Fast HILIC Method for Separation and Quantification of Non-Volatile Aromatic Compounds and Monosaccharides from Willow (Salix sp.) Bark Extract
Abstract
1. Introduction
2. Materials and Methods
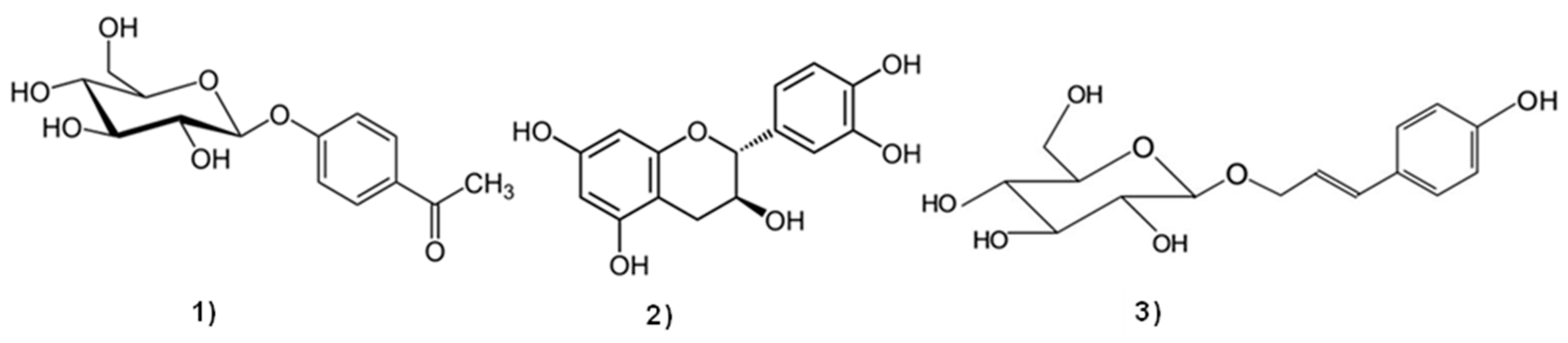
2.1. Materials
2.2. HILIC-UV/ELSD
3. Results and Discussion
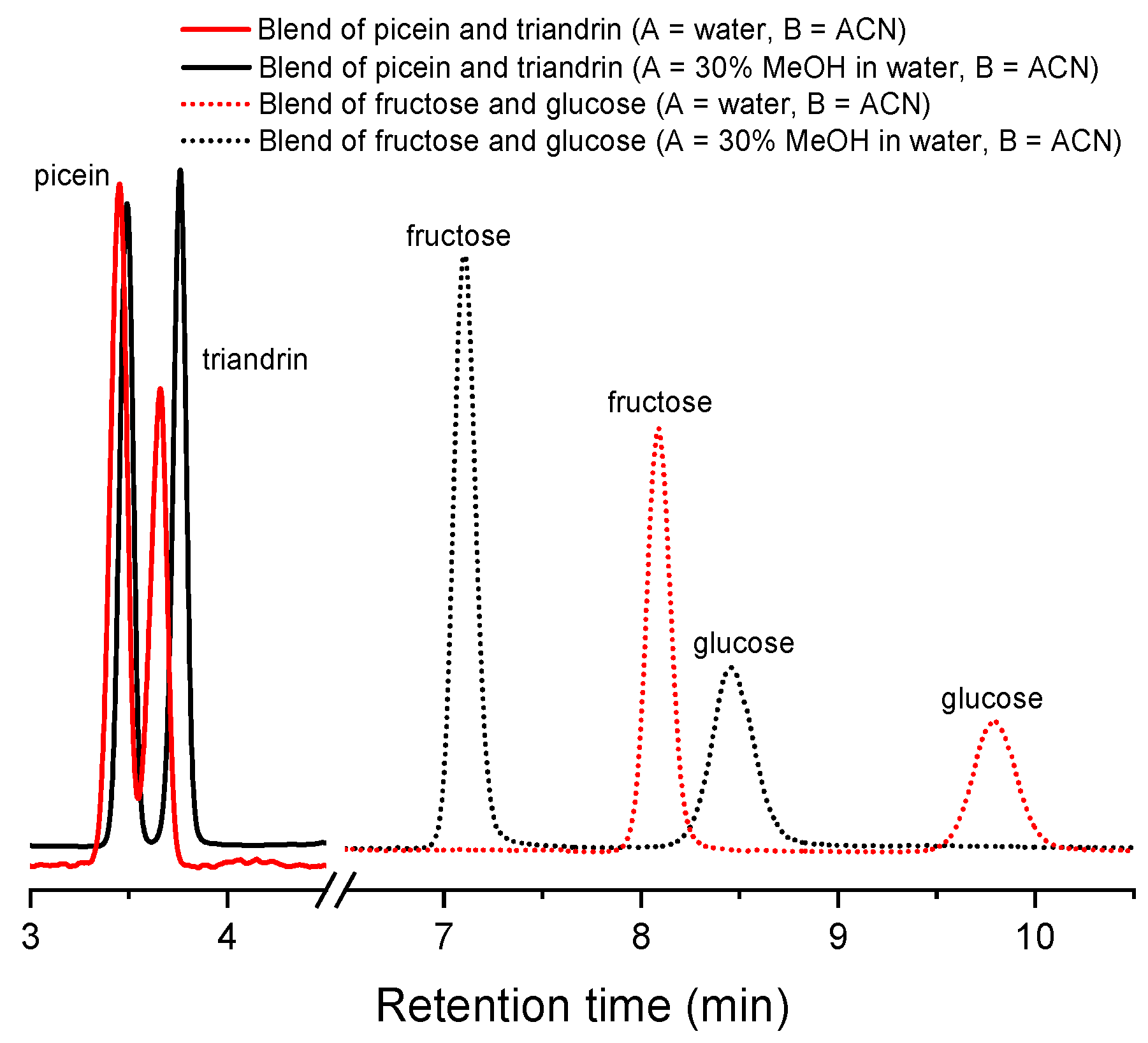
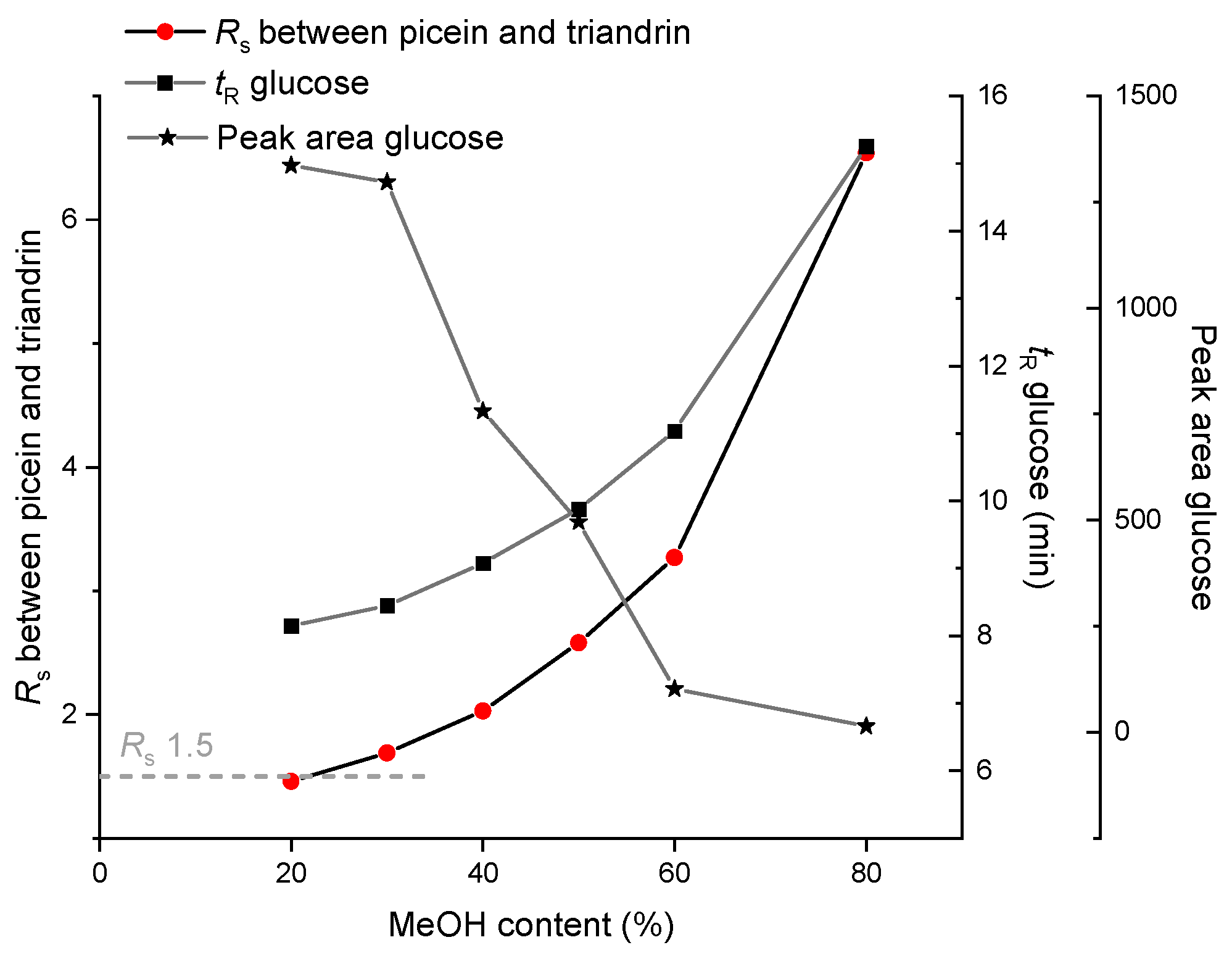
3.1. Optimization of Eluent Composition
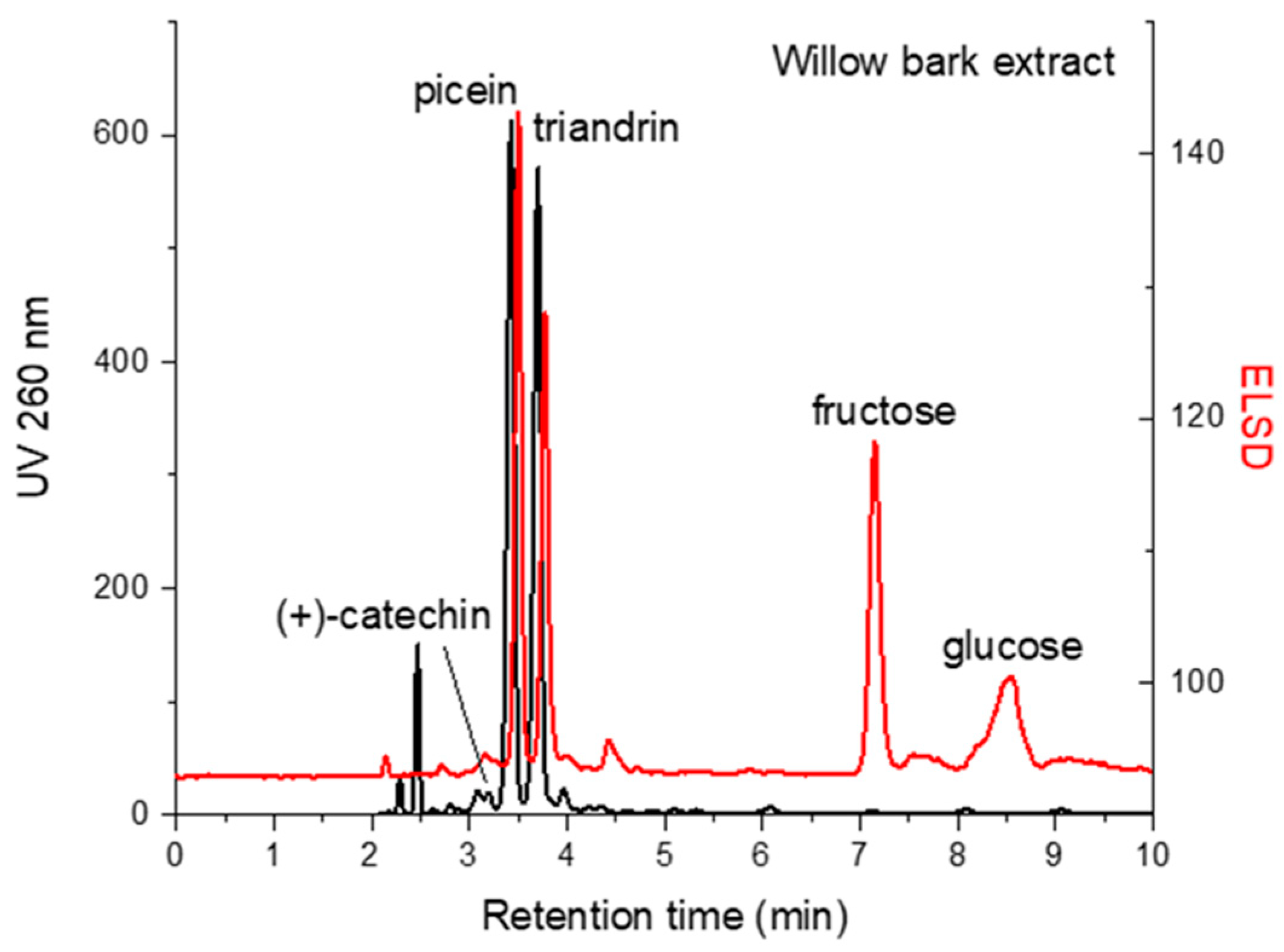
3.2. Quantification of Aromatic Compounds and Monosaccharides by HILIC-UV/ELSD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hedner, T.; Everts, B. The early clinical history of salicylates in rheumatology and pain. Clin. Rheumatol. 1998, 17, 17–25. [Google Scholar] [CrossRef]
- Dou, J.; Xu, W.; Koivisto, J.J.; Mobley, J.K.; Padmakshan, V.; Kögler, M.; Xu, C.; Willför, S.; Ralph, J.; Vuorinen, V. Characteristics of hot water extracts from the bark of cultivated willow (Salix sp.). ACS Sustain. Chem. Eng. 2018, 6, 5566–5573. [Google Scholar] [CrossRef]
- Noleto-Dias, C.; Wu, W.; Bellisai, A.; Macalpine, W.; Beale, H.M.; Ward, L.J. Phenylalkanoid glycosides (non-salicinoids) from wood chips of Salix triandra × dasyclados hybrid willow. Molecules 2019, 24, 1152. [Google Scholar] [CrossRef] [PubMed]
- Mageroy, M.H.; Parent, G.; Germanos, G.; Giguère, I.; Delvas, N.; Maaroufi, H.; Bauce, É.; Bohlmann, J.; Mackay, J.J. Expression of the β-glucosidase gene Pgβglu-1 underpins natural resistance of white spruce against spruce budworm. Plant J. 2015, 81, 68–80. [Google Scholar] [CrossRef]
- Kesari, K.K.; Dhasmana, A.; Shandilya, S.; Prabhakar, N.; Shaukat, A.; Dou, J.; Rosenholm, J.M.; Vuorinen, T.; Ruokolainen, J. Plant-derived natural biomolecule picein attenuates menadione induced oxidative stress on neuroblastoma cell mitochondria. Antioxidants 2020, 9, 552. [Google Scholar] [CrossRef]
- Kurkin, V.A. Phenylpropanoids as the biologically active compounds of the medicinal plants and phytopharmaceuticals. Adv. Biol. Chem. 2013, 3, 26–28. [Google Scholar] [CrossRef]
- Gadkari, P.V.; Balaraman, M. Catechins: Sources, extraction and encapsulation: A review. Food Bioprod. Process. 2015, 93, 122–138. [Google Scholar] [CrossRef]
- Kammerer, B.; Kahlich, R.; Biegert, C.; Gleiter, C.H.; Heide, L. HPLC-MS/MS analysis of willow bark extracts contained in pharmaceutical preparations. Phytochem. Anal. 2005, 16, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Brummer, Y.; Cui, S.W. Understanding carbohydrate analysis. In Food Carbohydrates—Chemistry, Physical Properties, and Applications; Cui, S.W., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 67–104. [Google Scholar]
- Lee, Y.C. Carbohydrate analyses with high-performance anion-exchange chromatography. J. Chromatogr. A 1996, 720, 137–149. [Google Scholar] [CrossRef]
- Corradini, C.; Cavazza, A.; Bignardi, C. High-performance anion-exchange chromatography coupled with pulsed electrochemical detection as a powerful tool to evaluate carbohydrates of food interest: Principles and applications. Int. J. Carbohydr. Chem. 2012, 2012, 487564. [Google Scholar] [CrossRef]
- Fu, Q.; Liang, T.; Li, Z.; Xu, X.; Ke, Y.; Jin, Y.; Liang, X. Separation of carbohydrates using hydrophilic interaction liquid chromatography. Carbohydr Res. 2013, 379, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Juvonen, M.; Kotiranta, M.; Jokela, J.; Tuomainen, P.; Tenkanen, M. Identification and structural analysis of cereal arabinoxylan-derived oligosaccharides by negative ionization HILIC-MS/MS. Food Chem. 2019, 275, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Noga, S. Hydrophilic interaction liquid chromatography (HILIC)—A powerful separation technique. Anal. Bioanal. Chem. 2012, 402, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Tobimatsu, Y.; Hosoya, T.; Hattori, T.; Ohnishi, J.; Takano, M.; Kamitakahara, H.; Nakatsubo, F. Studies on the dehydrogenative polymerizations of monolignol β-glycosides. Part 1. Syntheses of monolignol β-glycosides, (E)-isoconiferin, (E)-isosyringin, and (E)-triandrin. J. Wood Chem. Technol. 2006, 26, 215–229. [Google Scholar] [CrossRef]
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant activity, total phenolic content, individual phenolics and physicochemical parameters puitability for Romanian honey authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Multari, S.; Carafa, I.; Barp, L.; Caruso, M.; Licciardello, C.; Larcher, R.; Tuohy, K.; Martens, S. Effects of Lactobacillus spp. on the phytochemical composition of juices from two varieties of Citrus sinensis L. Osbeck: ‘Tarocco’ and ‘Washington navel’. LWT Food Sci. Technol. 2020, 125, 109205. [Google Scholar] [CrossRef]
- Dou, J.; Heinonen, J.; Vuorinen, T.; Xu, C.; Sainio, T. Chromatographic recovery and purification of natural phytochemicals from underappreciated willow bark water extracts. Sep. Purif. Technol. 2021, 261, 118247. [Google Scholar] [CrossRef]
- Hao, Z.; Xiao, B.; Weng, N. Impact of column temperature and mobile phase components on selectivity of hydrophilic interaction chromatography (HILIC). J. Sep. Sci. 2008, 31, 1449–1464. [Google Scholar] [CrossRef]
- Soukup, J.; Jandera, P. Comparison of nonaqueous hydrophilic interaction chromatography with aqueous normal-phase chromatography on hydrosilated silica-based stationary phases. J. Sep. Sci. 2013, 36, 2753–2759. [Google Scholar] [CrossRef] [PubMed]
- Peres, A.M.; Macedo, E.A. A modified UNIFAC model for the calculation of thermodynamic properties of aqueous and non-aqueous solutions containing sugars. Fluid Phase Equilib. 1997, 139, 47–74. [Google Scholar] [CrossRef]
- Greco, G.; Letzel, T. Main interactions and influences of the chromatographic parameters in HILIC separations. J. Chromatogr. Sci. 2013, 51, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Olsen, B.A.; Pack, B.W. (Eds.) Hydrophilic Interaction Chromatogaphy—A Guide for Practitioners; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Megoulas, N.C.; Koupparis, M.A. Twenty years of evaporative light scattering detection. Crit. Rev. Anal. Chem. 2005, 35, 301–316. [Google Scholar] [CrossRef]
| ELSD | UV | |||
|---|---|---|---|---|
| LOD | LOQ | LOD | LOQ | |
| Picein | 5 | 10 | 0.01 | 0.05 |
| Triandrin | 5 | 10 | 0.01 | 0.05 |
| Fructose | 10 | 50 | n.a. | n.a. |
| Glucose | 25 | 125 | n.a. | n.a. |
| Picein | Triandrin | (+)-catechin | Fructose | Glucose | |
|---|---|---|---|---|---|
| Willow bark extract | 46 ± 2 | 36 ± 1 | 32 ± 2 | 56 ± 9 | 114 ± 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meriö-Talvio, H.; Dou, J.; Vuorinen, T.; Pitkänen, L. Fast HILIC Method for Separation and Quantification of Non-Volatile Aromatic Compounds and Monosaccharides from Willow (Salix sp.) Bark Extract. Appl. Sci. 2021, 11, 3808. https://doi.org/10.3390/app11093808
Meriö-Talvio H, Dou J, Vuorinen T, Pitkänen L. Fast HILIC Method for Separation and Quantification of Non-Volatile Aromatic Compounds and Monosaccharides from Willow (Salix sp.) Bark Extract. Applied Sciences. 2021; 11(9):3808. https://doi.org/10.3390/app11093808
Chicago/Turabian StyleMeriö-Talvio, Heidi, Jinze Dou, Tapani Vuorinen, and Leena Pitkänen. 2021. "Fast HILIC Method for Separation and Quantification of Non-Volatile Aromatic Compounds and Monosaccharides from Willow (Salix sp.) Bark Extract" Applied Sciences 11, no. 9: 3808. https://doi.org/10.3390/app11093808
APA StyleMeriö-Talvio, H., Dou, J., Vuorinen, T., & Pitkänen, L. (2021). Fast HILIC Method for Separation and Quantification of Non-Volatile Aromatic Compounds and Monosaccharides from Willow (Salix sp.) Bark Extract. Applied Sciences, 11(9), 3808. https://doi.org/10.3390/app11093808






