Structural Evolution of Nanophase Separated Block Copolymer Patterns in Supercritical CO2
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
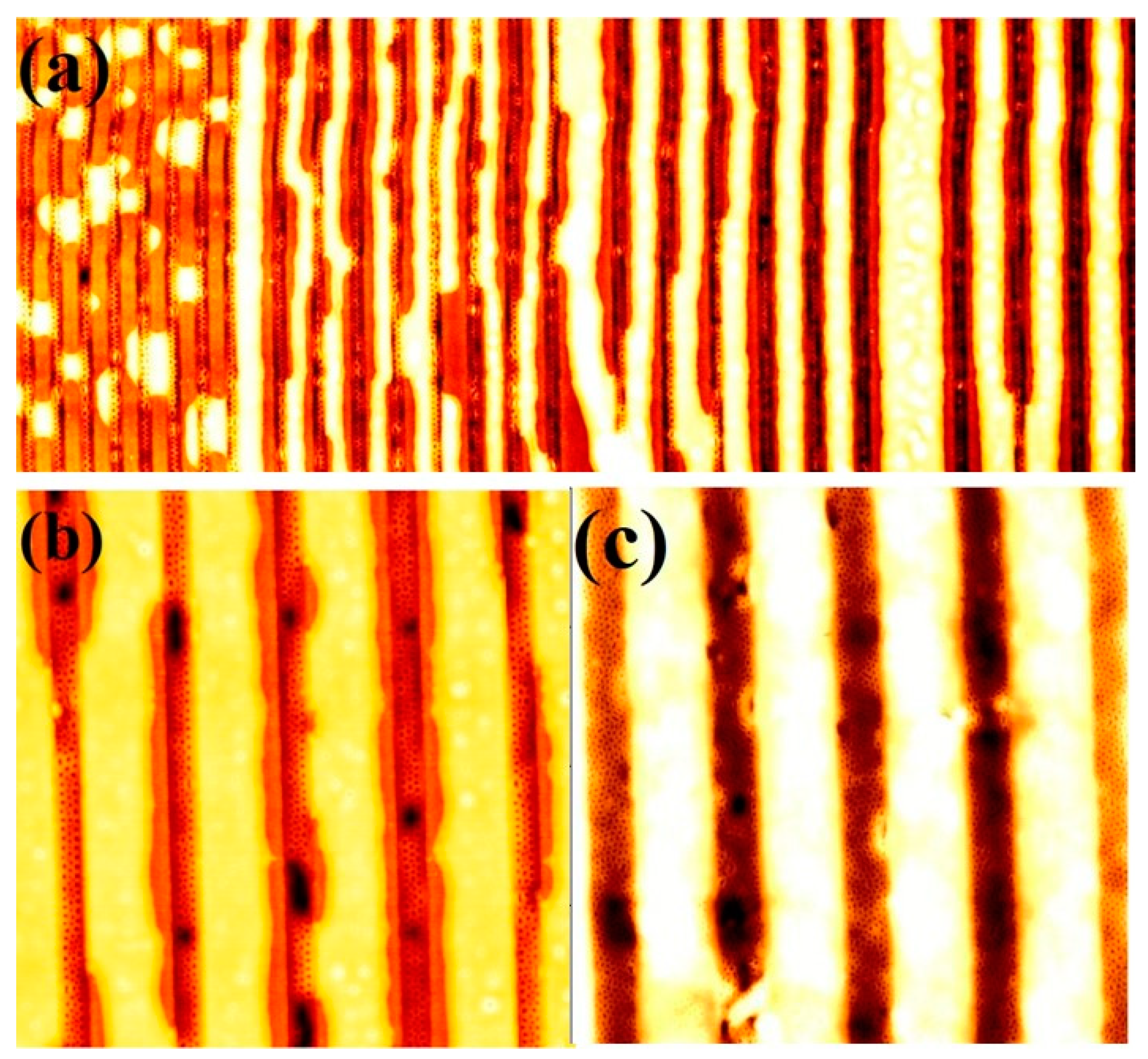
3.1. Dimensional and Structural Control of Block Copolymer Nanopatterns
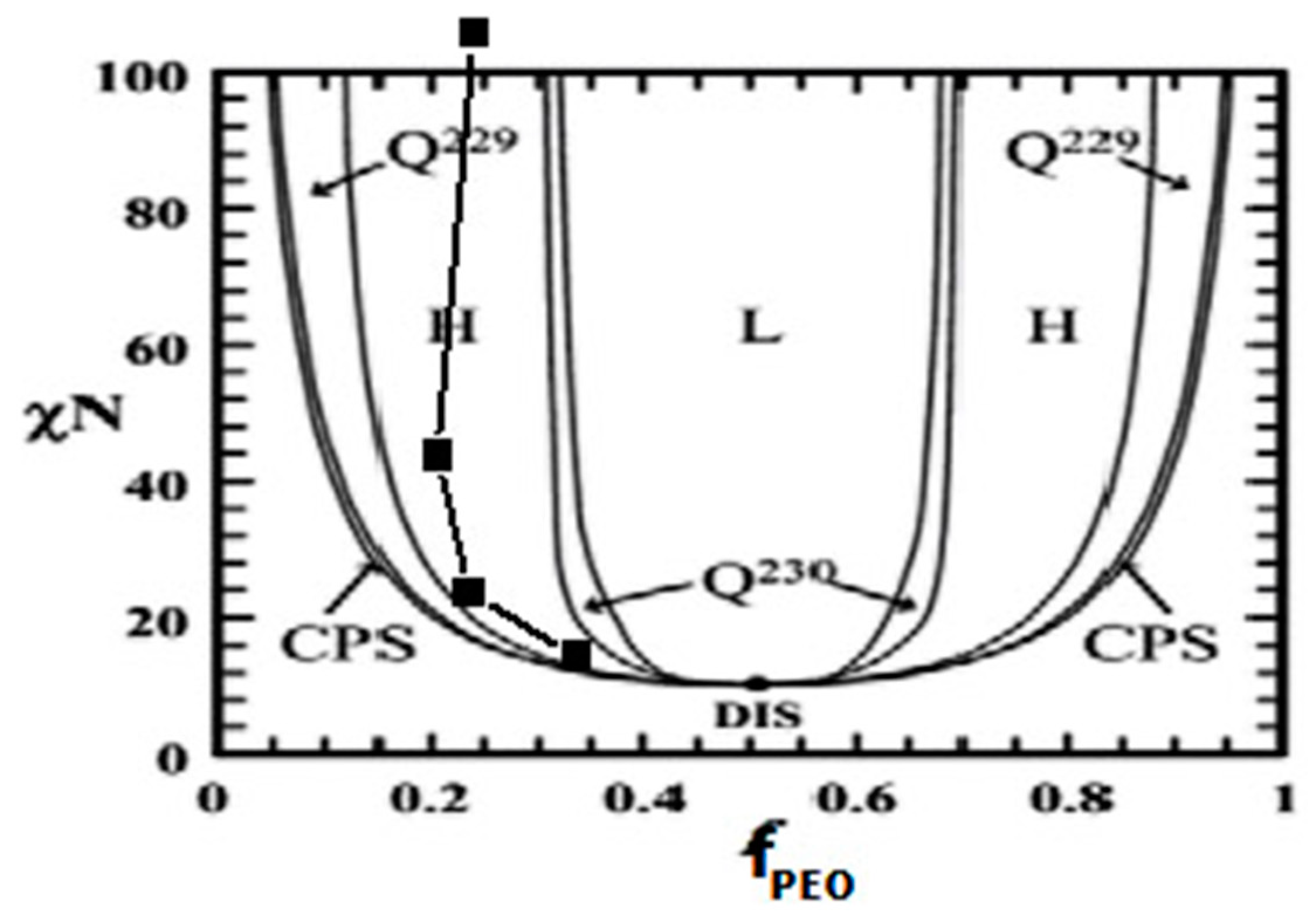
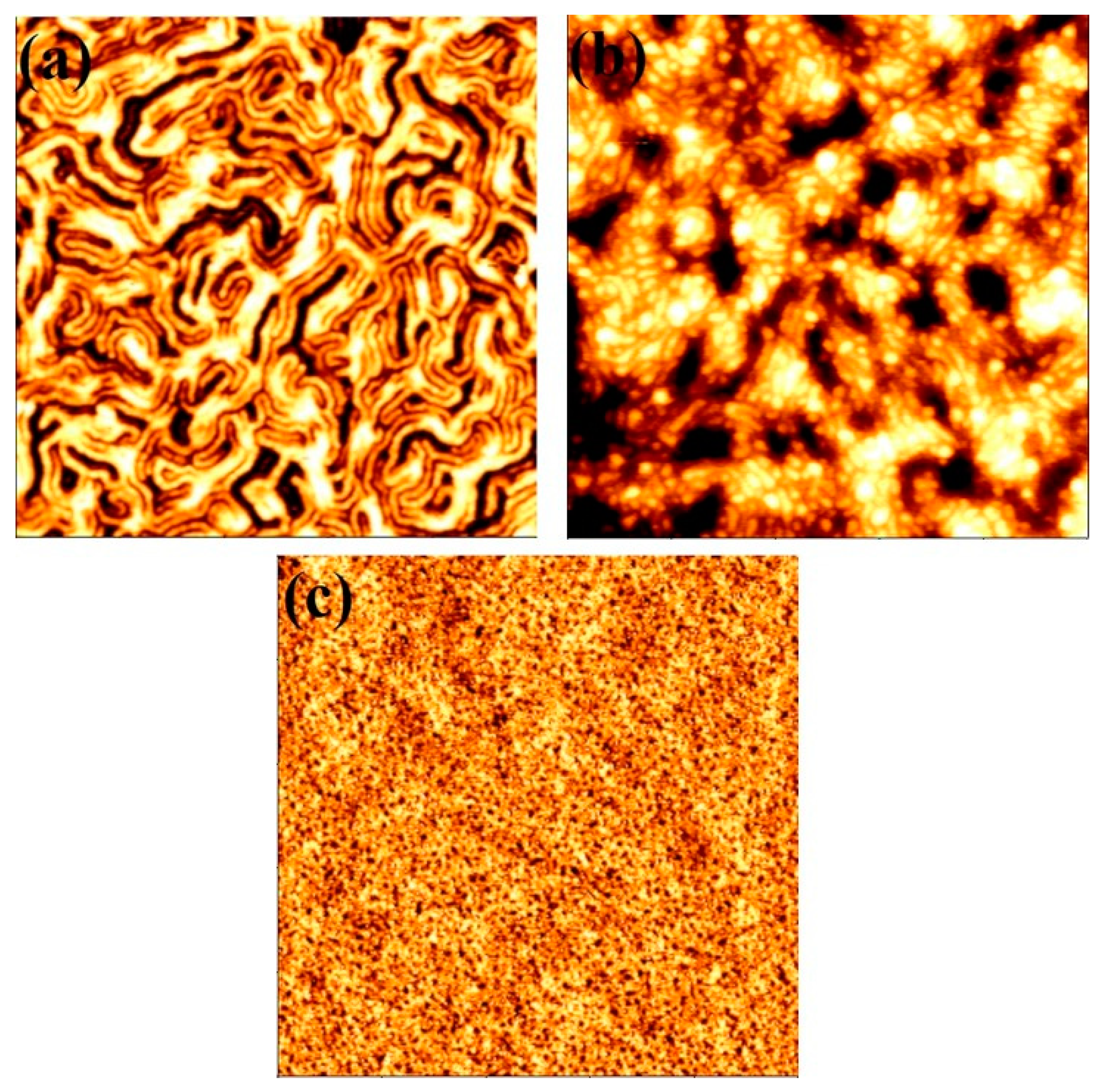
3.2. Mechanism of Microphase Separation for Different BCP Systems
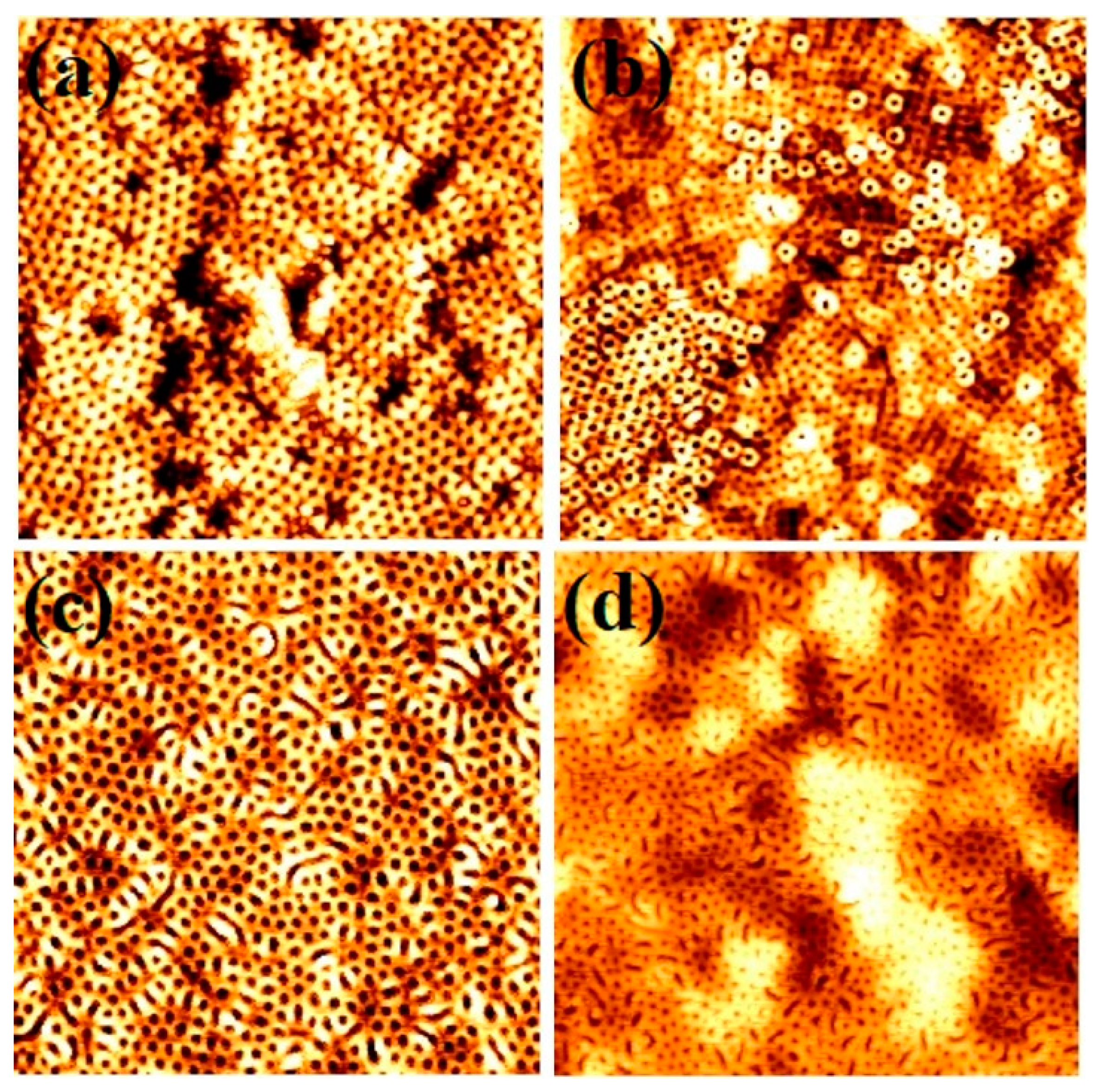
3.3. Formation of BCP Nanopatterns at Different SCF Annealing Temperatures
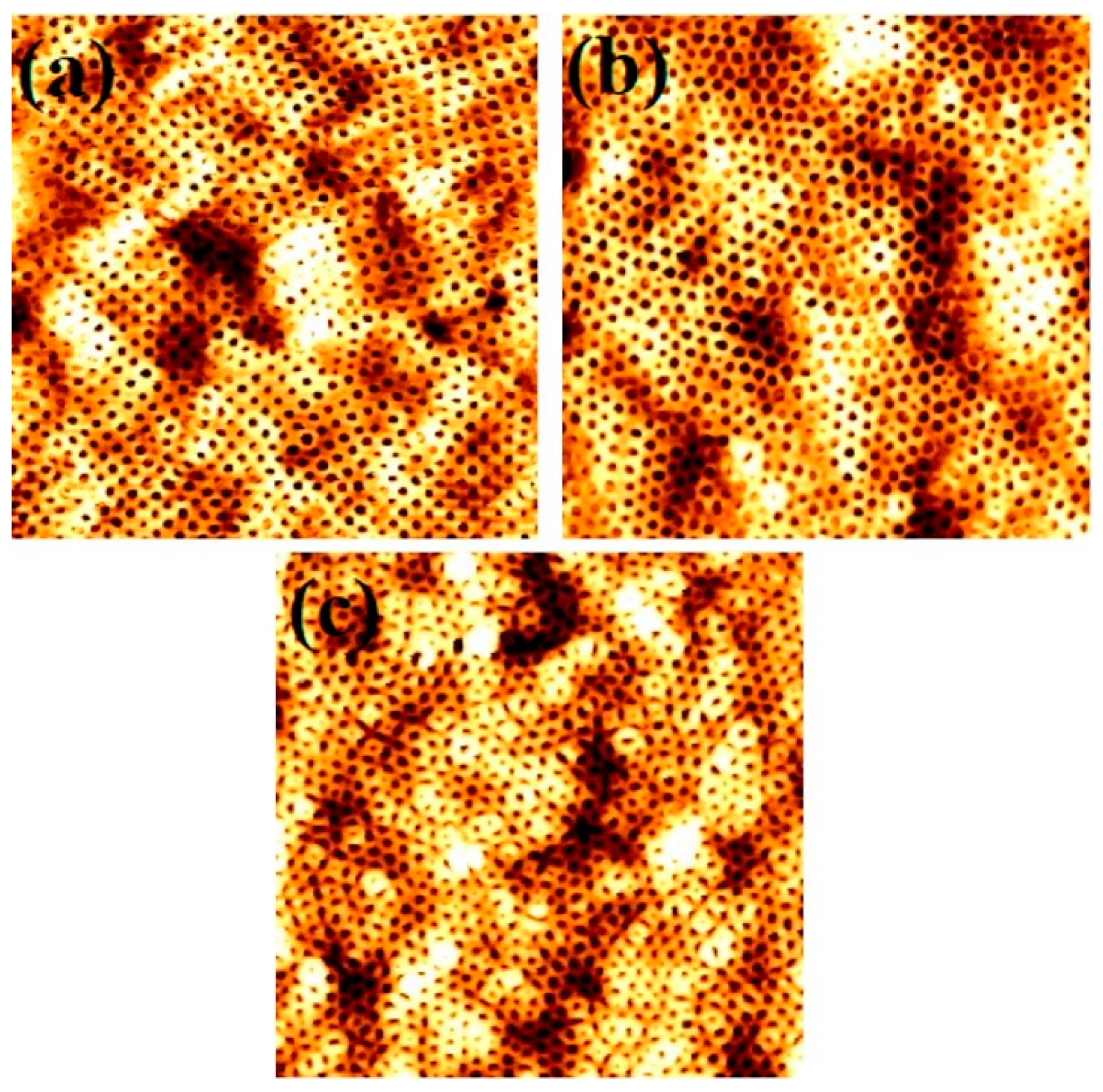
3.4. Effects of Film Thicknesses by Varying Spin Coating Speed
3.5. Effects of Depressurisation Rate on the Self Assembly
3.6. Fabrication of BCP Nanopatterns on Graphoepitaxial Substrate
3.7. Exploring SCF Annealing for Other BCP Composition
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hamley, I.W. Introduction to Block Copolymers. Dev. Block Copolym. Sci. Technol. 2004, 1–29. [Google Scholar] [CrossRef]
- Segalman, R.A. Patterning with Block Copolymer Thin Films Mater. Sci. Eng. R. Rep. 2005, 48, 191–226. [Google Scholar] [CrossRef]
- Fasolka, M.J.; Mayes, A.M. Block Copolymer Thin Films: Physics and Applications. Annu. Rev. Mater. Sci. 2001, 31, 323–355. [Google Scholar] [CrossRef]
- Hamley, I.W. Ordering in Thin Films of Block Copolymers: Fundamentals to Potential Applications. Prog. Polym. Sci. 2009, 34, 1161–1210. [Google Scholar] [CrossRef]
- Mokarian-Tabari, P.; Collins, T.W.; Holmes, J.D.; Morris, M.A. Cyclical “Flipping” of Morphology in Block Copolymer Thin Films. ACS Nano 2011, 5, 4617–4623. [Google Scholar] [CrossRef]
- Majewski, P.W.; Yager, K.G. Rapid Ordering of Block copolymer Thin Films. J. Phys. Condens. Matter. 2016, 28, 403002. [Google Scholar] [CrossRef] [PubMed]
- Gotrik, K.W.; Ross, C.A. Solvothermal Annealing of Block Copolymer Thin Films. Nano Lett. 2013, 13, 5117–5122. [Google Scholar] [CrossRef]
- Brassat, K.; Lindner, J.K.N. Nanoscale Block Copolymer Self-Assembly and Microscale Polymer Film Dewetting: Progress in Understanding the Role of Interfacial Energies in the Formation of Hierarchical Nanostructures. Adv. Mater. Interfaces 2020, 7, 1901565. [Google Scholar] [CrossRef]
- Huang, W.H.; Chen, P.Y.; Tung, S.H. Effects of Annealing Solvents on the Morphology of Block Copolymer-Based Supramolecular Thin Films. Macromolecules 2012, 45, 1562–1569. [Google Scholar] [CrossRef]
- Padinger, F.; Brabec, C.J.; Fromherz, T.; Hummelen, J.C.; Sariciftci, N.S. Fabrication of Large Area Photovoltaic Devices Containing Various Blends of Polymer and Fullerene Derivatives by using the Doctor Blade Technique. Opto-Electron. Rev. 2000, 8, 280–283. [Google Scholar]
- Koegler, W.S.; Patrick, C.; Cima, M.J.; Griffith, L.G. Carbon Dioxide Extraction of Residual Chloroform from Biodegradable Polymers. J. Biomed. Mater. Res. 2002, 63, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, T.; Senthamaraikannan, R.; Shaw, M.T.; Holmes, J.D.; Morris, M.A. ‘Insitu’ Hard Mask Materials: A New Methodology on Creation of Vertical Silicon Nanopillar and Nanowire Arrays. Nanoscale 2012, 4, 7743–7752. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, T.; Fleming, P.G.; Holmes, J.D.; Morris, M.A. The Stability of “Ce2O3” Nanodots in Ambient Conditions: A Study using Block Copolymer Templated Structures. J. Mater. Chem. 2012, 22, 22949–22957. [Google Scholar] [CrossRef]
- Kendall, J.L.; Canelas, D.A.; Young, J.L.; DeSimone, J.M. Polymerizations in Supercritical Carbon Dioxide. Chem. Rev. 1999, 99, 543–564. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Sanchez, I.C.; Johnston, K.P.; Green, P.F. Ordering in Asymmetric Block Copolymer Films by a Compressible Fluid. J. Phys. Chem. B 2007, 111, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Tomasko, D.L.; Li, H.; Liu, D.; Han, X.; Wingert, M.J.; Lee, L.J.; Koelling, K.W. A Review of CO2 Applications in the Processing of Polymers. Ind. Eng. Chem. Res. 2003, 42, 6431–6456. [Google Scholar] [CrossRef]
- Wissinger, R.G.; Paulaitis, M.E. Glass Transitions in Polymer/CO2 Mixtures at Elevated Pressures. J. Polym. Sci. Part B Polym. Phys. 1991, 29, 631–633. [Google Scholar] [CrossRef]
- Wissinger, R.G.; Paulaitis, M.E. Swelling and Sorption in Polymer–CO2 Mixtures at Elevated Pressures. J. Polym. Sci. Part B Polym. Phys. 1987, 25, 2497–2510. [Google Scholar] [CrossRef]
- Koga, T.; Akashige, E.; Reinstein, A.; Brönner, M.; Seo, Y.-S.; Shin, K.; Rafailovich, M.; Sokolov, J.; Chu, B.; Satija, S. The effect of density fluctuations in supercritical fluids: New science and technology for polymer thin films. Phys. B: Condens. Matter 2005, 357, 73–79. [Google Scholar] [CrossRef]
- Raudino, A.; Celso, L.F.; Triolo, A.; Triolo, R. Pressure-Induced Formation of Diblock Copolymer “Micelles” in Supercritical Fluids. A Combined Study by Small Angle Scattering Experiments and Mean-Field Theory. I. The Critical Micellization Density Concept. J. Chem. Phys. 2004, 120, 3489–3498. [Google Scholar] [CrossRef]
- Kiran, E. Supercritical Fluids and Polymers-The Year in Review-2014. J. Supercrit. Fluids 2016, 110, 126–153. [Google Scholar] [CrossRef]
- Farrell, R.A.; Fitzgerald, T.G.; Borah, D.; Holmes, J.D.; Morris, M.A. Chemical Interactions and their Role in the Microphase Separation of Block Copolymer Thin Films. Int. J. Mol. Sci. 2009, 10, 3671–3712. [Google Scholar] [CrossRef]
- Arceo, A.; Green, P.F. Ordering Transition of Block Copolymer Films. J. Phys. Chem. B 2005, 109, 6958–6962. [Google Scholar] [CrossRef]
- Chen, Y.; Koberstein, J.T. Fabrication of Block Copolymer Monolayers by Adsorption from Supercritical Fluids: A Versatile Concept for Modification and Functionalization of Polymer Surfaces. Langmuir 2008, 24, 10488–10493. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, B.M.D.; Griffiths, G.H.; Matsen, M.W.; Hamley, I.W. Structure Variation and Evolution in Microphase-Separated Grafted Diblock Copolymer Films. Macromolecules 2011, 44, 8527–8536. [Google Scholar] [CrossRef]
- Zhang, R.; Yokoyama, H. Fabrication of Nanoporous Structures in Block Copolymer using Selective Solvent Assisted with Compressed Carbon Dioxide. Macromolecules 2009, 42, 3559–3564. [Google Scholar] [CrossRef]
- Ghoshal, T.; Biswas, S.; O’Regan, C.; Holmes, J.D.; Morris, M.A. Nanophase Separation and Structural evolution of Block Copolymer Films: A “Green” And “Clean” Supercritical Fluid Approach. Nano Res. 2015, 84, 1279–1292. [Google Scholar] [CrossRef]
- Ghoshal, T.; Maity, T.; Godsell, J.F.; Roy, S.; Morris, M.A. Large Scale Monodisperse Hexagonal Arrays o Superparamagnetic Iron Oxides Nanodots: A Facile Block Copolymer Inclusion Method. Adv. Mater. 2012, 4, 7743. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, T.; Shaw, M.T.; Bolger, C.T.; Holmes, J.D.; Morris, M.A. A General Method for Controlled Nanopatterning of Oxide Dots: A Microphase Separated Block Copolymer Platform. J. Mater. Chem. 2012, 22, 12083–12089. [Google Scholar] [CrossRef]
- Wang, H.; Djurisic, A.B.; Chan, W.K.; Xie, M.H. Factors affecting phase and height contrastof diblock copolymer PS-b-PEO thin filmsin dynamic force modeatomic force microscopy. Appli. Surf. Sci. 2005, 252, 1092–1100. [Google Scholar] [CrossRef]
- Zhang, Y.; Gangwani, K.K.; Lemert, R.M. Sorption and swelling of block copolymers in the presence of supercritical fluid carbon dioxide. J. Supercrit. Fluids 1997, 11, 115–134. [Google Scholar] [CrossRef]
- Weidner, E.; Wiesmet, V.; Knez, Ž.; Škerget, M. Phase Equilibrium (Solid-Liquid-Gas) in Polyethyleneglycol-Carbon Dioxide Systems. J. Supercrit. Fluids 1997, 10, 139–147. [Google Scholar] [CrossRef]
- Guadagno, T.; Kazarian, S.G. High-Pressure CO2-Expanded Solvents: Simultaneous Measurement of CO2 Sorption and Swelling of Liquid Polymers with In-Situ Near-IR Spectroscopy. J. Phys. Chem. B 2004, 108, 13995–13999. [Google Scholar] [CrossRef]
- Gourgouillon, D.; Da Ponte, M.N. High Pressure Phase Equilibria for Poly(Ethylene Glycol)S + CO2: Experimental Results and Modelling. Phys. Chem. Chem. Phys. 1999, 1, 5369–5375. [Google Scholar] [CrossRef]
- Ghoshal, T.; Senthamaraikannan, R.; Shaw, M.T.; Holmes, J.D.; Morris, M.A. Fabrication of Ordered, Large Scale, Horizontally-Aligned Si Nanowire Arrays based on an Insitu Hard Mask Block Copolymer Approach. Adv. Mater. 2014, 26, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, T.; Maity, T.; Senthamaraikannan, R.; Shaw, M.T.; Carolan, P.; Holmes, J.D.; Roy, S.; Morris, M.A. Size and space controlled hexagonal arrays of superparamagnetic iron oxide nanodots: Magnetic studies and application. Sci. Rep. 2013, 3, srep02772. [Google Scholar] [CrossRef]
- García-Turiel, J.; Jérôme, B. Solvent Retention in Thin Polymer Films Studied by Gas Chromatography. Colloid Polym. Sci. 2007, 285, 1617–1623. [Google Scholar] [CrossRef]
- Wang, W.-C.V.; Kramer, E.J.; Sachse, W.H. Effects of high-pressure CO2 on the glass transition temperature and mechanical properties of polystyrene. J. Polym. Sci. Polym. Phys. Ed. 1982, 20, 1371–1384. [Google Scholar] [CrossRef]
- Ghoshal, T.; Holmes, J.D.; Morris, M.A. Development of Ordered, Porous (Sub-25 nm Dimensions) Surface Membrane Structures Using a Block Copolymer Approach. Sci. Rep. 2018, 8, 7252. [Google Scholar] [CrossRef]
- Rasappa, S.; Schulte, L.; Borah, D.; Hulkkonen, H.; Ndoni, S.; Salminen, T.; Senthamaraikanan, R.; Morris, M.A.; Niemi, T. Morphology evolution of PS-b-PDMS block copolymer and its hierarchical directed self-assembly on block copolymer templates. Microelectron. Eng. 2018, 192, 1–7. [Google Scholar] [CrossRef]
- Cummins, C.; Tabari, P.M.; Andreazza, P.; Sinturel, C.; Morris, M.A. Solvothermal Vapor Annealing of Lamellar Poly(styrene)-block-poly(d,l-lactide) Block Copolymer Thin Films for Directed Self-Assembly Application. ACS Appl. Mater. Interfaces 2016, 8, 8295–8304. [Google Scholar] [CrossRef] [PubMed]
- Cummins, C.; Mokarian-Tabari, P.; Holmes, J.D.; Morris, M. A Selective etching of polylactic acid in poly(styrene)-block-poly(D,L)lactide diblock copolymer for nanoscale patterning. J. Appl. Polymer Sci. 2014, 131, 40795. [Google Scholar] [CrossRef]
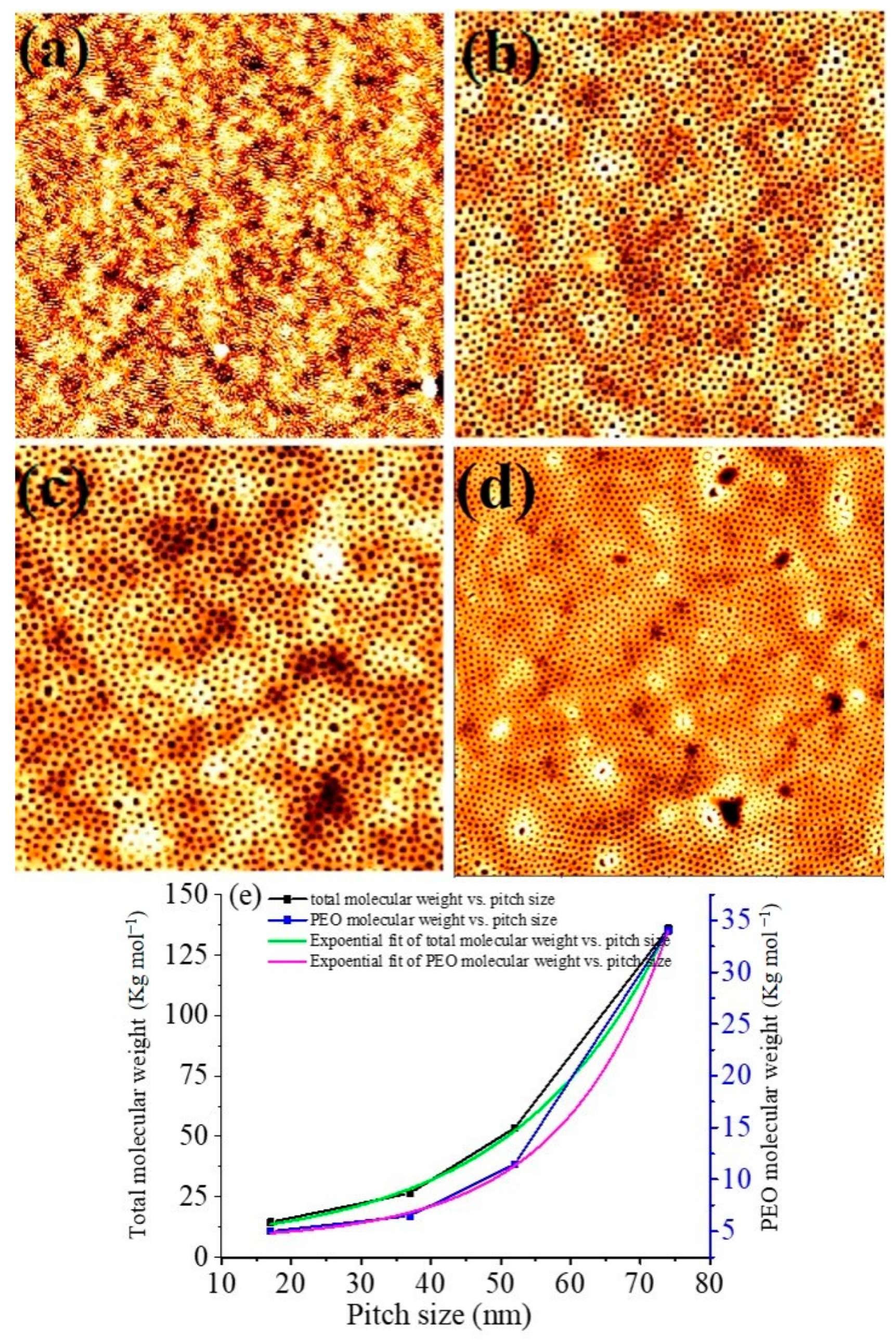

| PS-b-PEO (Molecular Weight) | χN | fPEO | SCF Annealing Pressure (psi) | Film Thickness (nm) | Pitch Size (nm) | PEO Cylinder Diameter (nm) |
|---|---|---|---|---|---|---|
| 9500–5000 | 11.21 | 0.341 | 1300 | 19 | 17 | 7 |
| 20,000–6500 | 20.54 | 0.242 | 1300 | 36 | 37 | 22 |
| 42,000–11,500 | 41.49 | 0.212 | 1200 | 52 | 52 | 28 |
| 102,000–34,000 | 105.42 | 0.247 | 1200 | 72 | 74 | 38 |
| Spin Coating Speed (rpm) | Film Thickness (nm) | PEO Cylinder Diameter (nm) in Black Region | PEO Cylinder Diameter (nm) in White Region |
|---|---|---|---|
| 1000 | 59 | 27 | 25 |
| 2000 | 55 | 29 | 27 |
| 3000 | 52 | 28 | 28 |
| 4000 | 50 | 30 | 26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghoshal, T.; Collins, T.W.; Biswas, S.; A. Morris, M.; Holmes, J.D. Structural Evolution of Nanophase Separated Block Copolymer Patterns in Supercritical CO2. Nanomaterials 2021, 11, 669. https://doi.org/10.3390/nano11030669
Ghoshal T, Collins TW, Biswas S, A. Morris M, Holmes JD. Structural Evolution of Nanophase Separated Block Copolymer Patterns in Supercritical CO2. Nanomaterials. 2021; 11(3):669. https://doi.org/10.3390/nano11030669
Chicago/Turabian StyleGhoshal, Tandra, Timothy W. Collins, Subhajit Biswas, Michael A. Morris, and Justin D. Holmes. 2021. "Structural Evolution of Nanophase Separated Block Copolymer Patterns in Supercritical CO2" Nanomaterials 11, no. 3: 669. https://doi.org/10.3390/nano11030669
APA StyleGhoshal, T., Collins, T. W., Biswas, S., A. Morris, M., & Holmes, J. D. (2021). Structural Evolution of Nanophase Separated Block Copolymer Patterns in Supercritical CO2. Nanomaterials, 11(3), 669. https://doi.org/10.3390/nano11030669






