Phototransformation of Three Psychoactive Drugs in Presence of Sedimental Water Extractable Organic Matter
Abstract
:1. Introduction
2. Results and Discussion
2.1. Main Characteristics of the Sediments
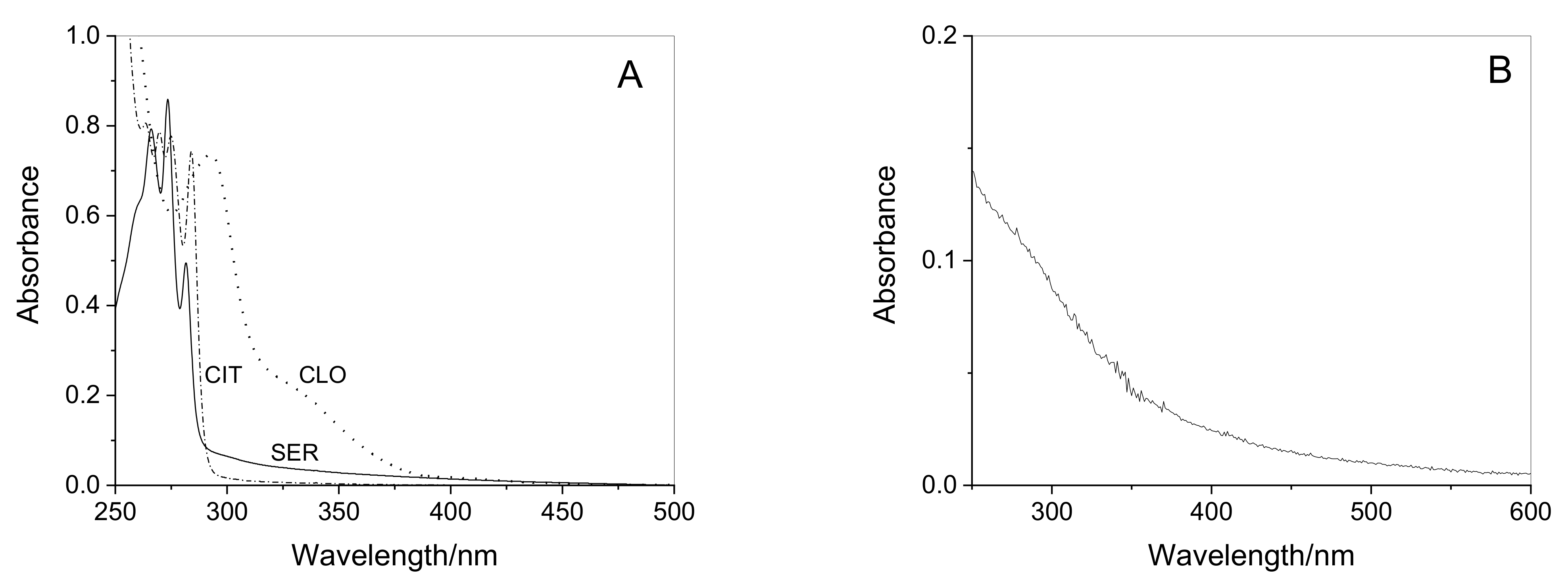
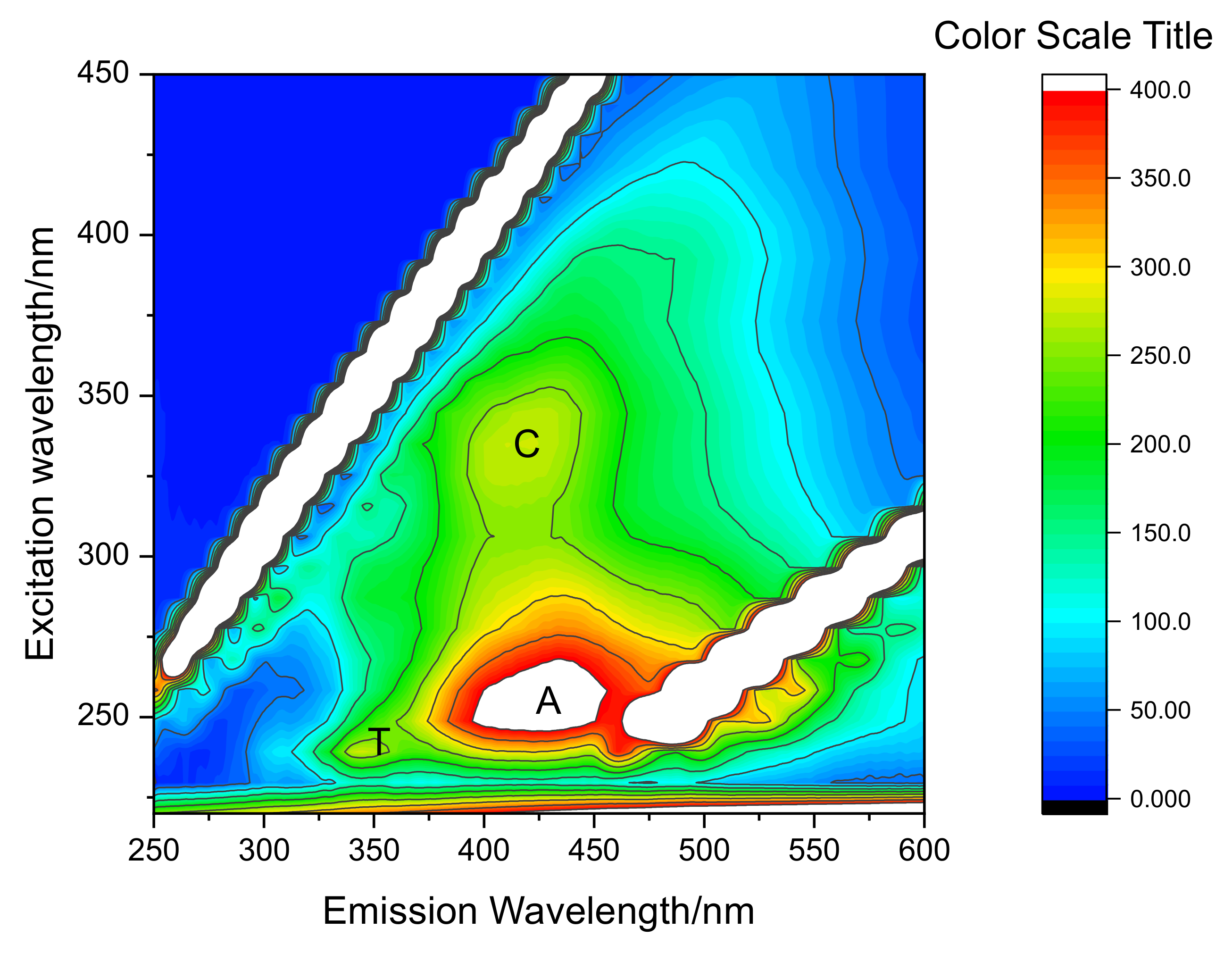
2.2. Extraction and Characterization of WEOM from Sediments
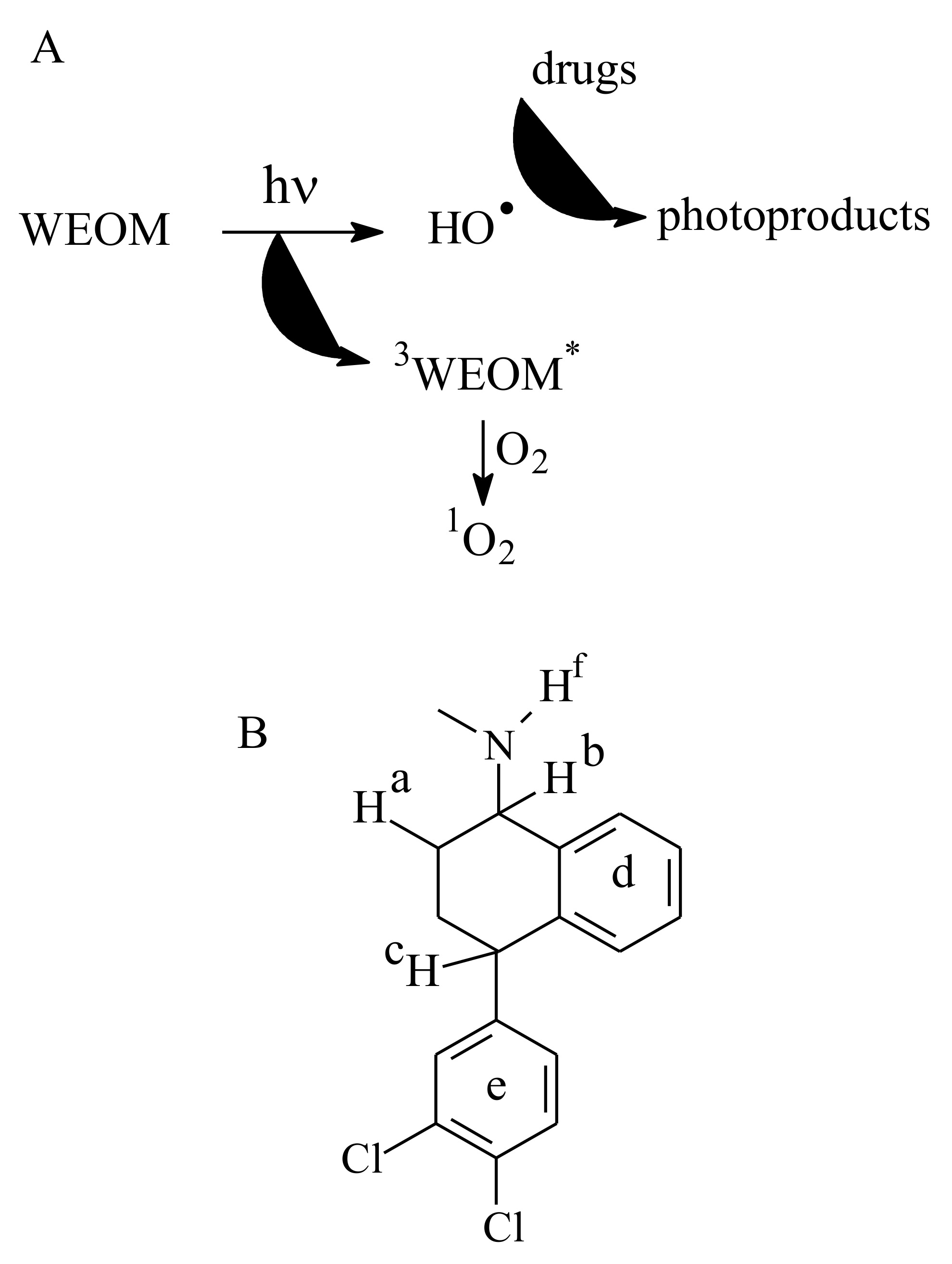
2.3. Photoproduction of Reactive Species upon WEOM Irradiation
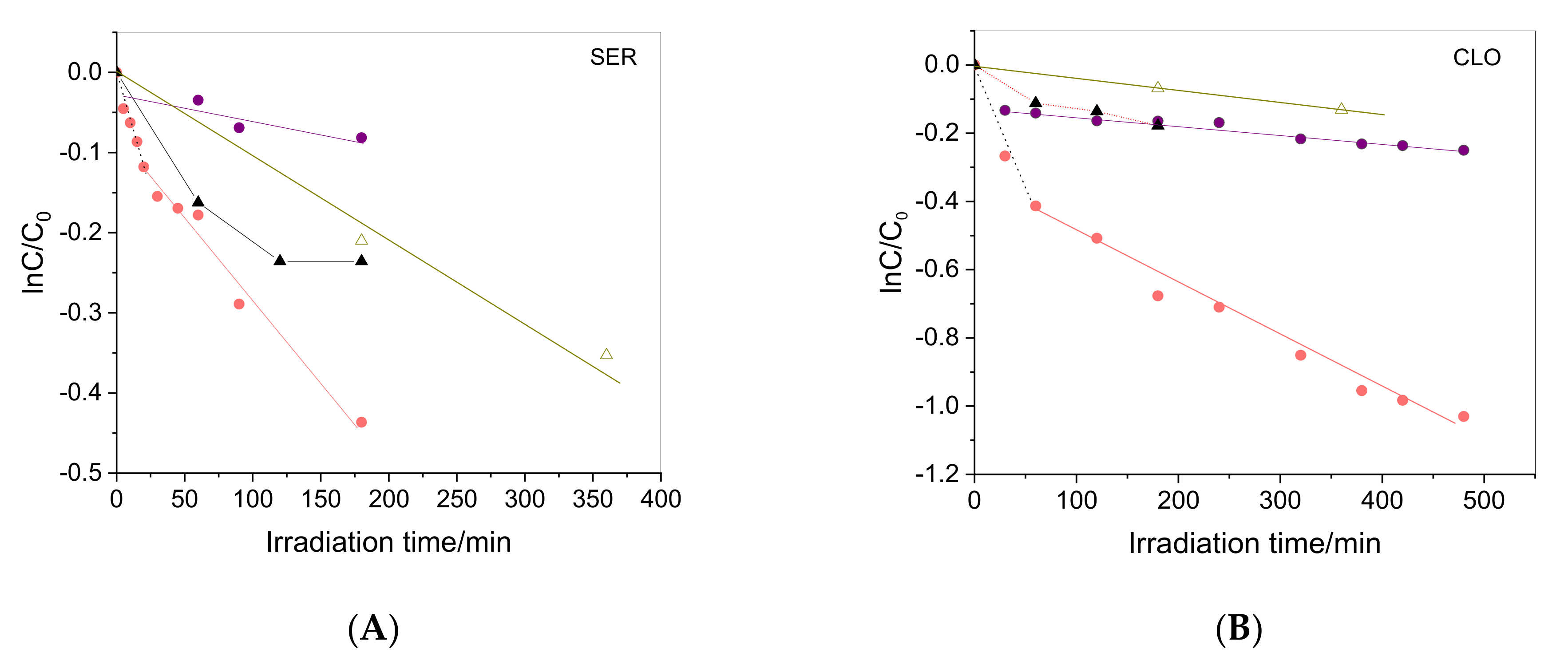
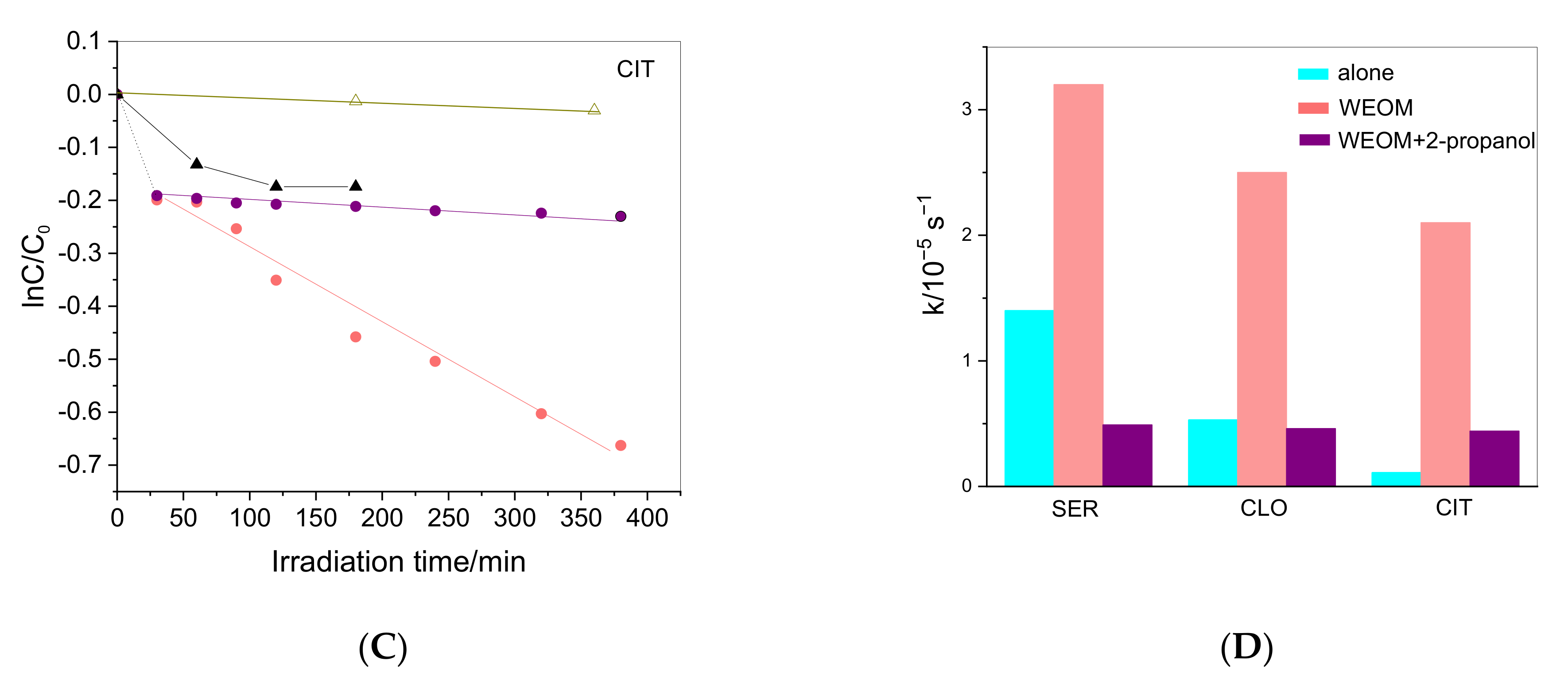
2.4. Irradiation of Drugs in the Presence of WEOM
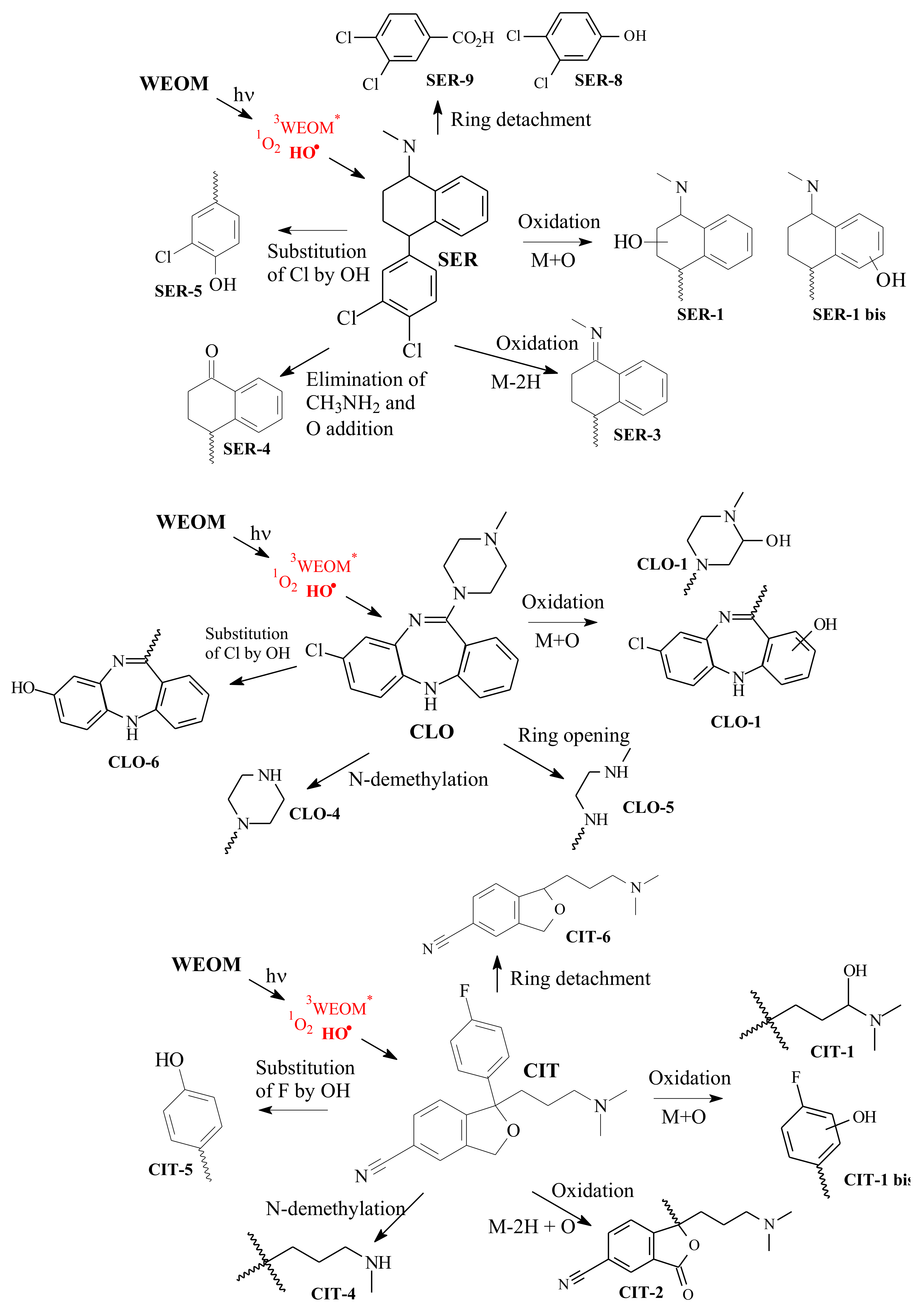
2.5. Drugs Photoproducts
2.6. Prediction of Ecotoxicity Assessment
3. Experimental
3.1. Sediment Sampling and Analysis
3.2. Chemicals and Preparation of Solutions
3.3. Water Extractable Organic Matter Extraction
3.4. DOC Analyses
3.5. Optical Analyses
3.6. Irradiations
3.7. HPLC Analyses
3.8. Ecotoxicity Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Calisto, V.; Esteves, V. Psychiatric pharmaceuticals in the environment. Chemosphere 2009, 77, 1257–1274. [Google Scholar] [CrossRef] [PubMed]
- Skibiński, R.; Trawiński, J.; Komsta, Ł.; Bajda, K. Characterization of forced degradation products of clozapine by LC-DAD/ESI-Q-TOF. J. Pharm. Biomed. Anal. 2016, 131, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Trawiński, J.; Skibiński, R. Studies on photodegradation process of psychotropic drugs: A review. Environ. Sci. Pollut. Res. 2017, 24, 1152–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trawiński, J.; Skibiński, R. Rapid degradation of clozapine by heterogeneous photocatalysis. Comparison with direct photolysis, kinetics, identification of transformation products and scavenger study. Sci. Total. Environ. 2019, 665, 557–567. [Google Scholar] [CrossRef]
- Delorenzo, M.; Fleming, J. Individual and mixture effects of selected pharmaceuticals and personal care products on the marine phytoplankton species Dunaliella tertiolecta. Arch. Environ. Contam. Toxicol. 2008, 54, 203–210. [Google Scholar] [CrossRef]
- Dietrich, S.; Ploessl, F.; Bracher, F.; Laforsch, C. Single and combined toxicity of pharmaceuticals at environmentally relevan concentrations in Daphnia magna—A multigenerational study. Chemosphere. 2010, 79, 60–66. [Google Scholar] [CrossRef]
- Godoy, A.; de Oliveira, A.; Silva, J.; Azevedo, C.; Domingues, I.; Nogueira, A.; Kummrow, F. Single and mixture toxicity of four pharmaceuticals of environmental concern to aquatic organisms, including a behavioral assessment. Chemosphere 2019, 235, 373–382. [Google Scholar] [CrossRef]
- Hossain, M.; Kubec, J.; Guo, W.; Roje, S.; Ložek, F.; Grabicová, K.; Randák, T.; Kouba, A.; Buřič, M. A combination of six psychoactive pharmaceuticals at environmental concentrations alter the locomotory behaviour of clonal marbled crayfish. Sci. Total. Environ. 2021, 751, 141383. [Google Scholar] [CrossRef]
- Gonzales-Rey, M.; Mattos, J.; Piazza, C.; Bainy, A.; Bebianno, M. Effects of active pharmaceutical ingredients mixtures in mussel Mytilus galloprovincialis. Aquat. Toxicol. 2014, 153, 12–26. [Google Scholar] [CrossRef]
- Franzellitti, S.; Buratti, S.; Bowen, D.; Haddad, S.; Chambliss, C.; Brooks, B. A multibiomarker approach to explore interactive effects of propranolol and fluoxetine in marine mussels. Environ. Pollut. 2015, 205, 60–69. [Google Scholar] [CrossRef]
- Ding, J.; Lu, G.; Li, Y. Interactive effects of selected pharmaceutical mixtures on bioaccumulation and biochemical status in crucian carp (Carassius auratus). Chemosphere 2016, 148, 21–31. [Google Scholar] [CrossRef]
- Villain, J.; Minguez, L.; Halm-Lemeille, M.; Durrieu, G.; Bureau, R. Acute toxicities of pharmaceuticals toward green algae. mode of action, biopharmaceutical drug disposition classification system and quantile regression models. Ecotoxicol. Environ. Saf. 2016, 124, 337–343. [Google Scholar] [CrossRef]
- Calza, P.; Jiménez-Holgado, C.; Coha, M.; Chrimatopoulos, C.; dal Bello, F.; Medana, C.; Sakkas, V. Study of the Photoinduced Transformations of Sertraline in Aqueous Media. Sci. Tot. Environ. 2021, 756, 143805. [Google Scholar] [CrossRef]
- Kwon, J.W.; Armbrust, K.L. Degradation of citalopram by simulated sunlight. Environ. Toxicol. Chem. 2005, 24, 1618–1623. [Google Scholar] [CrossRef]
- Gornik, T.; Vozic, A.; Heath, E.; Trontelj, J.; Roskar, R.; Zigon, D.; Vione, D.; Kosjek, T. Determination and photodegradation of sertraline residues in aqueous environment. Environ. Pollut. 2020, 256, 113431. [Google Scholar] [CrossRef]
- Jakimska, A.; Śliwka Kaszyńska, M.; Nagórski, P.; Kot Wasik, A.; Namieśnik, J. Environmental Fate of Two Psychiatric Drugs, Diazepam and Sertraline: Phototransformation and Investigation of their Photoproducts in Natural Waters. J. Chromatogr. Sep. Tech. 2014, 5, 1000253/1–1000253/12. [Google Scholar] [CrossRef] [Green Version]
- Cooper, W.J.; Zika, R.G.; Petasne, R.G.; Plane, J.M. Photochemical formation of hydrogen peroxide in natural waters exposed to sunlight. Environ. Sci. Technol. 1988, 22, 1156–1160. [Google Scholar] [CrossRef]
- Zepp, R.G.; Schlotzhauer, P.F.; Sink, R.M. Photosensitized Transformations Involving Electronic Energy Transfer in Natural Waters: Role of Humic Substances. Environ. Sci. Technol. 1985, 19, 74–81. [Google Scholar] [CrossRef]
- Peterson, B.M.; McNally, A.M.; Cory, R.M.; Thoemke, J.D.; Cotner, J.B.; McNeill, K. Spatial and temporal distribution of singlet oxygen in Lake Superior. Environ. Sci. Technol. 2012, 46, 7222–7229. [Google Scholar] [CrossRef]
- Canonica, S.; Urs, J.; Stemmler, K.; Hoigné, J. Transformation Kinetics of Phenols in Water: Photosensitization. Environ. Sci. Technol. 1995, 29, 1822–1831. [Google Scholar] [CrossRef]
- Vaughan, P.P.; Blough, N.V. Photochemical formation of hydroxyl radical by constituents of natural waters. Environ. Sci. Technol. 1998, 32, 2947–2953. [Google Scholar] [CrossRef]
- Mopper, K.; Zhou, X. Hydroxyl radical photoproduction in the sea and its potential impact on marine processes. Science 1990, 250, 661–664. [Google Scholar] [CrossRef]
- Vione, D.; Minella, M.; Maurino, V.; Minero, C. Indirect photochemistry in sunlit surface waters: Photoinduced production of reactive transient species. Chem. A Eur. J. 2014, 20, 10590–10606. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Prevo, L. Production of dissolved organic carbon in aquatic sediment suspensions. Water Res. 2003, 37, 2217–2222. [Google Scholar] [CrossRef]
- Chen, M.; Hur, J. Pre-treatments, characteristics, and biogeochemical dynamics of dissolved organic matter in sediments: A review. Water Res. 2015, 79, 10–25. [Google Scholar] [CrossRef]
- Nkhili, E.; Boguta, P.; Bejger, R.; Guyot, G.; Sokołowska, Z.; Richard, C. Photosensitizing properties of water-extractable organic matter from soils. Chemosphere 2014, 95, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, J.C. Organic Matter Content in Sediments of Three Navigation Pools along the Upper Mississippi River. J. Freshw. Ecol. 1988, 4, 343–349. [Google Scholar] [CrossRef]
- Romero, J.; Kagalou, I.; Imberger, J.; Hela, D.; Kotti, M.; Bartzokas, A.; Albanis, T.; Evmirides, N.; Karkabounas, S.; Papagiannis, J.; et al. Seasonal water quality of shallow and eutrophic Lake Pamvotis, Greece: Implications for restoration. Hydrobiologia 2002, 474, 91–105. [Google Scholar] [CrossRef]
- Touka, A.; Vareli, K.; Igglezou, M.; Monokrousos, N.; Alivertis, D.; Halley, J.; Hadjikakou, S.; Frillingos, S.; Sainis, I. Ancient European Lakes: Reservoirs of Hidden Microbial Diversity? The Case of Lake Pamvotis (NW Greece). Open J. Ecol. 2008, 8, 537–578. [Google Scholar] [CrossRef] [Green Version]
- Frogley, M.R.; Huw, I.G.; Heaton, T.H.E. Historical Biogeography and Late Quaternary Environmental Change of Lake Pamvotis, Ioannina (North-Western Greece): Evidence from Ostracods. J. Biogeogr. 2001, 28, 745–756. [Google Scholar] [CrossRef]
- Daskalou, V.; Polona, V.; Gregor, M.; Constantine, S. Recent Environmental Changes in the Shallow Lake Pamvotis (NW Greece): Evidence from Sedimentary Organic Matter, Hydrocarbons, and Stable Isotopes. Arch. Environ. Contam. Toxicol. 2009, 57, 21–31. [Google Scholar] [CrossRef]
- Cole, R.A.; Weigmann, D.L. Relationships among Zoobenthos, Sediments, and Organic Matter in Littoral Zones of Western Lake Erie and Saginaw Bay. J. Great Lakes Res. 1983, 9, 568–581. [Google Scholar] [CrossRef]
- Lara, R.J.; Gomez, E.A.; Pucci, A.E. Organic Matter, Sediment Particle Size and Nutrient Distributions in a Sewage Affected Shallow Channel. Mar. Pollut. Bull. 1985, 16, 360–364. [Google Scholar] [CrossRef]
- Fu, P.; Wu, F.; Liu, C.Q.; Wei, Z.; Bai, Y.; Liao, H. Spectroscopic characterization and molecular weight distribution of dissolved organic matter in sediment porewaters from Lake Erhai, Southwest China. Biogeochemistry 2006, 81, 179–189. [Google Scholar] [CrossRef]
- Coble, P.G. Marine optical biogeochemistry: The chemistry of ocean color. Chem. Rev. 2007, 107, 402–418. [Google Scholar] [CrossRef]
- Xu, H.; Zou, L.; Guan, D.; Li, W.; Jiang, H. Molecular Weight-Dependent Spectral and Metal Binding Properties of Sediment Dissolved Organic Matter from Different Origins. Sci. Tot. Environ. 2019, 665, 828–835. [Google Scholar] [CrossRef]
- Rosario-Ortiz, F.L.; Canonica, S. Probe Compounds to Assess the Photochemical Activity of Dissolved Organic Matter. Environ. Sci. Technol. 2016, 50, 12532–12547. [Google Scholar] [CrossRef] [Green Version]
- McKay, G.; Huang, W.; Romera-Castillo, C.; Crouch, J.E.; Rosario-Ortiz, F.L.; Jaffé, R. Predicting Reactive Intermediate Quantum Yields from Dissolved Organic Matter Photolysis Using Optical Properties and Antioxidant Capacity. Environ. Sci. Technol. 2017, 51, 5404–5413. [Google Scholar] [CrossRef]
- Haag, W.R.; Hoigné, J.R.; Gassman, E.; Braun, A.M. Singlet oxygen in surface waters Part I: Furfuryl alcohol as a trapping agent. Chemosphere 1984, 13, 631–640. [Google Scholar] [CrossRef]
- Wilkinson, F.; Helman, W.P.; Ross, A.B. Rate constants for the decay and reactions of the lowest electronically excited singlet state of molecular oxygen in solution. An expanded and revised compilation. J. Phys. Chem. Ref. Data 1995, 24, 663–677. [Google Scholar] [CrossRef] [Green Version]
- Page, S.E.; Arnold, W.A.; McNeill, K. Terephthalate as a probe for photochemically generated hydroxyl radical. J. Environ. Monit. 2010, 12, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants fro reactions of hydrated electrons, hydrogen atoms and hydroxyl radicas in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.Q.; Beach, E.S.; Xiang, Y.; Tshudy, D.J.; Khanina, N.; Horwitz, C.P.; Bier, M.E.; Collins, T.J. Rapid Biomimetic Degradation in Water of the Persistent Drug Sertraline by TAML Catalysts and Hydrogen Peroxide. Environ. Sci. Technol. 2011, 45, 7882–7887. [Google Scholar] [CrossRef] [PubMed]
- Osawa, R.A.; Carvalho, A.P.; Monteiro, O.C.; Oliveira, M.C.; Florêncio, M.H. Transformation products of citalopram: Identification, wastewater analysis and in silico toxicological assessment. Chemosphere 2019, 217, 858–868. [Google Scholar] [CrossRef]
- Hörsing, M.; Kosjek, T.; Andersen, H.R.; Heath, E.; Ledin, A. Fate of Citalopram during Water Treatment with O3, ClO2, UV and Fenton Oxidation. Chemosphere 2012, 89, 129–135. [Google Scholar] [CrossRef]
- United Nations. Globally Harmonized System of Classification and Labelling of Chemical (GHS), 4th ed.; United Nations Publications: New York, NY, USA, 2011. [Google Scholar]
- Brown, A.; Challis, J.; Wong, C.; Hanson, M. Selective serotonin reuptake inhibitors and β-blocker transformation products may not pose a significant risk of toxicity to aquatic organisms in wastewater effluent-dominated receiving waters. Integr. Environ. Assess. Manag. 2015, 11, 618–639. [Google Scholar] [CrossRef]
- Dimitra, G.H.; Lambropoulou, D.A.; Konstantinou, I.K.; Albanis, T.A. Environmental Monitoring and Ecological Risk Assessment for Pesticide Contamination and Effects. in Lake Pamvotis, northwestern Greece. Environ. Toxicol. Chem. 2005, 24, 1548–1556. [Google Scholar] [CrossRef]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of Specific Ultraviolet Absorbance as an Indicator of the Chemical Composition and Reactivity of Dissolved Organic Carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef]
- Lou, T.; Xie, H. Photochemical Alteration of the Molecular Weight of Dissolved Organic Matter. Chemosphere 2006, 65, 2333–2342. [Google Scholar] [CrossRef]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric Characterization of Dissolved Organic Matter for Indication of Precursor Organic Material and Aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.M.; Parlanti, E. Properties of Fluorescent Dissolved Organic Matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- Kouras-Hadef, S.; de Sainte Claire, P.; ter Halle, A.; Amine-Khodja, A.; Richard, C. The Role of Triplet State Keto-Enol Tautomerism in the Photodeamination of Metamitron. J. Phys. Chem. A 2011, 115, 14397–14406. [Google Scholar] [CrossRef]
- Mayo-Bean, K.; Moran, K.; Meylan, B. Methodology Document for the ECOlogical Structure-Activity Relationship Model (ECOSAR) Class Program; US-EPA: Washington, DC, USA, 2012. [Google Scholar]
| Peak Code | Elemental Formula of [M + H+]+ | m/z | TIC Peak Area | Chemical Modifications vs. SER |
|---|---|---|---|---|
| SER | C17H18Cl2N | 306.0803 308.0771 310.0734 | ||
| SER-1 Several peaks | C17H18Cl2NO | 322.0753 324.0722 326.0687 | 3.2 × 108 | SER + O fragment M − H2O |
| SER-1bis Several peaks | C17H18Cl2NO | 322.0753 324.0722 326.0687 | SER + O | |
| SER-2 Several peaks | C17H18Cl2NO2 | 338.0699 340.0670 342.0641 | 1.7 × 107 | SER + 2O fragment M − H2O for some of the peaks |
| SER-3 2 peaks | C17H16Cl2N | 304.0647 306.0615 308.0581 | 4.3 × 107 | SER − 2H |
| SER-4 | C16H13Cl2O | 291.0330 293.0300 295.0266 | 9.8 × 107 | SER − CH3NH2 + O |
| SER-5 2 peaks | C17H19ClNO | 288.1146 290.1110 | 3.7 × 107 | SER − Cl + OH |
| SER-6 | C17H20NO2 | 270.1481 | 1.8 × 106 | SER − 2Cl + OH + OH |
| SER-7 | C16H14ClO | 257.0721 259.0690 | 1.8 × 107 | SER − CH3NH2 + O − Cl + H |
| Elemental formula of [M-H+]− | m/z | TIC peak Area | Chemical modifications vs. SER | |
| SER-8 | C6H3Cl2O | 160.9553 162.9524 164.9495 | 5.7 × 106 | 3,4-dichlorophenol |
| SER-9 | C7H3Cl2O2 | 188.9507 190.9275 192.9279 | 2.6 × 106 | 3,4-dichlorobenzoic acid |
| Peak Code | Elemental Formula of [M + H+]+ | m/z | TIC Peak Area | Chemical Modification vs. CLO |
|---|---|---|---|---|
| CLO | C18H20ClN4 | 327.1375 329.1341 | ||
| CLO-1 Several peaks | C18H20ClN4O | 343.1323 345.1291 | 1.4 × 108 | ClO + O |
| CLO-2 Several peaks | C18H20ClN4O2 | 359.1273 361.1238 | 1.5 × 107 | CLO + 20 |
| CLO-3 Several peaks | C18H20ClN4O3 | 375.1226 377.1193 | 2 × 106 | CLO + 3O |
| CLO-4 | C17H18ClN4 | 313.1218 315.1186 | 5.6 × 107 | CLO − CH2 |
| CLO-5 | C16H18ClN4 | 301.1216 | 6.8 × 107 | CLO − C2H2 |
| CLO-6 | C18H21N4O | 309.1716 | 1 × 106 | CLO − Cl + OH |
| CLO-7 | C17H18ClN4O | 329.1166 331.1136 | 1.2 × 107 | CLO − Cl + H − CH2 |
| CLO-8 | C17H19N4O3 | 327.1455 | 3.2 × 106 | CLO − CH2 − Cl + OH + 2O |
| CLO-9 | C18H19N4O2 | 323.1504 | 4 × 106 | CLO − Cl + OH − 2H + O |
| Peak Code | Elemental Formula of [M + H+]+ | m/z | TIC peak Area | Chemical Modification vs. CIT |
|---|---|---|---|---|
| CIT | C20H22ON2F | 325.1714 | ||
| CIT-1 Several peaks | C20H22O2N2F | 341.1658 | 1.3 × 109 | CIT + O fragment M − H2O |
| CIT-1 bis Several peaks | C20H22O2N2F | 341.1658 | CIT + O | |
| CIT-2 | C20H20O2N2F | 339.1506 | 7.2 × 108 | CIT − 2H + O |
| CIT-3 | C20H20O3N2F | 355.1456 | 1.3 × 107 | CIT − 2H + 2O |
| CIT-4 | C19H20ON2F | 311.1560 | 2 × 107 | CIT − CH2 |
| CIT-5 | C20H23O2N2 | 323.1757 | 2.5 × 108 | CIT − F + OH |
| CIT-6 | C14H19ON2 | 231.1492 | 1 × 107 | CIT − aromatic ring |
| CIT-7 Several peaks | C14H17O2N2 | 245.1287 | 2.2 × 108 | CIT − aromatic ring-2H+O |
| CIT-8 | C19H20O2N2F | 327.1509 | 2 × 108 | CIT − CH2 + O |
| CIT-9 | C19H20O3N2F | 343.1456 | 1 × 107 | CIT − CH2 + 2O |
| CIT-10 | C19H18O2N2F | 325.1350 | 1.2 × 108 | CIT − CH2- − 2H + O |
| CIT-11 | C20H21O4N2 | 353.1495 | 8.3 × 106 | CIT − F-2H + OH + 2O |
| CIT-12 | C20H21O3N2 | 337.1546 | 1.1 × 107 | CIT − F+OH − 2H |
| CIT-13 | C20H23O3N2 | 339.1703 | 1.2 × 107 | CIT − F+OH +O |
| CIT-14 Several peaks | C19H21O4N2 | 341.1491 | 1 × 107 | CIT − CH2 − F+OH+2O |
| CIT-15 | C19H19O3N2 | 323.1393 | 1.3 × 106 | CIT − CH2 − F+OH − 2H+O |
| Toxicity Range [mg/L] | Class |
|---|---|
| LC50/EC50/ChV ≤ 1 | Very toxic |
| 1 > LC50/EC50/ChV ≤ 10 | Toxic |
| 10 > LC50/EC50/ChV ≤ 100 | Harmful |
| LC50/EC50/ChV > 100 | Not harmful |
| Acute Toxicity [mg/L] | Chronic Toxicity (ChV) [mg/L] | |||||
|---|---|---|---|---|---|---|
| Fish (LC50) | Daphnid (LC50) | Algae (EC50) | Fish (LC50) | Daphnid (LC50) | Algae (EC50) | |
| SER | 0.408 | 0.071 | 0.028 | 0.0074 | 0.0085 | 0.012 |
| SER-1 | 0.887 | 0.147 | 0.063 | 0.019 | 0.017 | 0.027 |
| SER-2 | 1.93 | 0.301 | 0.154 | 0.049 | 0.032 | 0.059 |
| SER-3 | 0.078 | 0.132 | 0.00072 | 0.0018 | 0.021 | 0.019 |
| SER-4 | 0.477 | 0.357 | 0.839 | 0.065 | 0.075 | 0.408 |
| SER-5 | 2.10 | 0.322 | 0.162 | 0.0057 | 0.034 | 0.064 |
| SER-6 | 10.8 | 1.45 | 0.942 | 0.435 | 0.134 | 0.341 |
| SER-7 | 1.59 | 1.13 | 2.07 | 0.201 | 0.201 | 0.879 |
| SER-8 | 6.24 | 3.88 | 0.566 | 0.671 | 0.489 | 1.46 |
| SER-9 | 5.96 | 3.92 | 5.31 | 0.692 | 0.572 | 1.92 |
| Acute Toxicity [mg/L] | Chronic Toxicity (ChV) [mg/L] | |||||
|---|---|---|---|---|---|---|
| Fish (LC50) | Daphnid (LC50) | Algae (EC50) | Fish (LC50) | Daphnid (LC50) | Algae (EC50) | |
| CLO | 17.7 | 2.32 | 1.58 | 0.764 | 0.210 | 0.563 |
| CLO-1 | 65.2 | 20.1 | 6.40 | 4.58 | 2.20 | 8.65 |
| CLO-2 | 141 | 15.9 | 14.6 | 9.71 | 1.24 | 4.67 |
| CLO-3 | 518 | 53.1 | 59.1 | 47.9 | 3.76 | 17.6 |
| CLO-4 | 23.3 | 2.98 | 2.13 | 1.09 | 0.263 | 0.746 |
| CLO-5 | 26.0 | 3.29 | 2.41 | 1.25 | 0.287 | 0.835 |
| CLO-6 | 155 | 17.2 | 16.4 | 11.3 | 1.31 | 5.17 |
| CLO-7 | 86.0 | 10.0 | 8.66 | 5.38 | 0.804 | 2.83 |
| CLO-8 | 1.65E+3 | 153 | 207 | 206 | 9.82 | 57.3 |
| CLO-9 | 683 | 67.7 | 80.5 | 69.8 | 4.65 | 23.4 |
| Acute Toxicity [mg/L] | Chronic Toxicity (ChV) [mg/L] | |||||
|---|---|---|---|---|---|---|
| Fish (LC50) | Daphnid (LC50) | Algae (EC50) | Fish (LC50) | Daphnid (LC50) | Algae (EC50) | |
| CIT | 4.47 | 0.652 | 0.360 | 0.140 | 0.065 | 0.138 |
| CIT-1 | 48.0 | 5.86 | 4.62 | 2.60 | 0.493 | 1.56 |
| CIT-2 | 21.3 | 2.77 | 1.93 | 0.954 | 0.247 | 0.680 |
| CIT-3 | 249 | 26.9 | 27.0 | 19.7 | 2.01 | 8.35 |
| CIT-4 | 5.88 | 0.838 | 0.486 | 0.199 | 0.082 | 0.183 |
| CIT-5 | 61.6 | 7.35 | 6.06 | 3.58 | 0.604 | 2.01 |
| CIT-6 | 37.2 | 4.50 | 3.62 | 2.08 | 0.374 | 1.21 |
| CIT-7 | 260 | 27.2 | 29.1 | 22.7 | 1.97 | 8.80 |
| CIT-8 | 56.7 | 6.81 | 5.54 | 3.23 | 0.564 | 1.85 |
| CIT-9 | 330 | 34.7 | 36.6 | 28.0 | 2.53 | 11.1 |
| CIT-10 | 28.1 | 3.56 | 2.60 | 1.36 | 0.310 | 0.903 |
| CIT-11 | 636 | 63.8 | 74.1 | 62.6 | 4.44 | 21.7 |
| CIT-12 | 294 | 31.2 | 32. | 24.4 | 2.29 | 9.88 |
| CIT-13 | 134 | 15.1 | 13.9 | 9.22 | 1.17 | 4.43 |
| CIT-14 | 918 | 89.3 | 110 | 99.2 | 6.02 | 31.6 |
| CIT-15 | 176 | 19.4 | 18.8 | 13.1 | 1.48 | 5.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Holgado, C.; Sakkas, V.; Richard, C. Phototransformation of Three Psychoactive Drugs in Presence of Sedimental Water Extractable Organic Matter. Molecules 2021, 26, 2466. https://doi.org/10.3390/molecules26092466
Jiménez-Holgado C, Sakkas V, Richard C. Phototransformation of Three Psychoactive Drugs in Presence of Sedimental Water Extractable Organic Matter. Molecules. 2021; 26(9):2466. https://doi.org/10.3390/molecules26092466
Chicago/Turabian StyleJiménez-Holgado, Cristina, Vasilios Sakkas, and Claire Richard. 2021. "Phototransformation of Three Psychoactive Drugs in Presence of Sedimental Water Extractable Organic Matter" Molecules 26, no. 9: 2466. https://doi.org/10.3390/molecules26092466
APA StyleJiménez-Holgado, C., Sakkas, V., & Richard, C. (2021). Phototransformation of Three Psychoactive Drugs in Presence of Sedimental Water Extractable Organic Matter. Molecules, 26(9), 2466. https://doi.org/10.3390/molecules26092466








