1. Introduction
Drought and heat are the major abiotic stresses affecting wheat productivity in the world. The global wheat area exposed to drought doubled between 1979 and 2006 [
1] and the future availability of water to meet crop requirements will be a major challenge in arid and semi-arid regions [
2]. Global wheat production is expected to decrease by 4.1% to 6.4% for every 1 °C increase in temperature as warming is already slowing yield gains in the majority of wheat production areas [
3,
4].
These two abiotic stresses can occur at different wheat growth stages and affect several morphological, physiological, and molecular plant processes [
5,
6] resulting in significant yield losses [
7,
8,
9,
10]. These complex changes in addition to the high genotype by environment interaction limit the effectiveness of empirical selection for drought and heat tolerance [
11,
12], especially under the fluctuations of Mediterranean environments [
11]. Therefore, the development of drought and heat tolerant wheat cultivars requires an integrated breeding strategy that includes screening techniques for physiological and morphological adaptive traits in addition to the molecular tools to harness diverse genetic resources [
6,
13,
14,
15,
16].
For instance, canopy temperature (CT) was reported to be useful to select for water-stressed environments and high correlation with grain yield (GY) was found for CT measured at anthesis halfway and grain filling stages [
17,
18]. CT is an integrative measurement that is correlated with other physiological mechanisms. Cooler CT was associated with root spreading in bread wheat [
19] and the its association with genetic gains in hot conditions was established [
20,
21]. CT is also influenced by the stomatal control of transpiration as stomatal closure under reduced water status results in an increase of CT [
22]. The negative correlation between CT and grain yield, plant height, and heading time suggests that a cooler canopy provides yield benefits at both drought and heat stresses. These benefits are more pronounced under heat stress [
23].
Drought and heat stresses affect the photosynthetic activity by reducing the chlorophyll content [
24,
25] and damaging the photosystem II reaction sites [
26,
27]. Chlorophyll content measurement provides information on the health status of the leaf [
28] while chlorophyll fluorescence informs about the effectiveness in using the energy absorbed by the chlorophyll to diagnose the onset of photo-inhibition [
29]. Both traits were reported to be valuable for wheat breeding. Chlorophyll fluorescence was found to be useful for evaluating yield performance under rainfed Mediterranean conditions when measured during grain filling period [
7,
30]. The maximum quantum yield of PSII (Fv/Fm) represents the most meaningful chlorophyll fluorescence reading [
27], as it measures the efficiency of light harvesting and the conversion to chemical energy [
31]. Fv/Fm was identified as a suitable trait to screen for tolerance to high temperatures [
31] and several quantitative trait loci (QTLs) were identified for fluorescence parameters under heat stress [
32]. Chlorophyll content showed a correlation with grain yield and thousand kernel weight under heat and drought stresses [
33,
34] and it was found negatively correlated with the heat susceptibility index [
35].
Broadening the genetic base for these physiological adaptive traits requires access to novel diversity, which can be supplied by crop wild relatives [
36]. Screening of wheat wild relatives identified several donors for useful traits in wheat breeding [
37].
Triticum monococcum subsp.
aegilopides showed more potential to improve relative water content for drought tolerance than tetraploid and hexaploid wheat species [
5].
Aegilops geniculata and
Triticum dicoccoides were identified as sources for chlorophyll and photosynthesis-related traits to improve drought and heat tolerance [
38,
39]. Screening under field conditions identified a higher level of heat tolerance during a vegetative stage in
Aegilops speltoides and
Aegilops tauschii [
40]. Nachit and Elouafi [
41] reported several durum wheat lines generated through interspecific hybridization combining adaptation to dry conditions with high yield potential. In addition to physiological and morphological mechanisms involved in drought tolerance, several indices were proposed to screen for drought adaptation. Fischer and Maurer [
42] suggested the drought susceptibility index (DSI), Rosielle, and Hamblin [
43] developed the mean productivity (MP) and tolerance (TOL) while the drought tolerance index (DTI) was suggested by Fernandez [
44]. Farshadfar and Sutka [
45] introduced a correction coefficient to DTI and proposed a modified stress tolerance index (MsSTI). All these indices consider the performance of the genotypes under optimal and stressed conditions to separate susceptible genotypes from tolerant genotypes.
These indices were used extensively to screen and select genotypes for drought tolerance [
46,
47,
48,
49]. Tolerance and susceptibility indices can select genotypes with specific adaptation to drought stressed or favorable environments, and also genotypes combining high yield under both types of environments. There is some variation in the ability of these indices to select effectively suitable genotypes for dry environments, which suggests that their combination can be useful to achieve higher selection efficiency [
50]. The objectives of this study are: (i) assess the heritability of some physiological traits and their association with grain yield under drought and heat stresses, (ii) examine the ability of tolerance indices and their combination to select the best genotypes for drought stress, and (iii) select pre-breeding lines from a durum wheat population derived from interspecific crosses with high yield potential and good levels of drought and tolerance.
4. Discussion
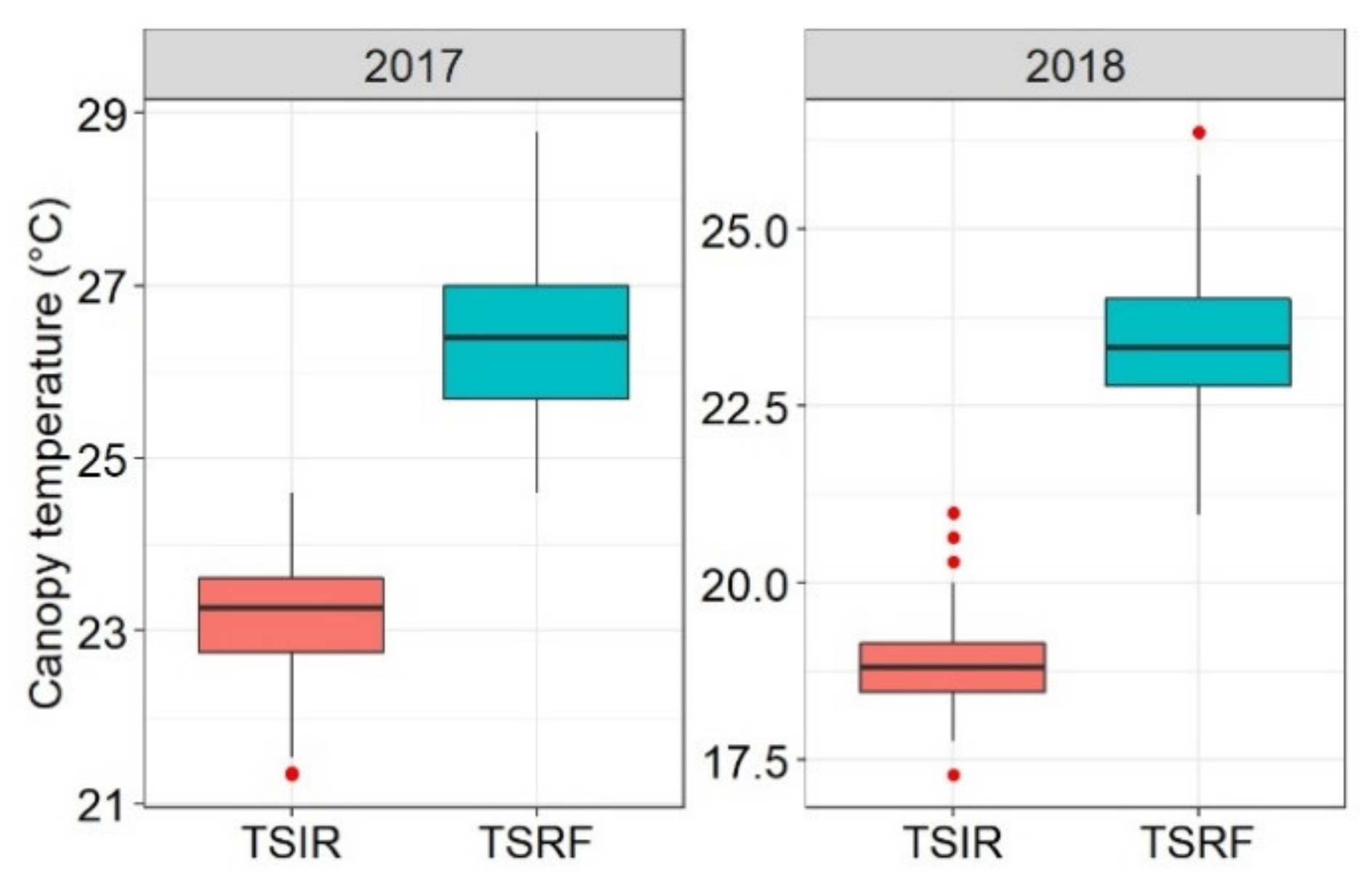
At Tessaout, the drought penalty on the yield varied with the season rainfall and was more severe in the first season, reflecting the unpredictable climate in which wheat is exposed to in the Mediterranean region. Under these conditions, varieties, which are drought tolerant and having high yield potential under more favorable conditions, are needed to ensure higher and more stable yields. The maximum temperature at WMD was consistently above 30 °C throughout the crop cycle, confirming the choice of this site as a research platform for heat tolerance breeding.
The results showed the potential to exploit the genotypic variation for CT to select for drought and heat tolerance. CT was more useful under heat than drought stress, as it is significantly correlated with both GY and BY and it had moderate heritability. The CT correlations with other traits under drought were not significant. However, it can be considered a secondary physiological trait to increase the probability of additive gene action for drought adaptation, as suggested by Reynolds et al. [
64]. Previous studies have reported an advantage of the CT measurement during grain filling for both genetic variations and correlations to GY under drought [
17,
47]. In fact, grain filling is the most vulnerable stage to drought stress [
65], which can allow to identify tolerant from susceptible genotypes.
Taking into consideration the balance between high genotypic variation and strong association to GY and BY, the present results suggest that early heading stage is suitable to use CT for heat tolerance screening. CTv can also be recommended because of its high association to GY and BY, which could be explained by the fact that, under continuous heat stress, heat damage increases through the cycle and genotypes that can avoid early thermal shock will accumulate less damage in later stages. Lines with higher biomass had cooler CT during all stages, which is important, as BY explained the highest variation in GY at WMD [
10]. Cooler CT and higher BY are associated with deeper root systems and root spreading, contributing to avoidance of heat stress as reported by previous studies [
19,
20,
66]. The importance of CT in heat tolerance was confirmed by many studies, which also identified several marker-trait associations and QTLs for canopy temperature under heat stress [
18,
67,
68,
69]. Some of these QTLs had pleiotropic effects on grain yield and other related traits.
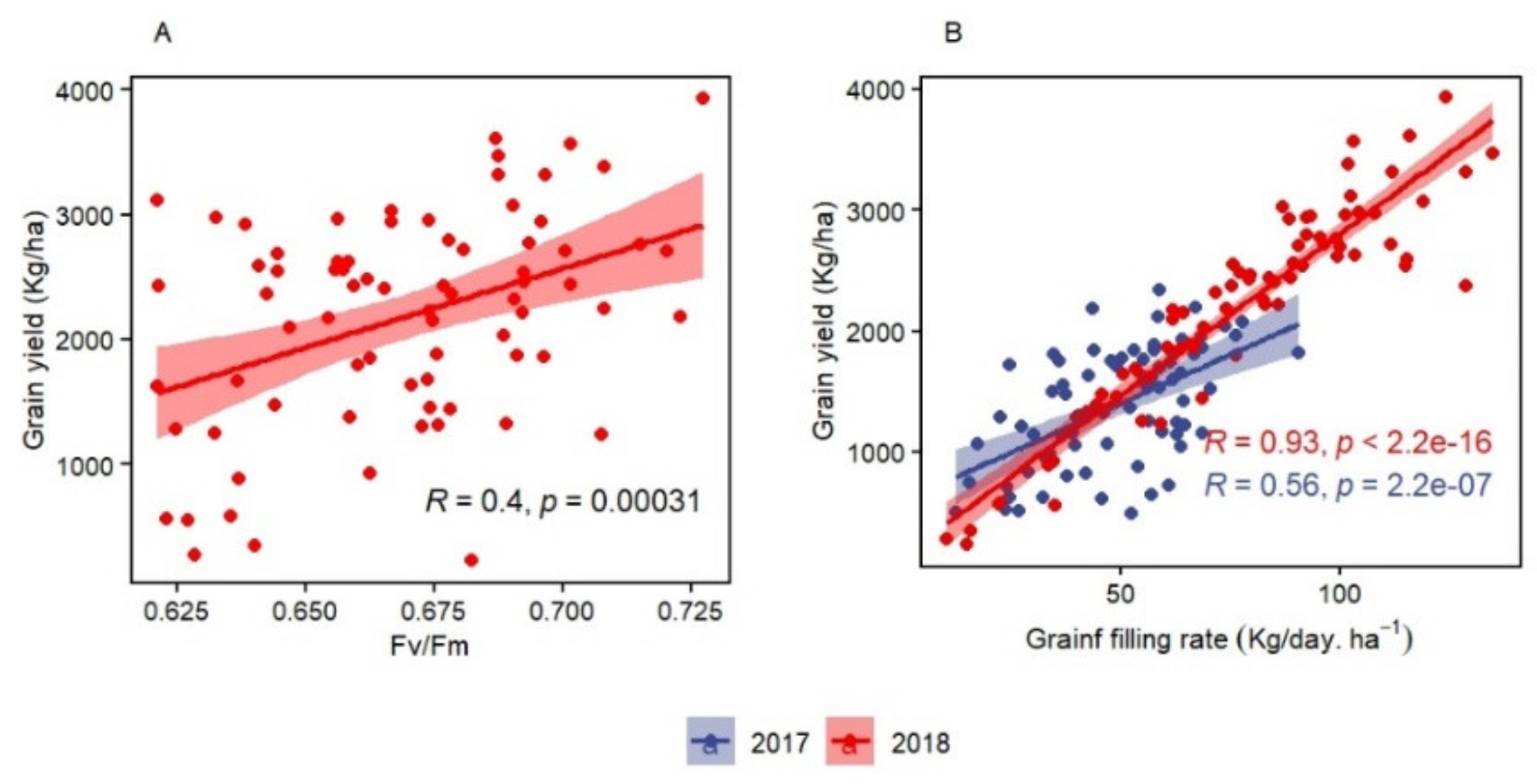
For chlorophyll fluorescence parameters, the correlation between Fv/Fm and GY under rainfed conditions at Tessaout and at WMD was significant, which suggests Fv/Fm as an effective selection criteria for both drought and heat tolerance. However, low and inconsistent heritability could be the major limitation for Fv/Fm to select in early generations. Low [
30] to moderate [
70] heritabilities were reported for Fv/Fm under drought stress with significant correlation to GY. However, high heritability was found at anthesis for terminal heat stress [
32] and under controlled (heat stressed) conditions at seedling stage [
71]. Drought was reported to damage the PSII and decrease Fv/Fm [
26,
72,
73]. In the present study, the limited genetic variation for Fv/Fm did not allow us to compare TSIR and TSRF during the same season in order to assess this damage. Chlorophyll fluorescence showed more potential to be used for heat than drought screening as the correlation with GY was higher at WMD. In addition, the Fv/Fm range at WMD was lower than what is reported for healthy plants [
74], confirming the effect of continuous heat stress on Fv/Fm [
35].
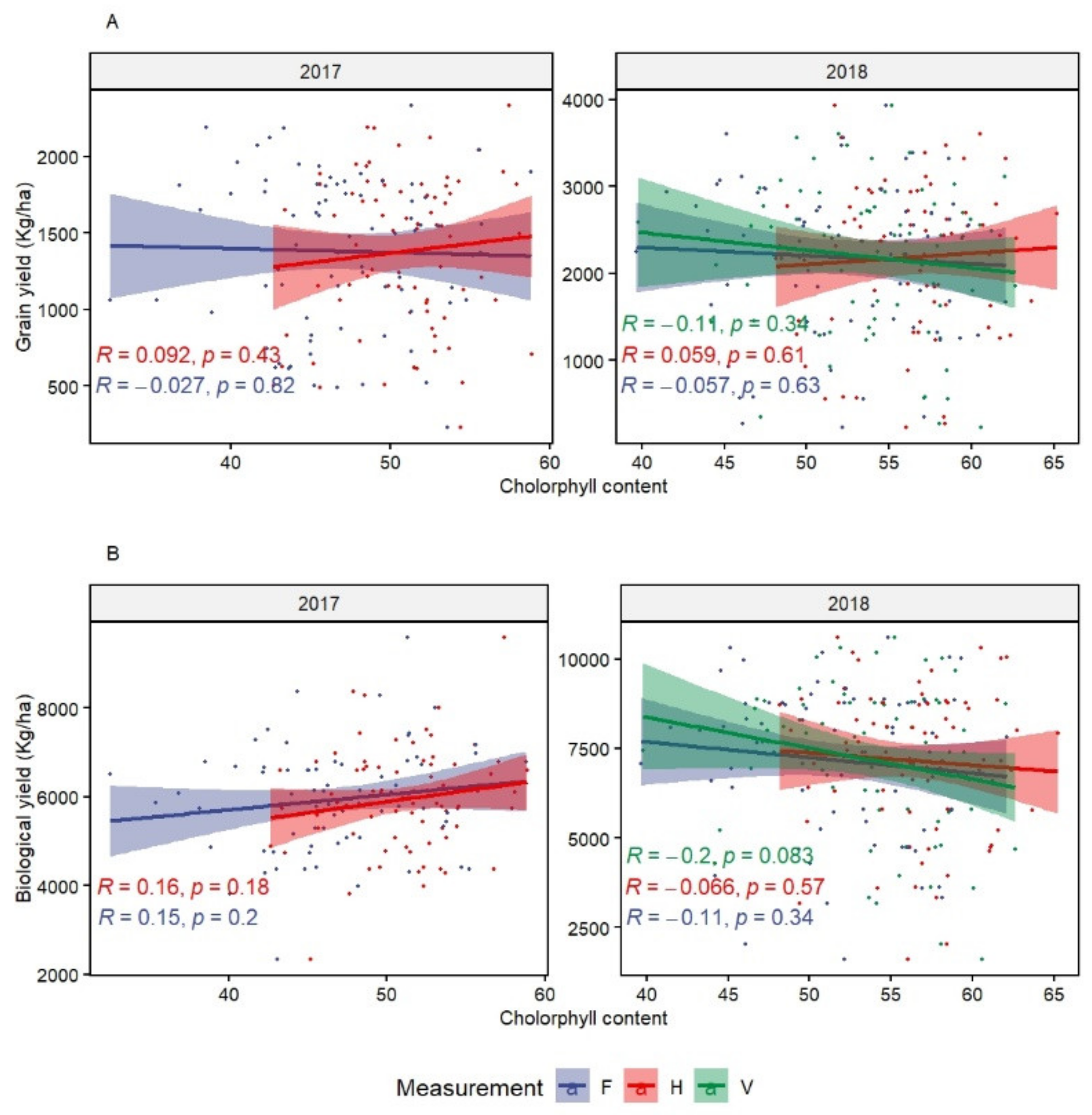
CHL showed high genetic variation at the heading stage. However, it did not correlate significantly with GY and the other traits. This could mean that chlorophyll content is not a determinant of the photosynthetic rate. In fact, Akter and Rafiqul Islam [
75] reported that several processes are involved in the determination of photosynthetic rate under heat stress. Despite the confirmed heat effect in reducing CHL [
34,
76,
77], selection using CHL under chronic heat stress should be used with caution. Its association with stay green [
24,
78], which could expose the plants to more stress during reproductive stage, leading to yield penalty.
The measurement of NDVI during the vegetative stage showed the possibility to identify and select heat tolerant genotypes. Measurements performed at later stages (NDVIh and NDVIf) did not present any advantage and NDVIf was the least effective in terms of correlation with all other major traits. The significant correlation of NDVIv with CTv and BY suggests the possibility to select lines integrating multiple tolerance mechanisms in early growth stages. Similar findings were reported for NDVI at the vegetative stage by Pinto et al. [
67] who identified several QTLs on A and B genomes. This supports the choice of using durum wheat direct progenitors (donors of A and B genome) from the primary gene pool to improve abiotic stress tolerance with polygenic inheritance, as suggested by Valkoun [
51]. Pradhan et al. [
18] also found a positive association between NDVI at heading and grain yield. They also identified several marker-trait associations (MTAs) under heat stress. It is, however, important to consider germplasm with similar phenology when collecting NDVI and avoid very late and winter/facultative genotypes in the analysis.
GFR can be recommended as efficient screening criteria for heat tolerance. It had the highest correlation to GY during both seasons. GFR can be easily computed for large breeding populations with accurate recording of anthesis and maturity time. The correlation of GFR and BY could be associated with the importance of assimilates availability to improve efficient translocation toward the grains. The usefulness of GFR is also derived from its ability to determine the final grain weight, which is a major yield component with high genetic plasticity [
79,
80,
81]. Knowing that BY and thousand kernel weight explained together more than 40% of GY variation under the same conditions of the present study at WMD [
10], GFR can increase the selection efficiency under chronic heat stress. A high filling rate can also compensate for the reduction of spike fertility and filling period, which are, in turn, reduced by heat stress [
8,
77,
82]. In addition, the contribution of GFR to grain yield was reported to be more important than filling duration under terminal heat stress [
83].
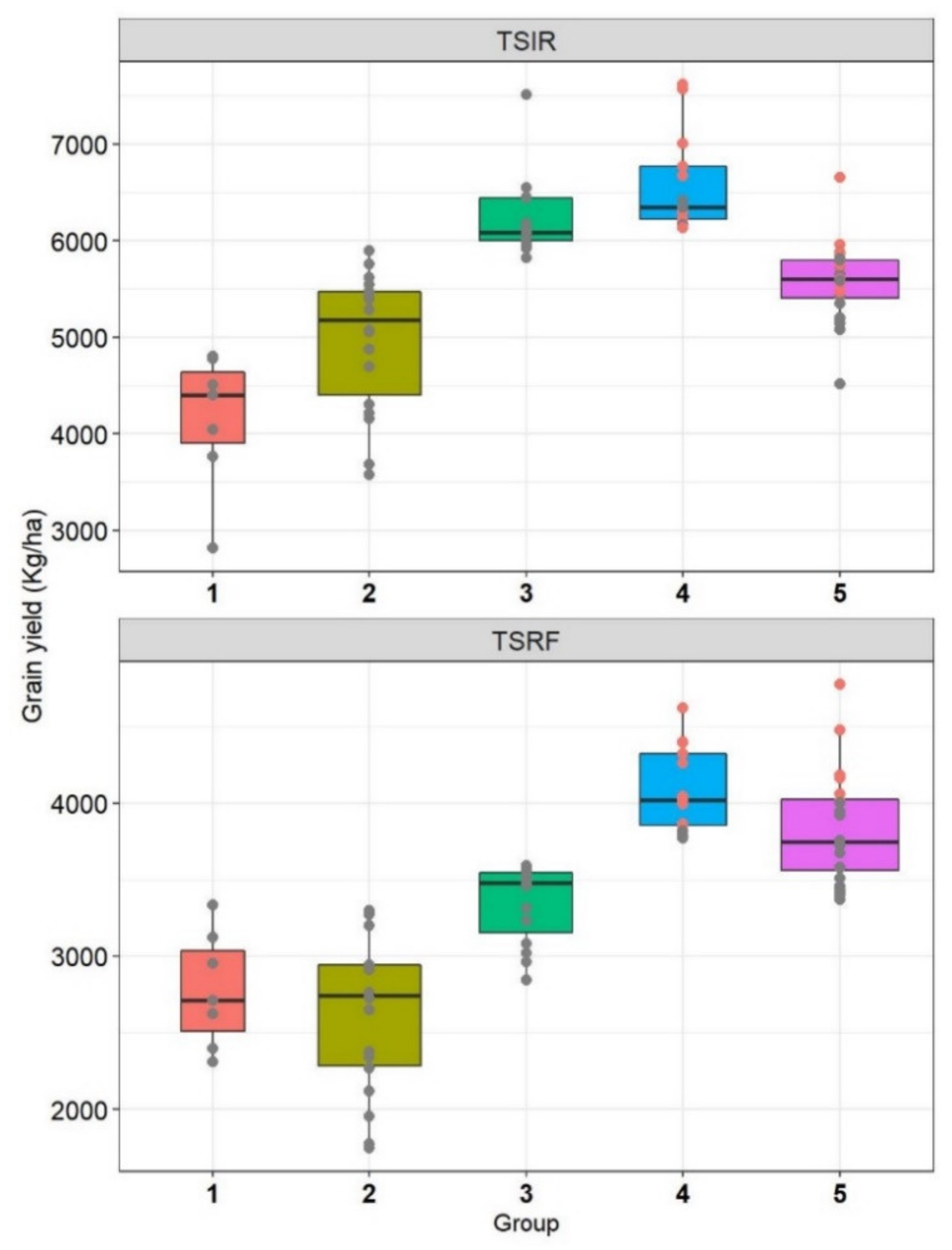
The drought tolerance and susceptibility indices showed different accuracies to select drought-adapted genotypes based on the groups identified by Fernandez [
44]. The susceptibility indices (TOL and DSI) were less effective than tolerance indices (DTI, MP, and MsSTI) to select suitable lines for drought stress. According to Mohammadi et al. [
46], MP and DTI can be used alternatively as they were correlated with each other and, therefore, selected the same genotypes. Using the scores, the present results showed that DTI is more efficient under drought stress while MP included some lines with moderate performance under drought stress. Selection based on MP is privileged in regions where drought stress occurs at equal frequencies as favorable conditions [
43], which is the case in the Mediterranean region. The level and duration of drought stress could be responsible for various results of correlation among indices. This explains the identification of different best suitable indices in multiple studies. For example, Farshadfar et al. [
84] suggested that DTI and TOL had the same selection ability while Gholinezhad et al. [
49] identified MsSTI, MP, and DTI as the most efficient screening indices under moderate and severe drought stress. In another study by Farshadfar et al. [
85], TOL and DTI were clustered in different groups and MsSTI was identified as an efficient selection index. These differences show that the ideal index to use could be environment-specific depending on the stage, duration, and severity of drought stress. Therefore, combining different indices provides breeders with the ability to increase the selection efficiency depending on the targeted environment. Thiry et al. [
50] computed MSI based on the scores of five indices (DSI, TOL, MP, GMP, and STI), which resulted in a determination coefficient of 0.98 with GY under heat stress. In the present study, MSI was computed including only the effective indices at Tessaout (MP, DTI, DSI, and MsSTI). The efficiency of MSI is shown in the coefficient of determination with GY at TSRF (0.92). MSI selected efficiently the genotypes with a high yield under both optimal and stressed conditions. Therefore, the use of scores and adapted MSI can be recommended as a flexible tool to use the drought tolerance/susceptibility indices.
The results also showed the potential of crop wild relatives to increase genetic variation for the physiological traits involved in heat and drought tolerance. For instance, the line 142001 (Haurani*2/
T. urartu) can be a source for cooler CT during the vegetative stage and higher CHL under heat. This line was previously recommended for its earliness at WMD [
10], which means that it can combine both escaping and avoidance mechanisms to continuous heat stress. Under drought stress, several lines derived from
T. urartu,
T. aegilopides, and
T. dicoccoides were characterized by cooler CT. An improvement in yield under terminal drought stress was also reported with lines derived from crosses with
T. dicoccoides [
41]. By selecting wild parents originating from heat and drought-prone environments, the expected derived lines can lead to drought and heat tolerant germplasm. Therefore, for further selection of wild parental germplasm, the aridity index can be an important factor. Peleg et al. [
86] also reported the potential of
Triticum dicoccoides collected from hot and/or dry environments to improve drought tolerance in durum wheat. This supports use of wild relatives, mainly from the primary gene pool, for drought and heat tolerance breeding.