The Effect of Virtual Reality Rehabilitation on Balance in Patients with Parkinson’s Disease: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion Criteria
2.3. Outcome Measures
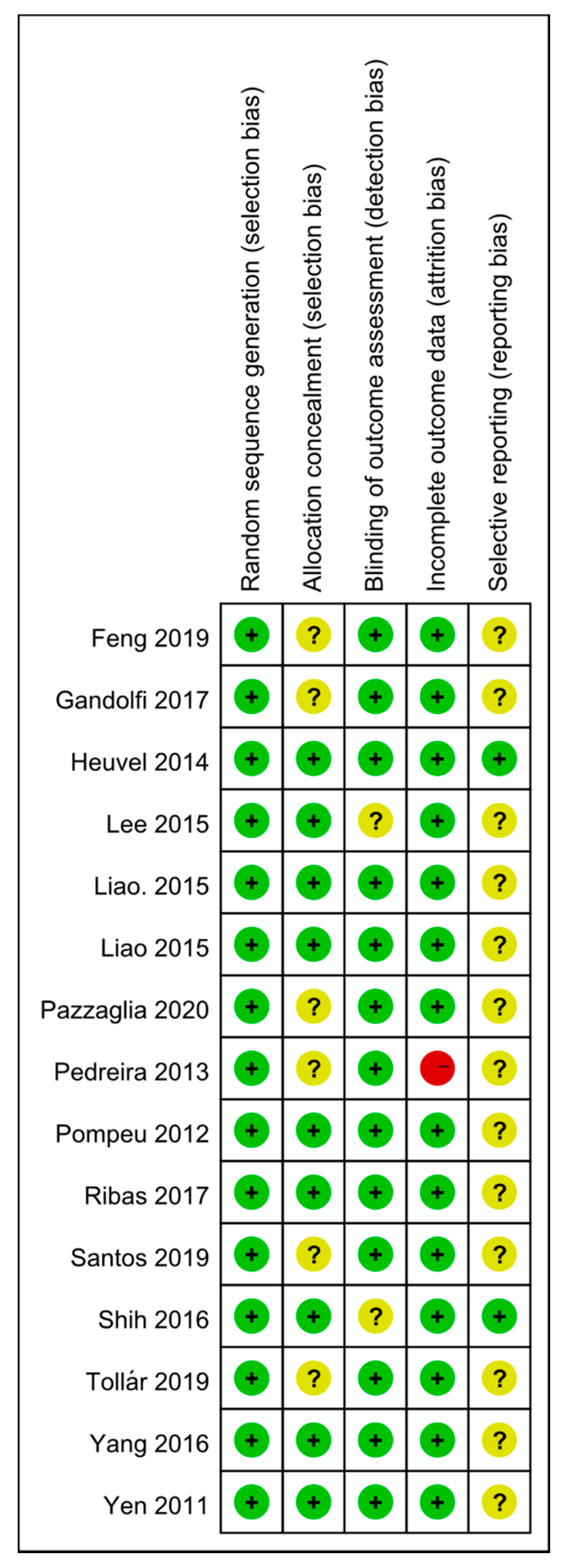
2.4. Data Extraction and Quality Assessment
2.5. Statistical Analysis
3. Results
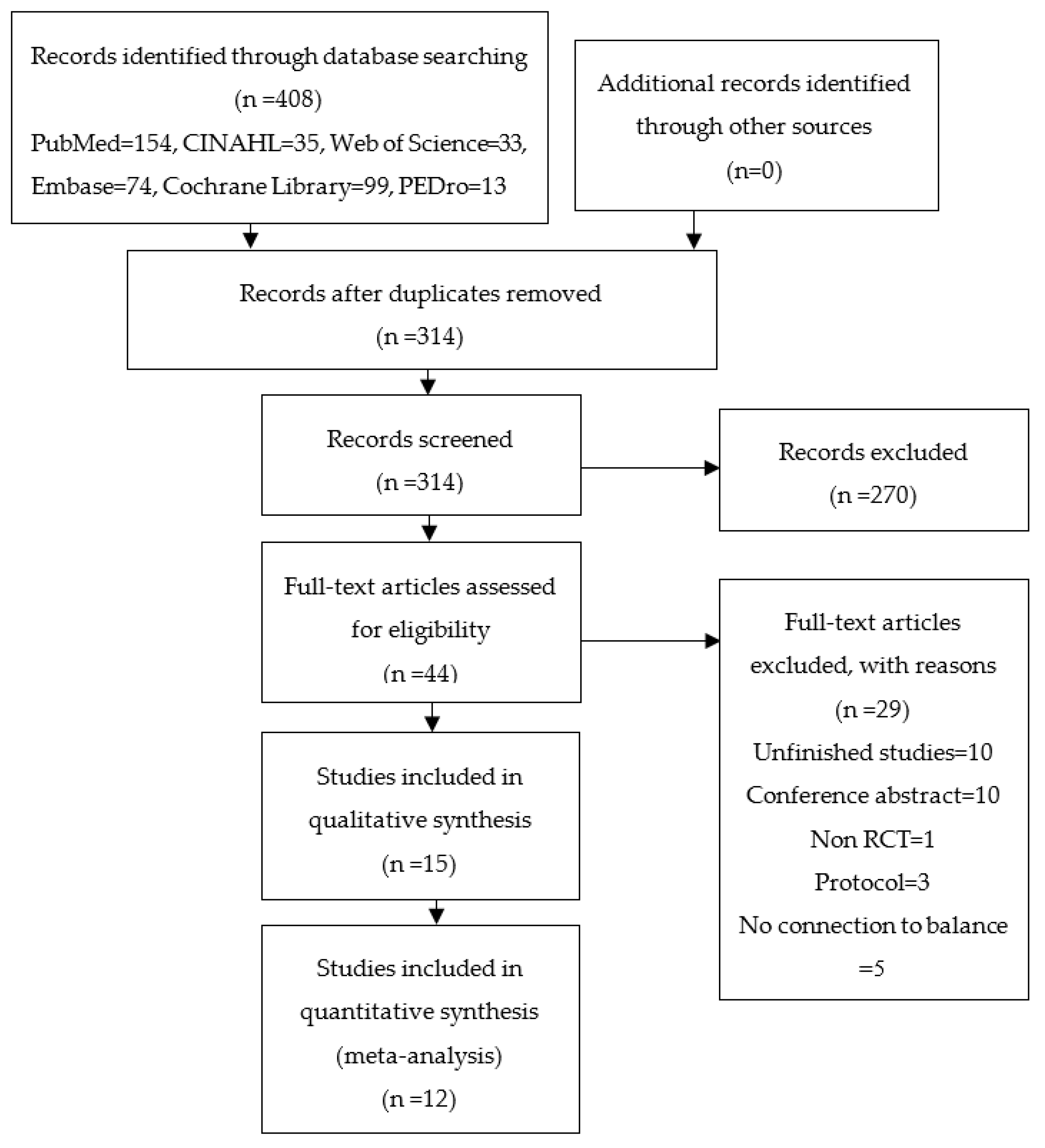
3.1. Study Selection and Quality Assessment
3.2. Main Characteristics
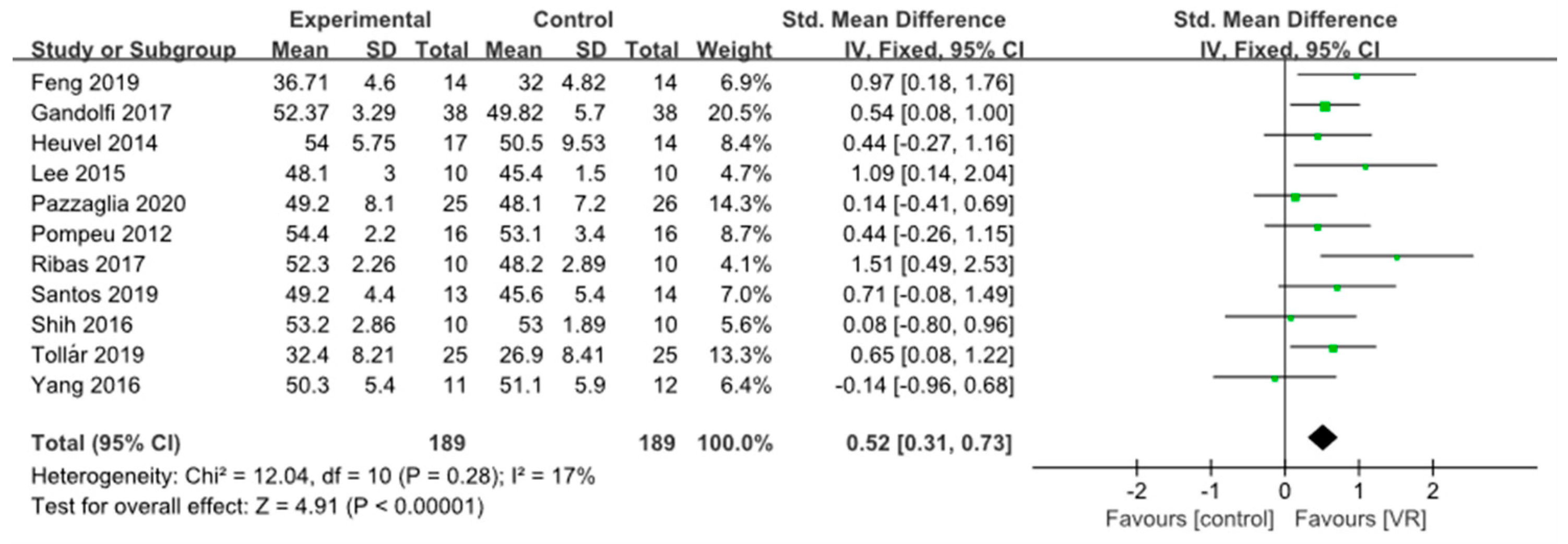
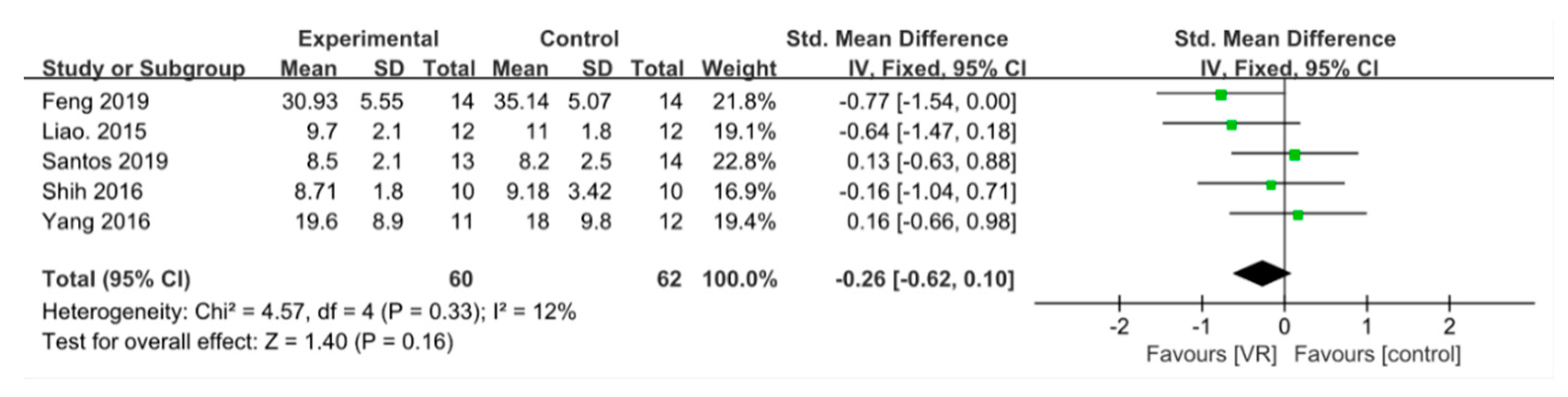
3.3. Results of Main Outcomes
3.3.1. Static Balance
3.3.2. Dynamic Balance
3.3.3. Confidence in Balance
3.3.4. Quality of Life
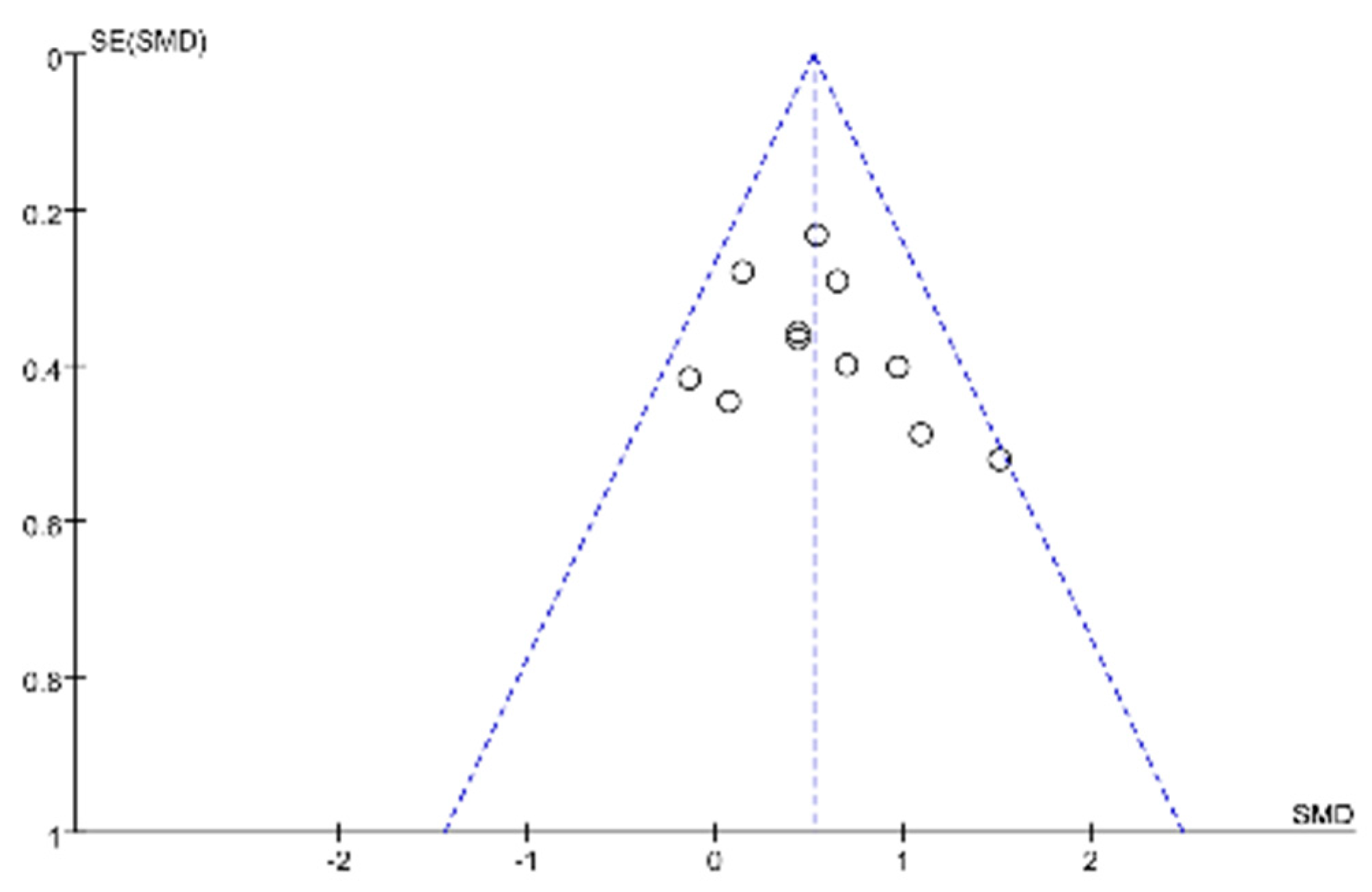
3.4. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, N.E.; Sherrington, C.; Paul, S.S.; Canning, C.G. Balance and falls in Parkinson’s disease: A meta-analysis of the effect of exercise and motor training. Mov. Disord. 2011, 26, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Sofuwa, O.; Nieuwboer, A.; Desloovere, K.; Willems, A.-M.; Chavret, F.; Jonkers, I. Quantitative Gait Analysis in Parkinson’s Disease: Comparison With a Healthy Control Group. Arch. Phys. Med. Rehabil. 2005, 86, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Abbruzzese, G.; Marchese, R.; Avanzino, L.; Pelosin, E. Rehabilitation for Parkinson’s disease: Current outlook and future challenges. Parkinsonism Relat. Disord. 2016, 22, S60–S64. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.C.; Nutt, J.G.; Holford, N.H.G. Progression of motor and nonmotor features of Parkinson’s disease and their response to treatment. Br. J. Clin. Pharmacol. 2012, 74, 267–283. [Google Scholar] [CrossRef]
- Klamroth, S.; Steib, S.; Devan, S.; Pfeifer, K. Effects of exercise therapy on postural instability in Parkinson disease: A meta-analysis. J. Neurol. Phys. Ther. 2016, 40, 3–14. [Google Scholar] [CrossRef]
- Hirsch, M.A.; Toole, T.; Maitland, G.G.; Rider, R.A. The effects of balance training and high-intensity resistance training on persons with idiopathic Parkinson’s disease. Arch. Phys. Med. Rehabil. 2003, 84, 1109–1117. [Google Scholar] [CrossRef]
- Schultheis, M.T.; Rizzo, A.A. The Application of Virtual Reality Technology in Rehabilitation. Rehabil. Psychol. 2001, 46, 296–311. [Google Scholar] [CrossRef]
- Ryan, M.-L. Narrative as Virtual Reality 2: Revisiting Immersion and Interactivity in Literature and Electronic Media; JHU Press: Baltimore, MD, USA, 2015; Volume 2. [Google Scholar]
- Slater, M.; Linakis, V.; Usoh, M.; Kooper, R. Immersion, presence and performance in virtual environments: An experiment with tri-dimensional chess. In Proceedings of the ACM Symposium on Virtual Reality Software and Technology, Hong Kong, China, 1–4 July 1996. [Google Scholar]
- Lewis, G.N.; Rosie, J.A. Virtual reality games for movement rehabilitation in neurological conditions: How do we meet the needs and expectations of the users? Disabil. Rehabil. 2012, 34, 1880–1886. [Google Scholar] [CrossRef]
- Mujber, T.; Szecsi, T.; Hashmi, M. Virtual reality applications in manufacturing process simulation. J. Mater. Process. Technol. 2004, 155–156, 1834–1838. [Google Scholar] [CrossRef]
- Mirelman, A.; Maidan, I.; Deutsch, J.E. Virtual reality and motor imagery: Promising tools for assessment and therapy in Parkinson’s disease. Mov. Disord. 2013, 28, 1597–1608. [Google Scholar] [CrossRef]
- Sveistrup, H. Motor rehabilitation using virtual reality. J. Neuroeng. Rehabil. 2004, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Mendes, F.A.; Pompeu, J.E.; Lobo, A.M.; Da Silva, K.G.; Oliveira, T.D.P.; Zomignani, A.P.; Piemonte, M.E.P. Motor learning, retention and transfer after virtual-reality-based training in Parkinson’s disease—Effect of motor and cognitive demands of games: A longitudinal, controlled clinical study. Physiotherapy 2012, 98, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Joo, L.Y.; Yin, T.S.; Xu, D.; Thia, E.; Fen, C.P.; Kuah, C.; Kong, K. A feasibility study using interactive commercial off-the-shelf computer gaming in upper limb rehabilitation in patients after stroke. J. Rehabil. Med. 2010, 42, 437–441. [Google Scholar]
- Studenski, S. Gait Speed and Survival in Older Adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shen, M.; Wang, Y.-X.; He, Z.-W.; Chi, S.-Q.; Yang, Z.-H. Effect of virtual reality on balance and gait ability in patients with Parkinson’s disease: A systematic review and meta-analysis. Clin. Rehabil. 2019, 33, 1130–1138. [Google Scholar] [CrossRef]
- Lei, C.; Sunzi, K.; Dai, F.; Liu, X.; Wang, Y.; Zhang, B.; He, L.; Ju, M. Effects of virtual reality rehabilitation training on gait and balance in patients with Parkinson’s disease: A systematic review. PLoS ONE 2019, 14, e0224819. [Google Scholar] [CrossRef]
- Weiss, P.L.T.; Katz, N. The potential of virtual reality for rehabilitation. J. Rehabil. Res. Dev. 2004, 41, 7–10. [Google Scholar]
- Riva, G.; Mantovani, F.; Gaggioli, A. Presence and rehabilitation: Toward second-generation virtual reality applications in neuropsychology. J. Neuroeng. Rehabil. 2004, 1, 9. [Google Scholar] [CrossRef]
- Schoneburg, B.; Mancini, M.; Horak, F.; Nutt, J.G. Framework for understanding balance dysfunction in Parkinson’s disease. Mov. Disord. 2013, 28, 1474–1482. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2; Updated February 2021; Cochrane: Chichester, UK, 2021. [Google Scholar]
- Yen, C.-Y.; Lin, K.-H.; Hu, M.-H.; Wu, R.-M.; Lu, T.-W.; Lin, C.-H. Effects of Virtual Reality–Augmented Balance Training on Sensory Organization and Attentional Demand for Postural Control in People With Parkinson Disease: A Randomized Controlled Trial. Phys. Ther. 2011, 91, 862–874. [Google Scholar] [CrossRef]
- Yang, W.-C.; Wang, H.-K.; Wu, R.-M.; Lo, C.-S.; Lin, K.-H. Home-based virtual reality balance training and conventional balance training in Parkinson’s disease: A randomized controlled trial. J. Formos. Med Assoc. 2016, 115, 734–743. [Google Scholar] [CrossRef]
- Van den Heuvel, M.R.C.; Kwakkel, G.; Beek, P.J.; Berendse, H.W.; Daffertshofer, A.; van Wegen, E.E. Effects of augmented visual feedback during balance training in Parkinson’s disease: A pilot randomized clinical trial. Park. Relat. Disord. 2014, 20, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Tollár, J.; Nagy, F.; Hortobágyi, T. Vastly Different Exercise Programs Similarly Improve Parkinsonian Symptoms: A Randomized Clinical Trial. Gerontology 2019, 65, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.-C.; Wang, R.-Y.; Cheng, S.-J.; Yang, Y.-R. Effects of a balance-based exergaming intervention using the Kinect sensor on posture stability in individuals with Parkinson’s disease: A single-blinded randomized controlled trial. J. Neuroeng. Rehabil. 2016, 13, 1–9. [Google Scholar] [CrossRef]
- Santos, P.; Machado, T.; Santos, L.; Ribeiro, N.; Melo, A. Efficacy of the Nintendo Wii combination with Conventional Exercises in the rehabilitation of individuals with Parkinson’s disease: A randomized clinical trial. Neurorehabilitation 2019, 45, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Ribas, C.G.; da Silva, L.A.; Corrêa, M.R.; Teive, H.G.; Valderramas, S. Effectiveness of exergaming in improving functional balance, fatigue and quality of life in Parkinson’s disease: A pilot randomized controlled trial. Park. Relat. Disord. 2017, 38, 13–18. [Google Scholar] [CrossRef]
- Pompeu, J.E.; Mendes, F.A.D.S.; Da Silva, K.G.; Lobo, A.M.; Oliveira, T.D.P.; Zomignani, A.P.; Piemonte, M.E.P. Effect of Nintendo Wii™-based motor and cognitive training on activities of daily living in patients with Parkinson’s disease: A randomised clinical trial. Physiotherapy 2012, 98, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Pedreira, G.; Prazeres, A.; Cruz, D.; Gomes, I.; Monteiro, L.; Melo, A. Virtual games and quality of life in Parkinson’s disease: A randomised controlled trial. Adv. Park. Dis. 2013, 2, 97–101. [Google Scholar] [CrossRef]
- Pazzaglia, C.; Imbimbo, I.; Tranchita, E.; Minganti, C.; Ricciardi, D.; Monaco, R.L.; Parisi, A.; Padua, L. Comparison of virtual reality rehabilitation and conventional rehabilitation in Parkinson’s disease: A randomised controlled trial. Physiotherapy 2020, 106, 36–42. [Google Scholar] [CrossRef]
- Liao, Y.-Y.; Yang, Y.-R.; Wu, Y.-R.; Wang, R.-Y. Virtual Reality-Based Wii Fit Training in Improving Muscle Strength, Sensory Integration Ability, and Walking Abilities in Patients with Parkinson’s Disease: A Randomized Control Trial. Int. J. Gerontol. 2015, 9, 190–195. [Google Scholar] [CrossRef]
- Liao, Y.-Y.; Yang, Y.-R.; Cheng, S.-J.; Wu, Y.-R.; Fuh, J.-L.; Wang, R.-Y. Virtual Reality–Based Training to Improve Obstacle-Crossing Performance and Dynamic Balance in Patients With Parkinson’s Disease. Neurorehabilit. Neural Repair 2014, 29, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.-Y.; Lee, D.-K.; Song, H.-S. Effect of virtual reality dance exercise on the balance, activities of daily living, and depressive disorder status of Parkinson’s disease patients. J. Phys. Ther. Sci. 2015, 27, 145–147. [Google Scholar] [CrossRef]
- Gandolfi, M.; Geroin, C.; Dimitrova, E.; Boldrini, P.; Waldner, A.; Bonadiman, S.; Picelli, A.; Regazzo, S.; Stirbu, E.; Primon, D.; et al. Virtual Reality Telerehabilitation for Postural Instability in Parkinson’s Disease: A Multicenter, Single-Blind, Randomized, Controlled Trial. BioMed Res. Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Li, C.; Liu, J.; Wang, L.; Ma, J.; Li, G.; Gan, L.; Shang, X.; Wu, Z. Virtual Reality Rehabilitation Versus Conventional Physical Therapy for Improving Balance and Gait in Parkinson’s Disease Patients: A Randomized Controlled Trial. Med. Sci. Monit. 2019, 25, 4186–4192. [Google Scholar] [CrossRef]
- Rose, T.; Nam, C.S.; Chen, K.B. Immersion of virtual reality for rehabilitation—Review. Appl. Ergon. 2018, 69, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, L.; Jacky, M.; Lünenburger, L.; Riener, R.; Bolliger, M. Increasing Patient Engagement During Virtual Reality-Based Motor Rehabilitation. Arch. Phys. Med. Rehabil. 2013, 94, 1737–1746. [Google Scholar] [CrossRef]
- Mirelman, A.; Rochester, L.; Maidan, I.; Del Din, S.; Alcock, L.; Nieuwhof, F.; Rikkert, M.O.; Bloem, B.R.; Pelosin, E.; Avanzino, L.; et al. Addition of a non-immersive virtual reality component to treadmill training to reduce fall risk in older adults (V-TIME): A randomised controlled trial. Lancet 2016, 388, 1170–1182. [Google Scholar] [CrossRef]
- Lewis, G.N.; Woods, C.; Rosie, J.A.; McPherson, K.M. Virtual reality games for rehabilitation of people with stroke: Perspectives from the users. Disabil. Rehabil. Assist. Technol. 2010, 6, 453–463. [Google Scholar] [CrossRef]
- Berg, K.; Wood-Dauphinee, S.; Williams, J.I. The Balance Scale: Reliability assessment with elderly residents and patients with an acute stroke. Scand. J. Rehabil. Med. 1995, 27, 27–36. [Google Scholar]
- Schlenstedt, C.; Brombacher, S.; Hartwigsen, G.; Weisser, B.; Möller, B.; Deuschl, G. Comparison of the Fullerton Advanced Balance Scale, Mini-BESTest, and Berg Balance Scale to Predict Falls in Parkinson Disease. Phys. Ther. 2016, 96, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.P.; Leddy, A.L.; Cavanaugh, J.T.; Dibble, L.E.; Ellis, T.D.; Ford, M.P.; Foreman, K.B.; Earhart, G.M. Balance differences in people with Parkinson disease with and without freezing of gait. Gait Posture 2015, 42, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Nocera, J.R.; Stegemöller, E.L.; Malaty, I.A.; Okun, M.S.; Marsiske, M.; Hass, C.J. Using the Timed Up & Go Test in a Clinical Setting to Predict Falling in Parkinson’s Disease. Arch. Phys. Med. Rehabil. 2013, 94, 1300–1305. [Google Scholar] [PubMed]
- Zampieri, C.; Salarian, A.; Carlson-Kuhta, P.; Aminian, K.; Nutt, J.G.; Horak, F.B. An instrumented timed up and go test characterizes gait and postural transitions in untreated Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2009. [Google Scholar] [CrossRef]
- Auriel, E.; Hausdorff, J.; Talia, H.; Simon, E.S.; Giladi, N. Effects of methylphenidate on cognitive function and gait in patients with Parkinson’s disease: A pilot study. Clin. Neuropharmacol. 2006, 29, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Rossi-Izquierdo, M.; Basta, D.; Rubio-Rodríguez, J.P.; Santos-Pérez, S.; Ernst, A.; Sesar-Ignacio, A.; Alberte-Woodward, M.; Amo, M.G.-D.; Estany-Gestal, A.; Román-Rodríguez, E.S.; et al. Is posturography able to identify fallers in patients with Parkinson’s disease? Gait Posture 2014, 40, 53–57. [Google Scholar] [CrossRef]
- Mancini, M.; Horak, F.B. The relevance of clinical balance assessment tools to differentiate balance deficits. Eur. J. Phys. Rehabil. Med. 2010, 46, 239–248. [Google Scholar] [PubMed]
- Salarian, A.; Zampieri, C.; Horak, F.B.; Carlson-Kuhta, P.; Nutt, J.G.; Aminian, K. Analyzing 180° turns using an inertial system reveals early signs of progression of parkinson’s disease. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MS, USA, 3–6 September 2009; Institute of Electrical and Electronics Engineers (IEEE): Minneapolis, MS, USA, 2009; Volume 2009, pp. 224–227. [Google Scholar]

| Study | Outcomes | Findings | PEDro |
|---|---|---|---|
| Yang 2016 | BBS TUG PDQ-39 | No significant differences were found in BBS, TUG, PDQ-39 | 7 |
| Tollár 2019 | PDQ-39 BBS | VR group showed a greater improvement in BBS compared with the control group | 6 |
| Heuvel 2014 | BBS SLS FES PDQ-39 | No significant differences were found in FRT, BBS, SLS, FES, PDQ-39 | 8 |
| Shih 2016 | LOS OLS BBS TUG | Significant improvement in LOS, OLS, BBS, TUG | 6 |
| Santos 2019 | BBS TUG PDQ-39 | Significant improvement in BBS, TUG, PDQ-39 | 6 |
| Ribas 2017 | BBS PDQ-39 | Significant improvement in BBS, but this benefit was not sustained in the long-term | 7 |
| Pompeu 2012 | BBS UST | No significant differences were found in BBS, UST | 5 |
| Pazzaglia 2020 | BBS | VR group showed a greater improvement in BBS compared with the control group | 5 |
| Liao. 2015a | TUG LOS SOT FES PDQ-39 | Significant improvement in TUG, LOS, SOT, FES, and PDQ-39 | 7 |
| Lee 2015 | BBS | Significant improvement in BBS | 4 |
| Gandolfi 2017 | BBS ABC | Significant improvement in BBS and ABC | 6 |
| Feng 2019 | BBS TUG | Significant improvement in BBS, TUG | 6 |
| Pedreira 2013 | PDQ-39 | VR group showed greater improvement in the PDQ-39 than the control group | 4 |
| Liao 2015b | SOT | No significant difference was found in SOT between the two groups | 7 |
| Yen 2011 | SOT | VR group showed greater improvement in SOT-6 than control group | 8 |
| Study | Sample | Intervention | Dosage | ||
|---|---|---|---|---|---|
| Diagnosis Hoehn and Yahr | Size M/F | Experimental Group | Control Group | ||
| Yang 2016 | IPD 2–3 | 23 14/9 | VR balance training (n = 11) | Conventional home balance training (n = 12) | 50 min/d 2 d/w 6 w |
| Tollár 2019 | PD 2–3 | 74 36/38 | Exergaming (n = 25) | Stationary cycling (CYC) (n = 25) Waitlist (n = 24) | 60 min/d 5 d/w 5 w |
| Heuvel 2014 | IPD 2–3 | 33 20/13 | Augmented visual feedback (VFT) (n = 17) | Conventional training (n = 14) | 60 min/d 2 d/w 5 w |
| Shih 2016 | IPD 1–3 | 20 16/4 | Exergaming group (n = 10) | Balance training group (n = 10) | 50 min/d 2 d/w 8 w |
| Santos 2019 | PD 1–3 | 45 31/14 | NW (n = 15) NW + CE (n = 15) | Conventional exercise (CE) (n = 15) | 50 min/d 2 d/w 8 w |
| Ribas 2017 | PD 1–3 | 20 8/12 | Exergaming Wii fit games (n = 10) | Conventional exercise (n = 10) | 30 m/d 2 d/w 12 w |
| Pompeu 2012 | IPD 1–2 | 32 17/15 | Wii-based motor training (n = 16) | Traditional balance exercise (n = 16) | 60 min/d 2 d/w 7 w |
| Pazzaglia 2020 | PD NA | 51 35/16 | VR rehabilitation (n = 25) | Conventional program (n = 26) | 40 min/d 3 d/w 6 w |
| Liao 2015a | IPD 1–3 | 36 17/19 | VR-based Wii Fit exercise (n = 12) | Traditional exercise (n = 12) Control group (n = 12) | 1 h/d 2 d/w 6 w |
| Lee 2015 | PD NA | 20 10/10 | VR + NDT and functional electrical stimulation (n = 10) | NDT and functional electrical stimulation (n = 10) | 45 min/d 5 d/w 6 w |
| Gandolfi 2017 | PD 2.5–3 | 76 51/25 | VR telerehabilitation (n = 38) | Sensory integration balance training (n = 38) | 50 min/d, 3 d/w 7 w |
| Feng 2019 | PD 2.5–4 | 30 17/13 | VR training (n = 14) | Conventional therapy(n = 14) | 45 min/d 5 d/w 12 w |
| Pedreira 2013 | PD 1–3 | 31 22/9 | Nintendo Wii virtual games (n = 16) | Traditional exercise (n = 15) | 40 min/d 3 d/w 4 w |
| Liao 2015b | IPD 1–3 | 36 17/19 | VR–based Wii Fit exercise (n = 12) | Traditional exercise (n = 12) Control group (n = 12) | 60 min/d 2 d/w 6 w |
| Yen 2011 | IPD 2–3 | 42 33/9 | VR balance training (n = 14) | Conventional balance training (n = 14) Control group (n = 14) | 30 min/d 2 d/w 6 w |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Wong, S.S.-l.; Lai, F.H.-y. The Effect of Virtual Reality Rehabilitation on Balance in Patients with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Electronics 2021, 10, 1003. https://doi.org/10.3390/electronics10091003
Wang W, Wong SS-l, Lai FH-y. The Effect of Virtual Reality Rehabilitation on Balance in Patients with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Electronics. 2021; 10(9):1003. https://doi.org/10.3390/electronics10091003
Chicago/Turabian StyleWang, Wenjing, Sharon Sui-lam Wong, and Frank Ho-yin Lai. 2021. "The Effect of Virtual Reality Rehabilitation on Balance in Patients with Parkinson’s Disease: A Systematic Review and Meta-Analysis" Electronics 10, no. 9: 1003. https://doi.org/10.3390/electronics10091003
APA StyleWang, W., Wong, S. S.-l., & Lai, F. H.-y. (2021). The Effect of Virtual Reality Rehabilitation on Balance in Patients with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Electronics, 10(9), 1003. https://doi.org/10.3390/electronics10091003






