatures? J. Mol. Evol. 1995, 41, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.; Russell, M.J. On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Phil. Trans. Roy. Soc. Lond. B 2002, 385, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.J.; Arndt, N.T. Geodynamic and metabolic cycles in the Hadean. Biogeosciences 2005, 2, 97–111. [Google Scholar] [CrossRef]
- Russell, M.J.; Hall, A.J. The emergence of life from iron monosulphide bubbles at a submarine hydrothermal redox and pH front. J. Geol. Soc. Lond. 1997, 154, 377–402. [Google Scholar] [CrossRef]
- Vlassov, A.V.; Kazakov, S.A.; Johnston, B.H.; Landweber, L.F. The RNA World on ice: a new scenario for the emergence of RNA information. J. Mol. Evol. 2005, 61, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Bahn, P.R.; Pappelis, A.; Grubbs, R. The role of heat in the origin of life. In Life in the Universe; Seckbach, J., Ed.; Kluwer: Dordrecht, The Netherlands, 2004; pp. 119–120. [Google Scholar]
- Fox, S.W.; Dose, K. Molecular Evolution and the Origin of Life; Marcel Dekker: New York, NY, USA, 1977. [Google Scholar]
- Buford Price, P. Microbial life in glacial ice and implications for a cold origin of life. FEMS Microbiol. Ecol. 2007, 59, 213–231. [Google Scholar]
- Trinks, H.; Schroeder, W.; Biebricher, C.K. Ice and the origin of life. Orig. Life Evol. Biosph. 2005, 35, 429–445. [Google Scholar] [CrossRef] [PubMed]
- Halliday, A.N. Terrestrial accretion rates and the formation of the Moon. Earth Plan. Science Lett. 2000, 176, 17–30. [Google Scholar] [CrossRef]
- Chyba, C.F. The violent environment of the origin of life: progress and uncertainties. Geochim. Cosmochim. Acta 1993, 57, 3351–3358. [Google Scholar] [CrossRef]
- Cohen, B.A.; Swindle, T.D.; King, D.A. Support for the lunar cataclysm hypothesis from lunar meteorite impact melt ages. Science 2000, 290, 1754–1756. [Google Scholar] [CrossRef] [PubMed]
- Ryder, G. Lunar samples, lunar accretion and the end of the early bombardment of the Moon. Eos 1990, 71, 322–323. [Google Scholar] [CrossRef]
- Sleep, N.H.; Zahnle, K.J.; Kasting, J.F.; Morowitz, H.J. Annihilation of ecosystems by large asteroid impacts on the early Earth. Nature 1989, 342, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Horneck, G.; Stöffler, D.; Ott, S.; Hornemann, U.; Cockell, C.S.; Moeller, R.; Meyer, C.; De Vera, J.-P.; Fritz, J.; Schade, S.; Artemieva, A. Microbial inhabitants survive hypervelocity impacts on Mars-like host planets: first phase of lithopanspermia experimentally tested. Astrobiology 2008, 8, 17–43. [Google Scholar] [CrossRef] [PubMed]
- Stöffler, D.; Horneck, G.; Ott, S.; Hornemann, U.; Cockell, C.S.; Moeller, R.; Meyer, C.; De Vera, J.-P.; Fritz, J.; Artemieva, A. Experimental evidence for the potential impact ejection of viable microorganisms from Mars and Mars-like planets. Astrobiology 2008, 8, 585–588. [Google Scholar] [CrossRef]
- Khalil, M.A.K. Non-CO2 greenhouse gases in the atmosphere. Annu. Rev. Energ. Environ. 1999, 24, 645–661. [Google Scholar] [CrossRef]
- Tajika, E. Faint young Sun and the carbon cycle: implications for the Proterozoic global glaciations. Earth Planet. Sci. Lett. 2003, 214, 443–453. [Google Scholar] [CrossRef]
- Gough, D.O. Solar interior structure and luminosity variations. Sol. Phys. 1981, 74, 21–34. [Google Scholar] [CrossRef]
- Bada, J.L.; Bigham, C.; Miller, S.L. Impact melting of frozen oceans on the early Earth: implications for the origin of life. Proc. Nat. Acad. Sci. USA 1994, 91, 1248–1250. [Google Scholar] [CrossRef] [PubMed]
- Sagan, C.; Mullen, G. Earth and Mars: evolution of atmospheres and surface temperatures. Science 1972, 177, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Valley, J.W.; Peck, W.H.; King, E.M.; Wilde, S.A. A cool early Earth. Geology 2002, 30, 351–354. [Google Scholar] [CrossRef]
- Kharecha, P.; Kasting, J.F.; Siefert, J.L. A coupled atmosphere-ecosystem model of the Early Archean Earth. Geobiology 2005, 3, 53–76. [Google Scholar] [CrossRef]
- Thauer, R.K. Biochemistry of methanogenesis: a tribute to Marjorie Stephenson. Microbiology 1998, 144, 2377–2406. [Google Scholar] [CrossRef] [PubMed]
- Kasting, J.F.; Howard, M.T. Atmospheric composition and climate on the early Earth. Phil. Trans. R. Soc. B 2006, 361, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Kasting, J.F.; Ono, S. Palaeoclimates: the first two billion years. Phil. Trans. R. Soc. B 2006, 361, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Young, G.M.; von Brunn, V.; Gold, D.J.C.; Minter, W.E.L. Earth’s oldest reported glaciation; physical and chemical evidence from the Archean Mozaan Group (~2.9 Ga) of South Africa. J. Geol. 1998, 106, 523–538. [Google Scholar] [CrossRef]
- Evans, D.A.; Beukes, N.J.; Kirschvink, J.L. Low-latitude glaciation in the Palaeoproterozoic era. Nature 1997, 386, 262–266. [Google Scholar] [CrossRef]
- Kasting, J.F.; Eggler, D.H.; Raeburn, S.P. Mantle redox evolution and the oxidation state of the Archean atmosphere. J. Geol. 1993, 101, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Schwartzman, D.; Caldeira, K.; Pavlov, A. Cyanobacterial emergence at 2.8 Gya and greenhouse feedbacks. Astrobiology 2008, 8, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Bekker, A.; Holland, H.D.; Wang, P.-L.; Rumble, D.; Stein, H.J.; Hannah, J.L.; Coetzee, L.L.; Beukes, N.J. Dating the rise of atmospheric oxygen. Nature 2004, 427, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Knoll, A.H.; Javaux, E.J.; Hewitt, D.; Cohen, P. Eukaryotic organisms in Proterozoic oceans. Phil. Trans. R. Soc. B 2006, 361, 1023–1038. [Google Scholar] [CrossRef] [PubMed]
- Huston, D.L.; Logan, G.A. Barite, BIFs and bugs, evidence for the evolution of the Earth’s early atmosphere. Earth Planet. Sci. Lett. 2004, 220, 41–55. [Google Scholar] [CrossRef]
- Slack, J.F.; Grenne, T.; Bekker, A.; Rouxel, O.J.; Lindberg, P.A. Suboxic deep seawater in the late Paleoproterozoic: evidence from hematitic chert and iron formation related to seafloor-hydrothermal sulfide deposits, central Arizona, USA. Earth Planet. Sci. Lett. 2007, 255, 243–256. [Google Scholar] [CrossRef]
- Armstrong, J.C.; Wells, L.E.; Gonzales, G. Rummaging through Earth’s attic for remains of ancient life. Icarus 2002, 160, 183–196. [Google Scholar] [CrossRef]
- Vekshin, N.L. Thermal-model of coupling ATP synthesis with electron-transfer. Biofizika 1991, 36, 994–999. [Google Scholar]
- Matsuno, K. Physics underlying the formation of protocells. J. Biol. Phys. 1995, 20, 117–121. [Google Scholar] [CrossRef]
- Matsuno, K. A possible prebiotic heat engine. Orig. Life Evol. Biosph. 1996, 26, 458. [Google Scholar]
- Imai, E.; Honda, H.; Brack, A.; Matsuno, K. Elongation of oligopeptides in a simulated submarine hydrothermal system. Science 2005, 283, 831–833. [Google Scholar] [CrossRef]
- Kompanichenko, V.; Frisman, E.; Fishman, B.; Savenkova, E.; Shlufman, K. Exploration of thermodynamic fluctuations in the probable hydrothermal medium for the origin of life (on the example of Mutnovsky hydrothermal system in Kamchatka). Int. J. Astrobiology 2008, 6, 74. [Google Scholar]
- Purcell, E.M. Life at low Reynolds number. Am. J. Phys. 1977, 45, 3–11. [Google Scholar] [CrossRef]
- Richardson, S.M. Fluid Mechanics; Hemisphere Publishing: New York, NY, USA, 1989; p. 202. [Google Scholar]
- Howard, J. Mechanics of Motor Proteins and the Cytoskeleton; Sinauer: Sunderland, MA, USA, 2001. [Google Scholar]
- Friedel, M.; Baumketner, A.; Shea, J.-E. Effects of surface tethering on protein folding mechanisms. Proc. Natl Acad. Sci. USA 2006, 103, 8396–8401. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H.A. Brownian motion in a field of force and the diffusion model of chemical reactions. Physica 1940, 7, 284–304. [Google Scholar] [CrossRef]
- Frauenfelder, H.; Fenimore, P.W.; Chen, G.; McMahon, B.H. Protein folding is slaved to solvent motions. Proc. Nat. Acad. Sci. USA 2006, 103, 15469–15472. [Google Scholar] [CrossRef] [PubMed]
- Doebeli, M.; Dieckmann, U. Speciation along environmental gradients. Nature 2003, 421, 259–264. [Google Scholar] [CrossRef] [PubMed]
- White, S.H.; VonHeijne, G. How translocons select transmembrane helices. Annu. Rev. Biophys. 2008, 37, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, G.R. The type III secretion injectisome. Nat. Rev. Microbiol. 2006, 4, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Galan, J.E.; Wolf-Watz, H. Protein delivery into eukaryotic cells by type III secretion machineries. Nature 2006, 444, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Holland, I.B. Translocation of bacterial proteins—an overview. Biochim. Biophys. Acta 2004, 1694, 5–16. [Google Scholar] [CrossRef] [PubMed]
- MacNab, R.M. Type III flagellar protein export and flagellar assembly. Biochim. Biophys. Acta 2004, 1694, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Wickner, W.; Schekman, R. Protein translocation across biological membranes. Science 2005, 310, 1452–1456. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, I.Z.; Oplatka, A.; Katchalsky, A. Mechanochemical engines. Nature 1966, 210, 568–571. [Google Scholar] [CrossRef]
- Hofmann, B.A.; Farmer, J.D.; Von Blanckenburg, F.; Fallick, A.E. Subsurface filamentous fabrics: an evaluation of origins based on morphological and geochemical criteria, with implications for exopaleontology. Astrobiology 2008, 8, 87–117. [Google Scholar] [CrossRef] [PubMed]
- Reysenbach, A.-L.; Cady, S.L. Microbiology of ancient and modern hydrothermal systems. Trends Microbiol. 2001, 9, 79–86. [Google Scholar] [CrossRef]
- Schopf, J.W. Fossil evidence of Archaean life. Phil. Trans. R. Soc. B 2006, 361, 869–885. [Google Scholar] [CrossRef] [PubMed]
- Santelli, C.M.; Orcutt, B.N.; Banning, E.; Bach, W.; Moyer, C.L.; Sogin, M.L.; Staudigel, H.; Edwards, K.J. Abundance and diversity of microbial life in ocean crust. Nature 2008, 453, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.A. Deep-ocean life where oxygen is scarce. Am. Scientist 2002, 90, 436–444. [Google Scholar] [CrossRef]
- Horita, J. Some perspectives on isotope biosignatures for early life. Chem. Geol. 2005, 218, 171–186. [Google Scholar] [CrossRef]
- Schulz, H.N.; Jørgensen, B.B. Big bacteria. Annu. Rev. Microbiol. 2001, 55, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Jannasch, H.W.; Wirsen, C.O. Morphological survey of microbial mats near deep-sea thermal vents. Appl. Environm. Microbiol. 1981, 41, 528–538. [Google Scholar]
- Kalanetra, K.M.; Huston, S.L.; Nelson, D.C. Novel, attached, sulphur-oxidizing bacteria at shallow hydrothermal vents possess vacuoles not involved in respiratory nitrate accumulation. Appl. Environm. Microbiol. 2004, 70, 7487–7496. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.C.; Wirson, C.O.; Jannasch, H.W. Characterization of large, autotrophic Beggiatoa spp. abundant at hydrothermal vents of the Guaymas Basin. Appl. Environm. Microbiol. 1989, 55, 2909–2917. [Google Scholar]
- Moriyama, Y.; Hiyama, A.; Asai, H. High-speed video cinematographic demonstration of stalk and zooid contraction of Vorticella convallaria. Biophys. J. 1998, 74, 487–491. [Google Scholar] [CrossRef]
- De Groot, S.R.; Mazur, P. Non-equilibrium Thermodynamics; North-Holland Publishing: Amsterdam, The Netherlands, 1962. [Google Scholar]
- Haase, R. Thermodynamik der irreversiblen Prozesse; Dietrich Steinkopf: Darmstadt, Germany, 1963. [Google Scholar]
- Baker, J.E. Free energy transduction in a chemical motor model. J. Theor. Biol. 2004, 228, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.S.; Koeppe, R.E. Bilayer thickness and protein function: an energetic perspective. Annu. Rev. Biophys. Mol. Struct. 2007, 36, 107–130. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.J. The alkaline solution to the emergence of life: energy, entropy and early evolution. Acta Biotheor. 2007, 55, 133–179. [Google Scholar] [CrossRef] [PubMed]
- Jagendorf, A.T.; Uribe, E. ATP formation caused by acid-base transition of spinach chloroplasts. Proc. Nat. Acad. Sci. USA 1966, 55, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.D.; Finney, W.H. The effect of acids and their salts on heat contraction of rat tendon. Q. J. Exp. Physiol. 1930, 20, 115–123. [Google Scholar] [CrossRef]
- Angevine, C.M.; Herold, K.A.G.; Fillingame, R.H. Aqueous access pathways in subunit a of rotary ATP synthase extend to both sides of the membrane. Proc. Nat. Acad. Sci. USA 2003, 100, 13179–13183. [Google Scholar] [CrossRef] [PubMed]
- Steed, P.R.; Fillingame, R.H. Subunit a facilitates aqueous access to a membrane-embedded region of subunit c in Escherichia coli F1Fo ATP Synthase. J. Biol. Chem. 2008, 283, 12365–12372. [Google Scholar] [CrossRef] [PubMed]
- Vincent, O.D.; Schwem, B.E.; Steed, P.R.; Fillingame, R.H. Fluidity of structure and swiveling of helices in the subunit c ring of Escherichia coli ATP Synthase as revealed by cysteine-cysteine cross linking. J. Biol. Chem. 2007, 282, 33788–33794. [Google Scholar] [CrossRef] [PubMed]
- Vorburger, T.; Ebneter, J.Z.; Wiedenmann, A.; Morger, D.; Weber, G.; Diederichs, K.; Dimroth, P.; von Ballmoos, C. Arginine-induced conformational change in the c-ring/a-subunit interface of ATP Synthase. FEBS J. 2008, 275, 2137–2150. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, F.S.; Bencivenga, U.; Canciglia, P.; Rossi, S.; Mita, D.G. Temperature gradients and prebiological evolution. Cell Biochem. Biophys. 1987, 10, 103–125. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.; Simon, M. Flagellar rotation and the mechanism of bacterial motility. Nature 1974, 249, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Neidhart, F.C. Chemical composition of Escherichia coli. In Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology; Neidhart, F.C., Ingraham, J.L., Low, K.B., Magasanik, B., Schaechter, M., Umbarger, H.E., Eds.; American Society for Microbiology: Washington, DC, USA, 1987; pp. 3–6. [Google Scholar]
- Chng, C.-P.; Kitao, A. Thermal unfolding simulations of bacterial flagellin: insight into its refolding before assembly. Biophys. J. 2008, 94, 3858–3871. [Google Scholar] [CrossRef] [PubMed]
- MacNab, R.M. How bacteria assemble flagella. Annu. Rev. Microbiol. 2003, 57, 77–100. [Google Scholar] [CrossRef] [PubMed]
- Imada, K.; Minamino, T.; Tahara, A.; Namba, K. Structural similarity between the flagellar type III ATPase FliI and F1-ATPase subunits. Proc. Nat. Acad. Sci. USA 2007, 104, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.H.; Adler, J.; Gargus, J.J.; Hogg, R.W. Chemomechanical coupling without ATP: the source of energy for motility and chemotaxis in bacteria. Proc. Nat. Acad. Sci. USA 1974, 71, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- MacNab, R.M. An entropy-driven engine—the bacterial flagellar motor. In Biological Structures and Coupled Flows; Oplatka, A., Balaban, M., Eds.; Academic Press: New York, NY, USA, 1979; pp. 147–160. [Google Scholar]
- Webre, D.J.; Wolanin, P.M.; Stock, J.B. Bacterial chemotaxis. Curr. Biol. 2003, 13, R47–R49. [Google Scholar] [CrossRef]
- Brown, P.N.; Terrazas, M.; Paul, K.; Blair, D.F. Mutational analysis of the flagellar protein FliG: sites of interaction with FliM and implications for organization of the switch complex. J. Bacteriol. 2007, 189, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Ben-Lulu, G.N.; Francis, N.R.; Shimoni, E.; Noy, D.; Davidov, Y.; Prasad, K.; Sagi, Y.; Cecchini, G.; Johnston, R.M.; Eisenbach, M. The bacterial flagellar switch complex is getting more complex. EMBO J. 2008, 27, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Mashimo, T.; Hashimoto, M.; Yamaguchi, S.; Aizawa, S.-I. Temperature-hypersensitive sites of the flagellar switch component FliG in Salmonella enterica Serovar Typhimurium. J. Bacteriol. 2007, 189, 5153–5160. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Blair, D.F. Conformational change in the stator of the bacterial flagellar motor. Biochemistry 2001, 40, 13041–13050. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, R. Helix rotation of the flagellar rotary motor. Biophys. J. 2003, 85, 843–849. [Google Scholar] [CrossRef]
- Metzner, P. Die Bewegung und Reizbeantwortung der bipolar begeiβelten Spirillen. Jahrbücher f. Wissensch. Botanik 1920, 59, 325–412. [Google Scholar]
- Paster, E.; Ryu, W.S. The thermal impulse response of Escherichia coli. Proc. Nat. Acad. Sci. USA 2008, 105, 5373–5377. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Ochman, H. Stepwise formation of the bacterial flagellar system. Proc. Nat. Acad. Sci. USA 2007, 104, 7116–7121. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, E.; Kamiya, R.; Asakura, S. Thermal transitions in helical forms of Salmonella flagella. J. Mol. Biol. 1982, 160, 609–621. [Google Scholar] [CrossRef]
- Turner, L.; Caplan, S.R.; Berg, H.C. Temperature-induced switching of the bacterial flagellar motor. Biophys. J. 1996, 71, 2227–2233. [Google Scholar] [CrossRef]
- Wang, G.M.; Sevick, E.M.; Mittag, E.; Searles, D.J.; Evans, D.J. Experimental demonstrations of violations of the second law of thermodynamics for small systems and short time scales. Phys. Rev. Lett. 2002, 89, 0500601. [Google Scholar] [CrossRef]
- Ritort, F. Work fluctuations, transient fluctuations of the second law and free-energy recovery methods. Poincaré Seminar 2003, 2, 195–229. [Google Scholar]
- Filliger, R.; Reimann, P. Brownian gyrator, a minimal heat engine on the nanoscale. Phys. Rev. Lett. 2007, 99, 230602. [Google Scholar] [CrossRef] [PubMed]
- Chapman, V.J. The Algae; MacMillan: London, UK, 1962. [Google Scholar]
- Pringsheim, E.G. Farblose Algen. Ein Beitrag zur Evolutionsforschung; Gustav Fischer: Stuttgart, Germany, 1963. [Google Scholar]
- Seilacher, A. Biomat-related life styles in the Precambrian. Palaios 1999, 14, 86–93. [Google Scholar] [CrossRef]
- Schulze-Makuch, D.; Irwin, L.N. Energy cycling and hypothetical organisms in Europa’s ocean. Astrobiology 2002, 2, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Beynon, R.P.; Bahl, V.K.; Prendergast, B.D. Infective endocarditis. Br. Med. J. 2006, 333, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, C.B. Comparative physiology of suspension feeding. Annu. Rev. Physiol. 1975, 37, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, F.D. Systems and methods for tethered wind turbines. U.S.Patent 2005. [Google Scholar]
- Reimann, P.; Hänggi, P. Introduction to the physics of Brownian motors. Appl. Phys. A 2002, 75, 169–178. [Google Scholar] [CrossRef]
- Bao, J.-D. Directed current of Brownian ratchet randomly circulating between two thermal sources. Physica A 1999, 273, 286–293. [Google Scholar] [CrossRef]
- Li, Y.-X. Transport generated by fluctuating temperature. Physica A 1997, 238, 245–251. [Google Scholar] [CrossRef]
- Reimann, P.; Bartussek, R.; Häuβler, R.; Hänggi, P. Brownian motors driven by temperature oscillations. Phys. Lett. A 1996, 215, 26–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J. Investigation on a temporal asymmetric oscillation temperature ratchet. Physica A 2008, 387, 3443–3448. [Google Scholar] [CrossRef]
- Bennett, C.H. The thermodynamics of computation—a review. Int. J. Theor. Phys. 1982, 21, 905–940. [Google Scholar] [CrossRef]
- Bennett, C.H. Logical reversibility of computation. IBM J. Res. Dev. 1973, 6, 525–532. [Google Scholar] [CrossRef]
- Benioff, P.A. Quantum mechanical models of Turing Machines that dissipate no energy. Phys. Rev. Lett. 1982, 48, 1581–1585. [Google Scholar] [CrossRef]
- Headrick, M.V. Clock and Watch Escapement Mechanics. Available online: http: //www.abbeyclock.com/escpdf.html 1997 (accessed September 16, 2009).
- Huxley, A.F. Reflexions on Muscle; Princeton University Press: Princeton, MA, USA, 1981. [Google Scholar]
- Lowry, D.S.; Frasch, W.D. Interactions between βD372 and γ subunit N-terminal residues γK9 and γS12 are important to catalytic activity catalyzed by Escherichia coli F1Fo-ATP Synthase. Biochemistry 2005, 44, 7275–7281. [Google Scholar] [CrossRef] [PubMed]
- Schilstra, M.J.; Martin, S.R. An elastically tethered viscous load imposes a regular gait on the motion of myosin-V. Simulation of the effect of transient force relaxation on a stochastic process. J. Royal Soc. Interface 2006, 3, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Petzold, C. The Annotated Turing: a Guided Tour through Alan Turing’s Historic Paper on Computability and the Turing Machine; Wiley: Indianapolis, IN, USA, 2008. [Google Scholar]
- Chaitin, G.J. Thinking about Gödel and Turing; World Scientific: Singapore, 2007. [Google Scholar]
- Kleene, S.C. Mathematical Logic; Wiley: New York, NY, USA, 1967. [Google Scholar]
- Perlis, A.J. Epigrams on Programming. SIGPLAN Notices 1982, 17, 7–13. [Google Scholar] [CrossRef]
- Stepney, S. The neglected pillar of material computation. Physica D 2008, 237, 1157–1164. [Google Scholar] [CrossRef]
- Bennett, C.H. Dissipation-error tradeoff in proofreading. BioSystems 1979, 11, 85–91. [Google Scholar] [CrossRef]
- Von Neumann, J. Probabilistic logics and the synthesis of reliable organisms from unreliable computers. In Automata Studies; Shannon, C.E., McCarthy, J., Eds.; Princeton University Press: Princeton, MA, USA, 1956; pp. 43–98. [Google Scholar]
- Danchin, A. The Delphic Boat: What Genomes Tell Us; Harvard University Press: Cambridge, MA, USA, 1998. [Google Scholar]
- Berg, H.C. Touring machines. Curr. Biol. 1996, 6, 624. [Google Scholar] [CrossRef]
- Tomizaki, K.-Y.; Mihara, H. Phosphate-mediated molecular memory driven by two different protein kinases as information input elements. J. Am. Chem. Soc. 2007, 129, 8345–8352. [Google Scholar] [CrossRef] [PubMed]
- Teuscher, C.; Nemenman, I.; Alexander, F.J. Novel computing paradigms: Quo vadis? Physica D 2008, 237, v–viii. [Google Scholar] [CrossRef]
- Ben-Jacob, E. Bacterial wisdom. Physica A 1998, 249, 553–557. [Google Scholar] [CrossRef]
- Ben Jacob, E. Seeking the foundations of cognition in bacteria: from Schrödinger’s negative entropy to latent information. Physica A 2006, 359, 495–524. [Google Scholar] [CrossRef]
- Hellingwerf, K.J. Bacterial observations: a rudimentary form of intelligence. Trends Microbiol. 2005, 13, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Casadesús, J.; D’Ari, R. Memory in bacteria and phage. BioEssays 2002, 24, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.M.; Fontaine-Bodin, L.; Bischofs, I.; Price, G.; Keasling, J.; Arkin, A.P. Memory in microbes: quantifying history-dependent behaviour in a bacterium. PLoS ONE 2006, 3, 1–114. [Google Scholar] [CrossRef] [PubMed]
- Vellai, T.; Vida, G. The origin of eukaryotes: the difference between prokaryotic and eukaryotic cells. Proc. Roy. Soc. Lond. B 1999, 266, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Margulis, L. Origin of Eukaryotic Cells; Yale University Press: New Haven, CT, USA, 1970. [Google Scholar]
- Margulis, L. Symbiosis in Cell Evolution; Freeman: San Francisco, CA, USA, 1981. [Google Scholar]
- Mitchell, D.R.; Nakatsugawa, M. Bend propagation drives central pair rotation in Chlamydomonas reinhardtii flagella. J. Cell Biol. 2004, 166, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Omoto, C.K.; Kung, C. Rotation and twist of the central-pair microtubules in the cilia of Paramecium. J. Cell Biol. 1980, 87, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.; Müller, M. The hydrogen hypothesis for the first eukaryote. Nature 1998, 392, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.G.; Karlberg, O.; Canback, B.; Kurland, C.G. On the origin of mitochondria: a genomics perspective. Phil. Trans. Roy. Soc. B 2003, 358, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Cavalier-Smith, T. Origin of mitochondria by intracellular enslavement of a photosynthetic purple bacterium. Proc. Roy. Soc. B 2006, 273, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Brennen, C.; Winet, H. Fluid mechanics of propulsion by cilia and flagella. Annu. Rev. Fluid Mech. 1977, 9, 339–398. [Google Scholar] [CrossRef]
- Adleman, L.M. Molecular computation of solution to combinatorial problems. Science 1994, 266, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- Benenson, Y.; Paz-Elizur, T.; Adar, R.; Keinan, E.; Livneh, Z.; Shapiro, E. Programmable and autonomous computing machine made of biomolecules. Nature 2001, 414, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Faulhammer, D.; Cukras, A.R.; Lipton, R.J.; Landweber, L.F. Molecular computation: RNA solutions to chess problems. Proc. Nat. Acad. Sci. USA 2000, 97, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Bray, D. Protein molecules as computational elements in living cells. Nature 1995, 376, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Unger, R.; Moult, J. Towards computing with proteins. Proteins 2006, 63, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, D.R.; Moritz, M.; Albertz, B.M. The centrosome and cellular organization. Annu. Rev. Biochem. 1994, 63, 639–674. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, S.; Kamiya, R. Cyclical interactions between two outer doublet microtubules in split flagellar axonemes. Biophys. J. 2005, 89, 3261–3268. [Google Scholar] [CrossRef] [PubMed]
- Shingyoyi, C.; Higuchi, H.; Yoshimura, M.; Katayama, E.; Yanagida, T. Dynein arms are oscillatory force generators. Nature 1998, 393, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Snell, W. The primary cilium: keeper of the key to cell division. Cell 2007, 129, 1255–1257. [Google Scholar] [CrossRef] [PubMed]
- Pazour, G.J.; Witman, G.B. The vertebrate primary cilium is a sensory organelle. Curr. Opin. Cell Biol. 2003, 15, 105–110. [Google Scholar] [CrossRef]
- Quarmby, L.M.; Parker, J.D.K. Cilia and the cell cycle? J. Cell Biol. 2005, 169, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Mans, D.A.; Voest, E.E.; Giles, R.H. All along the watchtower: Is the cilium a tumor suppressor organelle? Biochim. Biophys. Acta 2008, 1786, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Jekely, G.; Arendt, D. Evolution of intraflagellar transport from coated vesicles and autogenous origin of the eukaryotic cilium. BioEssays 2006, 28, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Craddock, T.J.A.; Tuszynski, J.A. On the role of the microtubule in cognitive brain functions. NeuroQuantology 2007, 5, 32–57. [Google Scholar] [CrossRef]
- Hameroff, S.R.; Watt, R.C. Information processing in microtubules. J. Theor. Biol. 1982, 98, 549–561. [Google Scholar] [CrossRef]
- Hameroff, S. Quantum computation in brain microtubules? The Penrose-Hameroff ‘Orch OR’ model of consciousness. Phil. Trans. Roy. Soc. Lond. A 1998, 356, 1869–1896. [Google Scholar]
- Tuszynski, J.A.; Hameroff, S.; Sataric, M.V.; Trpisova, B.; Nip, M.L.A. Ferroelectric behaviour in microtubule dipole lattices: implications for information processing, signalling and assembly/disassembly. J. Theor. Biol. 1995, 174, 371–380. [Google Scholar] [CrossRef]
- Agar, J.N. Thermogalvanic cells. Adv. Electrochem. Electroeng. 1963, 3, 31–121. [Google Scholar]
- Han, T.M.; Runnegar, B. Megascopic algae from the 2.1 billion-year-old Negaunee Iron Formation, Michigan. Science 1992, 257, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.A. The Matter of Life. Philosophical Problems of Biology; Yale University Press: New Haven, CT, USA, 1971; pp. 151, 152, 161-162, 167. [Google Scholar]
- Gould, S.J. Is a new and general theory of evolution emerging? Paleobiology 1980, 6, 116–130. [Google Scholar]
- Gould, S.J. Tempo and mode in the macroevolutionary reconstruction of Darwinism. Proc. Nat. Acad. Sci. USA 1994, 91, 9413–9417. [Google Scholar] [CrossRef]
- Gould, S.J.; Eldredge, N. Punctuated equilibrium comes of age. Nature 1993, 366, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Stanley, S.M. Macroevolution: Process and Product; Freeman: San Francisco, CA, USA, 1979. [Google Scholar]
- Boyle, R.A.; Lenton, T.M. Fluctuation in the physical environment as a mechanism for reinforcing evolutionary transitions. J. Theor. Biol. 2006, 242, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Kashtan, N.; Noor, E.; Alon, U. Varying environments can speed up evolution. Proc. Nat. Acad. Sci. USA 2007, 104, 13711–13716. [Google Scholar] [CrossRef] [PubMed]
- Martin, W. Archaebacteria (Archaea) and the origin of the eukaryotic nucleus. Curr. Opin. Microbiol. 2005, 8, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Popper, C.R. Conjectures and Refutations: the Growth of Scientific Knowledge; Routledge: London, UK, 1963. [Google Scholar]
- Cockell, C.S.; Westall, F. A postulate to assess ‘habitability’. Int. J. Astrobiol. 2004, 3, 157–163. [Google Scholar] [CrossRef]
- 2This protein with its long evolutionary history is still of importance: during cancer it is underexpressed [98].
- 3Ancient organisms ‘put on ice’ in meteorites of terrestrial origin, either in orbit around the Sun or deposited in repositories on the Moon [144] (especial near the poles in crater interiors shaded from the Sun) or other celestial objects, could allow the testing of the models presented here. This refutes the objection against these models that they are in principle untestable.
- 4Biological use of a thermal gradient across a biomembrane has previously been proposed [186], but is considered less plausible. The temperature difference across a biomembrane can only be small, given its thickness of a few nm. As the difference between the thermal conductivities of a lipid membrane and water are expected to be small, a temperature gradient will not become concentrated in the membrane; in contrast, the high electrical conductivity of water does cause electric fields to be concentrated across the membrane.
- 5Except for its much smaller size, the structure would resemble a wind turbine hanging under a balloon [214].
- 7In a clock an escapement converts a rotary motion of the rotor (driven by a spring or weight) into a back-and-forth motion [223].
- 8Berg [235] has drawn attention to the similarity of tumbling in bacteria and human thinking:Is a similar strategy fundamental to the operation of our brains? Does the sort of irritability that enables E. coli to change directions enable us to change our minds? Is there a secret here, learned early in evolution, that helps us think?
- 10A progenitor of goose-flesh?
- 11In a transformed (tumour) cell the cellular computer could contain a changed program that results in a differently executed cell cycle: in that case cancer would be associated with a software bug. The difference between the normal and the transformed cell would then be very small, and hard to exploit by chemotherapy, as observed.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Muller, A.W.J. Emergence of Animals from Heat Engines – Part 1. Before the Snowball Earths. Entropy 2009, 11, 463-512. https://doi.org/10.3390/e11030463
Muller AWJ. Emergence of Animals from Heat Engines – Part 1. Before the Snowball Earths. Entropy. 2009; 11(3):463-512. https://doi.org/10.3390/e11030463
Chicago/Turabian StyleMuller, Anthonie W. J. 2009. "Emergence of Animals from Heat Engines – Part 1. Before the Snowball Earths" Entropy 11, no. 3: 463-512. https://doi.org/10.3390/e11030463
APA StyleMuller, A. W. J. (2009). Emergence of Animals from Heat Engines – Part 1. Before the Snowball Earths. Entropy, 11(3), 463-512. https://doi.org/10.3390/e11030463