Green Nanofabrication Opportunities in the Semiconductor Industry: A Life Cycle Perspective
Abstract
1. Introduction
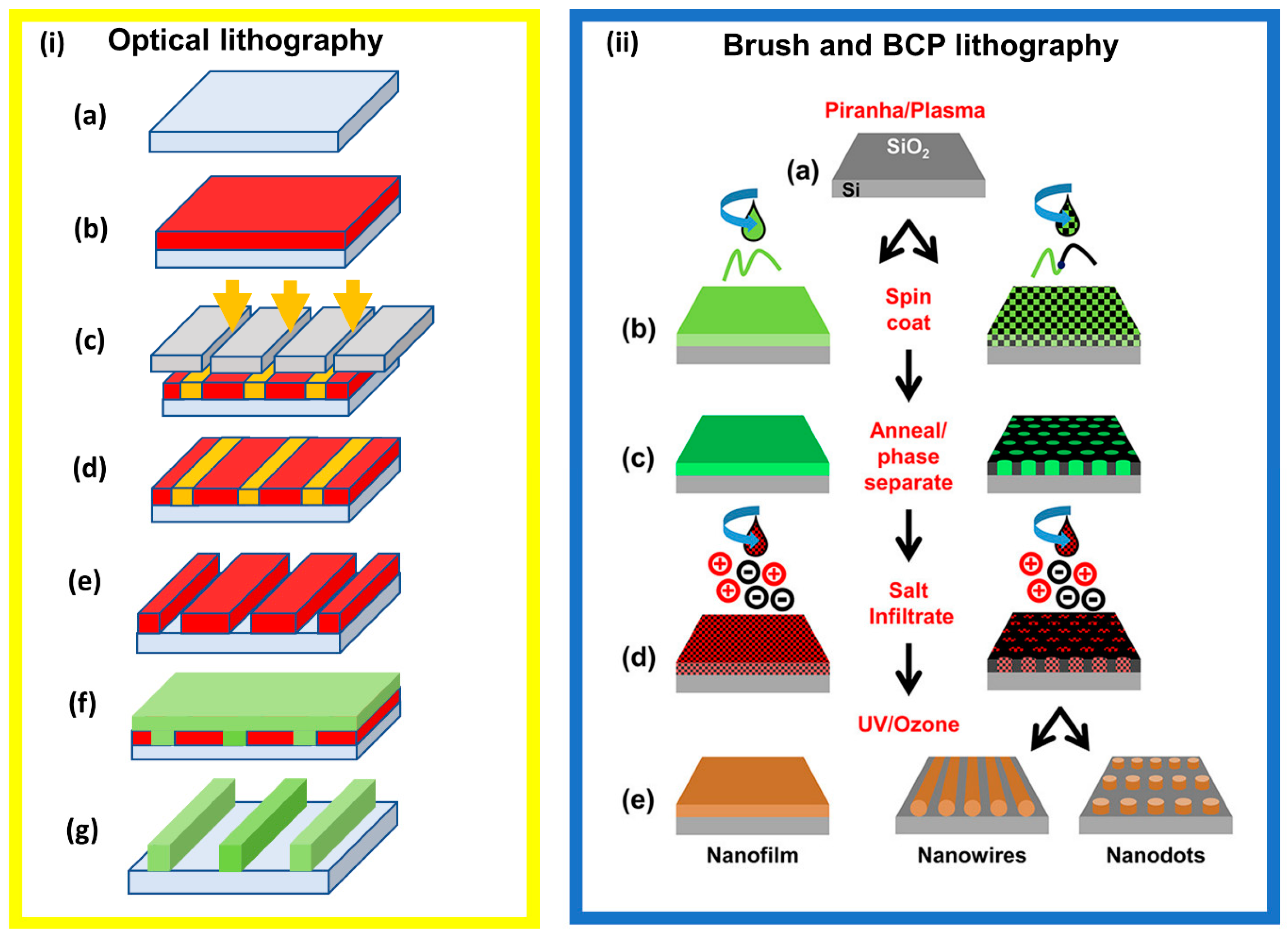
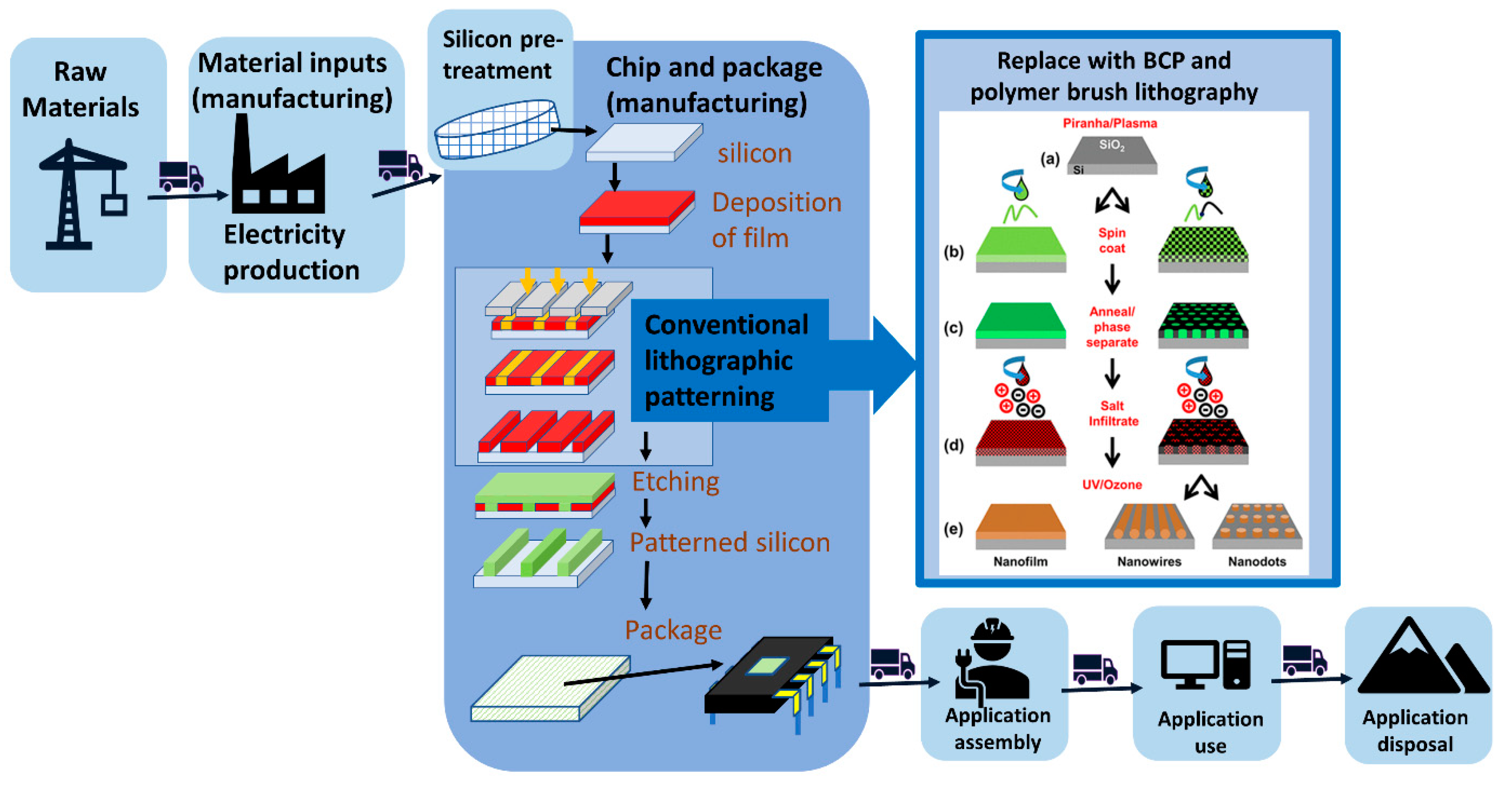
2. Bottom-Up versus Top-Down Lithography
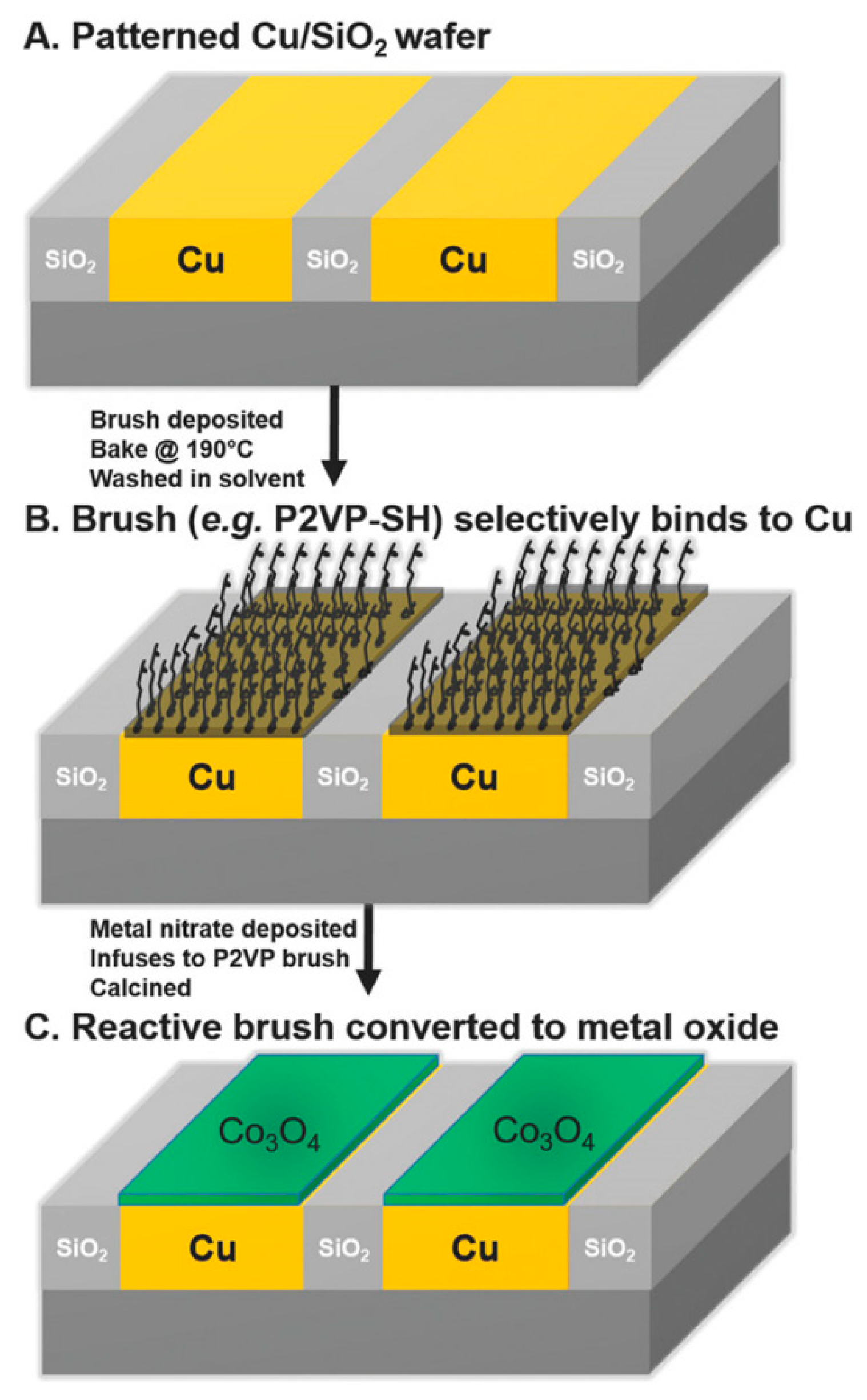
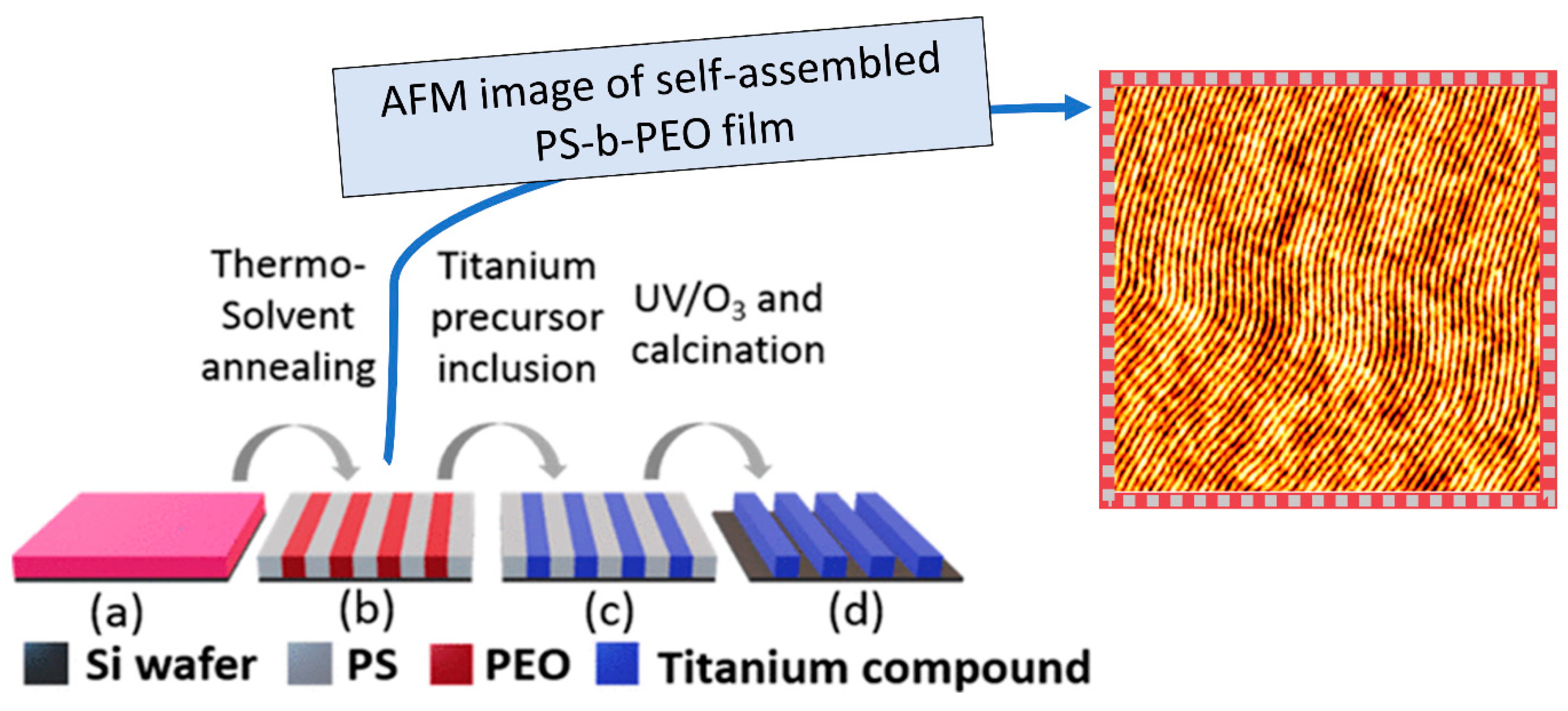
2.1. Block Copolymer and Polymer Brush Lithography
2.1.1. Polymer Selection
2.1.2. Polymer Deposition onto Substrates
2.1.3. Polymer-Based Pattern Formation
2.1.4. Metal Incorporation and Oxidative Removal of the Polymer
2.2. Comparison of the Next-Generation Lithographic Techniques
2.2.1. Optical/Photo Lithography
2.2.2. Extreme Ultraviolet Lithography
2.2.3. Nanoimprint Lithography
3. Material Challenges in Environmental, Safety, Health, and Sustainability in the Semiconductor Industry
3.1. Pressing Enviornmental Issues the Semicoductor Industry Faces
3.1.1. Reducing Waste
3.1.2. Reducing Power Consumption
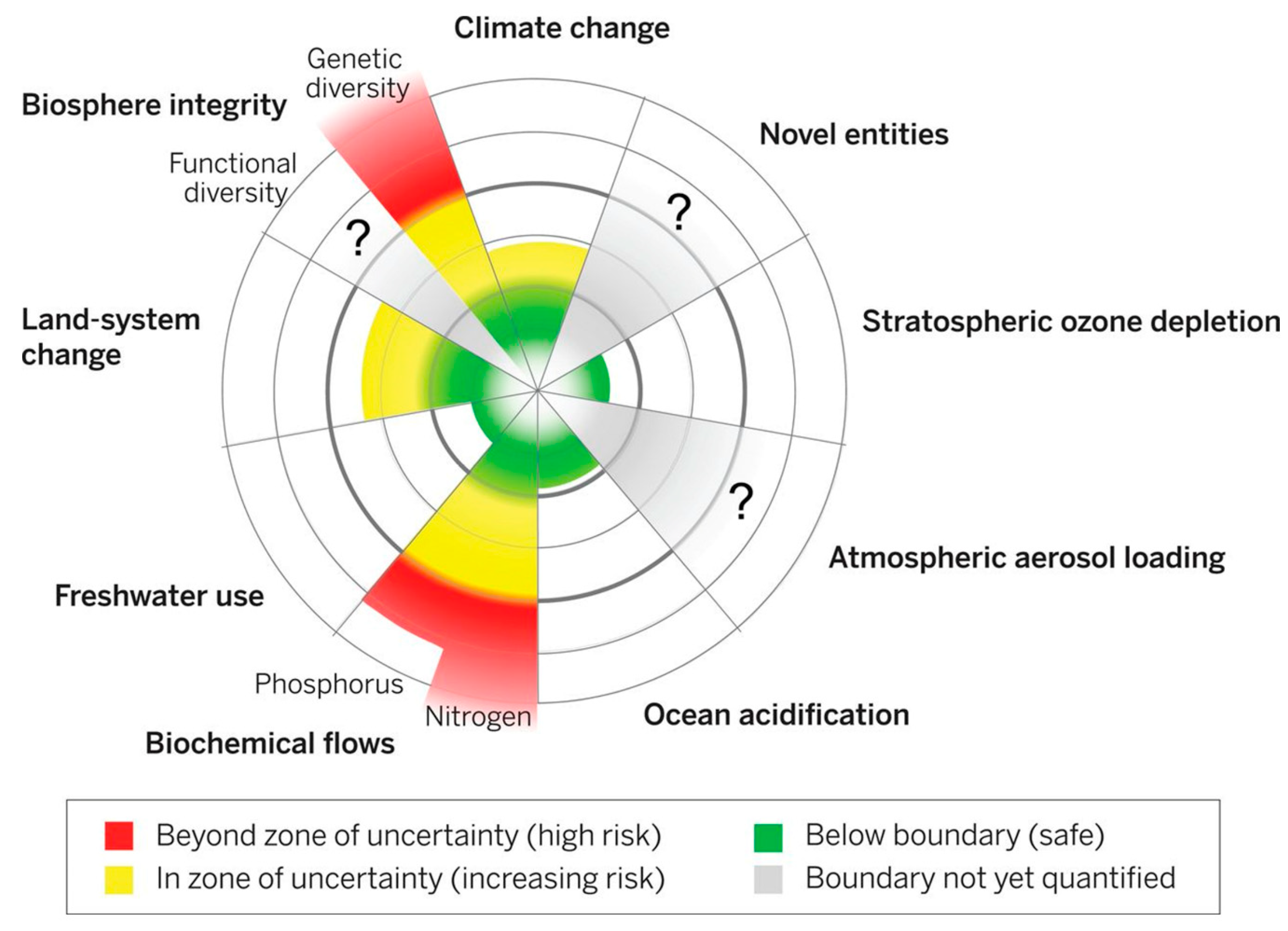
3.1.3. Avoiding Threats to Earth Systems
3.1.4. The Environmental Impact of Emerging Materials
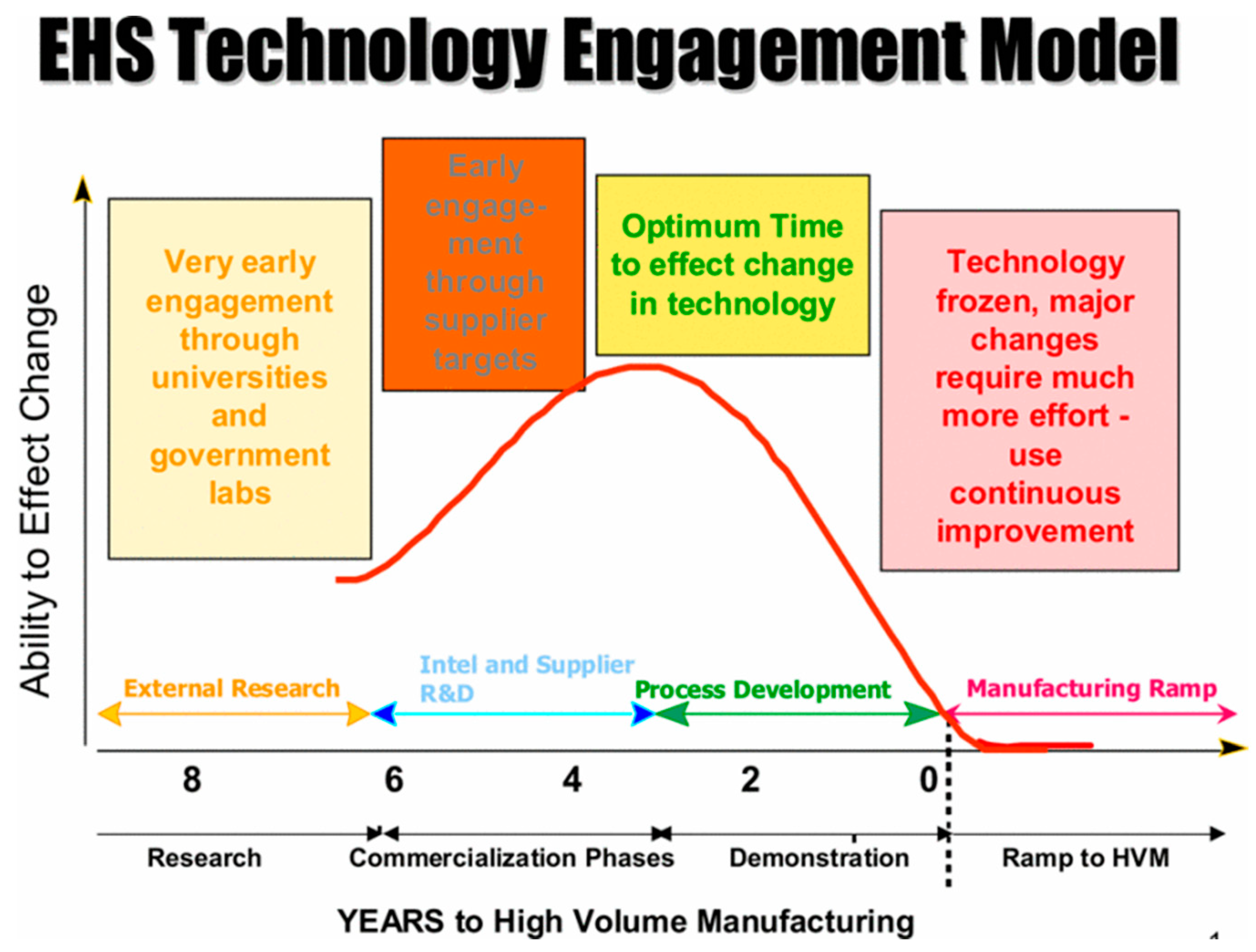
3.1.5. Developing Universally Accepted Guidelines for Assessing ESH/S Challenges
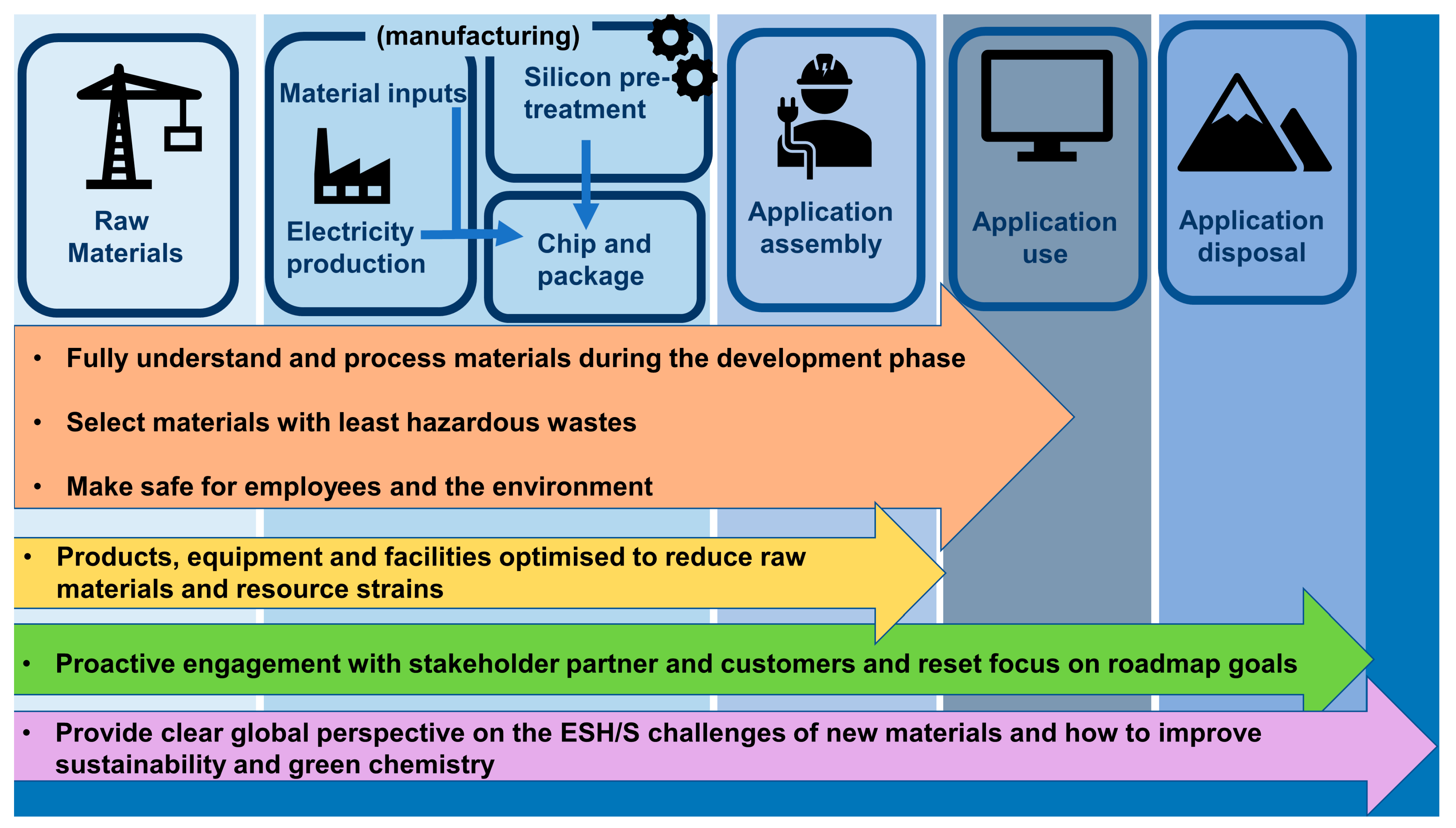
3.2. Green Chemistry and Environmental Sustainability in the Semiconductor Industry
3.2.1. Selecting Materials with the Least Hazardous Waste
3.2.2. Fully Understand the Materials and Process during the Development Phase
3.2.3. Factory and Industry Supply Chain Should Be Made Safe for Employees and the Environment
3.2.4. Selecting Products, Equipment and Facilities Optimised to Reduce Raw Materials and Resource Strains
3.2.5. Proactive Engagement with Stakeholders, Partners and Customers and Reset Focus on Roadmap Goals
3.2.6. Provide a Clear Global Perspective Concerning the ESH/S Challenges of New Materials, and How to Improve Sustainability and Green Chemistry
3.3. Are There Environmental Incentives to Pursue the Alternative Lithography Strategies?
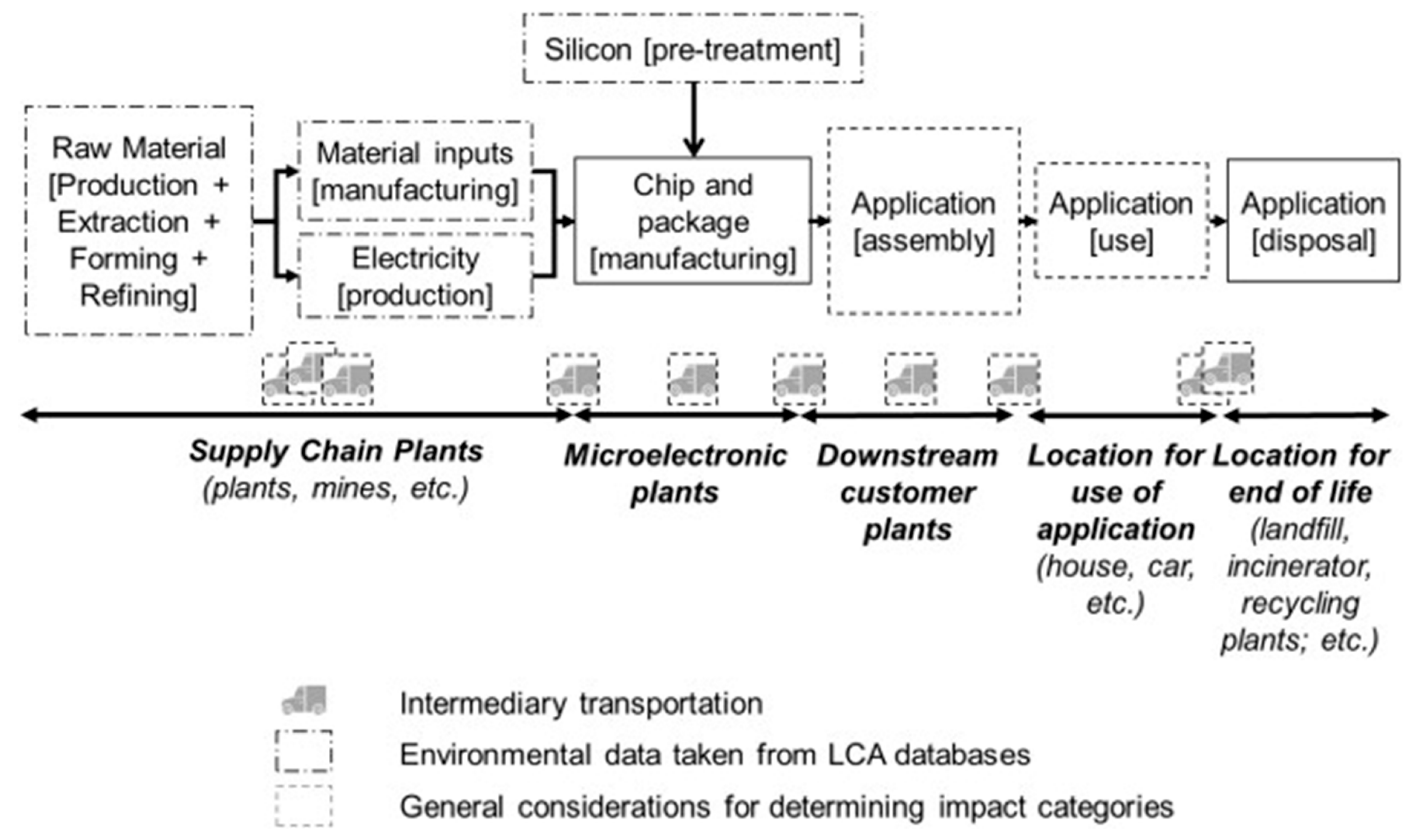
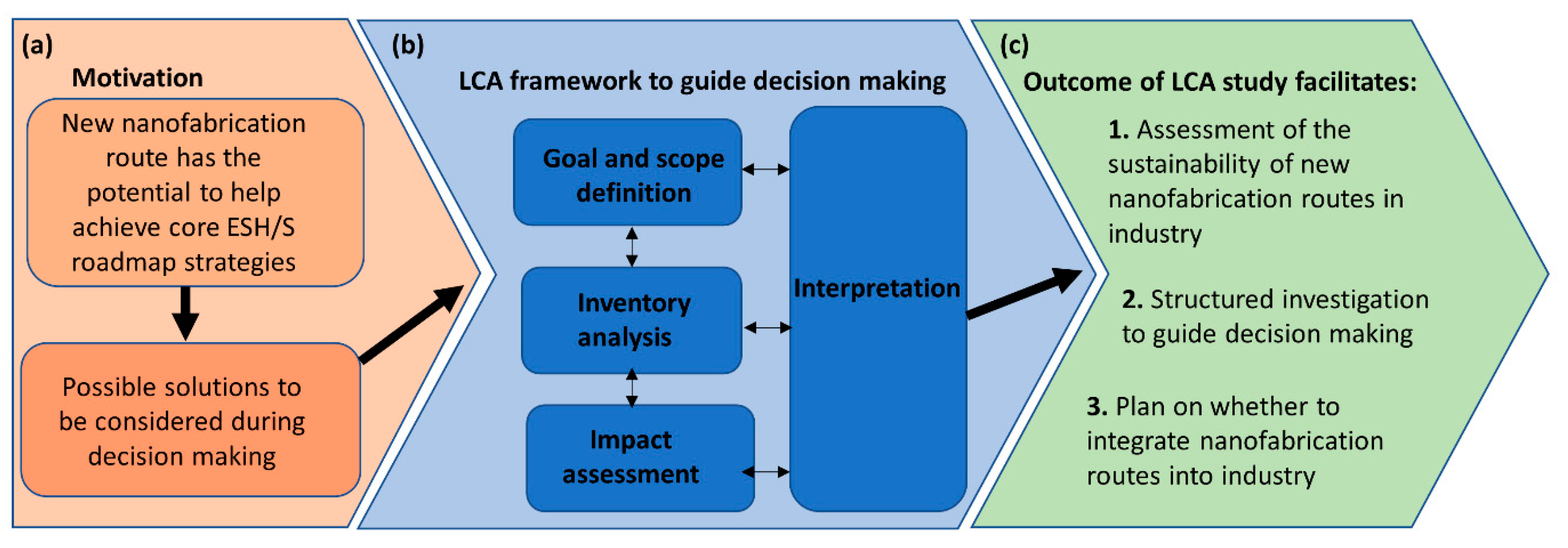
4. Life Cycle Analysis Framework to Assess the Viability of Green Lithographic Strategies
4.1. Goal Definition and Scoping
4.2. Life Cycle Inventory Analysis
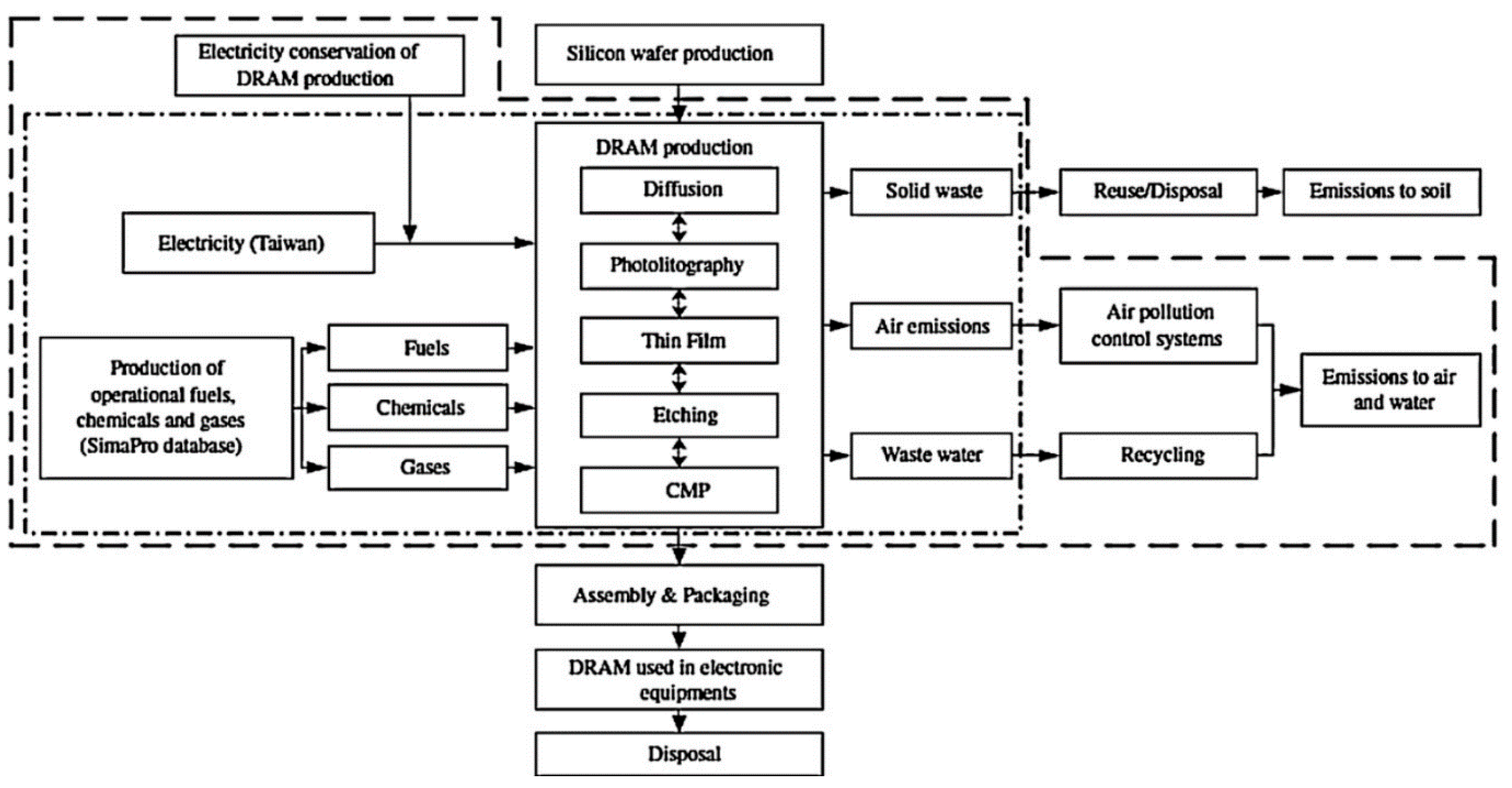
4.2.1. Data Collection for the Mature Technology
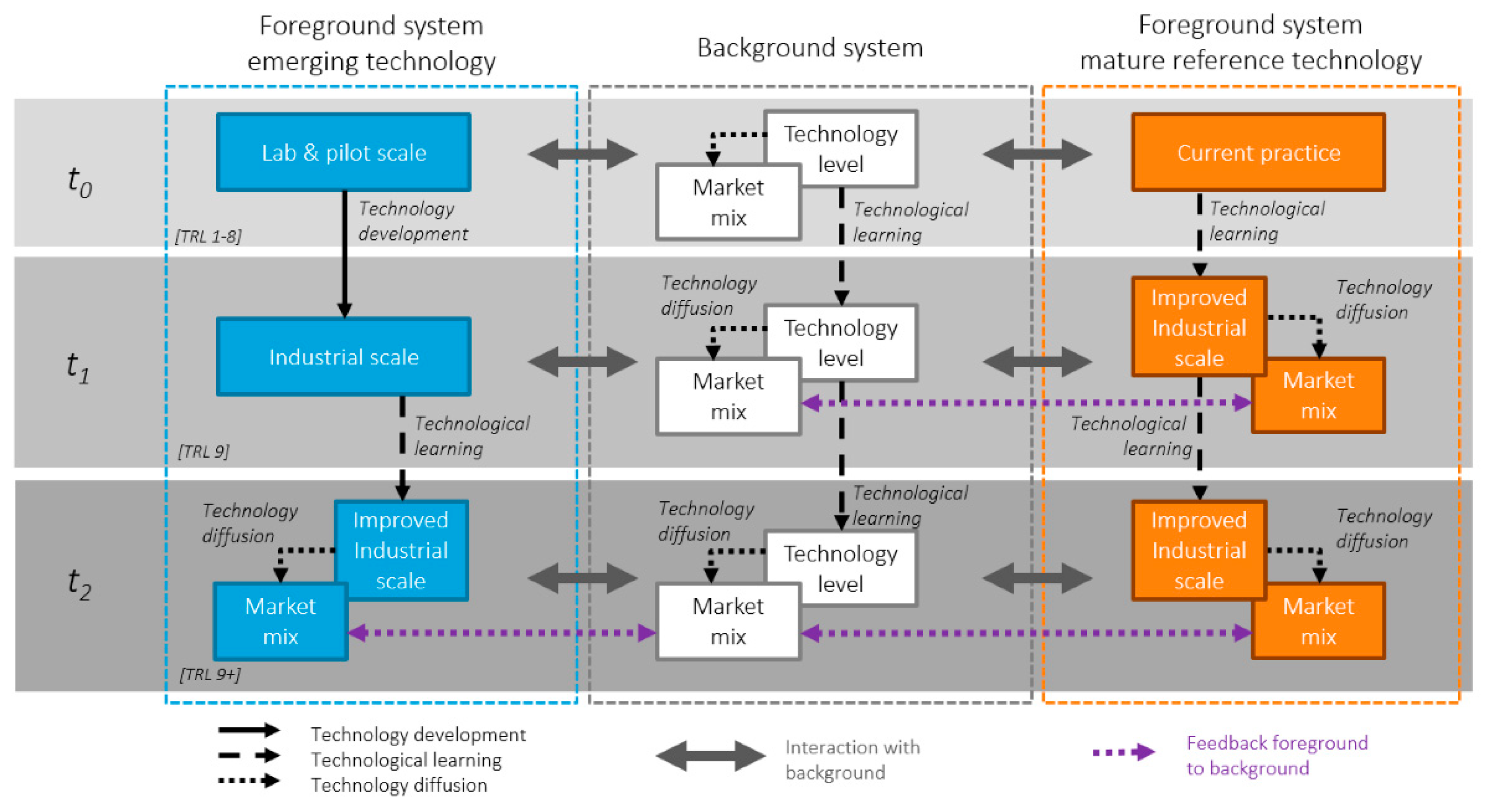
4.2.2. Data Collection for the Emerging Technology
4.2.3. Scaling to t1—Industrial Scale
4.2.4. Scaling to t2—Improved Industrial Scale and Market Mix
4.2.5. Quality of Data for Emerging and Mature Technologies
4.3. Life Cycle Impact Assessment
4.4. Interpretation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pinto-Gómez, C.; Pérez-Murano, F.; Bausells, J.; Villanueva, L.G.; Fernández-Regúlez, M. Directed self-assembly of block copolymers for the fabrication of functional devices. Polymers (Basel) 2020, 12, 2432. [Google Scholar] [CrossRef]
- Liddle, J.A.; Gallatin, G.M. Nanomanufacturing: A Perspective. ACS Nano 2016, 10, 2995–3014. [Google Scholar] [CrossRef]
- Shalf, J. The future of computing beyond Moore’s Law Subject Areas. Philos. Trans. R. Soc. 2020, 378, 1–14. [Google Scholar]
- Cummins, C.; Lundy, R.; Walsh, J.J.; Ponsinet, V.; Fleury, G.; Morris, M.A. Enabling future nanomanufacturing through block copolymer self-assembly: A review. Nano Today 2020, 35, 100936. [Google Scholar] [CrossRef]
- Sarangan, A. Nanofabrication. In Fundamentals and Applications of Nanophotonics; 2016; pp. 149–184. ISBN 9781782424642. Available online: https://www.elsevier.com/books/fundamentals-and-applications-of-nanophotonics/haus/978-1-78242-464-2 (accessed on 22 April 2021).
- Black, C.T. Polymer Self-Assembly as a Novel Extension to Optical Lithography. ACS Nano 2007, 1, 147–150. [Google Scholar] [CrossRef]
- Cummins, C.; Shaw, M.T.; Morris, M.A. Area Selective Polymer Brush Deposition. Macromol. Rapid Commun. 2017, 38, 1700252. [Google Scholar] [CrossRef]
- Cummins, C.; Gangnaik, A.; Kelly, R.A.; Borah, D.; O’Connell, J.; Petkov, N.; Georgiev, Y.M.; Holmes, J.D.; Morris, M.A. Aligned silicon nanofins via the directed self-assembly of PS-b-P4VP block copolymer and metal oxide enhanced pattern transfer. Nanoscale 2015, 7, 6712–6721. [Google Scholar] [CrossRef]
- Paripovic, D.; Klok, H.A. Polymer brush guided formation of thin gold and palladium/gold bimetallic films. ACS Appl. Mater. Interfaces 2011, 3, 910–917. [Google Scholar] [CrossRef]
- Jeong, S.-J.; Kim, J.Y.; Kim, B.H.; Moon, H.-S.; Kim, S.O. Directed self-assembly of block copolymers for next generation nanolithography. Mater. Today 2013, 16, 468–476. [Google Scholar] [CrossRef]
- Parsons, G.N.; Clark, R.D. Area-Selective Deposition: Fundamentals, Applications, and Future Outlook. Chem. Mater. 2020, 32, 4920–4953. [Google Scholar] [CrossRef]
- Cummins, C.; Alvarez-Fernandez, A.; Bentaleb, A.; Hadziioannou, G.; Ponsinet, V.; Fleury, G. Strategy for Enhancing Ultrahigh-Molecular-Weight Block Copolymer Chain Mobility to Access Large Period Sizes (>100 nm). Langmuir 2020, 36, 13872–13880. [Google Scholar] [CrossRef] [PubMed]
- Schacher, F.H.; Rupar, P.A.; Manners, I. Functional block copolymers: Nanostructured materials with emerging applications. Angew. Chem. Int. Ed. 2012, 51, 7898–7921. [Google Scholar] [CrossRef]
- Cummins, C.; Ghoshal, T.; Holmes, J.D.; Morris, M.A. Strategies for Inorganic Incorporation using Neat Block Copolymer Thin Films for Etch Mask Function and Nanotechnological Application. Adv. Mater. 2016, 28, 5586–5618. [Google Scholar] [CrossRef] [PubMed]
- Prochukhan, N.; Selkirk, A.; Lundy, R.; Giraud, E.C.; Ghoshal, T.; Downing, C.; Morris, M.A. Large-Area Fabrication of Vertical Silicon Nanotube Arrays via Toroidal Micelle Self-Assembly. Langmuir 2021, 37, 1932–1940. [Google Scholar] [CrossRef]
- IRDS International Roadmap For Devices and Systems 2020 Edition Lithography; IEEE: New York, NY, USA, 2020.
- Cummins, C.; Weingärtner, T.; Morris, M.A. Enabling Large-Area Selective Deposition on Metal-Dielectric Patterns using Polymer Brush Deactivation. J. Phys. Chem. C 2018, 122, 14698–14705. [Google Scholar] [CrossRef]
- Lundy, R.; Yadav, P.; Selkirk, A.; Mullen, E.; Ghoshal, T.; Cummins, C.; Morris, M.A. Optimizing Polymer Brush Coverage To Develop Highly Coherent Sub-5 nm Oxide Films by Ion Inclusion. Chem. Mater. 2019, 31, 9338–9345. [Google Scholar] [CrossRef]
- Mirzaee-sisan, M.; Sereshki, M.; Siadati, M.H.; Eslami-farsani, R. Metamaterials in the World of Materionics Overview of Fabrication Processes. Int. J. Eng. Sci. Invent. 2019, 8, 20–31. [Google Scholar]
- Chaudhari, A.; Ghoshal, T.; Shaw, M.T.; Cummins, C.; Borah, D.; Holmes, J.D.; Morris, M.A. Formation of sub-7 nm feature size PS-b-P4VP block copolymer structures by solvent vapour process. Adv. Patterning Mater. Process. XXXI 2014, 9051, 905110. [Google Scholar] [CrossRef]
- Lundy, R.; Yadav, P.; Prochukhan, N.; Giraud, E.C.; O’Mahony, T.F.; Selkirk, A.; Mullen, E.; Conway, J.; Turner, M.; Daniels, S.; et al. Precise Definition of a “Monolayer Point” in Polymer Brush Films for Fabricating Highly Coherent TiO2 Thin Films by Vapor-Phase Infiltration. Langmuir 2020, 36, 12394–12402. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dunn, A.; Lin, J.; Shi, D. Photothermal Effect of Nanomaterials for Efficient Energy Applications. In Novel Nanomaterials for Biomedical, Environmental and Energy Applications; Elsevier: Cincinnati, OH, USA, 2019; pp. 415–434. ISBN 9780128144978. [Google Scholar]
- Biswas, A.; Bayer, I.S.; Biris, A.S.; Wang, T.; Dervishi, E.; Faupel, F. Advances in top-down and bottom-up surface nanofabrication: Techniques, applications & future prospects. Adv. Colloid Interface Sci. 2012, 170, 2–27. [Google Scholar] [CrossRef] [PubMed]
- Teo, B.K.; Sun, X.H. From top-down to bottom-up to hybrid nanotechnologies: Road to nanodevices. J. Clust. Sci. 2006, 17, 529–540. [Google Scholar] [CrossRef]
- Iqbal, P.; Preece, J.A.; Mendes, P.M. Nanotechnology: The “Top-Down” and “Bottom-Up” Approaches. Supramol. Chem. 2012. [Google Scholar] [CrossRef]
- Steffen, W.; Richardson, K.; Rockstrom, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef] [PubMed]
- Sueyoshi, T.; Ryu, Y. Performance Assessment of the Semiconductor Industry: Measured by DEA Environmental Assessment. Energies 2020, 13, 5998. [Google Scholar] [CrossRef]
- Platzer, M.U.S. Semiconductor Manufacturing: Industry Trends, Global Competition, Federal Policy. Curr. Polit. Econ. U. S. Can. Mex. 2016, 19, 85. [Google Scholar]
- Choi, S.; Yoon, C.; Kim, S.; Kim, W.; Ha, K.; Jeong, J.; Kim, J.; Shin, J.; Park, D. Comprehensive evaluation of hazardous chemical exposure control system at a semiconductor manufacturing company in South Korea. Int. J. Environ. Res. Public Health 2018, 15, 1162. [Google Scholar] [CrossRef] [PubMed]
- Matarazzo, A.; Ingrao, C.; Clasadonte, M.T. Life Cycle Assessment Applied to the Sector of Microelectronic Devices. AIP Conf. Proc. 2016, 1758. [Google Scholar] [CrossRef]
- Kuo, C.H.; Hu, A.H.; Hung, L.H.; Yang, K.T.; Wu, C.H. Life cycle impact assessment of semiconductor packaging technologies with emphasis on ball grid array. J. Clean. Prod. 2020, 276, 124301. [Google Scholar] [CrossRef]
- Briones, R. California Semiconductor Industry Hazardous Waste Source Reduction Assesment Report. 2006. [Google Scholar]
- IRDS International Roadmap for Devices and Systems 2020 Edition Executive Summary. 2020.
- Jang, M.; Yoon, C.; Park, J.; Kwon, O. Evaluation of Hazardous Chemicals with Material Safety Data Sheet and By-products of a Photoresist Used in the Semiconductor-Manufacturing Industry. Saf. Health Work 2019, 10, 114–121. [Google Scholar] [CrossRef]
- Şengül, H.; Theis, T.L.; Ghosh, S. Toward Sustainable Nanoproducts. J. Ind. Ecol. 2008, 12, 329–359. [Google Scholar] [CrossRef]
- Liu, C.-C.; Franke, E.; Mignot, Y.; Xie, R.; Yeung, C.W.; Zhang, J.; Chi, C.; Zhang, C.; Farrell, R.; Lai, K.; et al. Directed self-assembly of block copolymers for 7 nanometre FinFET technology and beyond. Nat. Electron. 2018, 1, 562–569. [Google Scholar] [CrossRef]
- Giraud, E.C.; Mokarian-Tabari, P.; Toolan, D.T.W.; Arnold, T.; Smith, A.J.; Howse, J.R.; Topham, P.D.; Morris, M.A. Highly Ordered Titanium Dioxide Nanostructures via a Simple One-Step Vapor-Inclusion Method in Block Copolymer Films. ACS Appl. Nano Mater. 2018, 1, 3426–3434. [Google Scholar] [CrossRef]
- Nieuwlaar, E. Life Cycle Assessment and Energy Systems. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Verones, F.; Bare, J.; Bulle, C.; Frischknecht, R.; Hauschild, M.; Hellweg, S.; Henderson, A.; Jolliet, O.; Laurent, A.; Liao, X.; et al. LCIA framework and cross-cutting issues guidance within the UNEP-SETAC Life Cycle Initiative. J. Clean. Prod. 2017, 161, 957–967. [Google Scholar] [CrossRef]
- Harland, J.; Reichelt, T.; Yao, M. Environmental sustainability in the semiconductor industry. In Proceedings of the 2008 IEEE International Symposium on Electronics and the Environment, San Francisco, CA, USA, 19–22 May 2008; pp. 1–6. [Google Scholar]
- Cucurachi, S.; Van Der Giesen, C.; Guinée, J. Ex-ante LCA of emerging technologies. Procedia CIRP 2018, 69, 463–468. [Google Scholar] [CrossRef]
- Boyd, S.B.; Horvath, A.; Dornfeld, D. Life-Cycle Energy Demand and Global Warming Potential of Computational Logic. Environ. Sci. Technol. 2009, 43, 7303–7309. [Google Scholar] [CrossRef] [PubMed]
- Vijver, M.G.; Blanco, C.F.; Cucurachi, S.; Guin, J.B.; Peijnenburg, W.J.G.M.; Trattnig, R.; Heijungs, R. Assessing the sustainability of emerging technologies: A probabilistic LCA method applied to advanced photovoltaics. J. Clean. Prod. 2020, 259, 120968. [Google Scholar]
- Lee, K.-H.; Kim, J.-W. Integrating Suppliers into Green Product Innovation Development: An Empirical Case Study in the Semiconductor Industry. Bus. Strateg. Environ. 2011, 20, 527–538. [Google Scholar] [CrossRef]
- IRDS International Roadmap For Devices and Systems 2017 Environmental, Safety, Health, and Sustainability. 2017.
- Villard, A.; Lelah, A.; Brissaud, D.; Villard, A.; Lelah, A.; Brissaud, D.; Villard, A.; Lelah, A.; Brissaud, D. Drawing a chip environmental profile: Environmental indicators for the semiconductor industry. J. Clean. Prod. 2015, 86, 98–109. [Google Scholar] [CrossRef]
- Schmidt, M.; Hottenroth, H.; Schottler, M.; Fetzer, G.; Schlüter, B. Life cycle assessment of silicon wafer processingfor microelectronic chips and solar cells. Int. J. Life Cycle Assess. 2012, 17, 126–144. [Google Scholar] [CrossRef]
- Higgs, T.; Cullen, M.; Yao, M.; Stewart, S. Developing an overall CO2 footprint for semiconductor products. In Proceedings of the 2009 IEEE International Symposium on Sustainable Systems and Technology, Tempe, AZ, USA, 18–20 May 2009; pp. 1–6. [Google Scholar]
- Hu, S.C.; Shiue, A.; Chuang, H.C.; Xu, T. Life cycle assessment of high-technology buildings: Energy consumption and associated environmental impacts of wafer fabrication plants. Energy Build. 2013, 56, 126–133. [Google Scholar] [CrossRef]
- Murphy, C.F.; Laurent, J.; Allen, D.T. Semiconductor Manufacturing. 276–281.
- Hasan, R.M.M.; Luo, X. Promising Lithography Techniques for Next-Generation Logic Devices. Nanomanufacturing Metrol. 2018, 1, 67–81. [Google Scholar] [CrossRef]
- Harriott, L.R. Limits of lithography. Proc. IEEE 2001, 89, 366–374. [Google Scholar] [CrossRef]
- Mallik, A.; Vansumere, W.; Ryckaert, J.; Mercha, A.; Horiguchi, N.; Bömmels, J.; Zsolt, T.; Vandenberghe, G.; Ronse, K.; Thean, A.; et al. The need for EUV lithography at advanced technology for sustainable wafer cost. SPIE Adv. Lithogr. 2013, 8679, 1–10. [Google Scholar] [CrossRef]
- Hwang, B.; Huang, C.; Wu, C. A TOE Approach to Establish a Green Supply Chain Adoption Decision Model in the Semiconductor Industry. Sustainability 2016. [Google Scholar] [CrossRef]
- Krishnan, N.; Boyd, S.; Somani, A.; Raoux, S.; Clark, D.; Dornfeld, D. A hybrid life cycle inventory of nano-scale semiconductor manufacturing. Environ. Sci. Technol. 2008, 42, 3069–3075. [Google Scholar] [CrossRef] [PubMed]
- Hoefflinger, B. ITRS: The International Technology Roadmap for Semiconductors. In Chips 2020; Springer: Berlin/Heidelberg, Germany, 2011; pp. 161–174. [Google Scholar]
- Nie, Z.; Kumacheva, E. Patterning surfaces with functional polymers. Nat. Mater. 2008, 7, 277–290. [Google Scholar] [CrossRef]
- Mokarian-Tabari, P.; Senthamaraikannan, R.; Glynn, C.; Collins, T.W.; Cummins, C.; Nugent, D.; O’Dwyer, C.; Morris, M.A. Large Block Copolymer Self-Assembly for Fabrication of Subwavelength Nanostructures for Applications in Optics. Nano Lett. 2017, 17, 2973–2978. [Google Scholar] [CrossRef]
- Kim, H.-C.; Park, S.-M.; Hinsberg, W.D. Block Copolymer Based Nanostructures: Materials, Processes, and Applications to Electronics. Chem. Rev. 2010, 110, 146–177. [Google Scholar] [CrossRef]
- Brassat, K.; Kool, D.; Nallet, C.G.A.; Lindner, J.K.N. Understanding Film Thickness-Dependent Block Copolymer Self-Assembly by Controlled Polymer Dewetting on Prepatterned Surfaces. Adv. Mater. Interfaces 2020, 7, 1901605. [Google Scholar] [CrossRef]
- Löfstrand, A.; Svensson, J.; Wernersson, L.; Maximov, I. Feature size control using surface reconstruction temperature in block Feature size control using surface reconstruction temperature in block copolymer lithography for InAs nanowire growth. Nanotechnology 2020, 31, 325303. [Google Scholar] [CrossRef]
- Peng, Q.; Tseng, Y.-C.; Darling, S.B.; Elam, J.W. A Route to Nanoscopic Materials via Sequential Infiltration Synthesis on Block Copolymer Templates. ACS Nano 2011, 5, 4600–4606. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.A.; Berggren, K.K.; Cheng, J.Y.; Jung, Y.S.; Chang, J.B. Three-dimensional nanofabrication by block copolymer self-assembly. Adv. Mater. 2014, 26, 4386–4396. [Google Scholar] [CrossRef] [PubMed]
- Poelma, J.E.; Ono, K.; Miyajima, D.; Aida, T.; Satoh, K.; Hawker, C.J. Cyclic block copolymers for controlling feature sizes in block copolymer lithography. ACS Nano 2012, 6, 10845–10854. [Google Scholar] [CrossRef] [PubMed]
- Milner, S.T. Polymer Brushes. Science 1991, 251, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Snelgrove, M.; Zehe, C.; Lundy, R.; Yadav, P.; Rueff, J.; O’Connor, R.; Bogan, J.; Hughes, G.; McGlynn, E.; Morris, M.; et al. Surface characterization of poly-2-vinylpyridine—A polymer for area selective deposition techniques. J. Vac. Sci. Technol. A 2019, 37, 050601. [Google Scholar] [CrossRef]
- Hwang, D.H.; Nomura, A.; Kim, J.; Kim, J.H.; Cho, H.; Lee, C.; Ohno, K.; Tsujii, Y. Synthesis and characterization of polystyrene brushes for organic thin film transistors. J. Nanosci. Nanotechnol. 2012, 12, 4137–4141. [Google Scholar] [CrossRef]
- Oria, L.; Ruiz De Luzuriaga, A.; Alduncin, J.A.; Perez-Murano, F. Polystyrene as a brush layer for directed self-assembly of block co-polymers. Microelectron. Eng. 2013, 110, 234–240. [Google Scholar] [CrossRef]
- Durand, W.J.; Blachut, G.; Maher, M.J.; Sirard, S.; Tein, S.; Carlson, M.C.; Asano, Y.; Zhou, S.X.; Lane, A.P.; Bates, C.M.; et al. Design of High- v Block Copolymers for Lithography. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 344–352. [Google Scholar] [CrossRef]
- Nakajima, H.; Dijkstra, P.; Loos, K. The recent developments in biobased polymers toward general and engineering applications: Polymers that are upgraded from biodegradable polymers, analogous to petroleum-derived polymers, and newly developed. Polymers (Basel) 2017, 9, 523. [Google Scholar] [CrossRef]
- Scholten, P.B.V.; Moatsou, D.; Detrembleur, C.; Meier, M.A.R. Progress Toward Sustainable Reversible Deactivation Radical Polymerization. Macromol. Rapid Commun. 2020, 41, 2000266. [Google Scholar] [CrossRef]
- Gbabode, G.; Delvaux, M.; Schweicher, G.; Andreasen, J.W.; Nielsen, M.M.; Geerts, Y.H. Unique Crystal Orientation of Poly(ethylene oxide) Thin Films by Crystallization Using a Thermal Gradient. Macromolecules 2017, 50, 5877–5891. [Google Scholar] [CrossRef]
- Miyazaki, T.; Igarashi, K.; Matsumoto, Y.; Cabral, H. One-Pot Synthesis of PEG-Poly(amino acid) Block Copolymers Assembling Polymeric Micelles with PEG-Detachable Functionality. ACS Biomater. Sci. Eng. 2019, 5, 5727–5733. [Google Scholar] [CrossRef] [PubMed]
- Rabotyagova, O.S.; Cebe, P.; Kaplan, D.L. Protein-based block copolymers. Biomacromolecules 2011, 12, 269–289. [Google Scholar] [CrossRef] [PubMed]
- Banta, R.A.; Collins, T.W.; Curley, R.A.; Young, P.W.; Holmes, J.D.; Flynn, E.J. Nanopatterned protein-polysaccharide thin films by humidity regulated phase separation. J. Colloid Interface Sci. 2018, 532, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.W.; Park, S.; Bang, J.H.; Chae, S.; Song, K.; Lee, S.W. Nonpolar surface modification using fatty acids and its effect on calcite from mineral carbonation of desulfurized gypsum. Coatings 2018, 8, 43. [Google Scholar] [CrossRef]
- Micciulla, S.; Hayward, D.W.; Gerelli, Y.; Panzarella, A.; von Klitzing, R.; Gradzielski, M.; Chiappisi, L. One-step procedure for the preparation of functional polysaccharide/fatty acid multilayered coatings. Commun. Chem. 2019, 2. [Google Scholar] [CrossRef]
- Wang, S.; Vajjala Kesava, S.; Gomez, E.D.; Robertson, M.L. Sustainable thermoplastic elastomers derived from fatty acids. Macromolecules 2013, 46, 7202–7212. [Google Scholar] [CrossRef]
- Buzzacchera, I.; Vorobii, M.; Kostina, N.Y.; De Los Santos Pereira, A.; Riedel, T.; Bruns, M.; Ogieglo, W.; Möller, M.; Wilson, C.J.; Rodriguez-Emmenegger, C. Polymer Brush-Functionalized Chitosan Hydrogels as Antifouling Implant Coatings. Biomacromolecules 2017, 18, 1983–1992. [Google Scholar] [CrossRef]
- Mello, R.S.; Bedendo, G.C.; Nome, F.; Fiedler, H.D.; Laranjeira, M.C.M. Preparation of chitosan membranes for filtration and concentration of compounds under high pressure process. Polym. Bull. 2006, 56, 447–454. [Google Scholar] [CrossRef]
- Swain, S.N.; Biswal, S.M.; Nanda, P.K.; Nayak, P.L. Biodegradable Soy-Based Plastics: Opportunities and Challenges. J. Polym. Environ. 2004, 12, 35–42. [Google Scholar] [CrossRef]
- Sessa, D.J.; Mohamed, A.; Byars, J.A.; Hamaker, S.A.H.; Selling, G.W. Properties of films from corn zein reacted with glutaraldehyde. J. Appl. Polym. Sci. 2007, 105, 2877–2883. [Google Scholar] [CrossRef]
- Horniblow, R.D.; Dowle, M.; Iqbal, T.H.; Latunde-Dada, G.O.; Palmer, R.E.; Pikramenou, Z.; Tselepis, C. Alginate-iron speciation and its effect on in vitro cellular iron metabolism. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Braun, H.G.; Meyer, E. Structure formation of ultrathin PEO films at solid interfaces-complex pattern formation by dewetting and crystallization. Int. J. Mol. Sci. 2013, 14, 3254–3264. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, F.; Song, X. Preparation and characterization of lignin-based membrane material. BioResources 2015, 10, 5586–5595. [Google Scholar] [CrossRef]
- Shrotri, A.; Kobayashi, H.; Fukuoka, A. Catalytic Conversion of Structural Carbohydrates and Lignin to Chemicals. In Advances in Catalysis; Academic Press Inc.: Cambridge, MA, USA, 2017; Volume 60, pp. 59–123. [Google Scholar]
- Hong, M.; Chen, E.Y.X. Completely recyclable biopolymers with linear and cyclic topologies via ring-opening polymerization of γ-butyrolactone. Nat. Chem. 2016, 8, 42–49. [Google Scholar] [CrossRef]
- Nazarzadeh Zare, E.; Mansour Lakouraj, M.; Mohseni, M. Biodegradable polypyrrole/dextrin conductive nanocomposite: Synthesis, characterization, antioxidant and antibacterial activity. Synth. Met. 2014, 187, 9–16. [Google Scholar] [CrossRef]
- Sun, C.; Gao, L.; Wang, D.; Zhang, M.; Liu, Y.; Geng, Z.; Xu, W.; Liu, F.; Bian, H. Biocompatible polypyrrole-block copolymer-gold nanoparticles platform for determination of inosine monophosphate with bi-enzyme biosensor. Sens. Actuators B Chem. 2016, 230, 521–527. [Google Scholar] [CrossRef]
- Mondal, D.; Mollick, M.M.R.; Bhowmick, B.; Maity, D.; Bain, M.K.; Rana, D.; Mukhopadhyay, A.; Dana, K.; Chattopadhyay, D. Effect of poly(vinyl pyrrolidone) on the morphology and physical properties of poly(vinyl alcohol)/sodium montmorillonite nanocomposite films. Prog. Nat. Sci. Mater. Int. 2013, 23, 579–587. [Google Scholar] [CrossRef]
- Kumar, N.; Ravikumar, M.N.V.; Domb, A.J. Biodegradable block copolymers. Adv. Drug Deliv. Rev. 2001, 53, 23–44. [Google Scholar] [CrossRef]
- Kreiger, M.; Pearce, J.M. Environmental life cycle analysis of distributed three-dimensional printing and conventional manufacturing of polymer products. ACS Sustain. Chem. Eng. 2013, 1, 1511–1519. [Google Scholar] [CrossRef]
- Morão, A.; de Bie, F. Life Cycle Impact Assessment of Polylactic Acid (PLA) Produced from Sugarcane in Thailand. J. Polym. Environ. 2019, 27, 2523–2539. [Google Scholar] [CrossRef]
- Gironi, V.P.S.S.F. Chemical Recycling of PLA: A Great Opportunity Towards the Sustainable Development? J. Polym. Environ. 2013, 21, 640–647. [Google Scholar] [CrossRef]
- Erdmenger, T.; Guerrero-Sanchez, C.; Vitz, J.; Hoogenboom, R.; Schubert, U.S. Recent developments in the utilization of green solvents in polymer chemistry. Chem. Soc. Rev. 2010, 39, 3317–3333. [Google Scholar] [CrossRef] [PubMed]
- Renoud, P.; Toury, B.; Benayoun, S.; Attik, G.; Grosgogeat, B. Functionalization of titanium with chitosan via silanation: Evaluation of biological and mechanical performances. PLoS ONE 2012, 7, 39367. [Google Scholar] [CrossRef]
- Mayeda, M.K.; Hayat, J.; Epps, T.H.; Lauterbach, J. Metal oxide arrays from block copolymer thin film templates. J. Mater. Chem. A 2015, 3, 7822–7829. [Google Scholar] [CrossRef]
- Lundy, R.; Flynn, S.P.; Cummins, C.; Kelleher, S.M.; Collins, M.N.; Dalton, E.; Daniels, S.; Morris, M.A.; Enright, R. Controlled solvent vapor annealing of a high: χ block copolymer thin film. Phys. Chem. Chem. Phys. 2017, 19, 2805–2815. [Google Scholar] [CrossRef] [PubMed]
- Asatekin, A.; Barr, M.C.; Baxamusa, S.H.; Lau, K.K.S.; Tenhaeff, W.; Xu, J.; Gleason, K.K. Designing polymer surfaces via vapor deposition. Mater. Today 2010, 13, 26–33. [Google Scholar] [CrossRef]
- Moni, P.; Al-Obeidi, A.; Gleason, K.K. Vapor deposition routes to conformal polymer thin films. Beilstein J. Nanotechnol. 2017, 8, 723–735. [Google Scholar] [CrossRef]
- Pierson, H.O. Handbook of Chemical Vapor Deposition (CVD) Principles, Technology, and Applications, 2nd ed.; Noyes Publications: Park Ridge, NJ, USA; William Andrew Publishing, LLC Norwich: New York, NY, USA, 1999; ISBN 0815514328. [Google Scholar]
- Choy, K. Chemical vapour deposition of coatings. Prog. Mater. Sci. 2003, 48, 57–170. [Google Scholar] [CrossRef]
- Gleason, K.K. Nanoscale control by chemically vapour-deposited polymers. Nat. Rev. Phys. 2020, 2, 347–364. [Google Scholar] [CrossRef]
- Reeja-Jayan, B.; Kovacik, P.; Yang, R.; Sojoudi, H.; Ugur, A.; Kim, D.H.; Petruczok, C.D.; Wang, X.; Liu, A.; Gleason, K.K. A Route Towards Sustainability Through Engineered Polymeric Interfaces. Adv. Mater. Interfaces 2014, 1, 1400117. [Google Scholar] [CrossRef]
- Qiu, M.; Chen, X.; Fan, Y.; Xing, W. 1.11 Ceramic Membranes. Compr. Membr. Sci. Eng. 2017, 1, 270–297. [Google Scholar] [CrossRef]
- Peng, S.; Bhushan, B. Smart polymer brushes and their emerging applications. RSC Adv. 2012, 2, 8557–8578. [Google Scholar] [CrossRef]
- Ghoshal, T.; O’Connell, J.; Sinturel, C.; Andreazza, P.; Holmes, J.D.; Morris, M.A. Solvent mediated inclusion of metal oxide into block copolymer nanopatterns: Mechanism of oxide formation under UV-Ozone treatment. Polymer (Guildf) 2019, 173, 197–204. [Google Scholar] [CrossRef]
- Brassat, K.; Lindner, J.K.N. Nanoscale Block Copolymer Self-Assembly and Microscale Polymer Film Dewetting: Progress in Understanding the Role of Interfacial Energies in the Formation of Hierarchical Nanostructures. Adv. Mater. Interfaces 2020, 7. [Google Scholar] [CrossRef]
- Gu, X. Self-assembly of Block copolymers by solvent vapor annealing, mechanism and lithographic applications. Ph.D. Thesis, University of Massachusetts-Amherst, Amherst, MA, USA, 2014. [Google Scholar]
- Sinturel, C.; Vayer, M.; Morris, M.; Hillmyer, M.A. Solvent vapor annealing of block polymer thin films. Macromolecules 2013, 46, 5399–5415. [Google Scholar] [CrossRef]
- Gu, X.; Gunkel, I.; Hexemer, A.; Russell, T.P. Controlling Domain Spacing and Grain Size in Cylindrical Block Copolymer Thin Films by Means of Thermal and Solvent Vapor Annealing. Macromolecules 2016. [Google Scholar] [CrossRef]
- Höfer, R.; Bigorra, J. Green chemistry—a sustainable solution for industrial specialties applications. Green Chem. 2007, 9, 203–221. [Google Scholar] [CrossRef]
- Chavis, M.A.; Smilgies, D.M.; Wiesner, U.B.; Ober, C.K. Widely tunable morphologies in block copolymer thin films through solvent vapor annealing using mixtures of selective solvents. Adv. Funct. Mater. 2015, 25, 3057–3065. [Google Scholar] [CrossRef]
- Gotrik, K.W.; Ross, C.A. Solvothermal annealing of block copolymer thin films. Nano Lett. 2013, 13, 5117–5122. [Google Scholar] [CrossRef] [PubMed]
- Gotrik, K.W.; Hannon, A.F.; Son, J.G.; Keller, B.; Alexander-Katz, A.; Ross, C.A. Morphology control in block copolymer films using mixed solvent vapors. ACS Nano 2012, 6, 8052–8059. [Google Scholar] [CrossRef]
- Cummins, C.; Kelly, R.A.; Gangnaik, A.; Georgiev, Y.M.; Petkov, N.; Holmes, J.D.; Morris, M.A. Solvent vapor annealing of block copolymers in confined topographies: Commensurability considerations for nanolithography. Macromol. Rapid Commun. 2015, 36, 762–767. [Google Scholar] [CrossRef]
- Nikles, S.M.; Piao, M.; Lane, A.M.; Nikles, D.E. Ethyl lactate: A green solvent for magnetic tape coating. Green Chem. 2001, 3, 109–113. [Google Scholar] [CrossRef]
- Kua, Y.L.; Gan, S.; Ng, H.K.; Morris, A. The potential of ethyl lactate as a green solvent to extract carotenoids and vitamin e from crude palm oil. In Proceedings of the ISPFVF, University of Nottingham Malaysia Campus, Semenyih, Malaysia, 11–13 August 2014; pp. 244–251. [Google Scholar]
- Jessop, P.G. Searching for green solvents. Green Chem. 2011, 13, 1391–1398. [Google Scholar] [CrossRef]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 7. [Google Scholar] [CrossRef]
- Amelio, A.; Genduso, G.; Vreysen, S.; Luis, P.; Van Der Bruggen, B. Guidelines based on life cycle assessment for solvent selection during the process design and evaluation of treatment alternatives. Green Chem. 2014, 16, 3045–3063. [Google Scholar] [CrossRef]
- Mallakpour, S.; Rafiee, Z. Ionic Liquids as Environmentally Friendly Solvents in Macromolecules Chemistry and Technology, Part I. J. Polym. Environ. 2011, 19, 447–484. [Google Scholar] [CrossRef]
- Hulkkonen, H.; Salminen, T.; Niemi, T. Automated solvent vapor annealing with nanometer scale control of film swelling for block copolymer thin films. Soft Matter 2019, 15, 7909–7917. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Böker, A.; Tsarkova, L. Temperature-controlled solvent vapor annealing of thin block copolymer films. Polymers (Basel) 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Selkirk, A.; Prochukhan, N.; Lundy, R.; Cummins, C.; Gatensby, R.; Kilbride, R.; Parnell, A.; Vasquez, J.B.; Morris, M.; Mokarian-tabari, P. Optimization and Control of Large Block Copolymer Self-Assembly via Precision Solvent Vapor Annealing. Macromolecules 2021. [Google Scholar] [CrossRef]
- Wan, Z.; Lee, H.J.; Kim, H.G.; Jo, G.C.; Park, W.I.; Ryu, S.W.; Lee, H.; Kwon, S. Circular Double-Patterning Lithography Using a Block Copolymer Template and Atomic Layer Deposition. Adv. Mater. Interfaces 2018, 1800054, 1–9. [Google Scholar] [CrossRef]
- Singer, J.P.; Gotrik, K.W.; Lee, J.H.; Kooi, S.E.; Ross, C.A.; Thomas, E.L. Alignment and reordering of a block copolymer by solvent-enhanced thermal laser direct write. Polymer (Guildf) 2014, 55, 1875–1882. [Google Scholar] [CrossRef]
- Leniart, A.A.; Pula, P.; Sitkiewicz, A.; Majewski, P.W. Macroscopic Alignment of Block Copolymers on Silicon Substrates by Laser Annealing. ACS Nano 2020, 14, 4805–4815. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Cummins, C.; Morris, M.A. Using block copolymers as infiltration sites for development of future nanoelectronic devices: Achievements, barriers, and opportunities. Microelectron. Eng. 2018, 195, 74–85. [Google Scholar] [CrossRef]
- Subramanian, A.; Doerk, G.; Kisslinger, K.; Yi, D.H.; Grubbs, R.B.; Nam, C.-Y. Three-dimensional electroactive ZnO nanomesh directly derived from hierarchically self-assembled block copolymer thin films. Nanoscale 2019, 11, 9533–9546. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tiwale, N.; Doerk, G.; Kisslinger, K.; Nam, C.Y. Enhanced Hybridization and Nanopatterning via Heated Liquid-Phase Infiltration into Self-Assembled Block Copolymer Thin Films. ACS Appl. Mater. Interfaces 2020, 12, 1444–1453. [Google Scholar] [CrossRef]
- Snelgrove, M.; McFeely, C.; Mani-Gonzalez, P.G.; Lahtonen, K.; Lundy, R.; Hughes, G.; Valden, M.; McGlynn, E.; Yadav, P.; Saari, J.; et al. Aluminium oxide formation via atomic layer deposition using a polymer brush mediated selective infiltration approach. Appl. Surf. Sci. 2020, 515, 145987. [Google Scholar] [CrossRef]
- Ghoshal, T.; Shaw, M.T.; Holmes, J.D.; Morris, M.A. Development of a facile block copolymer method for creating hard mask patterns integrated into semiconductor manufacturing. Nano Res. 2016, 9, 3116–3128. [Google Scholar] [CrossRef]
- Park, J.T.; Koh, J.H.; Lee, K.J.; Seo, J.A.; Min, B.R.; Kim, J.H. Formation of silver nanoparticles created in situ in an amphiphilic block copolymer film. J. Appl. Polym. Sci. 2008, 110, 2352–2357. [Google Scholar] [CrossRef]
- Akinoglu, G.E.; Mir, S.H.; Gatensby, R.; Rydzek, G.; Mokarian-Tabari, P. Block Copolymer Derived Vertically Coupled Plasmonic Arrays for Surface-Enhanced Raman Spectroscopy. ACS Appl. Mater. Interfaces 2020, 12, 23410–23416. [Google Scholar] [CrossRef]
- Pease, R.F.; Chou, S.Y. Lithography and Other Patterning Techniques for Future Electronics. Proc. IEEE 2008, 96, 248–270. [Google Scholar] [CrossRef]
- IEEE International Roadmap for Devices and Systems 2017 - Emerging Research Matterials. 2018.
- Liddle, J.A.; Bowser, J.; Ilic, B.R.; Luciani, V. So, You Want to Have a Nanofab? Shared-Use Nanofabrication and Characterization Facilities: Cost-of-Ownership, Toolset, Utilization, and Lessons Learned. J. Res. Natl. Inst. Stand. Technol. 2020, 125, 125009. [Google Scholar] [CrossRef]
- Wan, L.; Ruiz, R.; Gao, H.; Patel, K.C.; Albrecht, T.R.; Yin, J.; Kim, J.; Cao, Y.; Lin, G. The Limits of Lamellae-Forming PS- b -PMMA Block Copolymers for Lithography. ACS Nano 2015, 9, 7506–7514. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, S.V. Nanoimprint lithography steppers for volume fabrication of leading-edge semiconductor integrated circuits. Microsyst. Nanoeng. 2017, 3, 17075. [Google Scholar] [CrossRef] [PubMed]
- Higashiki, T. Nanoimprint lithography and future patterning for semiconductor devices. J. Micro/Nanolithography MEMS MOEMS 2011, 10, 043008. [Google Scholar] [CrossRef]
- AXA XL Risk Consulting Semiconductor Manufacturing. Hartford, CT, USA. 2020.
- Naulleau, P. Optical Lithography. In Comprehensive Nanoscience and Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 387–398. [Google Scholar]
- Sanders, D.P. Advances in Patterning Materials for 193 nm Immersion Lithography. Chem. Rev. 2010, 110, 321–360. [Google Scholar] [CrossRef]
- Vigneswaran, N.; Samsuri, F.; Ranganathan, B. Padmapriya Recent Advances in Nano Patterning and Nano Imprint Lithography for Biological Applications. Procedia Eng. 2014, 97, 1387–1398. [Google Scholar] [CrossRef]
- Manipulation and Patterning of Surfaces (Nanolithography). In Fundamentals and Applications of Nano Silicon in Plasmonics and Fullerines; Elsevier: Amsterdam, The Netherlands, 2018; pp. 89–137.
- Mack, C.A. Field Guide to Optical Lithography; SPIE Press: Bellingham, WA, USA, 2006; Volume 6, ISBN 0819462071. [Google Scholar]
- Huang, S.Z.; Wu, K.Y. Health Risk Assessment of Photoresists Used in an Optoelectronic Semiconductor Factory. Risk Anal. 2019, 39, 2625–2639. [Google Scholar] [CrossRef]
- Museau, M.; Tichkiewitch, S. Integrated design of mems: Aiming at manufacturability. Int. J. Interact. Des. Manuf. (IJIDeM) 2007, 1, 127–134. [Google Scholar] [CrossRef]
- Chaniago, Y.D.; Hussain, A.; Andika, R.; Lee, M. Reactive Pressure-Swing Distillation toward Sustainable Process of Novel Continuous Ultra-High-Purity Electronic-Grade Propylene Glycol Monomethyl Ether Acetate Manufacture. ACS Sustain. Chem. Eng. 2019. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, F.; Lin, Y.; Duan, W.; Liu, Q. Chemosphere Four types of attenuation of phenol and cresols in microcosms under simulated marine conditions: A kinetic study. Chemosphere 2017, 185, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Bahadar, H.; Mostafalou, S.; Abdollahi, M. Current understandings and perspectives on non-cancer health effects of benzene: A global concern. Toxicol. Appl. Pharmacol. 2014, 276, 83–94. [Google Scholar] [CrossRef]
- van de Kerkhof, M.A.; Benschop, J.P.H.; Banine, V.Y. Lithography for now and the future. Solid. State. Electron. 2019, 155, 20–26. [Google Scholar] [CrossRef]
- Wong, A.K. Microlithography: Trends, challenges, solutions, and their impact on design. IEEE Micro 2003, 23, 12–21. [Google Scholar] [CrossRef]
- Chen, Y.; Xiong, S. Directed self-assembly of block copolymers for sub-10 nm fabrication. Int. J. Extrem. Manuf. 2020, 2. [Google Scholar]
- Wang, C.H.; Huang, C.Y.; Yak, H.K.; Hsieh, H.C.; Wang, J.L. Identifying an unknown compound in flue gas of semiconductor industry – Forensics of a perfluorocarbon. Chemosphere 2021, 264, 128504. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.B.; Chang, J.S.; Chang, M.B.; Chang, J.S. Abatement of PFCs from Semiconductor Manufacturing Processes by Nonthermal Plasma Technologies: A Critical Review. Ind. Eng. Chem. Res. 2006, 45, 4101–4109. [Google Scholar] [CrossRef]
- Mariussen, E.; Fonnum, F.; Mariussen, E. Critical Reviews in Toxicology Neurochemical Targets and Behavioral Effects of Organohalogen Compounds: An Update Neurochemical Targets and Behavioral Effects of Organohalogen Compounds: An Update. Crit. Rev. Toxicol. 2008, 8444. [Google Scholar] [CrossRef]
- Dietz, R.; Gustavson, K.; Sonne, C.; Desforges, J.; Rigét, F.F.; Pavlova, V.; Mckinney, M.A.; Letcher, R.J. Physiologically-based pharmacokinetic modelling of immune, re- productive and carcinogenic effects from contaminant exposure in polar bears (Ursus maritimus) across the Arctic. Environ. Res. 2015, 140, 45–55. [Google Scholar] [CrossRef]
- Murata, T.; Ohtsuka, M.; Hotta, K.; Fujisawa, M. Conversion from helium to nitrogen as a TEOS carrier gas in sub-atmospheric chemical vapor deposition. In Proceedings of the 2013 e-Manufacturing & Design Collaboration Symposium (eMDC), Hsinchu, Taiwan, 6 September 2013; pp. 1–4. [Google Scholar]
- Wu, B.; Kumar, A. Extreme ultraviolet lithography: A review. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 2007, 25, 1743. [Google Scholar] [CrossRef]
- Ghosh, S.; Satyanarayana, V.S.V.; Pramanick, B.; Sharma, S.K.; Pradeep, C.P.; Morales-Reyes, I.; Batina, N.; Gonsalves, K.E. Patterning highly ordered arrays of complex nanofeatures through EUV directed polarity switching of non chemically amplified photoresist. Sci. Rep. 2016, 6, 22664. [Google Scholar] [CrossRef] [PubMed]
- van de Kerkhof, M.; Jasper, H.; Levasier, L.; Peeters, R.; van Es, R.; Bosker, J.-W.; Zdravkov, A.; Lenderink, E.; Evangelista, F.; Broman, P.; et al. Enabling sub-10nm node lithography: Presenting the NXE:3400B EUV scanner. In Proceedings of the SPIE; Panning, E.M., Goldberg, K.A., Eds.; 2017; Volume 10143, p. 101430D. [Google Scholar]
- Choi, S.; Park, D.; Park, Y. Possibility of Benzene Exposure in Workers of a Semiconductor Industry Based on the Patent Resources, 1990–2010. Saf. Health Work 2021, 1990–2010. [Google Scholar] [CrossRef]
- Turkot, B.; Carson, S.; Lio, A. Continuing moore’s law with EUV lithography. Tech. Dig. Int. Electron Devices Meet. IEDM 2018, 14.4.1–14.4.3. [Google Scholar] [CrossRef]
- Van Schoot, J.; Schift, H. Next-generation lithography–an outlook on EUV projection and nanoimprint. Adv. Opt. Technol. 2017, 6, 159–162. [Google Scholar] [CrossRef]
- Itani, T.; Kozawa, T. Resist Materials and Processes for Extreme Ultraviolet Lithography. Jpn. J. Appl. Phys. 2013, 52, 010002. [Google Scholar] [CrossRef]
- De Silva, A.; Felix, N.M.; Ober, C.K. Molecular Glass Resists as High-Resolution Patterning Materials. Adv. Mater. 2008, 20, 3355–3361. [Google Scholar] [CrossRef]
- Mojarad, N.; Gobrecht, J.; Ekinci, Y. Beyond EUV lithography: A comparative study of efficient photoresists’ performance. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef]
- Barcelo, S.; Li, Z. Nanoimprint lithography for nanodevice fabrication. Nano Converg. 2016, 3, 21. [Google Scholar] [CrossRef]
- Havard, J.M.; Shim, S.Y.; Fréchet, J.M.J.; Lin, Q.; Medeiros, D.R.; Grant Willson, C.; Byers, J.D. Design of photoresists with reduced environmental impact. 1. Water-soluble resists based on photo-cross-linking of poly(vinyl alcohol). Chem. Mater. 1999, 11, 719–725. [Google Scholar] [CrossRef]
- Tormen, M.; Sovernigo, E.; Pozzato, A.; Pianigiani, M.; Tormen, M. Sub-100 l s nanoimprint lithography at wafer scale. Microelectron. Eng. 2015, 141, 21–26. [Google Scholar] [CrossRef]
- Cox, L.M.; Martinez, A.M.; Blevins, A.K.; Sowan, N.; Ding, Y.; Bowman, C.N. Nanoimprint lithography: Emergent materials and methods of actuation. Nano Today 2020, 31, 100838. [Google Scholar] [CrossRef]
- Kwon, B.; Kim, J.H. Importance of Molds for Nanoimprint Lithography: Hard, Soft, and Hybrid Molds. J. Nanosci. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Schift, H. Nanoimprint lithography: An old story in modern times? A review. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 2008, 26, 458. [Google Scholar] [CrossRef]
- Nicaise, S.M.; Tavakkoli, K.G.A.; Berggren, K.K. Self-Assembly of Block Copolymers by Graphoepitaxy; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 9780081002506. [Google Scholar]
- Doble, M.; Kruthiventi, A.K. Industrial Examples. In Green Chemistry and Engineering; Elsevier: Amsterdam, The Netherlands, 2007; pp. 245–296. [Google Scholar]
- Iacopi, F.; McIntosh, M. Opportunities and perspectives for green chemistry in semiconductor technologies. Green Chem. 2019, 21, 3250–3255. [Google Scholar] [CrossRef]
- Shen, C.; Tran, P.; Minh Ly, P. Chemical Waste Management in the U.S. Semiconductor Industry. Sustainability 2018, 10, 1545. [Google Scholar] [CrossRef]
- Schischke, K.; Stutz, M.; Ruelle, J.-P.; Griese, H.; Reichl, H. Life cycle inventory analysis and identification of environmentally significant aspects in semiconductor manufacturing. In Proceedings of the 2001 IEEE International Symposium on Electronics and the Environment. 2001 IEEE ISEE (Cat. No.01CH37190), Denver, CO, USA, 9 May 2001; pp. 145–150. [Google Scholar]
- Hsu, L.; Huang, C.; Chuang, Y.; Chen, H.; Chan, Y. Accumulation of heavy metals and trace elements in fluvial sediments received effluents from traditional and semiconductor industries. Nat. Publ. Gr. 2016, 1–12. [Google Scholar] [CrossRef]
- Suzuki, Y.; Watanabe, I.; Oshida, T.; Chen, Y. Accumulation of trace elements used in semiconductor industry in Formosan squirrel, as a bio-indicator of their exposure, living in Taiwan. Chemosphere 2007, 68, 1270–1279. [Google Scholar] [CrossRef]
- Chaniago, Y.D.; Minh, L.Q.; Khan, M.S.; Koo, K.-K.; Bahadori, A.; Lee, M. Optimal design of advanced distillation configuration for enhanced energy efficiency of waste solvent recovery process in semiconductor industry. Energy Convers. Manag. 2015, 102, 92–103. [Google Scholar] [CrossRef]
- Sun, C.; Rose, T. Supply Chain Complexity in the Semiconductor Industry: Assessment from System View and the Impact of Changes. IFAC-PapersOnLine 2015, 48, 1210–1215. [Google Scholar] [CrossRef]
- Hu, S.-C.; Tsai, Y.-W.; Fu, B.-R.; Chang, C.-K. Assessment of the SEMI energy conversion factor and its application for semiconductor and LCD fabs. Appl. Therm. Eng. 2017, 121, 39–47. [Google Scholar] [CrossRef]
- Hu, S.-C.; Lin, T.; Huang, S.-H.; Fu, B.-R.; Hu, M.-H. Energy savings approaches for high-tech manufacturing factories. Case Stud. Therm. Eng. 2020, 17, 100569. [Google Scholar] [CrossRef]
- ITRS International Technology Roadmap for Semiconductors, 2003 Edition Enviornment, saftey and health. 2003.
- Denkena, B.; Abele, E.; Brecher, C.; Dittrich, M.-A.; Kara, S.; Mori, M. Energy efficient machine tools. CIRP Ann. 2020, 69, 646–667. [Google Scholar] [CrossRef]
- Wang, C.T.; Chiu, C.S. Competitive strategies for Taiwan’s semiconductor industry in a new world economy. Technol. Soc. 2014, 36, 60–73. [Google Scholar] [CrossRef]
- Zimmerman, J.B.; Anastas, P.T.; Erythropel, H.C.; Leitner, W. Designing for a green chemistry future. Science 2020, 367, 397–400. [Google Scholar] [CrossRef]
- Lin, A.Y.; Panchangam, S.C.; Lo, C. The impact of semiconductor, electronics and optoelectronic industries on downstream perfluorinated chemical contamination in Taiwanese rivers. Environ. Pollut. 2009, 157, 1365–1372. [Google Scholar] [CrossRef]
- Loganathan, B.G. Author’s personal copy. Int. Encycl. Public Health 2017. [Google Scholar] [CrossRef]
- Liu, S.; Xia, T. Continued Efforts on Nanomaterial-Environmental Health and Safety Is Critical to Maintain Sustainable Growth of Nanoindustry. Small 2020, 16, 2000603. [Google Scholar] [CrossRef]
- Adam, V.; Nowack, B. European country-specific probabilistic assessment of nanomaterial flows towards landfilling, incineration and recycling. Environ. Sci. Nano 2017, 4, 1961–1973. [Google Scholar] [CrossRef]
- Watjanatepin, P.; Castagnola, V.; Cetin, Y.; Linkov, I.; Skentelbery, C.; Prodanov, D. Workshop Report: Governance of Emerging Nanotechnology Risks in the Semiconductor Industry. Front. Public Health 2020, 8, 275. [Google Scholar] [CrossRef] [PubMed]
- Miseljic, M.; Olsen, S.I. Life-cycle assessment of engineered nanomaterials: A literature review of assessment status. J. Nanoparticle Res. 2014, 16. [Google Scholar] [CrossRef]
- Malakar, A.; Kanel, S.R.; Ray, C.; Snow, D.D.; Nadagouda, M.N. Nanomaterials in the environment, human exposure pathway, and health effects: A review. Sci. Total Environ. 2020, 143470. [Google Scholar] [CrossRef]
- Johnston, L.J.; Gonzalez-Rojano, N.; Wilkinson, K.J.; Xing, B. Key challenges for evaluation of the safety of engineered nanomaterials. NanoImpact 2020, 18, 100219. [Google Scholar] [CrossRef]
- Kim, S.; Yoon, C.; Ham, S.; Park, J.; Kwon, O.; Park, D.; Choi, S.; Kim, S.; Ha, K.; Kim, W. Chemical use in the semiconductor manufacturing industry. Int. J. Occup. Environ. Health 2018, 24, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, N.; Hösel, M.; Jørgensen, M.; Krebs, F.C. Large scale deployment of polymer solar cells on land, on sea and in the air. Energy Environ. Sci. 2014, 7, 855–866. [Google Scholar] [CrossRef]
- IRDS International Roadmap For Devices and Systems 2020 Edition Factory Integration. 2020.
- Anastas, P.T.; Kirchhoff, M.M. Origins, current status, and future challenges of green chemistry. Acc. Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef]
- Tufvesson, L.M.; Tufvesson, P.; Woodley, J.M.; Börjesson, P. Life cycle assessment in green chemistry: Overview of key parameters and methodological concerns. Int. J. Life Cycle Assess. 2013, 18, 431–444. [Google Scholar] [CrossRef]
- European Commission -- Joint Research Centre -- Institute for Environment and Sustainability International Reference Life Cycle Data System (ILCD) Handbook -- General guide for Life Cycle Assessment -- Detailed guidance. 2010; ISBN 978-92-79-19092-6.
- Choi, K.-H.; Kim, H.; Kim, M.-H.; Kwon, H.-J. Semiconductor Work and Adverse Pregnancy Outcomes Associated with Male Workers: A Retrospective Cohort Study. Ann. work Expo. Health 2019, 63, 870–880. [Google Scholar] [CrossRef]
- Plepys, A. The environmental impacts of electronics. Going beyond the walls of semiconductor fabs. IEEE Int. Symp. Electron. Environ. 2004, 159–165. [Google Scholar] [CrossRef]
- Steffen, W.; Rockström, J.; Richardson, K.; Lenton, T.M.; Folke, C.; Liverman, D. Trajectories of the Earth System in the Anthropocene. Proc. Natl. Acad. Sci. USA 2018, 115, 8252–8259. [Google Scholar] [CrossRef]
- Kim, J.H.; Jin, H.M.; Yang, G.G.; Han, K.H.; Yun, T.; Shin, J.Y.; Jeong, S.; Kim, S.O. Smart Nanostructured Materials based on Self-Assembly of Block Copolymers. Adv. Funct. Mater. 2020, 30, 1902049. [Google Scholar] [CrossRef]
- Su, J.C.P.; Wang, L.; Ho, J.C. The timing of green product introduction in relation to technological evolution. J. Ind. Prod. Eng. 2016, 1015, 1–11. [Google Scholar] [CrossRef]
- Ambec, S.; Lanoie, P. Does It Pay to Be Green? A Systematic Overview. Acad. Manag. Perspect. 2008, 22, 45–62. [Google Scholar]
- Kim, H.; Kwon, H.J.; Rhie, J.; Lim, S.; Kang, Y.D.; Eom, S.Y.; Lim, H.; Myong, J.P.; Roh, S. The relationship between spontaneous abortion and female workers in the semiconductor industry. Ann. Occup. Environ. Med. 2017, 29, 1–12. [Google Scholar] [CrossRef]
- Correa, A.; Gray, R.H.; Cohen, R.; Rothman, N.; Shah, F.; Seacat, H.; Corn, M. Ethylene glycol ethers and risks of spontaneous abortion and subfertility. Am. J. Epidemiol. 1996, 143, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Fomenkov, I.; Brandt, D.; Ershov, A.; Schafgans, A.; Tao, Y.; Vaschenko, G.; Rokitski, S.; Kats, M.; Vargas, M.; Purvis, M. Light sources for high-volume manufacturing EUV lithography: Technology, performance, and power scaling. Adv. Opt. Technol. 2017, 6, 173–186. [Google Scholar] [CrossRef]
- Tsai, W.-T. Environmental and health risks of chlorine trifluoride (ClF3), an alternative to potent greenhouse gases in the semiconductor industry. J. Hazard. Mater. 2011, 190, 1–7. [Google Scholar] [CrossRef]
- Jin, C.; Olsen, B.C.; Luber, E.J.; Buriak, J.M. Nanopatterning via Solvent Vapor Annealing of Block Copolymer Thin Films. Chem. Mater. 2017, 29, 176–188. [Google Scholar] [CrossRef]
- Eng, C.Y.; Yan, D.; Withanage, N.; Liang, Q.; Zhou, Y. Wastewater treatment and recycle from a semiconductor industry: A demo-plant study. Water Pract. Technol. 2019, 14, 371–379. [Google Scholar] [CrossRef]
- Chung, J.; Fleege, D.; Ong, S.K.; Lee, Y. Organic semiconductor wastewater treatment using a four-stage Bardenpho with membrane system. Environ. Technol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Y.; Fu, Q.S.; Robertson, A.P.; Criddle, C.S.; Leckie, J.O. Use of reverse osmosis membranes to remove perfluorooctane sulfonate (PFOS) from semiconductor wastewater. Environ. Sci. Technol. 2006, 40, 7343–7349. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Jiang, C.; Xu, T. Electrodialysis process for the recycling and concentrating of tetramethylammonium hydroxide (TMAH) from photoresist developer wastewater. Ind. Eng. Chem. Res. 2013, 52, 18356–18361. [Google Scholar] [CrossRef]
- Lin, C.C.; Yang, C.C.; Ger, J.; Deng, J.F.; Hung, D.Z. Tetramethylammonium hydroxide poisoning. Clin. Toxicol. 2010, 48, 213–217. [Google Scholar] [CrossRef]
- Chang, K.F.; Yang, S.Y.; You, H.S.; Pan, J.R. Anaerobic treatment of tetra-methyl ammonium hydroxide (TMAH) containing wastewater. IEEE Trans. Semicond. Manuf. 2008, 21, 486–491. [Google Scholar] [CrossRef]
- Kim, E.A.; Lee, H.E.; Ryu, H.W.; Park, S.H.; Kang, S.K. Cases series of malignant lymphohematopoietic disorder in Korean semiconductor industry. Saf. Health Work 2011, 2, 122–134. [Google Scholar] [CrossRef]
- Kim, M.-H.; Kim, H.; Paek, D. The health impacts of semiconductor production: An epidemiologic review. Int. J. Occup. Environ. Health 2014, 20, 95–114. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.F. An Introduction to Lithography; American Chemical Society: Washington, DC, USA, 1983; pp. 1–13. [Google Scholar]
- Puliyalil, H.; Cvelbar, U. Selective plasma etching of polymeric substrates for advanced applications. Nanomaterials 2016, 6. [Google Scholar] [CrossRef]
- Martinez, V.M.; Edgar, T.F. IEEE Control Systems Magazine. 2006; 46–55. [Google Scholar]
- Santillan, J.J.; Harumoto, M.; Motono, T.; Dos Santos, A.F.; Mori, C.; Tanaka, Y.; Stokes, H.; Asai, M.; Itani, T. Application of ethyltrimethylammonium hydroxide (ETMAH) as an alternative developer solution/process for semiconductor lithography. Jpn. J. Appl. Phys. 2021, 60. [Google Scholar] [CrossRef]
- Mori, I.C.; Arias-Barreiro, C.R.; Koutsaftis, A.; Ogo, A.; Kawano, T.; Yoshizuka, K.; Inayat-Hussain, S.H.; Aoyama, I. Toxicity of tetramethylammonium hydroxide to aquatic organisms and its synergistic action with potassium iodide. Chemosphere 2015, 120, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Field, J.A.; Paniego, R.; Pepel, R.D.; Chorover, J.; Abrell, L.; Sierra-alvarez, R. Bioconcentration potential and microbial toxicity of onium cations in photoacid generators PAG-2. Environ. Sci. Pollut. Res. 2021, 28, 8915–8921. [Google Scholar] [CrossRef] [PubMed]
- Ober, M.S.; Romer, D.R.; Etienne, J.; Thomas, P.J.; Jain, V.; Cameron, J.F.; Thackeray, J.W. Backbone Degradable Poly(aryl acetal) Photoresist Polymers: Synthesis, Acid Sensitivity, and Extreme Ultraviolet Lithography Performance. Macromolecules 2019. [Google Scholar] [CrossRef]
- Knaepen, W.; American, T.; For, S.; Mallik, A.; Vandenbroeck, N. Improved cost-effectiveness of the block co-polymer anneal process for DSA. In Proceedings of the Alternative Lithographic Technologies VIII, San Jose, CA, USA, 1 April 2016. [Google Scholar] [CrossRef]
- Louria, D.B.; Joselow, M.M.; Browder, A.A. The Human Toxicity of Certain Trace Elements. Ann. Intern. Med. 1972, 307–319. [Google Scholar] [CrossRef]
- Chung, S.; Chung, J.; Chung, C. Journal of Water Process Engineering Enhanced electrochemical oxidation process with hydrogen peroxide pretreatment for removal of high strength ammonia from semiconductor wastewater. J. Water Process Eng. 2020, 37, 101425. [Google Scholar] [CrossRef]
- Inoue, K. Heavy metal toxicity. J. Clin. Toxicol. 2013. [Google Scholar] [CrossRef]
- Klopffer, W. Background and Future Prospects in Life Cycle Assessment; LCA Compendium–The Complete World of Life Cycle Assessment; Klöpffer, W., Ed.; Springer: Dordrecht, The Netherlands, 2014; ISBN 978-94-017-8696-6. [Google Scholar]
- International Organization for Standardization ISO 14040: Environmental management—Life cycle assessment—Principles and framework. 2006; 20.
- Mutel, C.; Liao, X.; Patouillard, L.; Bare, J.; Fantke, P.; Frischknecht, R.; Hauschild, M.; Jolliet, O.; Maia de Souza, D.; Laurent, A.; et al. Overview and recommendations for regionalized life cycle impact assessment. Int. J. Life Cycle Assess. 2019, 24, 856–865. [Google Scholar] [CrossRef] [PubMed]
- García-Valverde, R.; Cherni, J.A.; Urbina, A. Life cycle analysis of organic photovoltaic technologies. Prog. Photovoltaics Res. Appl. 2010, 18, 535–558. [Google Scholar] [CrossRef]
- Moni, S.M.; Mahmud, R.; High, K.; Carbajales-Dale, M. Life cycle assessment of emerging technologies: A review. J. Ind. Ecol. 2020, 24, 52–63. [Google Scholar] [CrossRef]
- Tecchio, P.; Freni, P.; De Benedetti, B.; Fenouillot, F. Ex-ante Life Cycle Assessment approach developed for a case study on bio-based polybutylene succinate. J. Clean. Prod. 2016, 112, 316–325. [Google Scholar] [CrossRef]
- Buyle, M.; Audenaert, A.; Billen, P.; Boonen, K.; Van Passel, S. The Future of Ex-Ante LCA? Lessons Learned and Practical Recommendations. Sustainability 2019, 11, 5456. [Google Scholar] [CrossRef]
- Tsoy, N.; Steubing, B.; van der Giesen, C.; Guinée, J. Upscaling methods used in ex ante life cycle assessment of emerging technologies: A review. Int. J. Life Cycle Assess. 2020, 25, 1680–1692. [Google Scholar] [CrossRef]
- van der Giesen, C.; Cucurachi, S.; Guinée, J.; Kramer, G.J.; Tukker, A. A critical view on the current application of LCA for new technologies and recommendations for improved practice. J. Clean. Prod. 2020, 259, 120904. [Google Scholar] [CrossRef]
- Thonemann, N.; Schulte, A.; Maga, D. How to Conduct Prospective Life Cycle Assessment for Emerging Technologies? A Systematic Review and Methodological Guidance. Sustainability 2020, 12, 1192. [Google Scholar] [CrossRef]
- Kirchain, R.E.; Gregory, J.R.; Olivetti, E.A. Environmental life-cycle assessment. Nat. Mater. 2017, 16, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Göswein, V.; Habert, G.; Rodrigues, C.; König, J.; Silvestre, J.D.; Freire, F. Using anticipatory life cycle assessment to enable future sustainable construction. J. Ind. Ecol. 2021, 178–192. [Google Scholar] [CrossRef]
- Piccinno, F.; Hischier, R.; Seeger, S.; Som, C. From laboratory to industrial scale: A scale-up framework for chemical processes in life cycle assessment studies. J. Clean. Prod. 2016, 135, 1085–1097. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Yuan, Y.; Zhai, Q. Life Cycle Assessment on Wave and Tidal Energy Systems: A Review of Current Methodological Practice. Int. J. Environ. Res. Public Health 2020, 17, 1604. [Google Scholar] [CrossRef]
- International Organization for Standardization ISO 14044: Environmental Management: Life Cycle Assessment; Principles and Framework; ISO: Geneva, Switzerland, 2006; p. 20.
- Finnveden, G.; Hauschild, M.Z.; Ekvall, T.; Guinée, J.; Heijungs, R.; Hellweg, S.; Koehler, A.; Pennington, D.; Suh, S. Recent developments in Life Cycle Assessment. J. Environ. Manag. 2009, 91, 1–21. [Google Scholar] [CrossRef]
- Hauschild, M.Z.; Rosenbaum, R.K.; Olsen, S.I. LCA Cookbook. In Life Cycle Assessment; Hauschild, M.Z., Rosenbaum, R.K., Olsen, S.I., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 963–1048. ISBN 978-3-319-56474-6. [Google Scholar]
- Ramanathan, M.; Tseng, Y.C.; Ariga, K.; Darling, S.B. Emerging trends in metal-containing block copolymers: Synthesis, self-assembly, and nanomanufacturing applications. J. Mater. Chem. C 2013, 1, 2080–2091. [Google Scholar] [CrossRef]
- Clift, R.; Sim, S.; King, H.; Chenoweth, J.; Christie, I.; Clavreul, J.; Mueller, C.; Posthuma, L.; Boulay, A.-M.; Chaplin-Kramer, R.; et al. The Challenges of Applying Planetary Boundaries as a Basis for Strategic Decision-Making in Companies with Global Supply Chains. Sustainability 2017, 9, 279. [Google Scholar] [CrossRef]
- Hischier, R.; Salieri, B.; Pini, M. NanoImpact Most important factors of variability and uncertainty in an LCA study of nanomaterials–Findings from a case study with nano titanium dioxide. NanoImpact 2017, 7, 17–26. [Google Scholar] [CrossRef]
- Cooper, J.S. LeA Me! hodology with Case Studies Specifying Functional Units and Reference Flows for Comparable Alternatives. Int. J. Life Cycle Assess. 2002, 337–349. [Google Scholar]
- Bull, J.G.; Kozak, R.A. Comparative life cycle assessments: The case of paper and digital media. Environ. Impact Assess. Rev. 2014, 45, 10–18. [Google Scholar] [CrossRef]
- Korea, S. Critical Review System Boundary Selection in Life-Cycle Inventories Using Hybrid Approaches. Environ. Sci. Technol. 2006, 38, 657–664. [Google Scholar] [CrossRef]
- Suh, S.; Huppes, G. Methods in the Life Cycle Inventory of a Product. In Handbook of Input-Output Economics in Industrial Ecology; Springer: Dordrecht, The Netherlands, 2009; pp. 263–282. [Google Scholar] [CrossRef]
- Espinosa, N.; García-Valverde, R.; Krebs, F.C. Life-cycle analysis of product integrated polymer solar cells. Energy Environ. Sci. 2011, 4, 1547–1557. [Google Scholar] [CrossRef]
- Das, S.; Mao, E. The global energy footprint of information and communication technology electronics in connected Internet-of-Things devices. Sustain. Energy Grids Netw. 2020, 24, 100408. [Google Scholar] [CrossRef]
- Aymard, V.; Botta-Genoulaz, V. Normalisation in life-cycle assessment: Consequences of new European factors on decision-making. Supply Chain Forum 2017, 18, 76–83. [Google Scholar] [CrossRef]
- Lesage, P.; Muller, S. Life Cycle Inventory: An In-Depth Look at the Modeling, Data, and Available Tools; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, ISBN 9780124095489. [Google Scholar]
| Category | DSA of BCPs | ASD of Polymer Brushes | EUV Lithography | Nano-Imprint Lithography | Optical Lithography |
|---|---|---|---|---|---|
| Principle of operation | Microphase separation produces self-assembled architectures [15]. Structural modulation by selection of molecular composition and weight [12,125]. | Polymer brush’s metal binning sites facilitate deposition of inorganic films [18]. Thickness controlled by SVA and deposition process [124]. | Relies on a wavelength of 13.5 nm instead of 193 nm used in conventional optical lithography [144]. Principle of operation relies on reflection [51]. | Mask physically displaces photoresist to pattern it before cross linking [144,171]. Once resist is cured, mould is removed and patterned resist is used for manufacturing [171]. | UV light and mask to transfer patterns. photosensitive substrate selectively exposed. Reactive ion etch transfers the pattern to the substrate [169]. |
| Efficiency and energetic costs | Varies. Industrial SVA system requires development with fast, precise wafer scale processing [123,124,125]. | Grafting of polymer brushes to substrates in seconds [18]. Requires standardised industrial process. | Recent EUV tools have productive capacity of 125 wafers per hour [164]. Hot plasma 20-50 eV or accelerator [154]. technology is energetically expensive [16,154]. | Capable of producing over 40 wafers of 300 nm per hour with a defect rate less than 9 pcs/cm2 [171]. | Short time for pattern. E.g., 150-300-mm wafers per hour and 40-nm two-dimensional pattern resolution of a scanner with pixel throughput of 1.8T pixels per second approximately [144]. |
| low-temperature vapor phase deposition process cuts costs and improves environmental sustainability [21,137]. | |||||
| Wastes generated | Solvents, excess inorganic precursor for metal infiltration, excess polymer during deposition stage. | Solvents are typically volatile and flammable. Polymer matrix, photoactive compounds and cross-linkers in polymer photoresist are toxic and non-degradable [149,172]. | |||
| Tool complexity | Thickness and pattern formation control can be achieved with simple chambers consisting of temperature and gas flow systems [124,125]. Predicted low cost of ownership. These techniques do not require the use of photoresists and avoids acquisition costs. | Expensive and complex multilayer optics, hot plasma or acceleration technology and high vacuum. Cost of tool > $30 million [137,144,154]. | Depends: TE-NIL uses heat and pressure. SFIL uses capillary forces, pressure, and light exposure. Improved tool performance required [173,174,176]. | Acquisition cost of photolithographic resist coat system is $950,000 [139]. | |
| Disadvantages | High defect density of BCPs [138]. Poor etch contrast of polymer blocks [14]. | Improved range of metals that can be deposited [18]. | Obtaining high reflectivity [144], improved etch and deposition defect mitigation and repair of mask, installation and power costs [16,142,162,166,170]. | Needs reduced process steps and improved mould material, fabrication quality, mass production capacity, reduced overlay and defectivity [16,33,171]. | Photolithography can no longer be further optimised as it has an intrinsic resolution limit [51,137]. |
| Standardised methodology and further research required [7,138]. | |||||
| Advantages | No diffraction limit in resolution [137], directly pattern functional materials [137], efficient for 3D patterning [137]. | Reduction of processing steps [133]. self-aligned patterning with capability of extending to 3D [17]. | Improved: cycle time, increased number of patterning levels, line edge roughness, high etch resistance, increased sensitivity [33,138]. | Facilitates change in flash memory from scaling horizontally to vertically. Simple processing steps, high throughput, low cost and high resolution [51]. | Established technology being the dominate patterning technique since the beginning of IC production [51]. |
| Category | DSA of BCPs | ASD of Polymer Brushes | EUV Lithography | Nano-Imprint Lithography | Optical Lithography |
|---|---|---|---|---|---|
| Accuracy and Resolution | Needs research and development for:
| Stochastic issues leading to critical dimension variation, critical pattern defects and feature roughness [33,170]. | Limited by size of features on mould. high fidelity of pattern transfer [137]. Defect density and mask damage issues [137]. Suitable material for moulds [171,175]. | Resolution improvements reaching limits [52,144,154,155]. | |
| Size | BCP patterns 5 to 200 nm size range [123] with dot, line hole or lamellar patterns [126]. May improve feature control to sub 7 nm and pitch multiplication [138]. | Depends on the chemistry of the end-functional group and polymer deposition technique used. | Main candidate for sub10nm manufacturing [51,170]. 25 nm 1:1 line/space resolution with an approximate 2.7 nm line edge roughness [162]. | Allows for patterning features of sub-100 nm possibly with features as small as 10 nm [146,174]. | Smallest features approximately equal to wavelength of light used [52]. Wavelength of 193 nm or longer is generally used [144,177]. |
| Predicted or used in HMV in device type | 3 nm node logic [16]. | unknown | 1.5–7 nm node logic [16]. | Device type: 3D Flash Memory [16]. | Limited by wavelength. |
| Sustainability Issue | Conventional Lithography | Bottom-Up BCP and Polymer Brush Nanofabrication Routes | ||
|---|---|---|---|---|
| Process | Impact | Process | Impact | |
| Carbon emissions | NGL tools such as EUV require high source power [214]. | High power requirements increased strain on resources. | Predicted lower power requirements due to high-power optical set ups not being necessary and reduced number of processing steps [4,18]. | Predicted reduced power consumption costs and energy resource strain. |
| Organohalogens | Use of PFCs for etching and to clean CVD chambers [157,179,215]. | Enhances greenhouse effect [158,215]. | Versatility of bottom-up lithography may allow for exclusion of PFCs from initial stages of lithography but may still be required during etching processes. | When scaling to industrial-scale green fabrication route can be selected. Predicted impact requires further investigation. |
| Wastewater | Accumulation of various wastes from conventional lithographic processes in wastewater is of concern [192,218,220]. | Contamination of water systems, environmental toxicity and eutrophication [221,222,234] | BCP and polymer brush impact on wastewater is point of further research. | Unknown on an industrial scale. LCA studies may help predict impact. |
| Photoresists, photostrippers, BCP and polymer brush wastes | Wastes from photoresist and photostripper are of concern [34,180,228,230,231]. Photoresists after exposure to light decomposes into harmful chemicals [149]. This step is not required for bottom-up lithography. | Persistent, bio accumulative, toxic and bio accumulative and thus of substantial environmental concern [219,229,230]. | BCP and polymer brushes are removed via processes such as reactive ion etch or UV ozone [125,226]. Potential use of biopolymers may avoid harmful by products of etching processes. | Reduced etch steps have been reported for DSA [232]. Further research is required to evaluate environmental impact. |
| Acids, bases and solvent waste | Wet etching processing uses various acids, bases and solvents of environmental concern for example Sulfuric acid [157,223,234,225]. | Health risks and environmental toxicity [212,213,223,224,234]. | Acid, base, and solvent use depends on polymer and metals used in BCP and polymer brush lithography. | Unknown environmental impact on industrial scale. Capability of using green fabrication routes [104,179] |
| Trace elements and heavy metals | Trace elements such as Gallium and Indium have reported toxicity as high as metals and are deposited during lithography [183]. | Hazardous for both humans and wildlife [233,234,235]. | Metal use is required for lithographic patterning in the electronics industry. | Research required to determined more efficient and less wasteful than conventional lithography. |
| Material and Energy Consumption Used in Raw Materials Extraction/Acquisition, Processing, and Transportation |
|---|
| Material and energy resource consumption during the deposition process |
Quantitative data and chemical composition of the wastes:
|
| Quantitative data on chemical composition of recycled wastes |
| Qualitive data on chemical wastes disposed of off sight and process of disposal |
| Transportation emissions for off sight waste disposal |
| Damage Category | Impact Category | |
|---|---|---|
| Human Health | Human toxicity Respiratory inorganics Photochemical oxidation | Ionising radiations Ozone layer depletion |
| Ecosystem Quality | Aquatic ecotoxicity Terrestrial ecotoxicology Terrestrial acidification/nitrification | Aquatic acidification Aquatic eutrophication Land occupation |
| Climate change | Global warming | |
| Resources | Non-renewable energy | Mineral extraction |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mullen, E.; Morris, M.A. Green Nanofabrication Opportunities in the Semiconductor Industry: A Life Cycle Perspective. Nanomaterials 2021, 11, 1085. https://doi.org/10.3390/nano11051085
Mullen E, Morris MA. Green Nanofabrication Opportunities in the Semiconductor Industry: A Life Cycle Perspective. Nanomaterials. 2021; 11(5):1085. https://doi.org/10.3390/nano11051085
Chicago/Turabian StyleMullen, Eleanor, and Michael A. Morris. 2021. "Green Nanofabrication Opportunities in the Semiconductor Industry: A Life Cycle Perspective" Nanomaterials 11, no. 5: 1085. https://doi.org/10.3390/nano11051085
APA StyleMullen, E., & Morris, M. A. (2021). Green Nanofabrication Opportunities in the Semiconductor Industry: A Life Cycle Perspective. Nanomaterials, 11(5), 1085. https://doi.org/10.3390/nano11051085






