Neurological Disorders Associated with WWOX Germline Mutations—A Comprehensive Overview
Abstract
1. Introduction
2. Clinical Implications of WWOX Germline Variants
3. Molecular Characteristics
4. WWOX Expression in the Nervous System
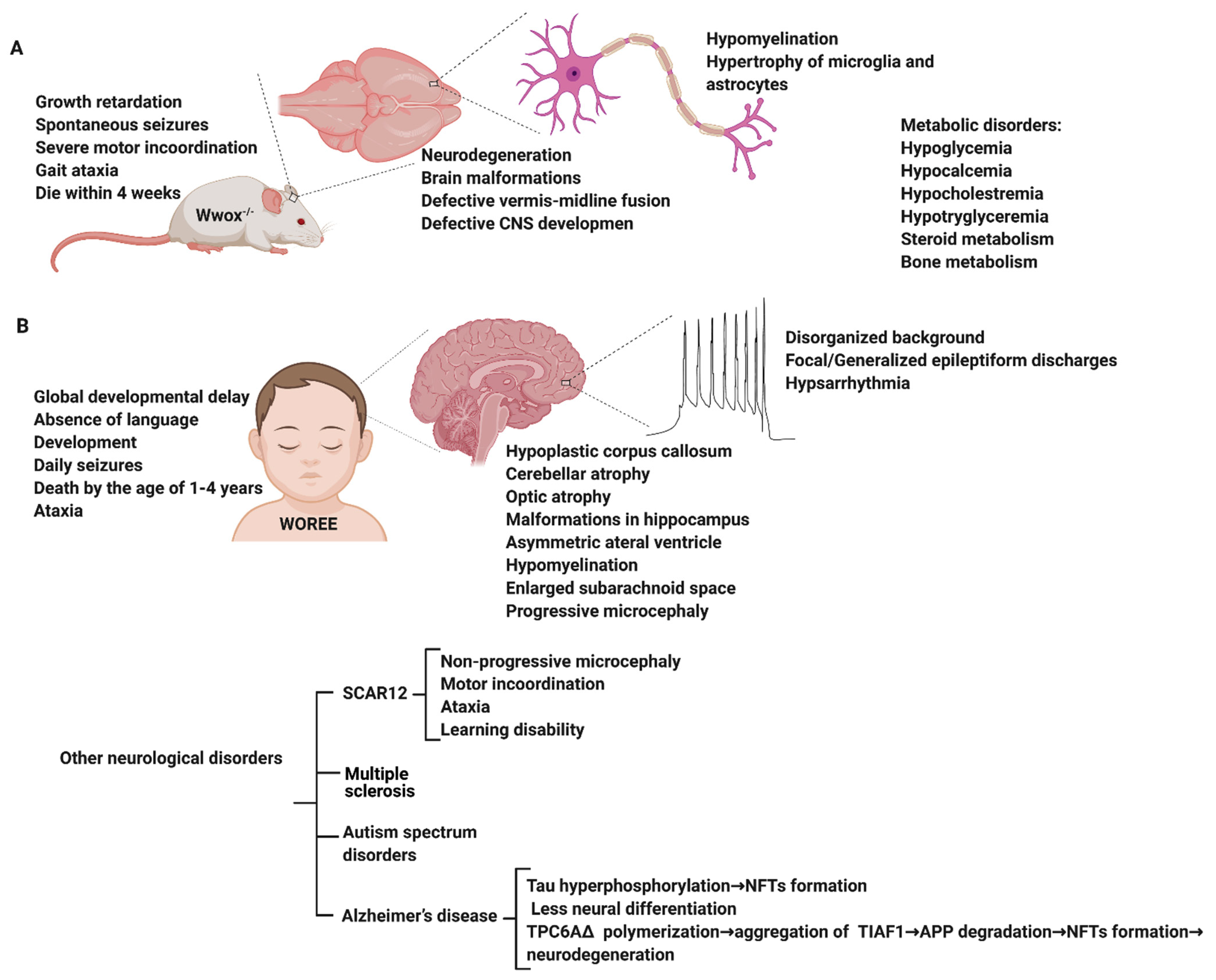
5. Modeling WWOX Deficiency in Rodents Reveals an Epileptic Phenotype
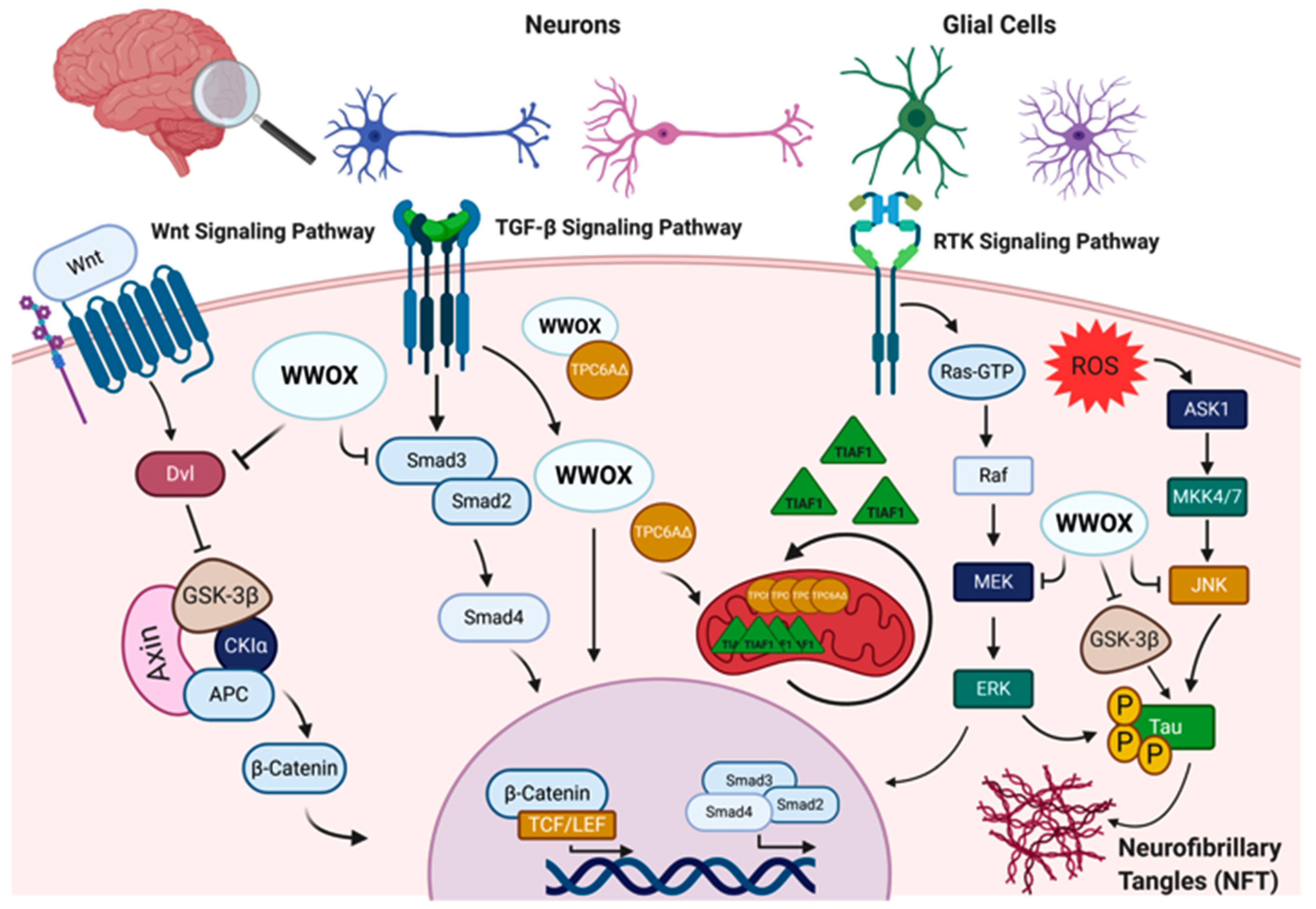
6. WWOX Impairment in Neurological Disorders
6.1. WWOX in Alzheimer’s Disease
6.2. WWOX in Multiple Sclerosis
6.3. WWOX in Autism Spectrum Disorders (ASD)
6.4. WWOX in Early Onset Epilepsy and Ataxia
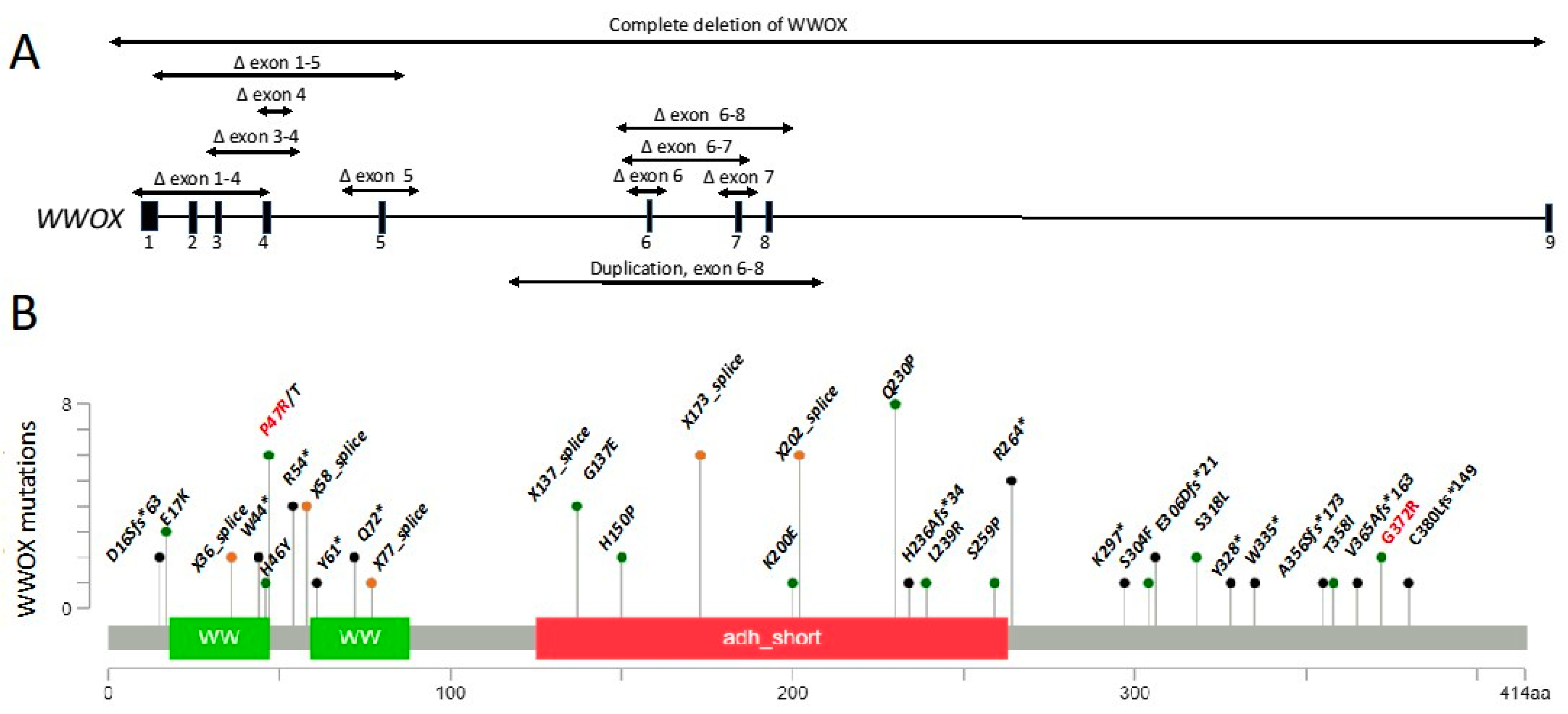
7. WWOX Variants and Mutations in Early Onset Epilepsy and Ataxia
7.1. Published WOREE Cases
7.2. Further Patient Details
7.3. Data Collected from ClinVar
7.4. Unpublished WWOX Cases
8. Future Possibilities for Personalized Medicine Interventions
9. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Howell, K.B.; Freeman, J.L.; Mackay, M.T.; Fahey, M.C.; Archer, J.; Berkovic, S.F.; Chan, E.; Dabscheck, G.; Eggers, S.; Hayman, M.; et al. The severe epilepsy syndromes of infancy: A population-based study. Epilepsia 2021, 62, 358–370. [Google Scholar] [CrossRef]
- Steward, C.A.; Roovers, J.; Suner, M.M.; Gonzalez, J.M.; Uszczynska-Ratajczak, B.; Pervouchine, D.; Fitzgerald, S.; Viola, M.; Stamberger, H.; Hamdan, F.F.; et al. Re-annotation of 191 developmental and epileptic encephalopathy-associated genes unmasks de novo variants in SCN1A. NPJ Genom. Med. 2019, 4, 31. [Google Scholar] [CrossRef]
- Shbarou, R.; Mikati, M.A. The Expanding Clinical Spectrum of Genetic Pediatric Epileptic Encephalopathies. Semin. Pediatr. Neurol. 2016, 23, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Mallaret, M.; Synofzik, M.; Lee, J.; Sagum, C.A.; Mahajnah, M.; Sharkia, R.; Drouot, N.; Renaud, M.; Klein, F.A.; Anheim, M.; et al. The tumour suppressor gene WWOX is mutated in autosomal recessive cerebellar ataxia with epilepsy and mental retardation. Brain 2014, 137, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Piard, J.; Hawkes, L.; Milh, M.; Villard, L.; Borgatti, R.; Romaniello, R.; Fradin, M.; Capri, Y.; Heron, D.; Nougues, M.C.; et al. The phenotypic spectrum of WWOX-related disorders: 20 additional cases of WOREE syndrome and review of the literature. Genet. Med. 2019, 21, 1308–1318. [Google Scholar] [CrossRef]
- Shaukat, Q.; Hertecant, J.; El-Hattab, A.W.; Ali, B.R.; Suleiman, J. West syndrome, developmental and epileptic encephalopathy, and severe CNS disorder associated with WWOX mutations. Epileptic Disord. 2018, 20, 401–412. [Google Scholar] [CrossRef] [PubMed]
- White, S.; Hewitt, J.; Turbitt, E.; van der Zwan, Y.; Hersmus, R.; Drop, S.; Koopman, P.; Harley, V.; Cools, M.; Looijenga, L.; et al. A multi-exon deletion within WWOX is associated with a 46, XY disorder of sex development. Eur. J. Hum. Genet. 2012, 20, 348–351. [Google Scholar] [CrossRef]
- Gribaa, M.; Salih, M.; Anheim, M.; Lagier-Tourenne, C.; H’Mida, D.; Drouot, N.; Mohamed, A.; Elmalik, S.; Kabiraj, M.; Al-Rayess, M.; et al. A new form of childhood onset, autosomal recessive spinocerebellar ataxia and epilepsy is localized at 16q21-q23. Brain 2007, 130, 1921–1928. [Google Scholar] [CrossRef]
- Bird, T.D. Hereditary Ataxia Overview. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Mirzaa, G., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Bednarek, A.K.; Laflin, K.J.; Daniel, R.L.; Liao, Q.; Hawkins, K.A.; Aldaz, C.M. WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3-24.1, a region frequently affected in breast cancer. Cancer Res. 2000, 60, 2140–2145. [Google Scholar]
- Del Mare, S.; Salah, Z.; Aqeilan, R.I. WWOX: Its genomics, partners, and functions. J. Cell. Biochem. 2009, 108, 737–745. [Google Scholar] [CrossRef]
- Abu-Remaileh, M.; Dodson, E.J.; Schueler-Furman, O.; Aqeilan, R.I. Pleiotropic Functions of Tumor Suppressor WWOX in Normal and Cancer Cells. J. Biol. Chem. 2015. [Google Scholar] [CrossRef] [PubMed]
- Salah, Z.; Aqeilan, R.; Huebner, K. WWOX gene and gene product: Tumor suppression through specific protein interactions. Future Oncol. 2010, 6, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Aqeilan, R.I.; Abu-Remaileh, M.; Abu-Odeh, M. The common fragile site FRA16D gene product WWOX: Roles in tumor suppression and genomic stability. Cell. Mol. Life Sci. 2014, 71, 4589–4599. [Google Scholar] [CrossRef] [PubMed]
- Aqeilan, R.I.; Croce, C.M. WWOX in biological control and tumorigenesis. J. Cell. Physiol. 2007, 212, 307–310. [Google Scholar] [CrossRef]
- Gardenswartz, A.; Aqeilan, R.I. WW domain-containing oxidoreductase’s role in myriad cancers: Clinical significance and future implications. Exp. Biol. Med. 2014, 239, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Aldaz, C.M.; Ferguson, B.W.; Abba, M.C. WWOX at the crossroads of cancer, metabolic syndrome related traits and CNS pathologies. Biochim. Biophys. Acta 2014. [Google Scholar] [CrossRef] [PubMed]
- Bouteille, N.; Driouch, K.; Hage, P.E.; Sin, S.; Formstecher, E.; Camonis, J.; Lidereau, R.; Lallemand, F. Inhibition of the Wnt/beta-catenin pathway by the WWOX tumor suppressor protein. Oncogene 2009. [Google Scholar] [CrossRef] [PubMed]
- Khawaled, S.; Nigita, G.; Distefano, R.; Oster, S.; Suh, S.S.; Smith, Y.; Khalaileh, A.; Peng, Y.; Croce, C.M.; Geiger, T.; et al. Pleiotropic tumor suppressor functions of WWOX antagonize metastasis. Signal Transduct. Target. Ther. 2020, 5, 43. [Google Scholar] [CrossRef]
- Kosla, K.; Pluciennik, E.; Styczen-Binkowska, E.; Nowakowska, M.; Orzechowska, M.; Bednarek, A.K. The WWOX Gene Influences Cellular Pathways in the Neuronal Differentiation of Human Neural Progenitor Cells. Front. Cell. Neurosci. 2019, 13, 391. [Google Scholar] [CrossRef]
- Ferguson, B.W.; Gao, X.; Zelazowski, M.J.; Lee, J.; Jeter, C.R.; Abba, M.C.; Aldaz, C.M. The cancer gene WWOX behaves as an inhibitor of SMAD3 transcriptional activity via direct binding. BMC Cancer 2013, 13, 593. [Google Scholar] [CrossRef]
- Aqeilan, R.I.; Donati, V.; Palamarchuk, A.; Trapasso, F.; Kaou, M.; Pekarsky, Y.; Sudol, M.; Croce, C.M. WW domain-containing proteins, WWOX and YAP, compete for interaction with ErbB-4 and modulate its transcriptional function. Cancer Res. 2005, 65, 6764–6772. [Google Scholar] [CrossRef]
- Aqeilan, R.I.; Donati, V.; Gaudio, E.; Nicoloso, M.S.; Sundvall, M.; Korhonen, A.; Lundin, J.; Isola, J.; Sudol, M.; Joensuu, H.; et al. Association of WWOX with ErbB4 in Breast Cancer. Cancer Res. 2007, 67, 9330–9336. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, B.J.; Bhat, V.; Mikles, D.C.; McDonald, C.B.; Sudol, M.; Farooq, A. Molecular Origin of the Binding of WWOX Tumor Suppressor to ErbB4 Receptor Tyrosine Kinase. Biochemistry 2013, 52, 9223–9236. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Qu, Z.; Yan, P.; Ishikawa, C.; Aqeilan, R.I.; Rabson, A.B.; Xiao, G. The tumor suppressor gene WWOX links the canonical and noncanonical NF-kappaB pathways in HTLV-I Tax-mediated tumorigenesis. Blood 2011, 117, 1652–1661. [Google Scholar] [CrossRef]
- Abu-Remaileh, M.; Aqeilan, R.I. Tumor suppressor WWOX regulates glucose metabolism via HIF1alpha modulation. Cell Death Differ. 2014, 21, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, L.V.; Colella, A.; Dayan, S.; Chen, Q.; Choo, A.; Jacob, R.; Price, G.; Venter, D.; Richards, R.I. Drosophila orthologue of WWOX, the chromosomal fragile site FRA16D tumour suppressor gene, functions in aerobic metabolism and regulates reactive oxygen species. Hum. Mol. Genet. 2011, 20, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Abu-Odeh, M.; Salah, Z.; Herbel, C.; Hofmann, T.G.; Aqeilan, R.I. WWOX, the common fragile site FRA16D gene product, regulates ATM activation and the DNA damage response. Proc. Natl. Acad. Sci. USA 2014, 111, E4716–E4725. [Google Scholar] [CrossRef]
- Aqeilan, R.I.; Pekarsky, Y.; Herrero, J.J.; Palamarchuk, A.; Letofsky, J.; Druck, T.; Trapasso, F.; Han, S.Y.; Melino, G.; Huebner, K.; et al. Functional association between WWOX tumor suppressor protein and p73, a p53 homolog. Proc. Natl. Acad. Sci. USA 2004, 101, 4401–4406. [Google Scholar] [CrossRef]
- Salah, Z.; Bar-mag, T.; Kohn, Y.; Pichiorri, F.; Palumbo, T.; Melino, G.; Aqeilan, R.I. Tumor suppressor WWOX binds to DeltaNp63alpha and sensitizes cancer cells to chemotherapy. Cell Death Dis. 2013, 4, e480. [Google Scholar] [CrossRef][Green Version]
- Liu, C.C.; Ho, P.C.; Lee, I.T.; Chen, Y.A.; Chu, C.H.; Teng, C.C.; Wu, S.N.; Sze, C.I.; Chiang, M.F.; Chang, N.S. WWOX Phosphorylation, Signaling, and Role in Neurodegeneration. Front. Neurosci. 2018, 12, 563. [Google Scholar] [CrossRef]
- Nunez, M.I.; Ludes-Meyers, J.; Aldaz, C.M. WWOX protein expression in normal human tissues. J. Mol. Histol. 2006, 37, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Chuang, J.I.; Wang, J.P.; Tsai, M.S.; Li, H.; Chang, N.S. Expression of WW domain-containing oxidoreductase WOX1 in the developing murine nervous system. Neuroscience 2004, 124, 831–839. [Google Scholar] [CrossRef]
- Aldaz, C.M.; Hussain, T. WWOX Loss of Function in Neurodevelopmental and Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 8922. [Google Scholar] [CrossRef] [PubMed]
- Tochigi, Y.; Takamatsu, Y.; Nakane, J.; Nakai, R.; Katayama, K.; Suzuki, H. Loss of WWOX Causes Defective Development of Cerebral Cortex with Hypomyelination in a Rat Model of Lethal Dwarfism with Epilepsy. Int. J. Mol. Sci. 2019, 20, 3596. [Google Scholar] [CrossRef]
- Suzuki, H.; Katayama, K.; Takenaka, M.; Amakasu, K.; Saito, K.; Suzuki, K. A spontaneous mutation of the WWOX gene and audiogenic seizures in rats with lethal dwarfism and epilepsy. Genes Brain Behav. 2009. [Google Scholar] [CrossRef] [PubMed]
- Aqeilan, R.I.; Hassan, M.Q.; de Bruin, A.; Hagan, J.P.; Volinia, S.; Palumbo, T.; Hussain, S.; Lee, S.H.; Gaur, T.; Stein, G.S.; et al. The WWOX tumor suppressor is essential for post-natal survival and normal bone metabolism. J. Biol. Chem. 2008, 283, 21629–21639. [Google Scholar] [CrossRef] [PubMed]
- Aqeilan, R.I.; Trapasso, F.; Hussain, S.; Costinean, S.; Marshall, D.; Pekarsky, Y.; Hagan, J.P.; Zanesi, N.; Kaou, M.; Stein, G.S.; et al. Targeted deletion of WWOX reveals a tumor suppressor function. Proc. Natl. Acad. Sci. USA 2007, 104, 3949–3954. [Google Scholar] [CrossRef]
- Cheng, Y.Y.; Chou, Y.T.; Lai, F.J.; Jan, M.S.; Chang, T.H.; Jou, I.M.; Chen, P.S.; Lo, J.Y.; Huang, S.S.; Chang, N.S.; et al. WWOX deficiency leads to neurodevelopmental and degenerative neuropathies and glycogen synthase kinase 3beta-mediated epileptic seizure activity in mice. Acta Neuropathol. Commun. 2020, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Raymond, A.A.; Fish, D.R.; Stevens, J.M.; Sisodiya, S.M.; Alsanjari, N.; Shorvon, S.D. Subependymal heterotopia: A distinct neuronal migration disorder associated with epilepsy. J. Neurol. Neurosurg. Psychiatry 1994, 57, 1195–1202. [Google Scholar] [CrossRef]
- Hussain, T.; Kil, H.; Hattiangady, B.; Lee, J.; Kodali, M.; Shuai, B.; Attaluri, S.; Takata, Y.; Shen, J.; Abba, M.C.; et al. WWOX deletion leads to reduced GABA-ergic inhibitory interneuron numbers and activation of microglia and astrocytes in mouse hippocampus. Neurobiol. Dis. 2019, 121, 163–176. [Google Scholar] [CrossRef]
- Iacomino, M.; Baldassari, S.; Tochigi, Y.; Kosla, K.; Buffelli, F.; Torella, A.; Severino, M.; Paladini, D.; Mandara, L.; Riva, A.; et al. Loss of WWOX Perturbs Neuronal Migration and Impairs Early Cortical Development. Front. Neurosci. 2020, 14, 644. [Google Scholar] [CrossRef] [PubMed]
- Kunkle, B.W.; Grenier-Boley, B.; Sims, R.; Bis, J.C.; Damotte, V.; Naj, A.C.; Boland, A.; Vronskaya, M.; van der Lee, S.J.; Amlie-Wolf, A.; et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Abeta, tau, immunity and lipid processing. Nat. Genet. 2019, 51, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Sze, C.I.; Su, M.; Pugazhenthi, S.; Jambal, P.; Hsu, L.J.; Heath, J.; Schultz, L.; Chang, N.S. Down-regulation of WW domain-containing oxidoreductase induces Tau phosphorylation in vitro. A potential role in Alzheimer’s disease. J. Biol. Chem. 2004, 279, 30498–30506. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Juo, L.I.; Lin, Y.T.; Hsiao, M.; Lin, J.T.; Tsai, C.H.; Tzeng, Y.H.; Chuang, Y.C.; Chang, N.S.; Yang, C.N.; et al. WW domain-containing oxidoreductase promotes neuronal differentiation via negative regulation of glycogen synthase kinase 3beta. Cell Death Differ. 2012, 19, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Lee, M.H.; Lin, S.R.; Yang, L.Y.; Sun, H.S.; Sze, C.I.; Hong, Q.; Lin, Y.S.; Chou, Y.T.; Hsu, L.J.; et al. Trafficking protein particle complex 6A delta (TRAPPC6ADelta) is an extracellular plaque-forming protein in the brain. Oncotarget 2015, 6, 3578–3589. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Chang, N.S. WWOX dysfunction induces sequential aggregation of TRAPPC6ADelta, TIAF1, tau and amyloid beta, and causes apoptosis. Cell Death Discov. 2015, 1, 15003. [Google Scholar] [CrossRef]
- Lee, M.H.; Lin, S.R.; Chang, J.Y.; Schultz, L.; Heath, J.; Hsu, L.J.; Kuo, Y.M.; Hong, Q.; Chiang, M.F.; Gong, C.X.; et al. TGF-beta induces TIAF1 self-aggregation via type II receptor-independent signaling that leads to generation of amyloid beta plaques in Alzheimer’s disease. Cell Death Dis. 2010, 1, e110. [Google Scholar] [CrossRef]
- Goldenberg, M.M. Multiple sclerosis review. Pharm. Ther. 2012, 37, 175–184. [Google Scholar]
- Podbielska, M.; Banik, N.L.; Kurowska, E.; Hogan, E.L. Myelin recovery in multiple sclerosis: The challenge of remyelination. Brain Sci. 2013, 3, 1282–1324. [Google Scholar] [CrossRef] [PubMed]
- Jakel, S.; Agirre, E.; Mendanha Falcao, A.; van Bruggen, D.; Lee, K.W.; Knuesel, I.; Malhotra, D.; Ffrench-Constant, C.; Williams, A.; Castelo-Branco, G. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 2019, 566, 543–547. [Google Scholar] [CrossRef]
- International Multiple Sclerosis Genetics, C.; Beecham, A.H.; Patsopoulos, N.A.; Xifara, D.K.; Davis, M.F.; Kemppinen, A.; Cotsapas, C.; Shah, T.S.; Spencer, C.; Booth, D.; et al. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat. Genet. 2013, 45, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Ziliotto, N.; Marchetti, G.; Scapoli, C.; Bovolenta, M.; Meneghetti, S.; Benazzo, A.; Lunghi, B.; Balestra, D.; Laino, L.A.; Bozzini, N.; et al. C6orf10 Low-Frequency and Rare Variants in Italian Multiple Sclerosis Patients. Front. Genet. 2019, 10, 573. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T.; Madireddy, L.; Sprenger, T.; Khankhanian, P.; Magon, S.; Naegelin, Y.; Caverzasi, E.; Lindberg, R.L.; Kappos, L.; Hauser, S.L.; et al. Genetic associations with brain cortical thickness in multiple sclerosis. Genes Brain Behav. 2015, 14, 217–227. [Google Scholar] [CrossRef] [PubMed]
- International Multiple Sclerosis Genetics, C. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science 2019, 365. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Zhang, J.; Ahn, M.; Jha, S.; Crowley, J.J.; Szatkiewicz, J.; Li, T.; Zou, F.; Zhu, H.; Hibar, D.; et al. Genome-wide association analysis identifies common variants influencing infant brain volumes. Transl. Psychiatry 2017, 7, e1188. [Google Scholar] [CrossRef] [PubMed]
- Peter, B.; Dinu, V.; Liu, L.; Huentelman, M.; Naymik, M.; Lancaster, H.; Vose, C.; Schrauwen, I. Exome Sequencing of Two Siblings with Sporadic Autism Spectrum Disorder and Severe Speech Sound Disorder Suggests Pleiotropic and Complex Effects. Behav. Genet. 2019, 49, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Bacchelli, E.; Cameli, C.; Viggiano, M.; Igliozzi, R.; Mancini, A.; Tancredi, R.; Battaglia, A.; Maestrini, E. An integrated analysis of rare CNV and exome variation in Autism Spectrum Disorder using the Infinium PsychArray. Sci. Rep. 2020, 10, 3198. [Google Scholar] [CrossRef]
- Leppa, V.M.; Kravitz, S.N.; Martin, C.L.; Andrieux, J.; Le Caignec, C.; Martin-Coignard, D.; DyBuncio, C.; Sanders, S.J.; Lowe, J.K.; Cantor, R.M.; et al. Rare Inherited and De Novo CNVs Reveal Complex Contributions to ASD Risk in Multiplex Families. Am. J. Hum. Genet. 2016, 99, 540–554. [Google Scholar] [CrossRef]
- Abdel-Salam, G.; Thoenes, M.; Afifi, H.H.; Korber, F.; Swan, D.; Bolz, H.J. The supposed tumor suppressor gene WWOX is mutated in an early lethal microcephaly syndrome with epilepsy, growth retardation and retinal degeneration. Orphanet J. Rare Dis. 2014, 9, 12. [Google Scholar] [CrossRef]
- Ben-Salem, S.; Al-Shamsi, A.M.; John, A.; Ali, B.R.; Al-Gazali, L. A novel whole exon deletion in WWOX gene causes early epilepsy, intellectual disability and optic atrophy. J. Mol. Neurosci. 2015, 56, 17–23. [Google Scholar] [CrossRef]
- Mignot, C.; Lambert, L.; Pasquier, L.; Bienvenu, T.; Delahaye-Duriez, A.; Keren, B.; Lefranc, J.; Saunier, A.; Allou, L.; Roth, V.; et al. WWOX-related encephalopathies: Delineation of the phenotypical spectrum and emerging genotype-phenotype correlation. J. Med. Genet. 2015, 52, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Valduga, M.; Philippe, C.; Lambert, L.; Bach-Segura, P.; Schmitt, E.; Masutti, J.P.; Francois, B.; Pinaud, P.; Vibert, M.; Jonveaux, P. WWOX and severe autosomal recessive epileptic encephalopathy: First case in the prenatal period. J. Hum. Genet. 2015, 60, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Elsaadany, L.; El-Said, M.; Ali, R.; Kamel, H.; Ben-Omran, T. W44X mutation in the WWOX gene causes intractable seizures and developmental delay: A case report. BMC Med. Genet. 2016, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Tabarki, B.; AlHashem, A.; AlShahwan, S.; Alkuraya, F.S.; Gedela, S.; Zuccoli, G. Severe CNS involvement in WWOX mutations: Description of five new cases. Am. J. Med. Genet. Part A 2015, 167, 3209–3213. [Google Scholar] [CrossRef]
- Tarta-Arsene, O.; Barca, D.; Craiu, D.; Iliescu, C. Practical clues for diagnosing WWOX encephalopathy. Epileptic Disord. 2017, 19, 357–361. [Google Scholar] [CrossRef]
- Rim, J.H.; Kim, S.H.; Hwang, I.S.; Kwon, S.S.; Kim, J.; Kim, H.W.; Cho, M.J.; Ko, A.; Youn, S.E.; Kim, J.; et al. Efficient strategy for the molecular diagnosis of intractable early-onset epilepsy using targeted gene sequencing. BMC Med. Genom. 2018, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, J.; Kortum, F.; Rosenberger, G.; Bokelmann, K.; Schirmer, M.A.; Denecke, J.; Santer, R. A novel missense variant in the SDR domain of the WWOX gene leads to complete loss of WWOX protein with early-onset epileptic encephalopathy and severe developmental delay. Neurogenetics 2018, 19, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Kothur, K.; Holman, K.; Farnsworth, E.; Ho, G.; Lorentzos, M.; Troedson, C.; Gupta, S.; Webster, R.; Procopis, P.G.; Menezes, M.P.; et al. Diagnostic yield of targeted massively parallel sequencing in children with epileptic encephalopathy. Seizure 2018, 59, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.; Markello, T.; Wolfe, L.A.; Chepa-Lotrea, X.; Tifft, C.J.; Gahl, W.A.; Malicdan, M.C.V. Early infantile-onset epileptic encephalopathy 28 due to a homozygous microdeletion involving the WWOX gene in a region of uniparental disomy. Hum. Mutat. 2019, 40, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Weisz-Hubshman, M.; Meirson, H.; Michaelson-Cohen, R.; Beeri, R.; Tzur, S.; Bormans, C.; Modai, S.; Shomron, N.; Shilon, Y.; Banne, E.; et al. Novel WWOX deleterious variants cause early infantile epileptic encephalopathy, severe developmental delay and dysmorphism among Yemenite Jews. Eur. J. Paediatr. Neurol. 2019, 23, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, Y.; Song, Z.; Yi, Z.; Li, F. Novel compound heterozygous mutations in the WWOX gene cause early infantile epileptic encephalopathy. Int. J. Dev. Neurosci. 2019, 79, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Burgess, R.; Wang, S.; McTague, A.; Boysen, K.E.; Yang, X.; Zeng, Q.; Myers, K.A.; Rochtus, A.; Trivisano, M.; Gill, D.; et al. The Genetic Landscape of Epilepsy of Infancy with Migrating Focal Seizures. Ann. Neurol. 2019, 86, 821–831. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhou, W.; Shi, J.; Zhang, B.; Wang, H. A Chinese patient with epilepsy and WWOX compound heterozygous mutations. Epileptic Disord. 2020, 22, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Yan, Y.; Xu, S.; Zhang, K.; Xu, S. Early onset epileptic encephalopathy caused by novel compound heterozygous mutation of WWOX gene. Int. J. Dev. Neurosci. 2020, 80, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Nashabat, M.; Al Qahtani, X.S.; Almakdob, S.; Altwaijri, W.; Ba-Armah, D.M.; Hundallah, K.; Al Hashem, A.; Al Tala, S.; Maddirevula, S.; Alkuraya, F.S.; et al. The landscape of early infantile epileptic encephalopathy in a consanguineous population. Seizure 2019, 69, 154–172. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Goji, A.; Toda, Y.; Ito, H.; Mori, K.; Kohmoto, T.; Imoto, I.; Kagami, S. A 16q22.2-q23.1 deletion identified in a male infant with West syndrome. Brain Dev. 2019, 41, 888–893. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Wang, Q.; Pierce-Hoffman, E.; Cummings, B.B.; Alfoldi, J.; Francioli, L.C.; Gauthier, L.D.; Hill, A.J.; O’Donnell-Luria, A.H.; Genome Aggregation Database Production, T.; Genome Aggregation Database, C.; et al. Landscape of multi-nucleotide variants in 125,748 human exomes and 15,708 genomes. Nat. Commun. 2020, 11, 2539. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alfoldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Lek, M.; Karczewski, K.J.; Minikel, E.V.; Samocha, K.E.; Banks, E.; Fennell, T.; O’Donnell-Luria, A.H.; Ware, J.S.; Hill, A.J.; Cummings, B.B.; et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016, 536, 285–291. [Google Scholar] [CrossRef]
- Marchetto, M.C.; Carromeu, C.; Acab, A.; Yu, D.; Yeo, G.W.; Mu, Y.; Chen, G.; Gage, F.H.; Muotri, A.R. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 2010, 143, 527–539. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Sidhaye, J.; Knoblich, J.A. Brain organoids: An ensemble of bioassays to investigate human neurodevelopment and disease. Cell Death Differ. 2020. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.J.; Saleem, A.; Repudi, S.R.; Banne, E.; Mahajnah, M.; Hanna, J.H.; Carlen, P.L.; Aqeilan, R.I. Modeling Genetic Epileptic Encephalopathies using Brain Organoids. bioRxiv 2020. [Google Scholar] [CrossRef]
- Denichenko, P.; Mogilevsky, M.; Clery, A.; Welte, T.; Biran, J.; Shimshon, O.; Barnabas, G.D.; Danan-Gotthold, M.; Kumar, S.; Yavin, E.; et al. Specific inhibition of splicing factor activity by decoy RNA oligonucleotides. Nat. Commun. 2019, 10, 1590. [Google Scholar] [CrossRef]
- Pattali, R.; Mou, Y.; Li, X.J. AAV9 Vector: A Novel modality in gene therapy for spinal muscular atrophy. Gene Ther. 2019, 26, 287–295. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Hong, W.; Huang, M.; Wu, M.; Zhao, X. Applications of genome editing technology in the targeted therapy of human diseases: Mechanisms, advances and prospects. Signal Transduct. Target. Ther. 2020, 5, 1. [Google Scholar] [CrossRef]
- Cota-Coronado, A.; Diaz-Martinez, N.F.; Padilla-Camberos, E.; Diaz-Martinez, N.E. Editing the Central Nervous System through CRISPR/Cas9 Systems. Front. Mol. Neurosci. 2019, 12, 110. [Google Scholar] [CrossRef]
- Maeder, M.L.; Stefanidakis, M.; Wilson, C.J.; Baral, R.; Barrera, L.A.; Bounoutas, G.S.; Bumcrot, D.; Chao, H.; Ciulla, D.M.; DaSilva, J.A.; et al. Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat. Med. 2019, 25, 229–233. [Google Scholar] [CrossRef]
- Ledford, H. CRISPR treatment inserted directly into the body for first time. Nature 2020, 579, 185. [Google Scholar] [CrossRef] [PubMed]
- Ehaideb, S.N.; Al-Bu Ali, M.J.; Al-Obaid, J.J.; Aljassim, K.M.; Alfadhel, M. Novel Homozygous Mutation in the WWOX Gene Causes Seizures and Global Developmental Delay: Report and Review. Transl. Neurosci. 2018, 9, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Serin, H.M.; Simsek, E.; Isik, E.; Gokben, S. WWOX-associated encephalopathies: Identification of the phenotypic spectrum and the resulting genotype-phenotype correlation. Neurol. Sci. 2018, 39, 1977–1980. [Google Scholar] [CrossRef] [PubMed]
| Name | Variant type | Protein change | Cases/references | Gender | Ethnicity | Zygosity | Seizure onset | Death |
|---|---|---|---|---|---|---|---|---|
| Spinocerebellar ataxia, autosomal recessive 12 | ||||||||
| NM_016373.4(WWOX):c.139C>A (p.Pro47Thr) | Missense | P47T | 4 siblings [4,8] | F/F/M/F | Saudi-Arabian | Homozygous | 12m/9m/9m/9m | 17-26 y |
| NM_016373.4(WWOX):c.1114G>C (p.Gly372Arg) | Missense | G372R | 2 siblings [4] | M/F | Israeli | Homozygous | <2 y/<2y | 5-10 y |
| Developmental and epileptic encephalopathy 28 | ||||||||
| NM_016373.4(WWOX):c.160C>T (p.Arg54Ter) | Nonsense | R54* | 1 [60] | F | Egyptian | Homozygous | 2m | 16m |
| WWOX-null alleles | 2 [63] | F/M | Turkish | 3 m/prenatal | 22m/Mtp | |||
| GRCh37/hg19 16q23.1(chr16:78180603-78208482)x0 | Deletion (Exon 5) | 1 [61] | M | Emirati | Homozygous | 2 w | 5 m | |
| NM_016373.4(WWOX):c.606-1G>A | Splicing variant | 5 patients from 2 families [65] | F/F/F/M/F | Saudi Arabian | Homozygous | 2m/3m/2m/2m/3m | <3 y/<3 y/<3 y/<3 y/<3 y | |
| NM_016373.4(WWOX):c.46_49del (p.Asp16fs) | Deletion (Exon 1)/Nonsense | D16fs | 2 [62] | M/F | Portuguese | Compound heterozygous | 5 m/5m | alive at 4y/3 y |
| NM_016373.4(WWOX):c.140C>G (p.Pro47Arg) | Missense | P47R | ||||||
| NM_016373.4(WWOX):c.1005G>A (p.Trp335Ter) | Nonsense | W335* | 1 [62] | F | Portuguese | Compound heterozygous | 7 w | 24 m |
| NM_016373.2(WWOX):c.517-?_605+?del | Deletion | |||||||
| NM_016373.3(WWOX):c.-366-?_516+?del | Deletion | 1 [62] | F | Portuguese | Compound heterozygous | 2m | alive at 3y | |
| NM_016373.4:c.517_1056del | Deletion (Exon 6 to Exon 8) | |||||||
| NM_016373.4(WWOX):c.889A>T (p.Lys297*) | Nonsense | 1[62] | F | Portuguese | Compound heterozygous | 2m | 38m | |
| deletion encompassing the WWOX locus (2.8 Mb del) | Deletion | |||||||
| NM_016373.4(WWOX):c.131G>A (p.Trp44Ter) | Nonsense | W44* | 2 [64] | F/F | Qatari | Homozygous | 7 w/7w | 7y/20 m |
| NM_016373.4(WWOX):c.918del (p.Glu306fs) | Deletion (Exon 8)/Nonsense | E306fs | 1 [66] | M | Romanian | Compound heterozygous | 4 w | <3 y |
| NM_016373.4(WWOX):c.173-1G>T | Splicing variant | Asp58Alafs*3 | ||||||
| NM_016373.4(WWOX):c.160C>T (p.Arg54Ter) | Nonsense | R54* | 2 [93] | M/F | Saudi Arabian | Homozygous | 2 m | alive at 3 m/18 m |
| NM_016373.4(WWOX):c.409+1G>T | Splicing variant | 1 [93] | M | Saudi Arabian | Homozygous | 2 m | alive at 21 m | |
| GRCh37/hg19 16q23.1(chr16:78143675-78149052) | Deletion (Exon 3 to Exon 4) | 1 [6] | M | Emirati | Homozygous | 5 w | alive at 2 y | |
| NM_016373.4(WWOX):c.606-1G>A | Splicing variant | 1 [6] | M | Emirati | Homozygous | 5 w | alive at 13 m | |
| NM_016373.4(WWOX):c.689A>C (p.Gln230Pro) | Missense | Q230P | 2 [68] | F/F | Afghan | Homozygous | 2 m/2 m | alive at 12 y/10 y |
| NM_016373.4(WWOX):c.716T>G (p.Leu239Arg) | Missense | L239R | 1 [94] | F | Turkish | Homozygous | 2 m | NA |
| NM_016373.4:c.1_409del | Deletion (Exon 1 to Exon 4) | 1 [5] | M | Pakistani | Homozygous | 7 m | 2 y | |
| NM_016373.4:c.49G>A (p.Glu17Lys) | Missense | E17K | 1 [5] | F | NA | Compound heterozygous | 11 w | NA |
| NM_016373.4:c.911C>T (p.Ser304Phe) | Missense | S304F | ||||||
| NM_016373.4(WWOX):c.160C>T (p.Arg54Ter) | Nonsense | R54* | 1 [5] | F | French | Compound heterozygous | 2.5 m | NA |
| NM_016373.4:c.1094_1095delTC (p.Val365AlafsTer163) | Deletion (Exon 9)/Nonsense | V365Afs*163 | ||||||
| NM_016373.4:c.231_409del (p.Asp77GlufsTer27) | Deletion (Exon 4) | D77Efs*27 | 1 [5] | F | Italian | Compound heterozygous | day 20 | NA |
| NM_016373.4:c.607_791+1del | Deletion (Exon 7 to Intron 7) | |||||||
| NM_016373.4(WWOX):c.410G>A (p.Gly137Glu) | Missense | G137E | 1 [5] | M | NA | Compound heterozygous | day 1 | NA |
| NM_016373.4(WWOX):c.953C>T (p.Ser318Leu) | Missense | S318L | ||||||
| NM_016373.4(WWOX):c.410G>A (p.Gly137Glu) | Missense | G137E | 1 [5] | M | British | Compound heterozygous | day 1 | 2 y6 m |
| NM_016373.4:c.517_791del | Deletion(Exon 6 to Exon 7 ) | |||||||
| NM_016373.4:c.517_791del | Deletion (Exon 6 to Exon 7) | 2 [5] | M/M | French | Compound heterozygous | 3 m/2.5m | NA/NA | |
| NM_016373.4:c.449A>C (p.His150Pro) | Missense | H150P | ||||||
| NM_016373.4:c.411_516+1del | Deletion(Exon 5 to Intron 5) | 1 [5] | F | French | Compound heterozygous | day 1 | 6 m | |
| NM_016373.4:c.607_791+1del | Deletion (Exon 7 to Intron 7) | |||||||
| NM_016373.4(WWOX):c.689A>C (p.Gln230Pro) | Missense | Q230P | 1 [5] | F | Moroccan | Homozygous | 2 m | 3 y 3 m |
| NM_016373.4(WWOX):c.689A>C (p.Gln230Pro) | Missense | Q230P | 1 [5] | F | French | Compound heterozygous | 3 w | NA |
| NM_016373.4:c.598A>G (p.Lys200Glu) | Missense | K200E | ||||||
| NM_016373.4(WWOX):c.1073C>T (p.Thr358Ile) | Missense | T358I | ||||||
| NM_016373.4(WWOX):c.689A>C (p.Gln230Pro) | Missense | Q230P | 1 [5] | F | French | Compound heterozygous | 3 m | NA |
| NM_016373.4:c.1138dupT (p.Cys380LeufsTer149) | Duplication/Nonsense | C380Lfs*149 | ||||||
| NM_016373.4(WWOX):c.689A>C (p.Gln230Pro) | Missense | Q230P | 1 [5] | F | Iranian | Homozygous | 2 m | NA |
| NM_016373.4(WWOX):c.790C>T (p.Arg264Ter) | Nonsense | R264* | 2 [5] | F/M | Indian | Homozygous | 7 w/NA | 8y 11 m/5y 2m |
| NM_016373.4(WWOX):c.173-1G>T | Asp58Alafs*3 | 1 [5] | F | French | Compound heterozygous | day 20 | NA | |
| NM_016373.4(WWOX):c.517_1056dup | Duplication | His173_Met352dup | ||||||
| NM_016373.4:c.517_1056del | Deletion (Exon 6 to Exon 8) | 1 [5] | F | Caucasian | Compound heterozygous state | day 2 | NA | |
| NM_016373.4(WWOX):c.953C>T (p.Ser318Leu) | Missense | S318L | ||||||
| NM_016373.4:c.517_1056del | Deletion (Exon 6 to Exon 8 ) | 1 [5] | M | SubSaharan | Homozygous | 1 m | 27 m | |
| NM_016373.4:c.517_1056del | Deletion (Exon 6 to Exon 8) | 1 [5] | F | British | Compound heterozygous | day 5 | 2y5m | |
| NM_016373.4(WWOX):c.705dup (p.His236fs) | Frameshift | |||||||
| NM_016373.2(WWOX):c.517-?_605+?del | Deletion | 1 [70] | F | USA | Homozygous | 2 w | 6 y | |
| NM_016373.4:c.517-2A>G | Splicing variant | 4 [71] | F/F/F/M | Yemenite Jews | Homozygous | 3m/3 w/1m/6w | 30 m/33 m/9 m/alive at 6 m | |
| NM_016373.4:c.517-2A>G | Splicing variant | 2 [71] | M/F | Yemenite Jews | Compound heterozygous | 2 w/3 w | alive at 4y/3 y | |
| NM_016373.4(WWOX):c.689A>C (p.Gln230Pro) | Missense | Q230P | ||||||
| NM_016373.4:c.173-2A>G | Splicing variant | 1 [72] | M | Chinese | Compound heterozygous | day 19 | NA | |
| NM_016373.4:c.775T>C (p.Ser259Pro) | Missense | S259P | ||||||
| NM_016373.3 (and GRCh37/hg19): deletion-chr16:78146639-78151289, chr16:78166192-78184119 | Deletion | 1 [73] | F | Australian | compound heterozygous | 2.5m | alive at 7y | |
| NM_016373.4:c.49G>A (p.Glu17Lys) | Missense | E17K | ||||||
| GRCh37/hg19: 16q22.2q23.1(71,689,186–78,530,357)x1 | 6.8 Mb Deletion | 1 [77] | M | Japanese | heterozygous | 16m | 4 y7m | |
| NM_016373.4:c.229_230+2delGAGT | Deletion (Exon 3 to Intron 3 ) | 1 [75] | F | Chinese | Compound heterozygous | 8 w | 5 m | |
| NM_016373.4:c.1065dupA (p.Ala356SerfsTer173) | Duplication/Nonsense | A356Sfs*173 | ||||||
| NM_016373.4(WWOX):c.790C>T (p.Arg264Ter) | Nonsense | R264* | 2 [42] | M/M | Sicily Italy | Homozygous | 45 days/prenatal | alive/Mtp |
| NM_016373.4(WWOX):c.984C>G (p.Tyr328Ter) | Nonsense | Y328* | 1 [74] | M | Chinese | compound heterozygous | 1 m | 1y |
| NM_016373.4(WWOX):c.172+1G>C | Splicing variant |
| Decipher ID | Location | Variant Change | Protein Change | Size | Cases | Zygosity | Pathogenicity | Inheritance | Sex |
|---|---|---|---|---|---|---|---|---|---|
| 407439 | 78098478-78259430 | Deletion | 160.95 kb | 2 | Homozygous | Pathogenic | Biparental | 46XY | |
| 384984 | 78133724-78133724 | c.49G > A | E17K | SNV | 2 | Compound heterozygous | Likely pathogenic | Unknown | 46XX |
| 78022830-78216167 | Deletion | 193.34 kb | Likely pathogenic | Maternally inherited, constitutive in mother | |||||
| ClinVar | |||||||||
| Name | Variant change | Protein Change | Cases | Zygosity | Pathogenicity | Phenotype(s) | GRCh37Chromosome | GRCh37Location | |
| NM_016373.4(WWOX):c.108-2A>T | Splice acceptor | 1 | Homozygous | Likely pathogenic | seizures, Developmental regression, global developmental delay, microcephaly, and Dystonia | 16 | 78142318 | ||
| NM_016373.4(WWOX):c.183C>A (p.Tyr61Ter) | Nonsense | Y61* | 1 | Compound heterozygous | Likely pathogenic | a phenotype consistent with the WWOX-associated disease. | 16 | 78143685 | |
| NM_016373.4(WWOX):c.918del (p.Glu306fs) | Deletion (exon 8) | E306fs | Likely pathogenic | 16 | 78466511 | ||||
| NM_016373.4(WWOX):c.214C>T (p.Gln72Ter) | Nonsense | Q72* | 1 | Compound heterozygous | Pathogenicity | seizures | 16 | 78143716 | |
| NC_000016.10:g.(?_78099759)_(78115174_?)del | Deletion (exons 1–4) | Pathogenicity | 16 | 78133656–78149071 | |||||
| NM_016373.4(WWOX):c.214C>T (p.Gln72Ter) | Nonsense | Q72* | 1 | Compound heterozygous | Pathogenicity | microcephaly, infantile spasms/seizures | 16 | 78143716 | |
| NC_000016.9:g.(?_78198060)_(78198206_?)del | Deletion (exon 5) | Pathogenicity | 16 | 78198060–78198206 | |||||
| NM_016373.4(WWOX):c.107+1G>A | Splicing donor | 1 | Compound heterozygous | Pathogenicity | developmental delay and early-onset epileptic encephalopathy. | 16 | 78133783 | ||
| NM_016373.4(WWOX):c.136C>T (p.His46Tyr) | Missinse | H46Y | Likely pathogenic | 16 | 78142348 | ||||
| NM_016373.4(WWOX):c.409+1G>C | Splice donor | 1 | Compound heterozygous | Likely pathogenic | seizures | 16 | 78149052 | ||
| NM_016373.4(WWOX):c.790C>T (p.Arg264Ter) | Nonsense | R264* | Pathogenicity | 16 | 78458951 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banne, E.; Abudiab, B.; Abu-Swai, S.; Repudi, S.R.; Steinberg, D.J.; Shatleh, D.; Alshammery, S.; Lisowski, L.; Gold, W.; Carlen, P.L.; et al. Neurological Disorders Associated with WWOX Germline Mutations—A Comprehensive Overview. Cells 2021, 10, 824. https://doi.org/10.3390/cells10040824
Banne E, Abudiab B, Abu-Swai S, Repudi SR, Steinberg DJ, Shatleh D, Alshammery S, Lisowski L, Gold W, Carlen PL, et al. Neurological Disorders Associated with WWOX Germline Mutations—A Comprehensive Overview. Cells. 2021; 10(4):824. https://doi.org/10.3390/cells10040824
Chicago/Turabian StyleBanne, Ehud, Baraa Abudiab, Sara Abu-Swai, Srinivasa Rao Repudi, Daniel J. Steinberg, Diala Shatleh, Sarah Alshammery, Leszek Lisowski, Wendy Gold, Peter L. Carlen, and et al. 2021. "Neurological Disorders Associated with WWOX Germline Mutations—A Comprehensive Overview" Cells 10, no. 4: 824. https://doi.org/10.3390/cells10040824
APA StyleBanne, E., Abudiab, B., Abu-Swai, S., Repudi, S. R., Steinberg, D. J., Shatleh, D., Alshammery, S., Lisowski, L., Gold, W., Carlen, P. L., & Aqeilan, R. I. (2021). Neurological Disorders Associated with WWOX Germline Mutations—A Comprehensive Overview. Cells, 10(4), 824. https://doi.org/10.3390/cells10040824







