Socio-Economic and Environmental Impacts of Biomass Valorisation: A Strategic Drive for Sustainable Bioeconomy
Abstract
:1. Introduction
2. Transformation of Biomass and Waste into Bioproducts
3. Sources of Waste/Production of Biomass
3.1. Agricultural Aspects
3.2. Industrial Aspects
3.3. Domestic Food Waste and Considerations
4. Characteristics and Composition of Biomass
5. Green Technologies for Biomass and Waste Valorisation
5.1. Fermentation Technology
5.2. Flow Technology
5.3. Gasification
5.4. Microbial Digestion
5.5. Microwave Technology
6. Applied Potentialities—A Drive towards Sustainability
6.1. Biofuels and Energy Production
6.2. Organic Acids and Chemicals
6.3. Enzymes and Lipids
6.4. Biological Macromolecules
6.5. Food/Feed Products
6.6. Bioelectricity via Microbial Fuel Cell (MFC) Technology
6.7. Paper and Pulp
6.8. Pharmaceutical
6.9. Miscellaneous
7. Conclusions, Bioeconomy Challenges, and Socio-Economic Impacts
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Junginger, H.; Jonker, J.; Faaij, A. Summary, Synthesis and Conclusions from IEA Bioenergy Task 40 Country Reports on International Bioenergy Trade; Copernicus Institute, Utrecht University: Utrecht, The Netherlands, 2011; pp. 1–25. Available online: https://dspace.library.uu.nl/handle/1874/212189 (accessed on 31 March 2021).
- Demirbas, M.F. Emissions of polychlorinated dibenzo-p-dioxins and dibenzofurans from biomass combustion and solid waste incineration. Energy Sources Part A Recover. Util. Environ. Eff. 2007, 29, 1041–1047. [Google Scholar] [CrossRef]
- Monien, B.H.; Herrmann, K.; Florian, S.; Glatt, H. Metabolic activation of furfuryl alcohol: Formation of 2-methylfuranyl DNA adducts in Salmonella typhimurium strains expressing human sulfotransferase 1A1 and in FVB/N mice. Carcinogenesis 2011, 32, 1533–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, D.O.; Rosillo-Calle, F.; Williams, R.H.; Woods, J. Biomass for energy: Supply prospects. In Biomass Energy Supply Prospect; Earthscan: London, UK, 1993; pp. 593–651. ISBN 1853831557. [Google Scholar]
- Brunner, P.H.; Rechberger, H. Practical Handbook of Material Flow Analysis; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9780203507209. [Google Scholar]
- Awasthi, M.K.; Zhao, J.; Soundari, P.G.; Kumar, S.; Chen, H.; Awasthi, S.K.; Duan, Y.; Liu, T.; Pandey, A.; Zhang, Z. Sustainable Management of Solid Waste. In Sustainable Resource Recovery and Zero Waste Approaches; Elsevier: Amsterdam, The Netherlands, 2019; pp. 79–99. [Google Scholar]
- Korhonen, J.; Honkasalo, A.; Seppälä, J. Circular Economy: The Concept and its Limitations. Ecol. Econ. 2018, 143, 37–46. [Google Scholar] [CrossRef]
- Agarwal, M.; Jareda, K.; Bajpai, M. A review on solid waste management for smart city. SSRG Int. J. Civil Eng. (SSRG-IJCE) 2016, 3, 109–112. [Google Scholar]
- Berndes, G.; Hoogwijk, M.; Van Den Broek, R. The contribution of biomass in the future global energy supply: A review of 17 studies. Biomass Bioenergy 2003, 25, 1–28. [Google Scholar] [CrossRef]
- Kopetz, H. Renewable resources: Build a biomass energy market. Nature 2013, 494, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Chum, H.L.; Overend, R.P. Biomass and renewable fuels. Fuel Process. Technol. 2001, 71, 187–195. [Google Scholar] [CrossRef]
- Saldarriaga-Hernández, S.; Velasco-Ayala, C.; Flores, P.L.I.; de Jesús Rostro-Alanis, M.; Parra-Saldivar, R.; Iqbal, H.M.; Carrillo-Nieves, D. Biotransformation of lignocellulosic biomass into industrially relevant products with the aid of fungi-derived lignocellulolytic enzymes. Int. J. Biol. Macromol. 2020, 161, 1099–1116. [Google Scholar] [CrossRef]
- Razik, A.H.A.; Khor, C.S.; Elkamel, A. A model-based approach for biomass-to-bioproducts supply Chain network planning optimization. Food Bioprod. Proc. 2019, 118, 293–305. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M. Recent Advancements in the Life Cycle Analysis of Lignocellulosic Biomass. Curr. Sustain. Renew. Energy Rep. 2020, 7, 100–107. [Google Scholar] [CrossRef]
- Demirbaş, A. Biomass resource facilities and biomass conversion processing for fuels and chemicals. Energy Convers. Manag. 2001, 42, 1357–1378. [Google Scholar] [CrossRef]
- Parikka, M. Global biomass fuel resources. Biomass Bioenergy 2004, 27, 613–620. [Google Scholar] [CrossRef]
- Arevalo-Gallegos, A.; Ahmad, Z.; Asgher, M.; Parra-Saldivar, R.; Iqbal, H.M. Lignocellulose: A sustainable material to produce value-added products with a zero waste approach—A review. Int. J. Biol. Macromol. 2017, 99, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- John, R.P.; Sukumaran, R.K.; Nampoothiri, K.M.; Pandey, A. Statistical optimization of simultaneous saccharification and l(+)-lactic acid fermentation from cassava bagasse using mixed culture of lactobacilli by response surface methodology. Biochem. Eng. J. 2007, 36, 262–267. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, N.; Baredar, P.; Shukla, A. A review on biomass energy resources, potential, conversion and policy in India. Renew. Sustain. Energy Rev. 2015, 45, 530–539. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. Recent trends in global production and utilization of bio-ethanol fuel. Appl. Energy 2009, 86, 2273–2282. [Google Scholar] [CrossRef]
- Deublein, D.; Steinhauser, A. Biogas from Waste and Renewable Resources: An Introduction, 2nd, Revised and Expanded Edition; Current Reviews for Academic Libraries; John Wiley & Sons: New Jersey, NJ, USA, 2010; p. 578. ISBN 978-3-527-32798-0. [Google Scholar]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Ma, Y.; Ma, L. Utilization of straw in biomass energy in China. Renew. Sustain. Energy Rev. 2007, 11, 976–987. [Google Scholar] [CrossRef]
- Mendu, V.; Harman-Ware, A.E.; Crocker, M.; Jae, J.; Stork, J.; Morton, S.; Placido, A.; Huber, G.; Debolt, S. Identification and thermochemical analysis of high-lignin feedstocks for biofuel and biochemical production. Biotechnol. Biofuels 2011, 4. [Google Scholar] [CrossRef] [Green Version]
- Mendu, V.; Shearin, T.; Campbell, J.E.; Stork, J.; Jae, J.; Crocker, M.; Huber, G.; DeBolt, S. Global bioenergy potential from high-lignin agricultural residue. Proc. Natl. Acad. Sci. USA 2012, 109, 4014–4019. [Google Scholar] [CrossRef] [Green Version]
- Welker, C.M.; Balasubramanian, V.K.; Petti, C.; Rai, K.M.; De Bolt, S.; Mendu, V. Engineering plant biomass lignin content and composition for biofuels and bioproducts. Energies 2015, 8, 7654–7676. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Amos, K.; Li, M.; Pu, Y.; DeBolt, S.; Ragauskas, A.J.; Shi, J. Fractionation and characterization of lignin streams from unique high-lignin content endocarp feedstocks. Biotechnol. Biofuels 2018, 11. [Google Scholar] [CrossRef]
- Harman-Ware, A.E.; Crocker, M.; Pace, R.B.; Placido, A.; Morton, S.; DeBolt, S. Characterization of Endocarp Biomass and Extracted Lignin Using Pyrolysis and Spectroscopic Methods. Bioenergy Res. 2015, 8, 350–368. [Google Scholar] [CrossRef]
- Dardick, C.D.; Callahan, A.M.; Chiozzotto, R.; Schaffer, R.J.; Piagnani, M.C.; Scorza, R. Stone formation in peach fruit exhibits spatial coordination of the lignin and flavonoid pathways and similarity to Arabidopsis dehiscence. BMC Biol. 2010, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, E.A.; Echavarri-Erasun, C. Chapter 3—Yeast biotechnology. In The Yeasts; Elsevier: San Diego, CA, USA, 2011; pp. 21–44. [Google Scholar] [CrossRef]
- Siqueira, P.F.; Karp, S.G.; Carvalho, J.C.; Sturm, W.; Rodríguez-León, J.A.; Tholozan, J.L.; Singhania, R.R.; Pandey, A.; Soccol, C.R. Production of bio-ethanol from soybean molasses by Saccharomyces cerevisiae at laboratory, pilot and industrial scales. Bioresour. Technol. 2008, 99, 8156–8163. [Google Scholar] [CrossRef]
- John, R.P.; Nampoothiri, K.M.; Pandey, A. Fermentative production of lactic acid from biomass: An overview on process developments and future perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.S.; Cameotra, S.S. Biosurfactant production by microorganisms on unconventional carbon sources. J. Surfactants Deterg. 1999, 2, 237–241. [Google Scholar] [CrossRef]
- Nigam, P.S.; Singh, A. Production of liquid biofuels from renewable resources. Prog. Energy Combust. Sci. 2011, 37, 52–68. [Google Scholar] [CrossRef]
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Bhutto, A.W.; Bazmi, A.A.; Zahedi, G. Greener energy: Issues and challenges for Pakistan-Biomass energy prospective. Renew. Sustain. Energy Rev. 2011, 15, 3207–3219. [Google Scholar] [CrossRef]
- Turan, N.G.; Çoruh, S.; Akdemir, A.; Ergun, O.N. Municipal solid waste management strategies in Turkey. Waste Manag. 2009, 29, 465–469. [Google Scholar] [CrossRef]
- Ionescu, G.; Rada, E.C.; Ragazzi, M.; Mǎrculescu, C.; Badea, A.; Apostol, T. Integrated municipal solid waste scenario model using advanced pretreatment and waste to energy processes. Energy Convers. Manag. 2013, 76, 1083–1092. [Google Scholar] [CrossRef]
- Kupper, T.; Bürge, D.; Bachmann, H.J.; Güsewell, S.; Mayer, J. Heavy metals in source-separated compost and digestates. Waste Manag. 2014, 34, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Bridgwater, A.V.; Meier, D.; Radlein, D. An overview of fast pyrolysis of biomass. Org. Geochem. 1999, 30, 1479–1493. [Google Scholar] [CrossRef]
- Sonesson, U.; Björklund, A.; Carlsson, M.; Dalemo, M. Environmental and economic analysis of management systems for biodegradable waste. Resour. Conserv. Recycl. 2000, 28, 29–53. [Google Scholar] [CrossRef]
- Kiyasudeen, K.; Ibrahim, M.H.; Quaik, S.; Ismail, S.A. An introduction to anaerobic digestion of organic wastes. In Prospects of Organic Waste Management and the Significance of Earthworms; Springer: Cham, Switzerland, 2016; pp. 23–44. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Khanal, S.; Manandhar, A.; Shah, A. Anaerobic digestion for bioenergy production: Global status, environmental and techno-economic implications, and government policies. Bioresour. Technol. 2018, 247, 1015–1026. [Google Scholar] [CrossRef]
- Yokoyama, S. The Asian Biomass Handbook A Guide for Biomass Production and Utilization Support Project for Building Asian-Partnership for Environmentally Conscious Agriculture; Entrusted by Ministry of Agriculture, Forestry, and Fisheries; The Japan Institute of Energy: Tokyo, Japan, 2008. [Google Scholar]
- Kircher, M. Sustainability of biofuels and renewable chemicals production from biomass. Curr. Opin. Chem. Biol. 2015, 29, 26–31. [Google Scholar] [CrossRef]
- Brar, S.K.; Dhillon, G.S.; Soccol, C.R. Biotransformation of Waste Biomass into High Value Biochemicals; Springer Science & Business Media: New York, NY, USA, 2013; ISBN 9781461480044. [Google Scholar]
- Duku, M.H.; Gu, S.; Hagan, E. Ben A comprehensive review of biomass resources and biofuels potential in Ghana. Renew. Sustain. Energy Rev. 2011, 15, 404–415. [Google Scholar] [CrossRef]
- Kögel-Knabner, I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol. Biochem. 2002, 34, 139–162. [Google Scholar] [CrossRef]
- Zhang, N.; Li, S.; Xiong, L.; Hong, Y.; Chen, Y. Cellulose-hemicellulose interaction in wood secondary cell-wall. Model. Simul. Mater. Sci. Eng. 2015, 23. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Maki, M.; Leung, K.T.; Qin, W. The prospects of cellulase-producing bacteria for the bioconversion of lignocellulosic biomass The prospects of cellulase-producing bacteria for the bioconversion of lignocellulosic biomass Page 2 sur 8. Int. J. Biol. Sci. 2013, 5, 1–8. [Google Scholar] [CrossRef]
- Ekman, A.; Börjesson, P. Environmental assessment of propionic acid produced in an agricultural biomass-based biorefinery system. J. Clean. Prod. 2011, 19, 1257–1265. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Iqbal, H.M.; Hu, H.; Zhang, X. Biotransformation of lignocellulosic materials into value-added products—A review. Int. J. Biol. Macromol. 2017, 98, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, C.; Champagne, P. Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers. Manag. 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Van der Stelt, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass upgrading by torrefaction for the production of biofuels: A review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Demirbas, A. Combustion characteristics of different biomass fuels. Prog. Energy Combust. Sci. 2004, 30, 219–230. [Google Scholar] [CrossRef]
- Jenkins, B.M.; Baxter, L.L.; Miles, T.R.; Miles, T.R. Combustion properties of biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F.; et al. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Koutinas, A.A.; Stamatelatou, K.; Mubofu, E.B.; Matharu, A.S.; Kopsahelis, N.; Pfaltzgraff, L.A.; Clark, J.H.; Papanikolaou, S.; Kwan, T.H.; et al. Current and future trends in food waste valorization for the production of chemicals, materials and fuels: A global perspective. Biofuels Bioprod. Biorefin. 2014, 8, 686–715. [Google Scholar] [CrossRef]
- Matharu, A.S.; de Melo, E.M.; Houghton, J.A. Food Supply Chain Waste: A Functional Periodic Table of Bio-Based Resources. In Waste Biorefinery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 219–236. [Google Scholar]
- Vlachokostas, C.; Achillas, C.; Diamantis, V.; Michailidou, A.V.; Baginetas, K.; Aidonis, D. Supporting decision making to achieve circularity via a biodegradable waste-to-bioenergy and compost facility. J. Environ. Manag. 2021, 285, 112215. [Google Scholar] [CrossRef] [PubMed]
- Vlachokostas, C.; Michailidou, A.V.; Achillas, C. Multi-Criteria Decision Analysis towards promoting Waste-to-Energy Management Strategies: A critical review. Renew. Sust. Energ. Rev. 2021, 138, 110563. [Google Scholar] [CrossRef]
- Gray, K.; Zhao, L.; Emptage, M. Bioethanol. Curr. Opin. Chem. Biol. 2006, 10, 141–146. [Google Scholar] [CrossRef]
- You, C.; Chen, H.; Myung, S.; Sathitsuksanoh, N.; Ma, H.; Zhang, X.Z.; Li, J.; Zhang, Y.H.P. Enzymatic transformation of nonfood biomass to starch. Proc. Natl. Acad. Sci. USA 2013, 110, 7182–7187. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Tanaka, S. Ethanol fermentation from biomass resources: Current state and prospects. Appl. Microbiol. Biotechnol. 2006, 69, 627–642. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of biomass: Deriving more value from waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef]
- Yue, D.; You, F.; Snyder, S.W. Biomass-to-bioenergy and biofuel supply chain optimization: Overview, key issues and challenges. Comput. Chem. Eng. 2014, 66, 36–56. [Google Scholar] [CrossRef]
- Richard, T.L. Challenges in scaling up biofuels infrastructure. Science 2010, 329, 793–796. [Google Scholar] [CrossRef] [Green Version]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Glasnov, T.N.; Kappe, C.O. The microwave-to-flow paradigm: Translating high-temperature batch microwave chemistry to scalable continuous-flow processes. Chem. A Eur. J. 2011, 17, 11956–11968. [Google Scholar] [CrossRef]
- Ruiz, J.A.; Juárez, M.C.; Morales, M.P.; Muñoz, P.; Mendívil, M.A. Biomass gasification for electricity generation: Review of current technology barriers. Renew. Sustain. Energy Rev. 2013, 18, 174–183. [Google Scholar] [CrossRef]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Mondal, P.; Dang, G.S.; Garg, M.O. Syngas production through gasification and cleanup for downstream applications—Recent developments. Fuel Process. Technol. 2011, 92, 1395–1410. [Google Scholar] [CrossRef]
- Lapuerta, M.; Hernández, J.J.; Pazo, A.; López, J. Gasification and co-gasification of biomass wastes: Effect of the biomass origin and the gasifier operating conditions. Fuel Process. Technol. 2008, 89, 828–837. [Google Scholar] [CrossRef]
- Tock, J.Y.; Lai, C.L.; Lee, K.T.; Tan, K.T.; Bhatia, S. Banana biomass as potential renewable energy resource: A Malaysian case study. Renew. Sustain. Energy Rev. 2010, 14, 798–805. [Google Scholar] [CrossRef]
- Gasafi, E.; Reinecke, M.Y.; Kruse, A.; Schebek, L. Economic analysis of sewage sludge gasification in supercritical water for hydrogen production. Biomass Bioenergy 2008, 32, 1085–1096. [Google Scholar] [CrossRef]
- Antoni, D.; Zverlov, V.V.; Schwarz, W.H. Biofuels from microbes. Appl. Microbiol. Biotechnol. 2007, 77, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Gumisiriza, R.; Mshandete, A.M.; Rubindamayugi, M.S.T.; Kansiime, F.; Kivaisi, A.K. Enhancement of anaerobic digestion of Nile perch fish processing wastewater. Afr. J. Biotechnol. 2009, 8, 328–333. [Google Scholar]
- Cirne, D.G.; Paloumet, X.; Björnsson, L.; Alves, M.M.; Mattiasson, B. Anaerobic digestion of lipid-rich waste-Effects of lipid concentration. Renew. Energy 2007, 32, 965–975. [Google Scholar] [CrossRef] [Green Version]
- Das Neves, L.C.M.; Converti, A.; Penna, T.C.V. Biogas production: New trends for alternative energy sources in rural and urban zones. Chem. Eng. Technol. 2009, 32, 1147–1153. [Google Scholar] [CrossRef]
- Gupta, P.; Gupta, A. Biogas production from coal via anaerobic fermentation. Fuel 2014, 118, 238–242. [Google Scholar] [CrossRef]
- Scarlat, N.; Motola, V.; Dallemand, J.F.; Monforti-Ferrario, F.; Mofor, L. Evaluation of energy potential of Municipal Solid Waste from African urban areas. Renew. Sustain. Energy Rev. 2015, 50, 1269–1286. [Google Scholar] [CrossRef]
- Titirici, M.M.; Thomas, A.; Antonietti, M. Back in the black: Hydrothermal carbonization of plant material as an efficient chemical process to treat the CO2 problem? New J. Chem. 2007, 31, 787–789. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [Green Version]
- Silveira, M.H.L.; Morais, A.R.C.; Da Costa Lopes, A.M.; Olekszyszen, D.N.; Bogel-Łukasik, R.; Andreaus, J.; Pereira Ramos, L. Current Pretreatment Technologies for the Development of Cellulosic Ethanol and Biorefineries. ChemSusChem 2015, 8, 3366–3390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, S.; Glaser, B.; Quicker, P. Technical, economical, and climate-related aspects of biochar production technologies: A literature review. Environ. Sci. Technol. 2011, 45, 9473–9483. [Google Scholar] [CrossRef]
- Richel, A.; Paquot, M. Conversion of Carbohydrates Under Microwave Heating. In Carbohydrates—Comprehensive Studies on Glycobiology and Glycotechnology; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef] [Green Version]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef]
- Guo, L.; Li, X.M.; Bo, X.; Yang, Q.; Zeng, G.M.; Liao, D.X.; Liu, J.J. Impacts of sterilization, microwave and ultrasonication pretreatment on hydrogen producing using waste sludge. Bioresour. Technol. 2008, 99, 3651–3658. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, C.; Zhang, Y.; Ren, N.; Tang, Y. Microwave-assisted hydrothermal synthesis of nanozeolites with controllable size. Microporous Mesoporous Mater. 2009, 119, 306–314. [Google Scholar] [CrossRef]
- Menéndez, J.A.; Inguanzo, M.; Pis, J.J. Microwave-induced pyrolysis of sewage sludge. Water Res. 2002, 36, 3261–3264. [Google Scholar] [CrossRef]
- Digman, B.; Joo, H.S.; Kim, D.S. Recent progress in gasification/ pyrolysis technologies for biomass conversion to energy. Environ. Prog. Sustain. Energy 2009, 28, 47–51. [Google Scholar] [CrossRef]
- Yin, C. Microwave-assisted pyrolysis of biomass for liquid biofuels production. Bioresour. Technol. 2012, 120, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Luque, R.; Menéndez, J.A.; Arenillas, A.; Cot, J. Microwave-assisted pyrolysis of biomass feedstocks: The way forward? Energy Environ. Sci. 2012, 5, 5481–5488. [Google Scholar] [CrossRef]
- Guiotoku, M.; Rambo, C.R.; Hansel, F.A.; Magalhães, W.L.E.; Hotza, D. Microwave-assisted hydrothermal carbonization of lignocellulosic materials. Mater. Lett. 2009, 63, 2707–2709. [Google Scholar] [CrossRef]
- Amr Sohby, J.C. Microwave-assisted biorefinery. Chem. Eng. Trans. 2010, 19, 211–212. [Google Scholar] [CrossRef]
- Nomanbhay, S.; Salman, B.; Hussain, R.; Ong, M.Y. Microwave pyrolysis of lignocellulosic biomass—A contribution to power Africa. Energy Sustain. Soc. 2017, 7, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Narzari, R.; Bordoloi, N.; Sarma, B.; Gogoi, L.; Gogoi, N.; Borkotoki, B.; Kataki, R. Fabrication of biochars obtained from valorization of biowaste and evaluation of its physicochemical properties. Bioresour. Technol. 2017, 242, 324–328. [Google Scholar] [CrossRef]
- Omer, A.M. Renewable building energy systems and passive human comfort solutions. Renew. Sustain. Energy Rev. 2008, 12, 1562–1587. [Google Scholar] [CrossRef]
- Yang, X.; Choi, H.S.; Park, C.; Kim, S.W. Current states and prospects of organic waste utilization for biorefineries. Renew. Sustain. Energy Rev. 2015, 49, 335–349. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H.; Öz, C. Progress in bioethanol processing. Prog. Energy Combust. Sci. 2008, 34, 551–573. [Google Scholar] [CrossRef]
- Limayem, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Vohra, M.; Manwar, J.; Manmode, R.; Padgilwar, S.; Patil, S. Bioethanol production: Feedstock and current technologies. J. Environ. Chem. Eng. 2014, 2, 573–584. [Google Scholar] [CrossRef]
- Ho, D.P.; Ngo, H.H.; Guo, W. A mini review on renewable sources for biofuel. Bioresour. Technol. 2014, 169, 742–749. [Google Scholar] [CrossRef] [Green Version]
- Zabed, H.; Faruq, G.; Sahu, J.N.; Azirun, M.S.; Hashim, R.; Nasrulhaq Boyce, A. Bioethanol production from fermentable sugar juice. Sci. World J. 2014, 2014, 957102. [Google Scholar] [CrossRef] [Green Version]
- Sindhu, R.; Gnansounou, E.; Binod, P.; Pandey, A. Bioconversion of sugarcane crop residue for value added products—An overview. Renew. Energy 2016, 98, 203–215. [Google Scholar] [CrossRef]
- Lee, R.A.; Lavoie, J.-M. From first- to third-generation biofuels: Challenges of producing a commodity from a biomass of increasing complexity. Anim. Front. 2013, 3, 6–11. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Suely, A.; Boyce, A.N.; Faruq, G. Bioethanol production from renewable sources: Current perspectives and technological progress. Renew. Sustain. Energy Rev. 2017, 71, 475–501. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: Challenges and opportunities. Bioresour. Technol. 2016, 199, 92–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabed, H.; Sahu, J.N.; Boyce, A.N.; Faruq, G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew. Sustain. Energy Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Wu, R.; Liu, D. Organosolv fractionating pre-treatment of lignocellulosic biomass for efficient enzymatic saccharification: Chemistry, kinetics, and substrate structures. Biofuels, Bioprod. Biorefin. 2017, 11, 567–590. [Google Scholar] [CrossRef]
- Wang, M.; Wu, M.; Huo, H. Life-cycle energy and greenhouse gas emission impacts of different corn ethanol plant types. Environ. Res. Lett. 2007, 2, 024001. [Google Scholar] [CrossRef]
- Wyman, C.E. Ethanol Production from Lignocellulosic Biomass (No. CONF-950336-); American Society of Mechanical Engineers: New York, NY, USA, 1995. [Google Scholar]
- Hassan, M.H.; Kalam, M.A. An overview of biofuel as a renewable energy source: Development and challenges. Procedia Eng. 2013, 56, 39–53. [Google Scholar] [CrossRef] [Green Version]
- Balat, M.; Balat, H. Progress in biodiesel processing. Appl. Energy 2010, 87, 1815–1835. [Google Scholar] [CrossRef]
- Musa, I.A. The effects of alcohol to oil molar ratios and the type of alcohol on biodiesel production using transesterification process. Egypt. J. Pet. 2016, 25, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Mishra, V.K.; Goswami, R. A review of production, properties and advantages of biodiesel. Biofuels 2018, 9, 273–289. [Google Scholar] [CrossRef]
- Christopher, L.P.; Kumar, H.; Zambare, V.P. Enzymatic biodiesel: Challenges and opportunities. Appl. Energy 2014, 119, 497–520. [Google Scholar] [CrossRef]
- Demirbaş, A. Diesel fuel from vegetable oil via transesterification and soap pyrolysis. Energy Sources 2002, 24, 835–842. [Google Scholar] [CrossRef]
- Srivastava, G.; Paul, A.K.; Goud, V.V. Optimization of non-catalytic transesterification of microalgae oil to biodiesel under supercritical methanol condition. Energy Convers. Manag. 2018, 156, 269–278. [Google Scholar] [CrossRef]
- Tran, D.T.; Chang, J.S.; Lee, D.J. Recent insights into continuous-flow biodiesel production via catalytic and non-catalytic transesterification processes. Appl. Energy 2017, 185, 376–409. [Google Scholar] [CrossRef]
- Chen, X.; Gu, Y.; Zhou, X.; Zhang, Y. Asparagus stem as a new lignocellulosic biomass feedstock for anaerobic digestion: Increasing hydrolysis rate, methane production and biodegradability by alkaline pretreatment. Bioresour. Technol. 2014, 164, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Li, X.; Li, L.; Yang, X.; He, Y. Enhancing anaerobic biogasification of corn stover through wet state NaOH pretreatment. Bioresour. Technol. 2009, 100, 5140–5145. [Google Scholar] [CrossRef] [PubMed]
- Sawatdeenarunat, C.; Nguyen, D.; Surendra, K.C.; Shrestha, S.; Rajendran, K.; Oechsner, H.; Xie, L.; Khanal, S.K. Anaerobic biorefinery: Current status, challenges, and opportunities. Bioresour. Technol. 2016, 215, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.L.; Gu, Y.; Liu, Z.; Shen, Z.; Chu, H.; Zhou, X. Enhancing methane production from rice straw by extrusion pretreatment. Appl. Energy 2014, 122, 34–41. [Google Scholar] [CrossRef]
- Wu, N.; Moreira, C.M.; Zhang, Y.; Doan, N.; Yang, S.; Phlips, E.J.; Svoronos, S.A.; Pullammanappallil, P.C. Techno-Economic Analysis of Biogas Production from Microalgae through Anaerobic Digestion. In Anaerobic Digestion; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Guo, X.M.; Latrille, E.; Trably, E.; Steyer, J.P.; Carrere, H. Predictive models of biohydrogen and biomethane production based on the compositional and structural features of lignocellulosic materials. Environ. Sci. Technol. 2012, 46, 12217–12225. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- He, Y.; Pang, Y.; Liu, Y.; Li, X.; Wang, K. Physicochemical characterization of rice straw pretreated with sodium hydroxide in the solid state for enhancing biogas production. Energy Fuels 2008, 22, 2775–2781. [Google Scholar] [CrossRef]
- Rajput, A.A.; Zeshan; Visvanathan, C. Effect of thermal pretreatment on chemical composition, physical structure and biogas production kinetics of wheat straw. J. Environ. Manag. 2018, 221, 45–52. [Google Scholar] [CrossRef]
- Zhu, J.; Wan, C.; Li, Y. Enhanced solid-state anaerobic digestion of corn stover by alkaline pretreatment. Bioresour. Technol. 2010, 101, 7523–7528. [Google Scholar] [CrossRef]
- Salehian, P.; Karimi, K.; Zilouei, H.; Jeihanipour, A. Improvement of biogas production from pine wood by alkali pretreatment. Fuel 2013, 106, 484–489. [Google Scholar] [CrossRef]
- Monlau, F.; Aemig, Q.; Barakat, A.; Steyer, J.P.; Carrère, H. Application of optimized alkaline pretreatment for enhancing the anaerobic digestion of different sunflower stalks varieties. Environ. Technol. 2013, 34, 2155–2162. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; He, M.; Ren, Y.; Ma, L.; Luo, Y.; Sheng, H.; Xiang, Y.; Zhang, H.; Li, Q.; An, L. Anaerobic digestion of poplar processing residues for methane production after alkaline treatment. Bioresour. Technol. 2013, 134, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Multi-response optimization of process parameters in biogas production from food waste using Taguchi—Grey relational analysis. Energy Convers. Manag. 2017, 141, 429–438. [Google Scholar] [CrossRef]
- Krishna, D.; Kalamdhad, A.S. Pre-treatment and anaerobic digestion of food waste for high rate methane production—A review. J. Environ. Chem. Eng. 2014, 2, 1821–1830. [Google Scholar]
- Karthikeyan, O.P.; Visvanathan, C. Effect of C/N ratio and ammonia-N accumulation in a pilot-scale thermophilic dry anaerobic digester. Bioresour. Technol. 2012, 113, 294–302. [Google Scholar] [CrossRef]
- Dahiya, S.; Kumar, A.N.; Shanthi Sravan, J.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Food waste biorefinery: Sustainable strategy for circular bioeconomy. Bioresour. Technol. 2018, 248, 2–12. [Google Scholar] [CrossRef]
- Venkata Mohan, S. Harnessing of biohydrogen from wastewater treatment using mixed fermentative consortia: Process evaluation towards optimization. Int. J. Hydrog. Energy 2009, 34, 7460–7474. [Google Scholar] [CrossRef]
- Chandra, R.; Nikhil, G.N.; Venkata Mohan, S. Single-stage operation of hybrid dark-photo fermentation to enhance biohydrogen production through regulation of system redox condition: Evaluation with real-field wastewater. Int. J. Mol. Sci. 2015, 16, 9540–9556. [Google Scholar] [CrossRef] [Green Version]
- Pasupuleti, S.B.; Venkata Mohan, S. Single-stage fermentation process for high-value biohythane production with the treatment of distillery spent-wash. Bioresour. Technol. 2015, 189, 177–185. [Google Scholar] [CrossRef]
- Cavinato, C.; Giuliano, A.; Bolzonella, D.; Pavan, P.; Cecchi, F. Bio-hythane production from food waste by dark fermentation coupled with anaerobic digestion process: A long-term pilot scale experience. Int. J. Hydrog. Energy 2012, 37, 11549–11555. [Google Scholar] [CrossRef]
- Stoeberl, M.; Werkmeister, R.; Faulstich, M.; Russ, W. Biobutanol from food wastes—Fermentative production, use as biofuel an the influence on the emissions. Procedia Food Sci. 2011, 1, 1867–1874. [Google Scholar] [CrossRef] [Green Version]
- Rodionova, M.V.; Poudyal, R.S.; Tiwari, I.; Voloshin, R.A.; Zharmukhamedov, S.K.; Nam, H.G.; Zayadan, B.K.; Bruce, B.D.; Hou, H.J.M.; Allakhverdiev, S.I. Biofuel production: Challenges and opportunities. Int. J. Hydrog. Energy 2017, 42, 8450–8461. [Google Scholar] [CrossRef]
- Huang, H.; Singh, V.; Qureshi, N. Butanol production from food waste: A novel process for producing sustainable energy and reducing environmental pollution. Biotechnol. Biofuels 2015, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qureshi, N.; Eller, F. Recovery of butanol from Clostridium beijerinckii P260 fermentation broth by supercritical CO2 extraction. J. Chem. Technol. Biotechnol. 2018, 93, 1206–1212. [Google Scholar] [CrossRef]
- Jain, S.; Jain, S.; Wolf, I.T.; Lee, J.; Tong, Y.W. A comprehensive review on operating parameters and different pretreatment methodologies for anaerobic digestion of municipal solid waste. Renew. Sustain. Energy Rev. 2015, 52, 142–154. [Google Scholar] [CrossRef]
- Zheng, X. A Traditional Fermentation Starter in China: Microbial Ecology and Functionality. Ph.D. Thesis, Wageningen University, Wageningen, The Netehrlands, 2015. [Google Scholar]
- Bolado-Rodríguez, S.; Toquero, C.; Martín-Juárez, J.; Travaini, R.; García-Encina, P.A. Effect of thermal, acid, alkaline and alkaline-peroxide pretreatments on the biochemical methane potential and kinetics of the anaerobic digestion of wheat straw and sugarcane bagasse. Bioresour. Technol. 2016, 201, 182–190. [Google Scholar] [CrossRef]
- Sträuber, H.; Bühligen, F.; Kleinsteuber, S.; Dittrich-Zechendorf, M. Carboxylic acid production from ensiled crops in anaerobic solid-state fermentation—Trace elements as pH controlling agents support microbial chain elongation with lactic acid. Eng. Life Sci. 2018, 18, 447–458. [Google Scholar] [CrossRef] [Green Version]
- Loh, C.W.; Fakhru’l-Razi, A.; Hassan, M.A.; Karim, M.I.A. Production of organic acids from kitchen wastes. Artif. Cells. Blood Substit. Immobil. Biotechnol. 1999, 27, 455–459. [Google Scholar] [CrossRef]
- Pileidis, F.D.; Titirici, M.M. Levulinic Acid Biorefineries: New Challenges for Efficient Utilization of Biomass. ChemSusChem 2016, 9, 562–582. [Google Scholar] [CrossRef]
- Saha, B.; Abu-Omar, M.M. Advances in 5-hydroxymethylfurfural production from biomass in biphasic solvents. Green Chem. 2014, 16, 24–38. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Teong, S.P.; Yi, G.; Zhang, Y. Hydroxymethylfurfural production from bioresources: Past, present and future. Green Chem. 2014, 16, 2015–2026. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, S.; Li, B.; Zhang, H. Advances in the Catalytic Production of Valuable Levulinic Acid Derivatives. ChemCatChem 2012, 4, 1230–1237. [Google Scholar] [CrossRef]
- Mulder, G.J. Untersuchungen über die Humussubstanzen. J. Prakt. Chemie 1840, 21, 203–240. [Google Scholar] [CrossRef] [Green Version]
- Ahlkvist, J.; Wärnå, J.; Salmi, T.; Mikkola, J.P. Heterogeneously catalyzed conversion of nordic pulp to levulinic and formic acids. React. Kinet. Mech. Catal. 2016, 119, 415–427. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Yu, J. An intensified reaction technology for high levulinic acid concentration from lignocellulosic biomass. Biomass Bioenergy 2016, 95, 214–220. [Google Scholar] [CrossRef]
- Heeres, H.; Handana, R.; Chunai, D.; Borromeus Rasrendra, C.; Girisuta, B.; Jan Heeres, H. Combined dehydration/(transfer)-hydrogenation of C6-sugars (D-glucose and D-fructose) to γ-valerolactone using ruthenium catalysts. Green Chem. 2009, 11, 1247–1255. [Google Scholar] [CrossRef] [Green Version]
- Djukić-Vuković, A.; Mladenović, D.; Ivanović, J.; Pejin, J.; Mojović, L. Towards sustainability of lactic acid and poly-lactic acid polymers production. Renew. Sustain. Energy Rev. 2019, 238–252. [Google Scholar] [CrossRef]
- Thakur, K.; Tomar, S.K.; De, S. Lactic acid bacteria as a cell factory for riboflavin production. Microb. Biotechnol. 2016, 9, 441–451. [Google Scholar] [CrossRef]
- Rastogi, M.; Shrivastava, S. Recent advances in second generation bioethanol production: An insight to pretreatment, saccharification and fermentation processes. Renew. Sustain. Energy Rev. 2017, 80, 330–340. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Vlysidis, A.; Pleissner, D.; Kopsahelis, N.; Lopez Garcia, I.; Kookos, I.K.; Papanikolaou, S.; Kwan, T.H.; Lin, C.S.K. Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem. Soc. Rev. 2014, 43, 2587–2627. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Salvachúa, D.; Sànchez, V.N.I.; Michener, W.E.; Bratis, A.D.; Dorgan, J.R.; Beckham, G.T. Propionic acid production from corn stover hydrolysate by Propionibacterium acidipropionici. Biotechnol. Biofuels 2017, 10. [Google Scholar] [CrossRef]
- Guan, N.; Du, B.; Li, J.; Shin, H.D.; Chen, R.R.; Du, G.; Chen, J.; Liu, L. Comparative genomics and transcriptomics analysis-guided metabolic engineering of Propionibacterium acidipropionici for improved propionic acid production. Biotechnol. Bioeng. 2018, 115, 483–494. [Google Scholar] [CrossRef]
- Zhang, A.; Yang, S.T. Engineering Propionibacterium acidipropionici for enhanced propionic acid tolerance and fermentation. Biotechnol. Bioeng. 2009, 104, 766–773. [Google Scholar] [CrossRef]
- Beauprez, J.J.; De Mey, M.; Soetaert, W.K. Microbial succinic acid production: Natural versus metabolic engineered producers. Process Biochem. 2010, 45, 1103–1114. [Google Scholar] [CrossRef]
- Ahn, J.H.; Jang, Y.S.; Lee, S.Y. Production of succinic acid by metabolically engineered microorganisms. Curr. Opin. Biotechnol. 2016, 42, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Pateraki, C.; Patsalou, M.; Vlysidis, A.; Kopsahelis, N.; Webb, C.; Koutinas, A.A.; Koutinas, M. Actinobacillus succinogenes: Advances on succinic acid production and prospects for development of integrated biorefineries. Biochem. Eng. J. 2016, 112, 285–303. [Google Scholar] [CrossRef]
- Meynial-Salles, I.; Dorotyn, S.; Soucaille, P. A new process for the continuous production of succinic acid from glucose at high yield, titer, and productivity. Biotechnol. Bioeng. 2008, 99, 129–135. [Google Scholar] [CrossRef]
- Yazdani, S.S.; Gonzalez, R. Anaerobic fermentation of glycerol: A path to economic viability for the biofuels industry. Curr. Opin. Biotechnol. 2007, 18, 213–219. [Google Scholar] [CrossRef]
- Vlysidis, A.; Binns, M.; Webb, C.; Theodoropoulos, C. Glycerol utilisation for the production of chemicals: Conversion to succinic acid, a combined experimental and computational study. Biochem. Eng. J. 2011, 58–59, 1–11. [Google Scholar] [CrossRef]
- Chi, Z.; Wang, Z.P.; Wang, G.Y.; Khan, I.; Chi, Z.M. Microbial biosynthesis and secretion of l-malic acid and its applications. Crit. Rev. Biotechnol. 2016, 36, 99–107. [Google Scholar] [CrossRef] [PubMed]
- West, T.P. Malic acid production from thin stillage by Aspergillus species. Biotechnol. Lett. 2011, 33, 2463–2467. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Shanmugam, K.T.; Ingram, L.O. L-malate production by metabolically engineered Escherichia coli. Appl. Environ. Microbiol. 2011, 77, 427–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taing, O.; Taing, K. Production of malic and succinic acids by sugar-tolerant yeast Zygosaccharomyces rouxii. Eur. Food Res. Technol. 2007, 224, 343–347. [Google Scholar] [CrossRef]
- Maslova, O.; Stepanov, N.; Senko, O.; Efremenko, E. Production of various organic acids from different renewable sources by immobilized cells in the regimes of separate hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentation (SFF). Bioresour. Technol. 2019, 272, 1–9. [Google Scholar] [CrossRef]
- Cao, N.; Du, J.; Gong, C.S.; Tsao, G.T. Simultaneous Production and Recovery of Fumaric Acid from Immobilized Rhizopus oryzae with a Rotary Biofilm Contactor and an Adsorption Column. Appl. Environ. Microbiol. 1996, 62, 2926–2931. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Yang, H.; Yang, F.; Ma, Y. Current progress on butyric acid production by fermentation. Curr. Microbiol. 2009, 59, 656–663. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Liang, S.; Cai, J.; Xu, Z.; Cen, P.; Yang, S.; Li, S. Enhanced butyric acid tolerance and bioproduction by Clostridium tyrobutyricum immobilized in a fibrous bed bioreactor. Biotechnol. Bioeng. 2011, 108, 31–40. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Liang, S.; Wang, X.; Cen, P.; Xu, Z. Butyric acid fermentation in a fibrous bed bioreactor with immobilized Clostridium tyrobutyricum from cane molasses. Bioresour. Technol. 2009, 100, 3403–3409. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Cai, J.; Wang, J.; Zhu, X.; Huang, L.; Yang, S.T.; Xu, Z. Efficient production of butyric acid from Jerusalem artichoke by immobilized Clostridium tyrobutyricum in a fibrous-bed bioreactor. Bioresour. Technol. 2011, 102, 3923–3926. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, Z.; Yang, S.T. Butyric acid production from acid hydrolysate of corn fibre by Clostridium tyrobutyricum in a fibrous-bed bioreactor. Process Biochem. 2002, 38, 657–666. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Y.; Yang, S.T. Butyric acid and hydrogen production by Clostridium tyrobutyricum ATCC 25755 and mutants. Enzyme Microb. Technol. 2006, 38, 521–528. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Exploitation of Food Industry Waste for High-Value Products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef] [Green Version]
- Soni, R. Production, Purification and Industrial Applications of Cellulase from Aspergillus sp. AMA. Ph.D. Thesis, Guru Nanak Dev University Amritsar, Punjab, India, 2013. [Google Scholar]
- Periyasamy, K.; Santhalembi, L.; Mortha, G.; Aurousseau, M.; Guillet, A.; Dallerac, D.; Sivanesan, S. Production, Partial Purification and Characterization of Enzyme Cocktail from Trichoderma citrinoviride AUKAR04 Through Solid-State Fermentation. Arab. J. Sci. Eng. 2017, 42, 53–63. [Google Scholar] [CrossRef]
- Ahmed, I.; Zia, M.A.; Hussain, M.A.; Akram, Z.; Naveed, M.T.; Nowrouzi, A. Bioprocessing of citrus waste peel for induced pectinase production by Aspergillus niger; its purification and characterization. J. Radiat. Res. Appl. Sci. 2016, 9, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, G. Energy and geo-environmental applications for Olive Mill Wastes. A review. Hell. J. Geosci. 2010, 45, 269–282. [Google Scholar]
- Uçkun Kiran, E.; Trzcinski, A.P.; Ng, W.J.; Liu, Y. Enzyme Production from Food Wastes Using a Biorefinery Concept. Waste Biomass Valoriz. 2014, 5, 903–917. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Iqbal, H.M.N.; Kamal, S.; Ahmed, I.; Naveed, M.T. Enhanced bio-catalytic and tolerance properties of an indigenous cellulase through xerogel immobilization. Adv. Biosci. Biotechnol. 2012, 03, 308–313. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, K.; Fernandez-Lafuente, R. Control of protein immobilization: Coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzyme Microb. Technol. 2011, 48, 107–122. [Google Scholar] [CrossRef]
- Ahmed, I.; Zia, M.A.; Iqbal, H.M.N. Detergent-compatible purified endoglucanase from the agro-industrial residue by Trichoderma harzianum under solid state fermentation. BioResources 2016, 11, 6393–6406. [Google Scholar] [CrossRef] [Green Version]
- Dasari, P.R.; Ramteke, P.W.; Kesri, S.; Kongala, P.R. Comparative Study of Cellulase Production Using Submerged and Solid-State Fermentation. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 37–52. [Google Scholar]
- Viniegra-González, G.; Favela-Torres, E.; Aguilar, C.N.; de Jesus Rómero-Gomez, S.; Díaz-Godínez, G.; Augur, C. Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochem. Eng. J. 2003, 13, 157–167. [Google Scholar] [CrossRef]
- Rodríguez-Couto, S. Fungal Laccase: A Versatile Enzyme for Biotechnological Applications. In Recent Advancement in White Biotechnology Through Fungi; Springer: Berlin/Heidelberg, Germany, 2019; pp. 429–457. [Google Scholar]
- Voudouris, P.; Tamayo Tenorio, A.; Lesschen, J.P.; Kyriakopoulou, K.; Sanders, J.P.M.; van der Goot, A.J.; Bruins, M.E. Sustainable Protein Technology: An Evaluation on the STW Protein Programme and an Outlook for the Future; Wageningen Food & Biobased Research (Wageningen Food & Biobased Research Report 1786): Wageningen, The Netherlands, 2017; ISBN 9789463432368-58. [Google Scholar]
- Nagai, T.; Suzuki, N. Isolation of collagen from fish waste material—Skin, bone and fins. Food Chem. 2000, 68, 277–281. [Google Scholar] [CrossRef]
- Wang, S.; Hou, H.; Hou, J.; Tao, Y.; Lu, Y.; Yang, X.; Li, B. Characterization of acid-soluble collagen from bone of pacific cod (gadus macrocephalus). J. Aquat. Food Prod. Technol. 2013, 22, 407–420. [Google Scholar] [CrossRef]
- Yadav, J.S.S.; Yan, S.; Pilli, S.; Kumar, L.; Tyagi, R.D.; Surampalli, R.Y. Cheese whey: A potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol. Adv. 2015, 33, 756–774. [Google Scholar] [CrossRef]
- Prabakaran, S.; Damodaran, S. Thermal Unfolding of β-Lactoglobulin: Characterization of Initial Unfolding Events Responsible for Heat-Induced Aggregation. J. Agric. Food Chem. 1997, 45, 4303–4308. [Google Scholar] [CrossRef]
- Cuellas, A.; Jagus, R.; Wagner, J. Optimization of Proteins Recovery Process from Cheese Whey. J. Agric. Sci. Technol. B 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Chen, X.F.; Xiong, L.; de Chen, X.; Ma, L.L.; Chen, Y. Single cell oil production from low-cost substrates: The possibility and potential of its industrialization. Biotechnol. Adv. 2013, 31, 129–139. [Google Scholar] [CrossRef]
- Merino, S.T.; Cherry, J.; Ave, D. Progress and Challenges in Enzyme Development for Biomass Utilization. Adv. Biochem. Eng. Biotechnol. 2007, 95–96. [Google Scholar]
- Iniya, K. Single cell oil production from Mortierella sp for generation of biodiesel feedstock- a feasibility study. Afr. J. Microbiol. Res. 2011, 5. [Google Scholar] [CrossRef]
- Berlin, A.; Maximenko, V.; Gilkes, N.; Saddler, J. Optimization of enzyme complexes for lignocellulose hydrolysis. Biotechnol. Bioeng. 2007, 97, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Muniraj, I.K.; Uthandi, S.K.; Hu, Z.; Xiao, L.; Zhan, X. Microbial lipid production from renewable and waste materials for second-generation biodiesel feedstock. Environ. Technol. Rev. 2015, 4, 1–16. [Google Scholar] [CrossRef]
- Ratledge, C. Fatty acid biosynthesis in microorganisms being used for Single Cell Oil production. Biochimie 2004, 86, 807–815. [Google Scholar] [CrossRef]
- Jin, M.; Slininger, P.J.; Dien, B.S.; Waghmode, S.; Moser, B.R.; Orjuela, A.; da Costa Sousa, L.; Balan, V. Microbial lipid-based lignocellulosic biorefinery: Feasibility and challenges. Trends Biotechnol. 2015, 33, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.C.; Mi, L.; Pontrelli, S.; Luo, S. Fuelling the future: Microbial engineering for the production of sustainable biofuels. Nat. Rev. Microbiol. 2016, 14, 288–304. [Google Scholar] [CrossRef]
- Huang, C.; Zong, M.H.; Wu, H.; Liu, Q.P. Microbial oil production from rice straw hydrolysate by Trichosporon fermentans. Bioresour. Technol. 2009, 100, 4535–4538. [Google Scholar] [CrossRef]
- Yu, X.; Zheng, Y.; Dorgan, K.M.; Chen, S. Oil production by oleaginous yeasts using the hydrolysate from pretreatment of wheat straw with dilute sulfuric acid. Bioresour. Technol. 2011, 102, 6134–6140. [Google Scholar] [CrossRef]
- Huang, C.; Chen, X.F.; Xiong, L.; Yang, X.Y.; de Chen, X.; Ma, L.L.; Chen, Y. Microbial oil production from corncob acid hydrolysate by oleaginous yeast Trichosporon coremiiforme. Biomass Bioenergy 2013, 49, 273–278. [Google Scholar] [CrossRef]
- Obruca, S.; Petrik, S.; Benesova, P.; Svoboda, Z.; Eremka, L.; Marova, I. Utilization of oil extracted from spent coffee grounds for sustainable production of polyhydroxyalkanoates. Appl. Microbiol. Biotechnol. 2014, 98, 5883–5890. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.V.; Paiva, A.; Lisboa, P.; Freitas, F.; Alves, V.D.; Simões, P.; Barreiros, S.; Reis, M.A.M. Production of polyhydroxyalkanoates from spent coffee grounds oil obtained by supercritical fluid extraction technology. Bioresour. Technol. 2014, 157, 360–363. [Google Scholar] [CrossRef]
- Reis, M.; Albuquerque, M.; Villano, M.; Majone, M. Mixed Culture Processes for Polyhydroxyalkanoate Production from Agro-Industrial Surplus/Wastes as Feedstocks. In Comprehensive Biotechnology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 6, pp. 669–683. ISBN 9780080885049. [Google Scholar]
- Khan, T.; Park, J.K.; Kwon, J.H. Functional biopolymers produced by biochemical technology considering applications in food engineering. Korean J. Chem. Eng. 2007, 24, 816–826. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Y.; Phillips, G.O.; Yang, G. Utilization of bacterial cellulose in food. Food Hydrocoll. 2014, 35, 539–545. [Google Scholar] [CrossRef]
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial cellulose as a material for wound treatment: Properties and modifications: A review. Biotechnol. Adv. 2015, 33, 1547–1571. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chemie Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.K. Bacterial nanocellulose: Present status, biomedical applications and future perspectives. Mater. Sci. Eng. C 2019, 104, 109963. [Google Scholar] [CrossRef]
- Albuquerque, P.B.S.; Malafaia, C.B. Perspectives on the production, structural characteristics and potential applications of bioplastics derived from polyhydroxyalkanoates. Int. J. Biol. Macromol. 2018, 107, 615–625. [Google Scholar] [CrossRef]
- Kuo, C.H.; Chen, J.H.; Liou, B.K.; Lee, C.K. Utilization of acetate buffer to improve bacterial cellulose production by Gluconacetobacter xylinus. Food Hydrocoll. 2016, 53, 98–103. [Google Scholar] [CrossRef]
- Huang, C.; Guo, H.J.; Xiong, L.; Wang, B.; Shi, S.L.; Chen, X.F.; Lin, X.Q.; Wang, C.; Luo, J.; Chen, X. De Using wastewater after lipid fermentation as substrate for bacterial cellulose production by Gluconacetobacter xylinus. Carbohydr. Polym. 2016, 136, 198–202. [Google Scholar] [CrossRef]
- Jozala, A.F.; Pértile, R.A.N.; dos Santos, C.A.; de Carvalho Santos-Ebinuma, V.; Seckler, M.M.; Gama, F.M.; Pessoa, A. Bacterial cellulose production by Gluconacetobacter xylinus by employing alternative culture media. Appl. Microbiol. Biotechnol. 2015, 99, 1181–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, K.C.; Catchmark, J.M.; Demirci, A. Effect of different additives on bacterial cellulose production by Acetobacter xylinum and analysis of material property. Cellulose 2009, 16, 1033–1045. [Google Scholar] [CrossRef]
- Hafid, H.S.; Rahman, N.A.; Md Shah, U.K.; Baharudin, A.S. Enhanced fermentable sugar production from kitchen waste using various pretreatments. J. Environ. Manag. 2015, 156, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Gong, C.; Wang, J.; Tian, S.; Zhang, Y. Effects of ultrasound pre-treatment on the amount of dissolved organic matter extracted from food waste. Bioresour. Technol. 2014, 155, 266–271. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Salmi, T.; Holmbom, B.; Willför, S.; Murzin, D.Y. Synthesis of sugars by hydrolysis of hemicelluloses—A review. Chem. Rev. 2011, 111, 5638–5666. [Google Scholar] [CrossRef]
- Miao, M.; Jiang, B.; Jin, Z.; BeMiller, J.N. Microbial Starch-Converting Enzymes: Recent Insights and Perspectives. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1238–1260. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Li, J.; Chen, X.; Wu, J.; Wang, P.; Ye, J.; Yao, J. Enzymatical hydrolysis of food waste and ethanol production from the hydrolysate. Renew. Energy 2011, 36, 1259–1265. [Google Scholar] [CrossRef]
- Moon, H.C.; Song, I.S.; Kim, J.C.; Shirai, Y.; Lee, D.H.; Kim, J.K.; Chung, S.O.; Kim, D.H.; Oh, K.K.; Cho, Y.S. Enzymatic hydrolysis of food waste and ethanol fermentation. Int. J. Energy Res. 2009, 33, 164–172. [Google Scholar] [CrossRef]
- Li, P.; Zeng, Y.; Xie, Y.; Li, X.; Kang, Y.; Wang, Y.; Xie, T.; Zhang, Y. Effect of pretreatment on the enzymatic hydrolysis of kitchen waste for xanthan production. Bioresour. Technol. 2017, 223, 84–90. [Google Scholar] [CrossRef]
- Qin, L.; Liu, Z.H.; Li, B.Z.; Dale, B.E.; Yuan, Y.J. Mass balance and transformation of corn stover by pretreatment with different dilute organic acids. Bioresour. Technol. 2012, 112, 319–326. [Google Scholar] [CrossRef]
- De Bhowmick, G.; Sarmah, A.K.; Sen, R. Lignocellulosic biorefinery as a model for sustainable development of biofuels and value added products. Bioresour. Technol. 2018, 247, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.K.; Patel, A.K.; Adsul, M.; Singhania, R.R. Cellulase adsorption on lignin: A roadblock for economic hydrolysis of biomass. Renew. Energy 2016, 98, 29–42. [Google Scholar] [CrossRef]
- Sung, B.K.; Hyun, J.K.; Chang, J.K. Enhancement of the enzymatic digestibility of waste newspaper using Tween. Appl. Biochem. Biotechnol. 2006, 130, 486–495. [Google Scholar]
- Kim, H.J.; Kim, S.B.; Kim, C.J. The effects of nonionic surfactants on the pretreatment and enzymatic hydrolysis of recycled newspaper. Biotechnol. Bioprocess Eng. 2007, 12, 147–151. [Google Scholar] [CrossRef]
- Wu, J.; Ju, L.K. Enhancing enzymatic saccharification of waste newsprint by surfactant addition. Biotechnol. Prog. 1998, 14, 649–652. [Google Scholar] [CrossRef]
- Sangkharak, K. Optimization of enzymatic hydrolysis for ethanol production by simultaneous saccharification and fermentation of wastepaper. Waste Manag. Res. 2011, 29, 1134–1144. [Google Scholar] [CrossRef]
- Kurakake, M.; Ide, N.; Komaki, T. Biological pretreatment with two bacterial strains for enzymatic hydrolysis of office paper. Curr. Microbiol. 2007, 54, 424–428. [Google Scholar] [CrossRef]
- Kojima, Y.; Yoon, S.L. Improved enzymatic hydrolysis of waste paper by ozone pretreatment. J. Mater. Cycles Waste Manag. 2008, 10, 134–139. [Google Scholar] [CrossRef]
- Kim, J.W.; Mazza, G. Optimization of phosphoric acid catalyzed fractionation and enzymatic digestibility of flax shives. Ind. Crops Prod. 2008, 28, 346–355. [Google Scholar] [CrossRef]
- Loow, Y.L.; Wu, T.Y.; Jahim, J.M.; Mohammad, A.W.; Teoh, W.H. Typical conversion of lignocellulosic biomass into reducing sugars using dilute acid hydrolysis and alkaline pretreatment. Cellulose 2016, 23, 1491–1520. [Google Scholar] [CrossRef]
- Liguori, R.; Amore, A.; Faraco, V. Waste valorization by biotechnological conversion into added value products. Appl. Microbiol. Biotechnol. 2013, 97, 6129–6147. [Google Scholar] [CrossRef] [PubMed]
- Zervakis, G.I.; Koutrotsios, G. Solid-State Fermentation of Plant Residues and Agro-industrial Wastes for the Production of Medicinal Mushrooms. In Medicinal Plants and Fungi: Recent Advances in Research and Development; Springer: Singapore, 2017; pp. 365–396. [Google Scholar]
- Gupta, V.K.; Kubicek, C.P.; Berrin, J.G.; Wilson, D.W.; Couturier, M.; Berlin, A.; Filho, E.X.F.; Ezeji, T. Fungal Enzymes for Bio-Products from Sustainable and Waste Biomass. Trends Biochem. Sci. 2016, 41, 633–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oshoma, C.; Eguakun-Owie, S. Conversion of Food waste to Single Cell Protein using Aspergillus Niger. J. Appl. Sci. Environ. Manag. 2018, 22, 350. [Google Scholar] [CrossRef]
- Mondal, A.K.; Sengupta, S.; Bhowal, J.; Bhattacharya, D.K. Utilization of Fruit Wastes in Producing Single Cell Protein. Int. J. Sci. Environ. Technol. 2012, 1, 430–438. [Google Scholar]
- Howard, R.L.; Abotsi, E.; Van Rensburg, E.L.J.; Howard, S. Lignocellulose biotechnology: Issues of bioconversion and enzyme production. Afr. J. Biotechnol. 2003, 2, 702–733. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Yadav, J.S.; Tripathi, J.P. Optimization of cultivation and nutrition conditions and substrate pretreatment for solid-substrate fermentation of wheat straw by Coriolus versicolor. Folia Microbiol. 1991, 36, 294–301. [Google Scholar] [CrossRef]
- Iqbal Zafar, S.; Sheeraa, Q.; Abdullah, N. Degradation of the lignocellulosic component on wheat straw-Coriolus versicolor solid state fermentation under nitrogen-starved conditions. Biol. Wastes 1989, 27, 67–70. [Google Scholar] [CrossRef]
- Tripathi, J.P.; Yadav, J.S. Optimisation of solid substrate fermentation of wheat straw into animal feed by Pleurotus ostreatus: A pilot effort. Anim. Feed Sci. Technol. 1992, 37, 59–72. [Google Scholar] [CrossRef]
- Moyson, E.; Verachtert, H. Growth of higher fungi on wheat straw and their impact on the digestibility of the substrate. Appl. Microbiol. Biotechnol. 1991, 36, 421–424. [Google Scholar] [CrossRef]
- Basu, S.; Gaur, R.; Gomes, J.; Sreekrishnan, T.R.; Bisaria, V.S. Effect of seed culture on solid-state bioconversion of wheat straw by Phanerochaete chrysosporium for animal feed production. J. Biosci. Bioeng. 2002, 93, 25–30. [Google Scholar] [CrossRef]
- Okano, K.; Ohkoshi, N.; Nishiyama, A.; Usagawa, T.; Kitagawa, M. Improving the nutritive value of madake bamboo, Phyllostachys bambusoides, for ruminants by culturing with the white-rot fungus Ceriporiopsis subvermispora. Anim. Feed Sci. Technol. 2009, 152, 278–285. [Google Scholar] [CrossRef]
- Shabtay, A.; Hadar, Y.; Eitam, H.; Brosh, A.; Orlov, A.; Tadmor, Y.; Izhaki, I.; Kerem, Z. The potential of Pleurotus-treated olive mill solid waste as cattle feed. Bioresour. Technol. 2009, 100, 6457–6464. [Google Scholar] [CrossRef]
- Bisaria, R.; Madan, M.; Vasudevan, P. Utilisation of agro-residues as animal feed through bioconversion. Bioresour. Technol. 1997, 59, 5–8. [Google Scholar] [CrossRef]
- Mandyam, K.; Njiti, V.; Singleton, R.; Nanjundaswamy, A.K. Fermentation Optimization of Macro-Fungus Pleurotus Sajor-Caju on Soymeal. Ferment. Technol. 2017, 6. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, B.; Thakur, S.; Khasa, Y.P.; Gupte, A.; Puniya, A.K.; Kuhad, R.C. White-rot fungal conversion of wheat straw to energy rich cattle feed. Biodegradation 2011, 22, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Ward, T.E.; Regan, J.M. Electricity production from cellulose in a microbial fuel cell using a defined binary culture. Environ. Sci. Technol. 2007, 41, 4781–4786. [Google Scholar] [CrossRef] [PubMed]
- Kannaiah Goud, R.; Venkata Mohan, S. Pre-fermentation of waste as a strategy to enhance the performance of single chambered microbial fuel cell (MFC). Int. J. Hydrog. Energy 2011, 36, 13753–13762. [Google Scholar] [CrossRef]
- Ishii, S.; Suzuki, S.; Norden-Krichmar, T.M.; Wu, A.; Yamanaka, Y.; Nealson, K.H.; Bretschger, O. Identifying the microbial communities and operational conditions for optimized wastewater treatment in microbial fuel cells. Water Res. 2013, 47, 7120–7130. [Google Scholar] [CrossRef]
- Wrana, N.; Sparling, R.; Cicek, N.; Levin, D.B. Hydrogen gas production in a microbial electrolysis cell by electrohydrogenesis. J. Clean. Prod. 2010, 18. [Google Scholar] [CrossRef]
- Rabaey, K.; Verstraete, W. Microbial fuel cells: Novel biotechnology for energy generation. Trends Biotechnol. 2005, 23, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.V.; Chandrasekhar, K. Solid phase microbial fuel cell (SMFC) for harnessing bioelectricity from composite food waste fermentation: Influence of electrode assembly and buffering capacity. Bioresour. Technol. 2011, 102, 7077–7085. [Google Scholar] [CrossRef] [PubMed]
- Butti, S.K.; Velvizhi, G.; Sulonen, M.L.K.; Haavisto, J.M.; Oguz Koroglu, E.; Yusuf Cetinkaya, A.; Singh, S.; Arya, D.; Annie Modestra, J.; Vamsi Krishna, K.; et al. Microbial electrochemical technologies with the perspective of harnessing bioenergy: Maneuvering towards upscaling. Renew. Sustain. Energy Rev. 2016, 53, 462–476. [Google Scholar] [CrossRef]
- Srikanth, S.; Venkata Mohan, S. Influence of terminal electron acceptor availability to the anodic oxidation on the electrogenic activity of microbial fuel cell (MFC). Bioresour. Technol. 2012, 123, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Tokash, J.C.; Zhang, F.; Liang, P.; Huang, X.; Logan, B.E. Oxygen-reducing biocathodes operating with passive oxygen transfer in microbial fuel cells. Environ. Sci. Technol. 2013, 47, 2085–2091. [Google Scholar] [CrossRef]
- Oh, S.E.; Logan, B.E. Hydrogen and electricity production from a food processing wastewater using fermentation and microbial fuel cell technologies. Water Res. 2005, 39, 4673–4682. [Google Scholar] [CrossRef]
- Freguia, S.; Teh, E.H.; Boon, N.; Leung, K.M.; Keller, J.; Rabaey, K. Microbial fuel cells operating on mixed fatty acids. Bioresour. Technol. 2010, 101, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Mohanakrishna, G.; Venkata Mohan, S.; Sarma, P.N. Utilizing acid-rich effluents of fermentative hydrogen production process as substrate for harnessing bioelectricity: An integrative approach. Int. J. Hydrog. Energy 2010, 35, 3440–3449. [Google Scholar] [CrossRef]
- Nam, J.Y.; Kim, H.W.; Lim, K.H.; Shin, H.S. Effects of organic loading rates on the continuous electricity generation from fermented wastewater using a single-chamber microbial fuel cell. Bioresour. Technol. 2010, 101, S33–S37. [Google Scholar] [CrossRef]
- Teng, S.X.; Tong, Z.H.; Li, W.W.; Wang, S.G.; Sheng, G.P.; Shi, X.Y.; Liu, X.W.; Yu, H.Q. Electricity generation from mixed volatile fatty acids using microbial fuel cells. Appl. Microbiol. Biotechnol. 2010, 87, 2365–2372. [Google Scholar] [CrossRef]
- Choi, J.D.R.; Chang, H.N.; Han, J.I. Performance of microbial fuel cell with volatile fatty acids from food wastes. Biotechnol. Lett. 2011, 33, 705–714. [Google Scholar] [CrossRef]
- Li, X.M.; Cheng, K.Y.; Wong, J.W.C. Bioelectricity production from food waste leachate using microbial fuel cells: Effect of NaCl and pH. Bioresour. Technol. 2013, 149, 452–458. [Google Scholar] [CrossRef]
- Xu, S.Y.; Lam, H.P.; Karthikeyan, O.P.; Wong, J.W.C. Optimization of food waste hydrolysis in leach bed coupled with methanogenic reactor: Effect of pH and bulking agent. Bioresour. Technol. 2011, 102, 3702–3708. [Google Scholar] [CrossRef]
- Logan, B.E. Chapter 3 Voltage Generation. In Microbial Fuel Cells; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; ISBN 9780470258590. [Google Scholar]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Fan, Y.; Sharbrough, E.; Liu, H. Quantification of the internal resistance distribution of microbial fuel cells. Environ. Sci. Technol. 2008, 42, 8101–8107. [Google Scholar] [CrossRef]
- Huang, J.; Sun, B.; Zhang, X. Electricity generation at high ionic strength in microbial fuel cell by a newly isolated Shewanella marisflavi EP1. Appl. Microbiol. Biotechnol. 2010, 85, 1141–1149. [Google Scholar] [CrossRef]
- Lefebvre, O.; Tan, Z.; Kharkwal, S.; Ng, H.Y. Effect of increasing anodic NaCl concentration on microbial fuel cell performance. Bioresour. Technol. 2012, 112, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cheng, S.; Logan, B.E. Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell. Environ. Sci. Technol. 2005, 39, 658–662. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Huang, Y.; Manohar, A.K.; Mansfeld, F. Effect of electrolyte pH on the rate of the anodic and cathodic reactions in an air-cathode microbial fuel cell. Bioelectrochemistry 2008, 74, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Raghavulu, S.V.; Mohan, S.V.; Goud, R.K.; Sarma, P.N. Effect of anodic pH microenvironment on microbial fuel cell (MFC) performance in concurrence with aerated and ferricyanide catholytes. Electrochem. Commun. 2009, 11, 371–375. [Google Scholar] [CrossRef]
- Behera, M.; Jana, P.S.; More, T.T.; Ghangrekar, M.M. Rice mill wastewater treatment in microbial fuel cells fabricated using proton exchange membrane and earthen pot at different pH. Bioelectrochemistry 2010, 79, 228–233. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, B.; Zhou, S.; Zhong, S.; Zhuang, L. Electrocatalytic activity of anodic biofilm responses to pH changes in microbial fuel cells. Bioresour. Technol. 2011, 102, 6887–6891. [Google Scholar] [CrossRef] [PubMed]
- Macoveanu, M.; Gavrilescu, M.; Gavrilescu, D. Energy from biomass in pulp and paper mills. Environ. Eng. Manag. J. 2008, 7, 537–546. [Google Scholar]
- Sakai, M.; Seto, T.; Kaneko, M.; Hada, M.; Kinomoto, T. Method For Producing Pulp From. U.S. Patent 5500086, 19 March 1996. [Google Scholar]
- Chao, K.P.; Su, Y.C.; Chen, C.S. Feasibility of utilizing Rhizoclonium in pulping and papermaking. J. Appl. Phycol. 2000, 12, 53–62. [Google Scholar] [CrossRef]
- Ververis, C.; Georghiou, K.; Danielidis, D.; Hatzinikolaou, D.G.; Santas, P.; Santas, R.; Corleti, V. Cellulose, hemicelluloses, lignin and ash content of some organic materials and their suitability for use as paper pulp supplements. Bioresour. Technol. 2007, 98, 296–301. [Google Scholar] [CrossRef]
- Sudhakar, M.P.; Kumar, B.R.; Mathimani, T.; Arunkumar, K. A review on bioenergy and bioactive compounds from microalgae and macroalgae-sustainable energy perspective. J. Clean. Prod. 2019, 228, 1320–1333. [Google Scholar] [CrossRef]
- Ramana, K.V.; Xavier, J.R. Recent Trends in Pharmaceutical Biotechnology Abstract. iMedPub J. 2017, 1, 1–10. [Google Scholar]
- Zhang, M.; Cui, S.W.; Cheung, P.C.K.; Wang, Q. Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol. 2007, 18, 4–19. [Google Scholar] [CrossRef]
- Philippoussis, A.; Diamantopoulou, P.; Israilides, C. Productivity of agricultural residues used for the cultivation of the medicinal fungus Lentinula edodes. Int. Biodeterior. Biodegrad. 2007, 59, 216–219. [Google Scholar] [CrossRef]
- Israilides, C.; Philippoussis, A. Bio-technologies of Recycling Agro-industrial Wastes for the Production of Commercially Important Fungal Polysaccharides and Mushrooms. Biotechnol. Genet. Eng. Rev. 2003, 20, 247–260. [Google Scholar] [CrossRef] [Green Version]
- Subbarao, K.; Kuchibhotla, J.; Kakkar, V.V. Pyridoxal 5′-phosphate-A new physiological inhibitor of blood coagulation and platelet function. Biochem. Pharmacol. 1979, 28, 531–534. [Google Scholar] [CrossRef]
- Borchers, A.T.; Stern, J.S.; Hackman, R.M.; Keen, C.L.; Gershwin, M.E. Minireview Mushrooms, Tumors, and Immunity. Proc. Soc. Exp. Biol. Med. 1999, 221, 281–293. [Google Scholar] [PubMed]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Chihara, G.; Hamuro, J.; Maeda, Y.Y.; Arai, Y.; Fukuoka, F. Fractionation and Purification of the Polysaccharides with Marked Antitumor Activity, Especially Lentinan, from Lentinus edodes (Berk.) Sing. (an Edible Mushroom). Cancer Res. 1970, 30, 2776–2781. [Google Scholar] [PubMed]
- Ren, G.; Xu, L.; Lu, T.; Yin, J. Structural characterization and antiviral activity of lentinan from Lentinus edodes mycelia against infectious hematopoietic necrosis virus. Int. J. Biol. Macromol. 2018, 115, 1202–1210. [Google Scholar] [CrossRef]
- Cooke, C.L.; Hyun, J.A.; Kim, J.; Solnick, J.V.; Lebrilla, C.B. Method for profiling mucin oligosaccharides from gastric biopsies of rhesus monkeys with and without Helicobacter pylori infection. Anal. Chem. 2007, 79, 8090–8097. [Google Scholar] [CrossRef]
- Borchers, A.T.; Keen, C.L.; Gershwin, M.E. Mushrooms, Tumors, and Immunity: An Update. Exp. Biol. Med. 2004, 229, 393–406. [Google Scholar] [CrossRef]
- Reis, F.S.; Martins, A.; Vasconcelos, M.H.; Morales, P.; Ferreira, I.C.F.R. Functional foods based on extracts or compounds derived from mushrooms. Trends Food Sci. Technol. 2017, 66, 48–62. [Google Scholar] [CrossRef]
- Wasser, S. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar]
- Liu, Q.; Duan, B.; Xu, X.; Zhang, L. Progress in rigid polysaccharide-based nanocomposites with therapeutic functions. J. Mater. Chem. B 2017, 5, 5690–5713. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure-function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, V.; Chawla, S.P.; Nair, P.M. Spray dried protein powder from threadfin bream: Preparation, properties and comparison with FPC type-B. J. Muscle Foods 1996, 7, 55–71. [Google Scholar] [CrossRef]
- Craig, S.; College, V.; Medicine, V.; Tech, V. Understanding fish nutrition, feeds, and feeding. Virginia Coop Ext. 2017. Available online: https://vtechworks.lib.vt.edu/bitstream/handle/10919/80712/FST-269.pdf (accessed on 11 February 2021).
- Benjakul, S.; Morrissey, M.T. Protein Hydrolysates from Pacific Whiting Solid Wastes. J. Agric. Food Chem. 1997, 45, 3423–3430. [Google Scholar] [CrossRef]
- Yathisha, U.G.; Bhat, I.; Karunasagar, I.; Mamatha, B.S. Antihypertensive activity of fish protein hydrolysates and its peptides. Crit. Rev. Food Sci. Nutr. 2019, 59, 2363–2374. [Google Scholar] [CrossRef]
- Rajapakse, N.; Jung, W.K.; Mendis, E.; Moon, S.H.; Kim, S.K. A novel anticoagulant purified from fish protein hydrolysate inhibits factor XIIa and platelet aggregation. Life Sci. 2005, 76, 2607–2619. [Google Scholar] [CrossRef]
- Hajfathalian, M.; Ghelichi, S.; García-Moreno, P.J.; Moltke Sørensen, A.D.; Jacobsen, C. Peptides: Production, bioactivity, functionality, and applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 3097–3129. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K.; Kim, Y.T.; Byun, H.G.; Nam, K.S.; Joo, D.S.; Shahidi, F. Isolation and characterization of antioxidative peptides from gelatin hydrolysate of Alaska pollack skin. J. Agric. Food Chem. 2001, 49, 1984–1989. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Byun, H.G.; Kim, S.K. Investigation of jumbo squid (Dosidicus gigas) skin gelatin peptides for their in vitro antioxidant effects. Life Sci. 2005, 77, 2166–2178. [Google Scholar] [CrossRef]
- Pal, G.K.; Suresh, P.V. Sustainable valorisation of seafood by-products: Recovery of collagen and development of collagen-based novel functional food ingredients. Innov. Food Sci. Emerg. Technol. 2016, 37, 201–215. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Marine peptides: Bioactivities and applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Kitazawa, H.; Adachi, I.; Horikoshi, I. Microdialysis assessment of microfibrous collagen containing a P-glycoprotein-mediated transport inhibitor, cyclosporine A, for local delivery of etoposide. Pharm. Res. 1996, 13, 1565–1569. [Google Scholar] [CrossRef] [PubMed]
- Aytekin, A.O.; Morimura, S.; Kida, K. Synthesis of chitosan-caffeic acid derivatives and evaluation of their antioxidant activities. J. Biosci. Bioeng. 2011, 111, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Xing, R.; Liu, S.; Zhong, Z.; Li, P. Synthesis and hydroxyl radicals scavenging activity of quaternized carboxymethyl chitosan. Carbohydr. Polym. 2008, 73, 173–177. [Google Scholar] [CrossRef]
- Zhong, Z.; Ji, X.; Xing, R.; Liu, S.; Guo, Z.; Chen, X.; Li, P. The preparation and antioxidant activity of the sulfanilamide derivatives of chitosan and chitosan sulfates. Bioorganic Med. Chem. 2007, 15, 3775–3782. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Yusof, N.L.B.M.; Wee, A.; Lim, L.Y.; Khor, E. Flexible chitin films as potential wound-dressing materials: Wound model studies. J. Biomed. Mater. Res. Part. A 2003, 66, 224–232. [Google Scholar] [CrossRef]
- Hassan, S.A.; Al-Sabagh, A.M.; Shalaby, N.H.; Hanafi, S.A.; Hassan, H.A. Various characteristics of multi-modified rice husk silica-anchored Ni or Pt nanoparticles as swift catalytic systems in some petrochemical processes. Rev. Mex. Urol. 2016, 76, 484–495. [Google Scholar] [CrossRef]
- Harish, B.S.; Uppuluri, K.B.; Anbazhagan, V. Synthesis of fibrinolytic active silver nanoparticle using wheat bran xylan as a reducing and stabilizing agent. Carbohydr. Polym. 2015, 132, 104–110. [Google Scholar] [CrossRef]
- Patel, K.G.; Misra, N.M.; Vekariya, R.H.; Shettigar, R.R. One-pot multicomponent synthesis in aqueous medium of 1,4-dihydropyrano [2,3-c]pyrazole-5-carbonitrile and derivatives using a green and reusable nano-SiO2catalyst from agricultural waste. Res. Chem. Intermed. 2018, 44, 289–304. [Google Scholar] [CrossRef]
- Fang, K.; Chen, J.; Zhou, X.; Mei, C.; Tian, Q.; Xu, J.; Wong, C.P. Decorating biomass-derived porous carbon with Fe2O3 ultrathin film for high-performance supercapacitors. Electrochim. Acta 2018, 261, 198–205. [Google Scholar] [CrossRef]
- Devi, L.L.; Basavapoornima, C.; Venkatramu, V.; Babu, P.; Jayasankar, C.K. Synthesis of Ca2SiO4:Dy3+ phosphors from agricultural waste for solid state lighting applications. Ceram. Int. 2017, 43, 16622–16627. [Google Scholar] [CrossRef]
- Pandit, P.R.; Fulekar, M.H. Egg shell waste as heterogeneous nanocatalyst for biodiesel production: Optimized by response surface methodology. J. Environ. Manag. 2017, 198, 319–329. [Google Scholar] [CrossRef]
- Yan, D.; Zhang, H.; Chen, L.; Zhu, G.; Wang, Z.; Xu, H.; Yu, A. Supercapacitive properties of Mn3O4 nanoparticles bio-synthesized from banana peel extract. RSC Adv. 2014, 4, 23649–23652. [Google Scholar] [CrossRef]
- Ali, S.M. Fabrication of a nanocomposite from an agricultural waste and its application as a biosorbent for organic pollutants. Int. J. Environ. Sci. Technol. 2018, 15, 1169–1178. [Google Scholar] [CrossRef]
- Adoki, A. Factors affecting yeast growth and protein yield production from orange, plantain and banana wastes processing residues using Candida sp. Afr. J. Biotechnol. 2008, 7, 290–295. [Google Scholar]
- Yabaya, A.; Ado, S.A. Mycelial protein production by Aspergillus niger using banana peels. World 2008, 3, 9–12. [Google Scholar] [CrossRef]
- Lenihan, P.; Orozco, A.; O’Neill, E.; Ahmad, M.N.M.; Rooney, D.W.; Walker, G.M. Dilute acid hydrolysis of lignocellulosic biomass. Chem. Eng. J. 2010, 156, 395–403. [Google Scholar] [CrossRef]
- Kaparaju, P.; Serrano, M.; Thomsen, A.B.; Kongjan, P.; Angelidaki, I. Bioethanol, biohydrogen and biogas production from wheat straw in a biorefinery concept. Bioresour. Technol. 2009, 100, 2562–2568. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Gu, Y.; Zhou, X. A physicochemical method for increasing methane production from rice straw: Extrusion combined with alkali pretreatment. Appl. Energy 2015, 160, 39–48. [Google Scholar] [CrossRef]
- Zheng, L.; Zheng, P.; Sun, Z.; Bai, Y.; Wang, J.; Guo, X. Production of vanillin from waste residue of rice bran oil by Aspergillus niger and Pycnoporus cinnabarinus. Bioresour. Technol. 2007, 98, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Rózsenberszki, T.; Koók, L.; Hutvágner, D.; Nemestóthy, N.; Bélafi-Bakó, K.; Bakonyi, P.; Kurdi, R.; Sarkady, A. Comparison of anaerobic degradation processes for bioenergy generation from liquid fraction of pressed solid waste. Waste Biomass Valoriz. 2015, 6, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Koók, L.; Rózsenberszki, T.; Nemestóthy, N.; Bélafi-Bakó, K.; Bakonyi, P. Bioelectrochemical treatment of municipal waste liquor in microbial fuel cells for energy valorization. J. Clean. Prod. 2016, 112, 4406–4412. [Google Scholar] [CrossRef] [Green Version]
- Scarlat, N.; Dallemand, J.F.; Monforti-Ferrario, F.; Nita, V. The role of biomass and bioenergy in a future bioeconomy: Policies and facts. Environ. Dev. 2015, 15, 3–34. [Google Scholar] [CrossRef]
- Imbert, E. Food waste valorization options: Opportunities from the bioeconomy. Open Agric. 2017, 2, 195–204. [Google Scholar] [CrossRef]
- Rodas-Zuluaga, L.I.; Castañeda-Hernández, L.; Castillo-Vacas, E.I.; Gradiz-Menjivar, A.; López-Pacheco, I.Y.; Castillo-Zacarías, C.; Iqbal, H.M.N.; Parra-Saldívar, R. Bio-capture and influence of CO2 on the growth rate and biomass composition of the microalgae Botryococcus braunii and Scenedesmus sp. J. CO2 Util. 2021, 43, 101371. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.F.; Banja, M. Possible impact of 2020 bioenergy targets on European Union land use. A scenario-based assessment from national renewable energy action plans proposals. Renew. Sustain. Energy Rev. 2013, 18, 595–606. [Google Scholar] [CrossRef]
- Eickhout, B.B.; Gjaltema, J.; de Jong, F. A strategy for a bio-based economy. Green New Deal Ser. 2012, 9, 1–52. [Google Scholar]
- Lin, J.; Zuo, J.; Gan, L.; Li, P.; Liu, F.; Wang, K.; Chen, L.; Gan, H. Effects of mixture ratio on anaerobic co-digestion with fruit and vegetable waste and food waste of China. J. Environ. Sci. 2011, 23, 1403–1408. [Google Scholar] [CrossRef]
- Kretschmer, B.; Smith, C.; Watkins, E.; Allen, B.; Buckwell, A.; Desbarats, J.; Kieve, D. Technology options for feeding 10 billion people. Recycling agricultural, forestry & food wastes and residues for sustainable bioenergy and biomaterials. Food Eng. 2013, 1–149. [Google Scholar] [CrossRef]
- De Besi, M.; McCormick, K. Towards a bioeconomy in Europe: National, regional and industrial strategies. Sustainability 2015, 7, 10461–10478. [Google Scholar] [CrossRef] [Green Version]
- Azar, C.; Lindgren, K.; Andersson, B.A. Global energy scenarios meeting stringent CO2 constraints—Cost-effective fuel choices in the transportation sector. Energy Policy 2003, 31, 961–976. [Google Scholar] [CrossRef]
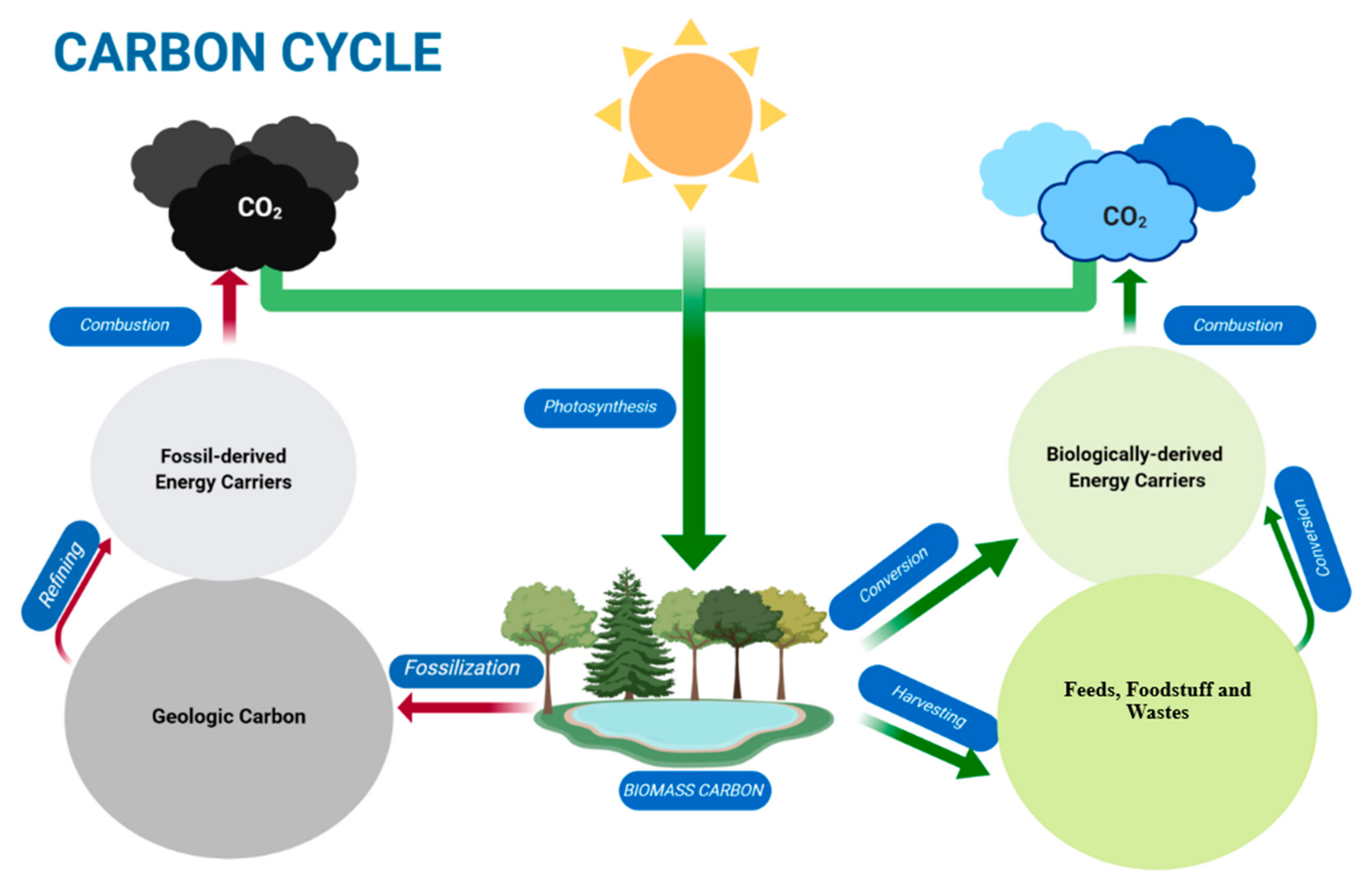
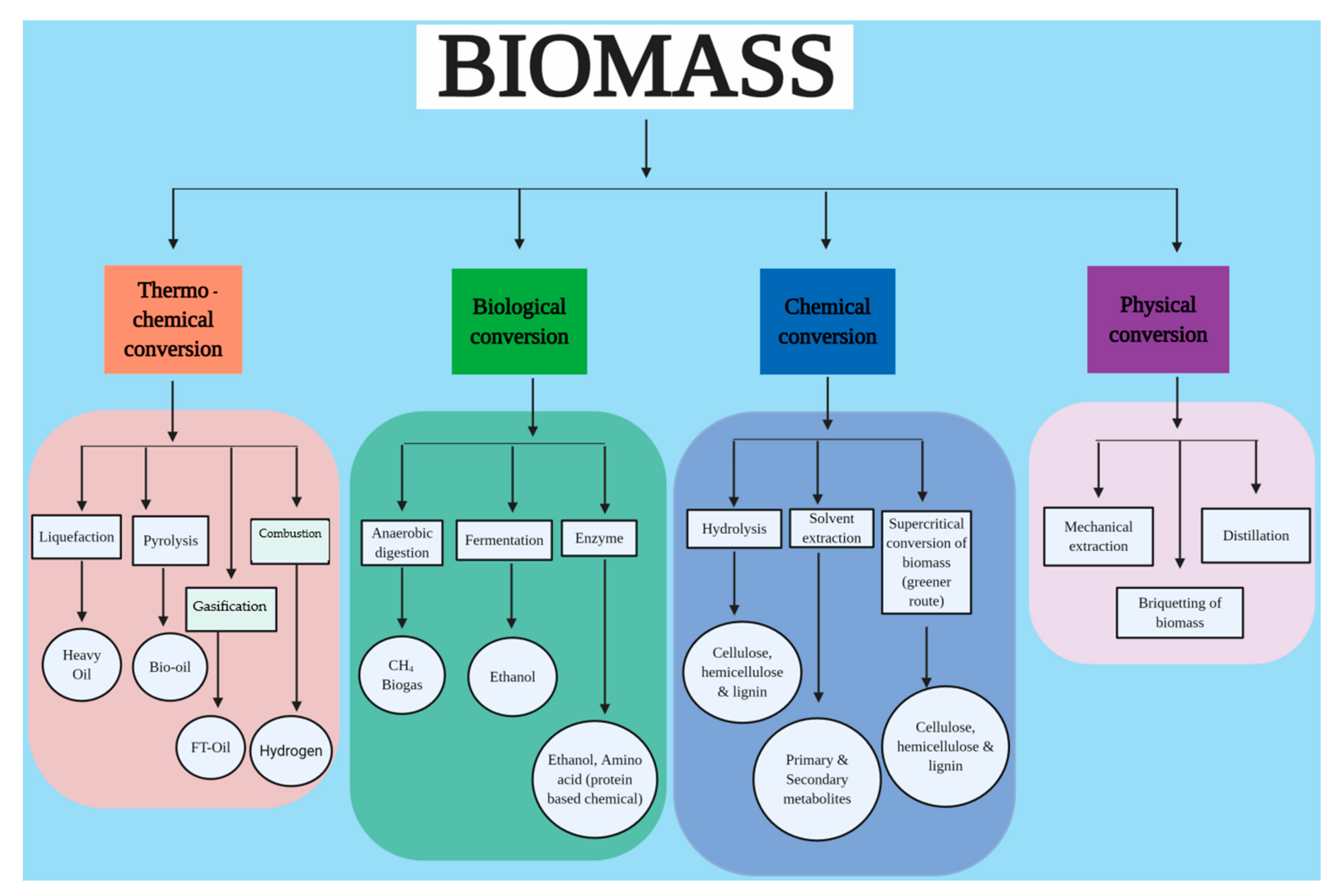
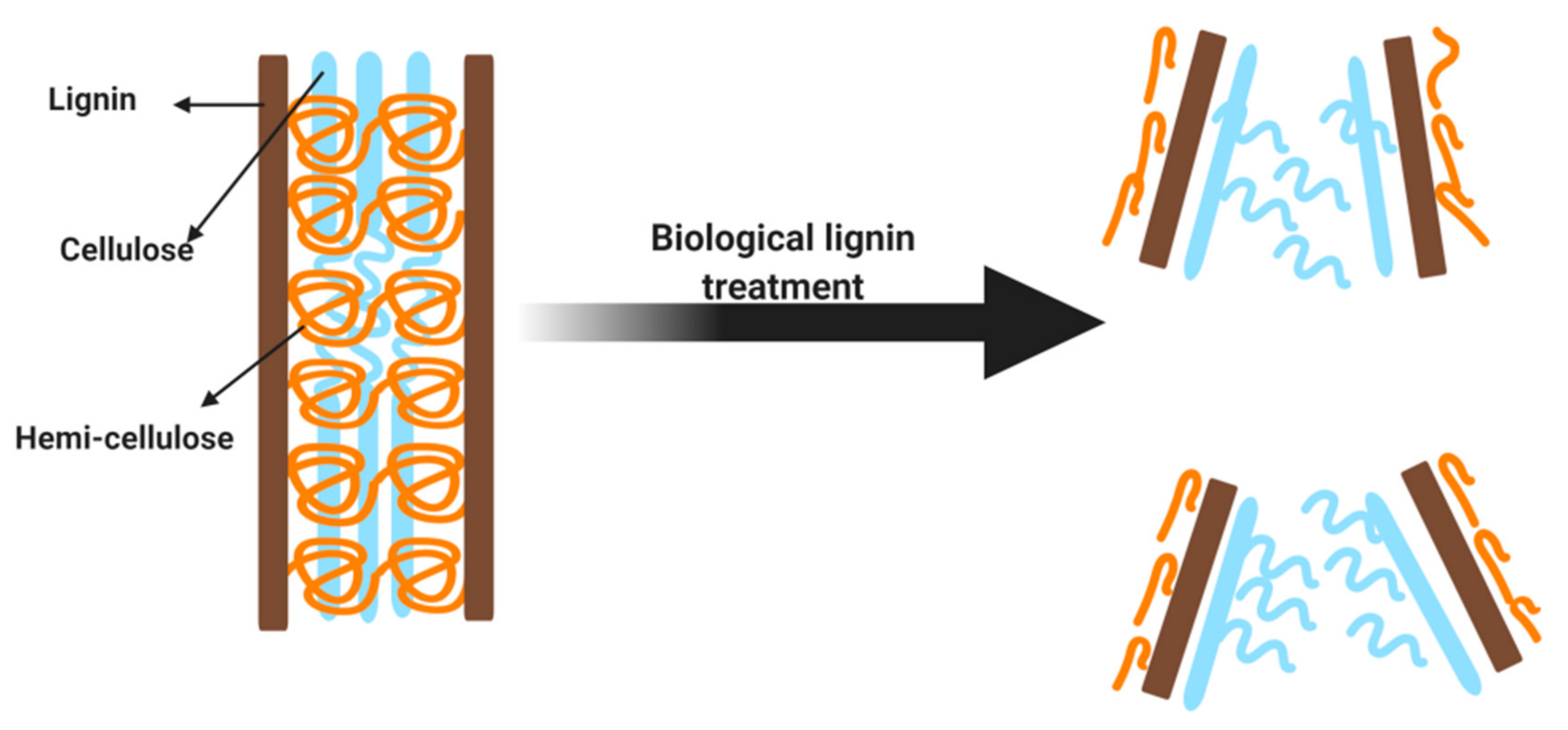
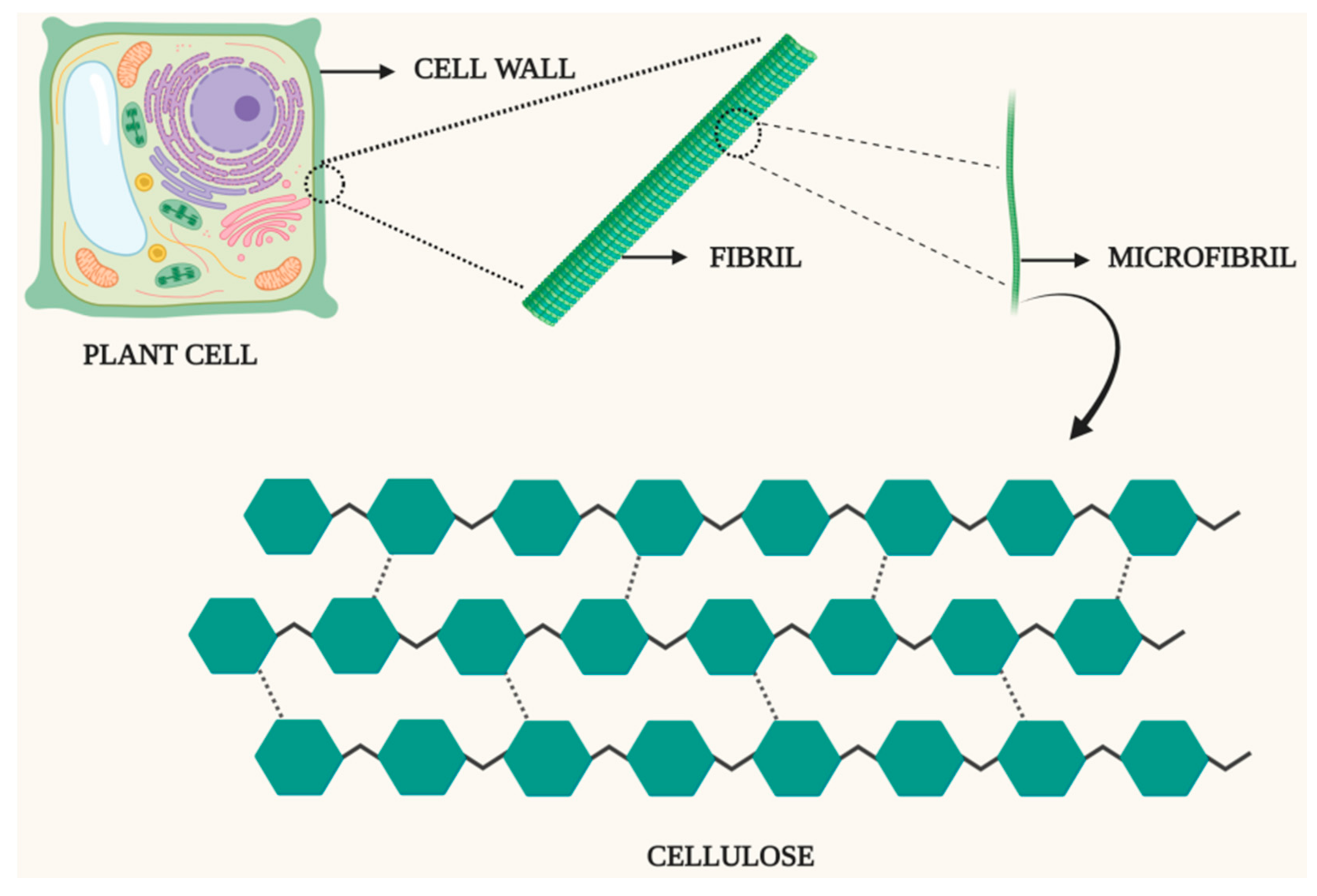
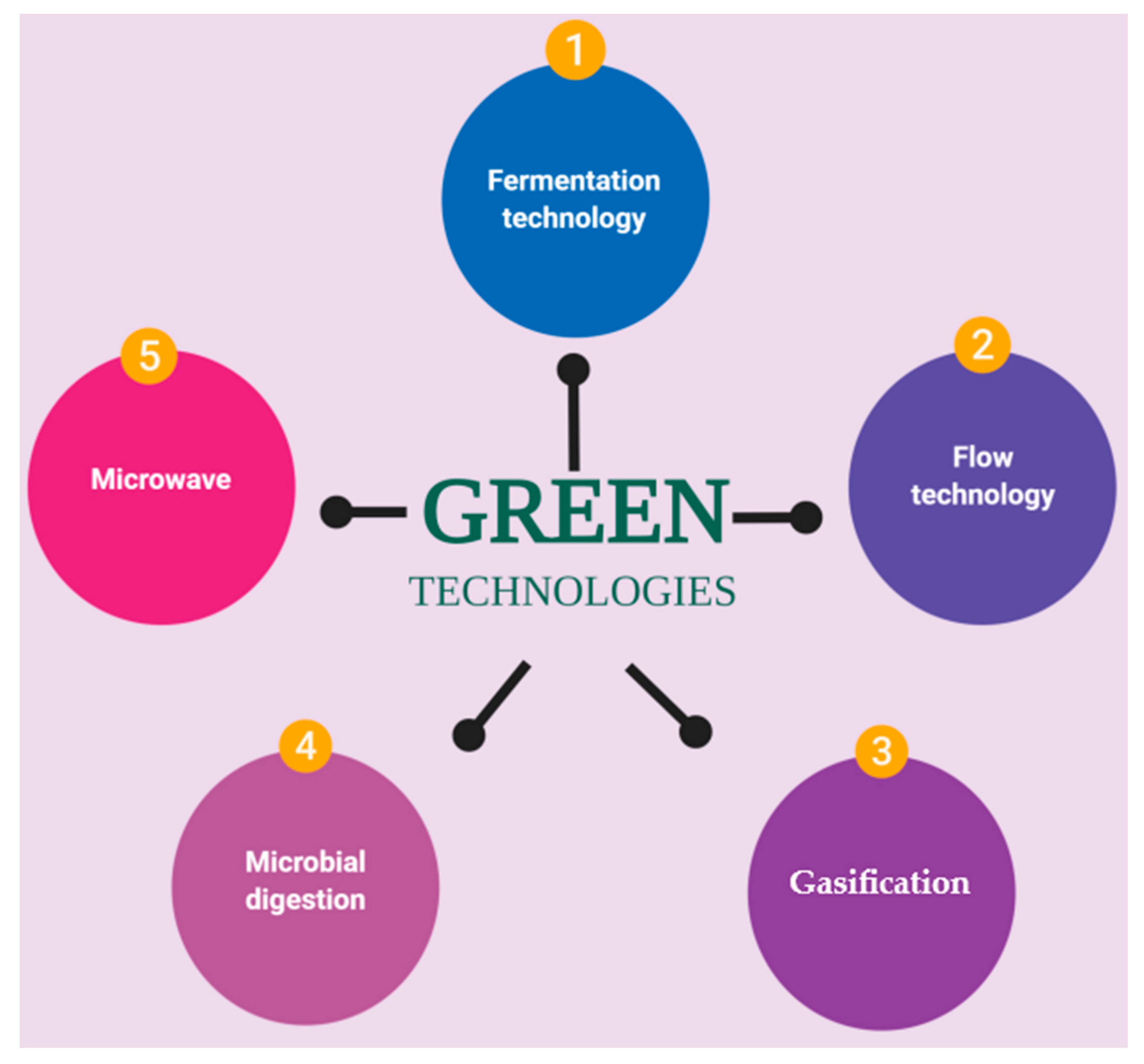
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, I.; Zia, M.A.; Afzal, H.; Ahmed, S.; Ahmad, M.; Akram, Z.; Sher, F.; Iqbal, H.M.N. Socio-Economic and Environmental Impacts of Biomass Valorisation: A Strategic Drive for Sustainable Bioeconomy. Sustainability 2021, 13, 4200. https://doi.org/10.3390/su13084200
Ahmed I, Zia MA, Afzal H, Ahmed S, Ahmad M, Akram Z, Sher F, Iqbal HMN. Socio-Economic and Environmental Impacts of Biomass Valorisation: A Strategic Drive for Sustainable Bioeconomy. Sustainability. 2021; 13(8):4200. https://doi.org/10.3390/su13084200
Chicago/Turabian StyleAhmed, Ishtiaq, Muhammad Anjum Zia, Huma Afzal, Shaheez Ahmed, Muhammad Ahmad, Zain Akram, Farooq Sher, and Hafiz M. N. Iqbal. 2021. "Socio-Economic and Environmental Impacts of Biomass Valorisation: A Strategic Drive for Sustainable Bioeconomy" Sustainability 13, no. 8: 4200. https://doi.org/10.3390/su13084200







