Novel Multifunctional Ascorbic Triazole Derivatives for Amyloidogenic Pathway Inhibition, Anti-Inflammation, and Neuroprotection
Abstract
:1. Introduction
2. Results and Discussion
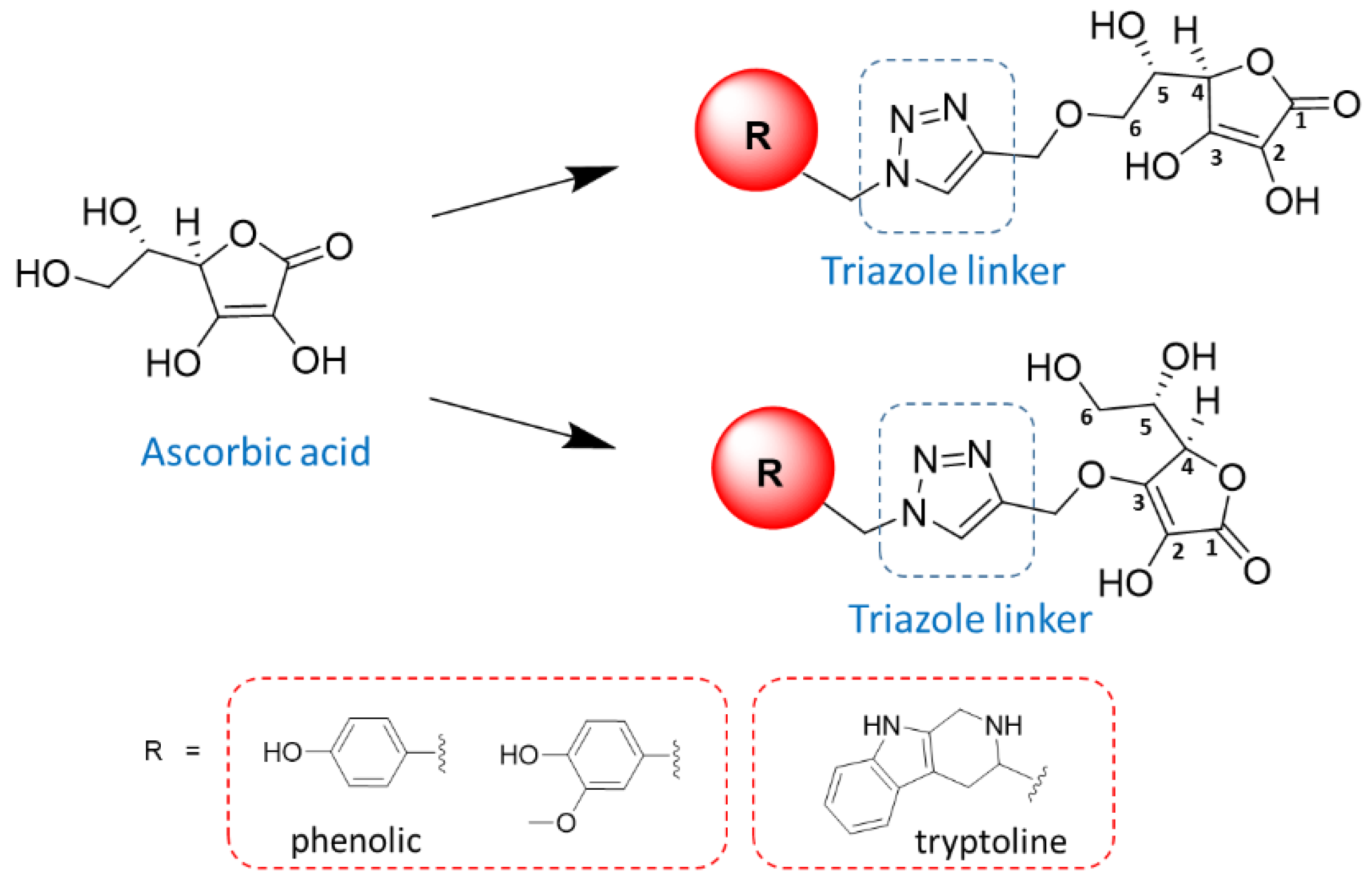
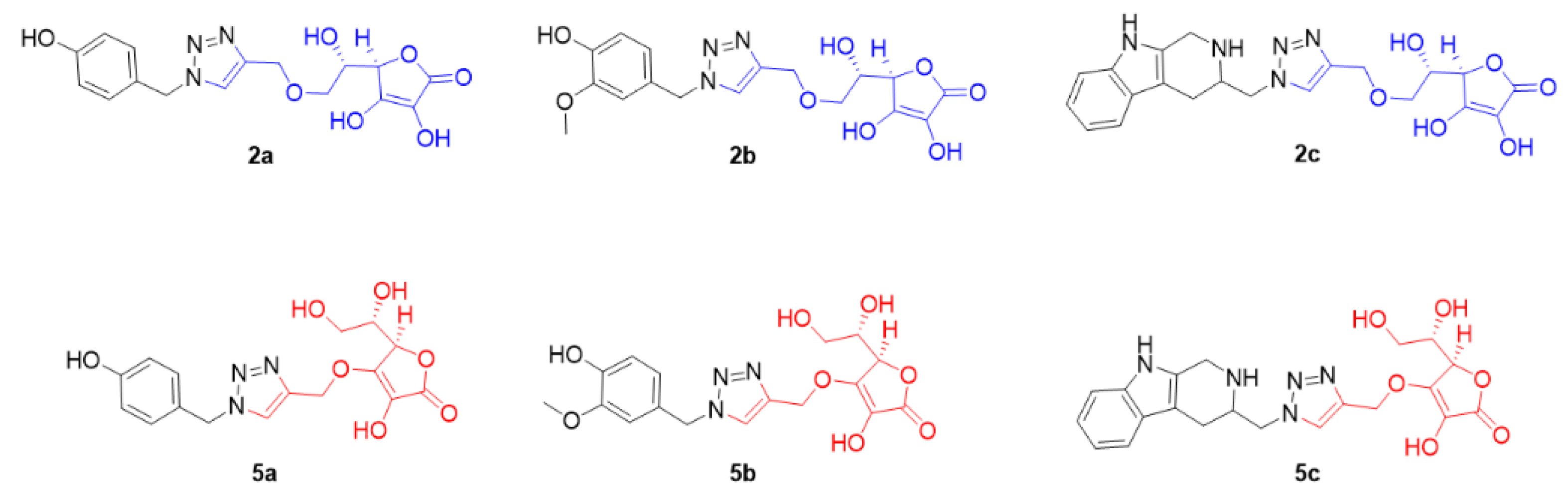
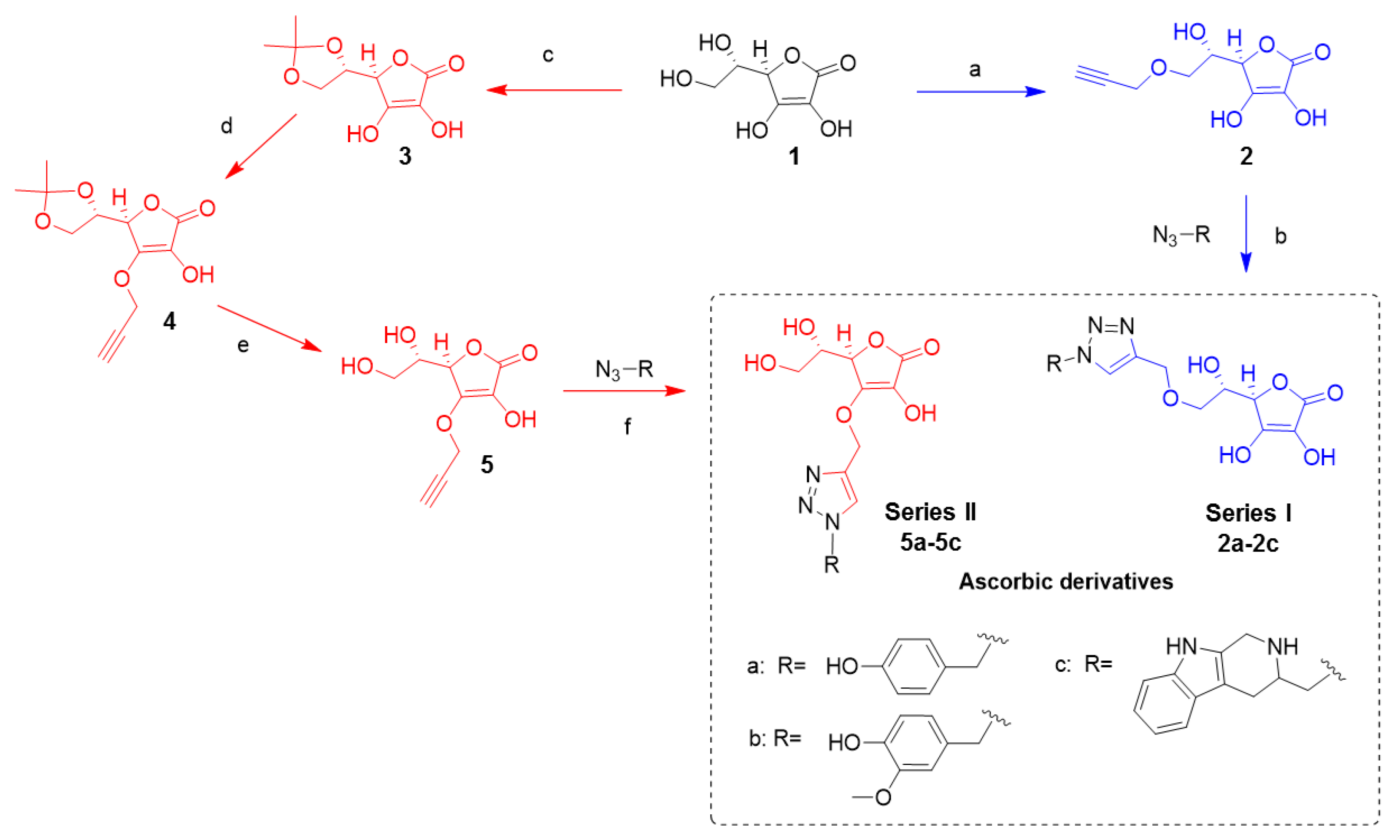
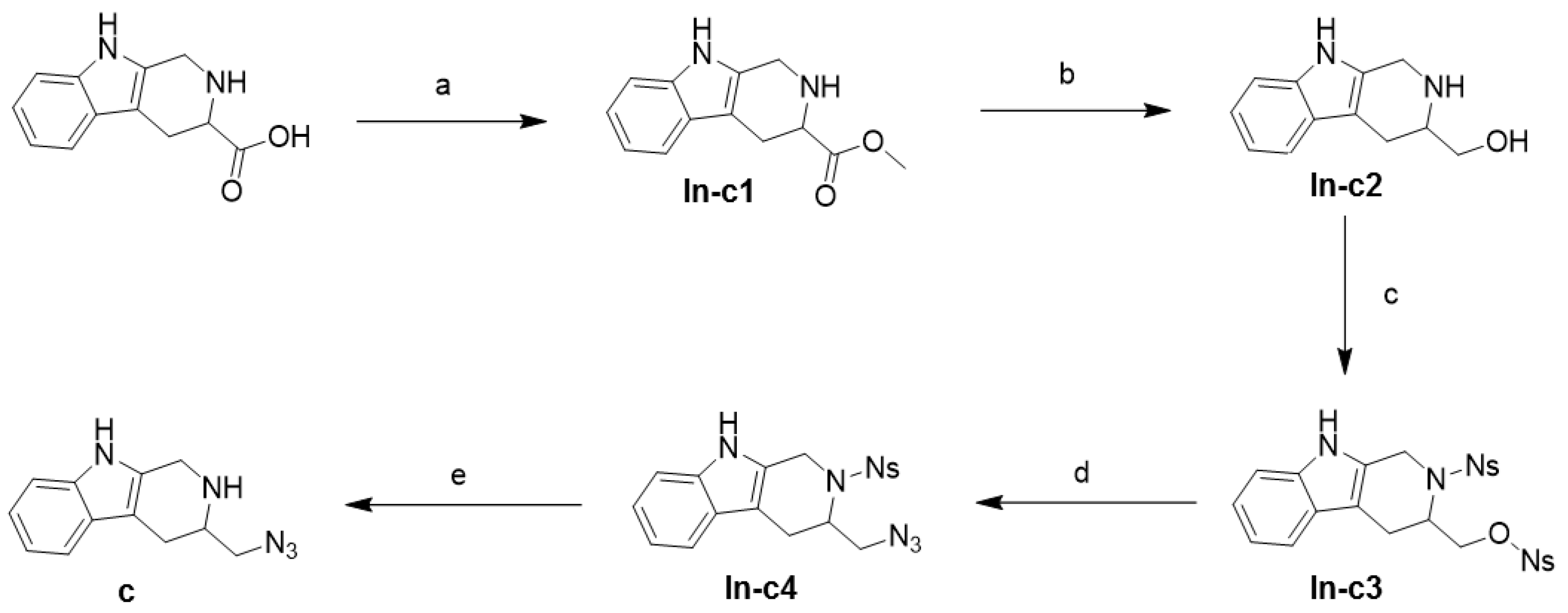
2.1. Synthesis
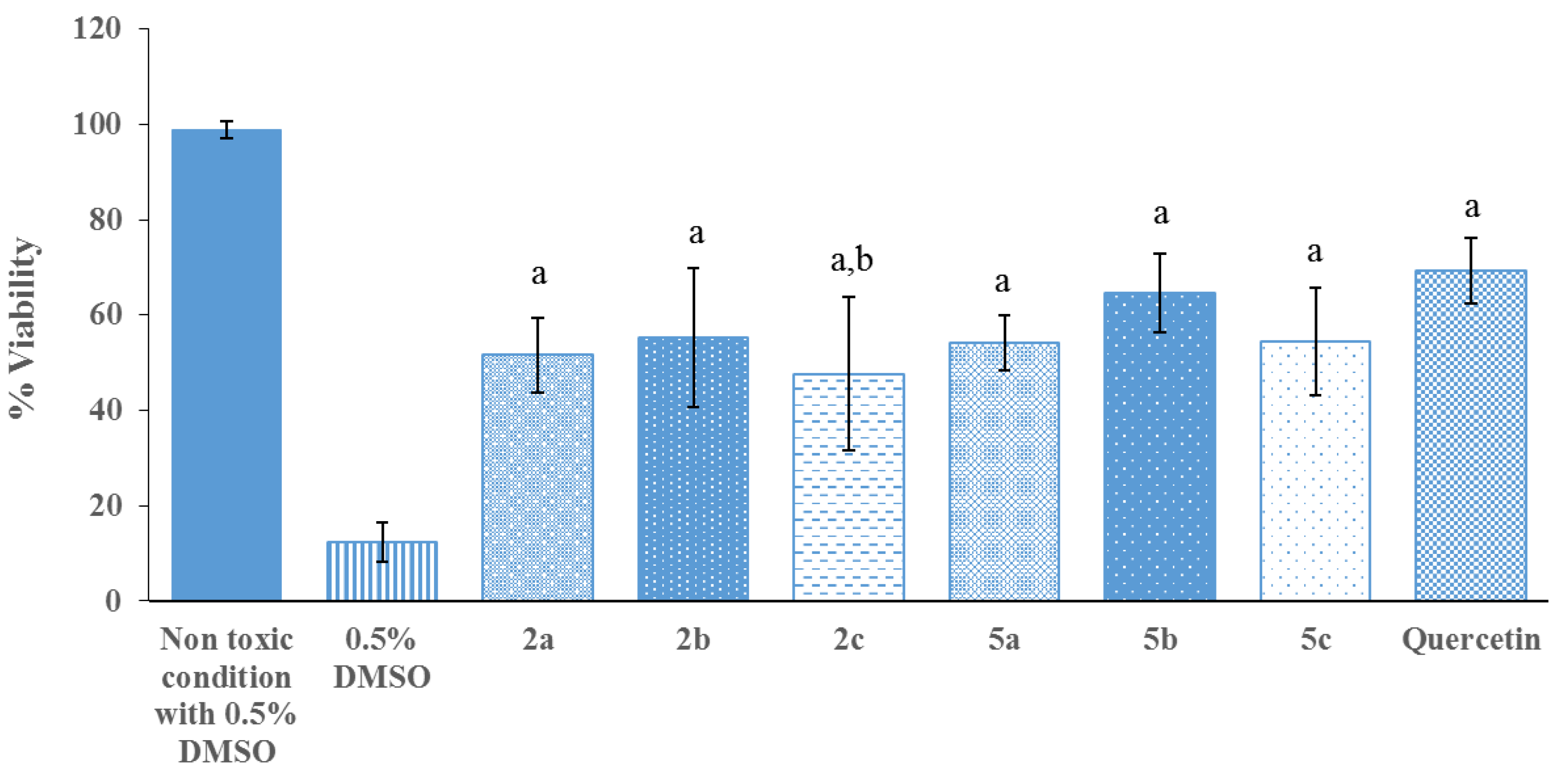
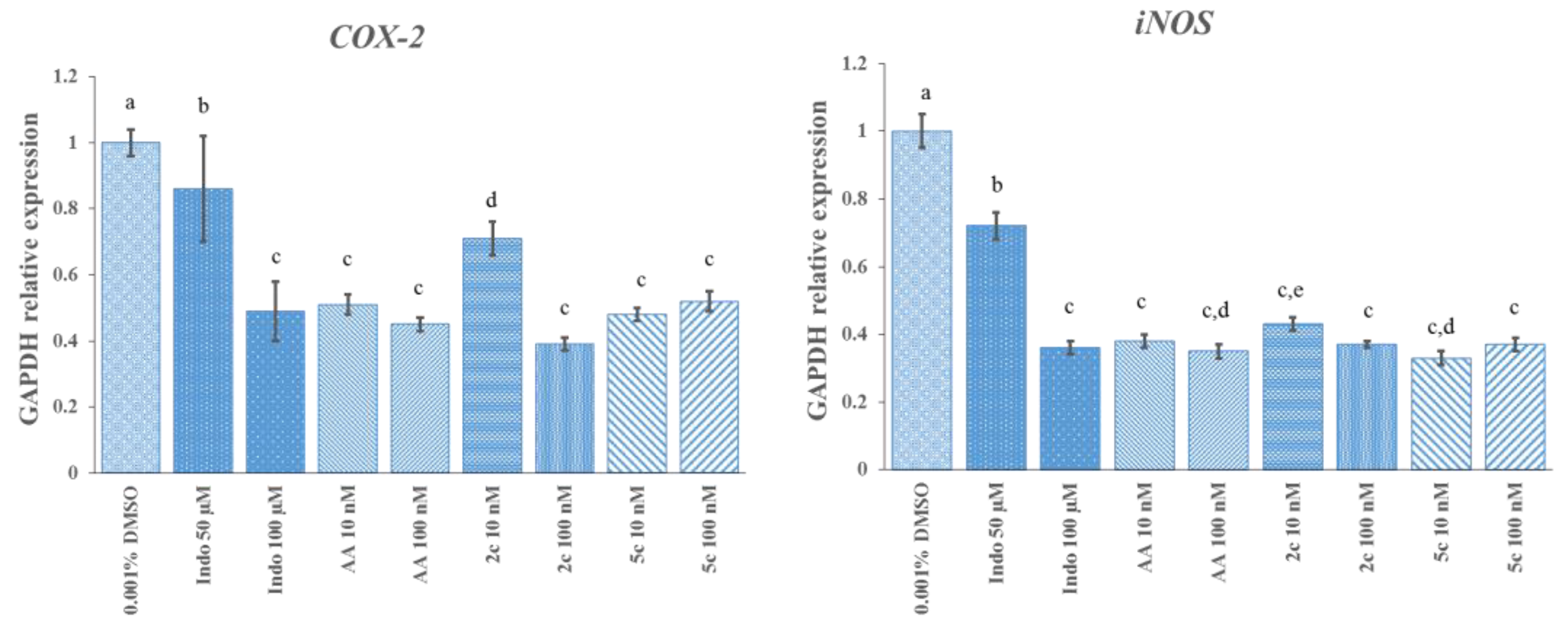
2.2. Biological Activity Assays
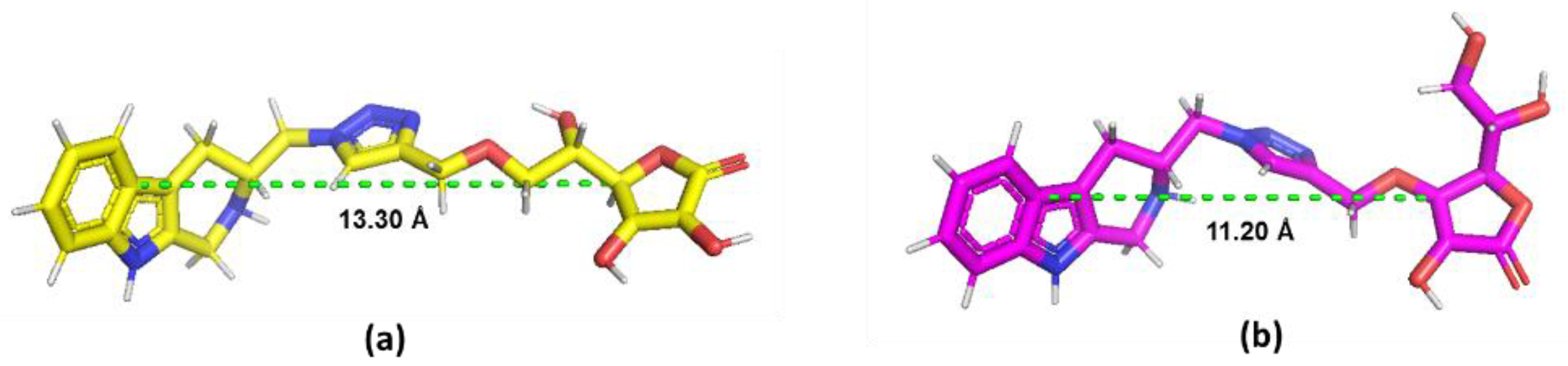
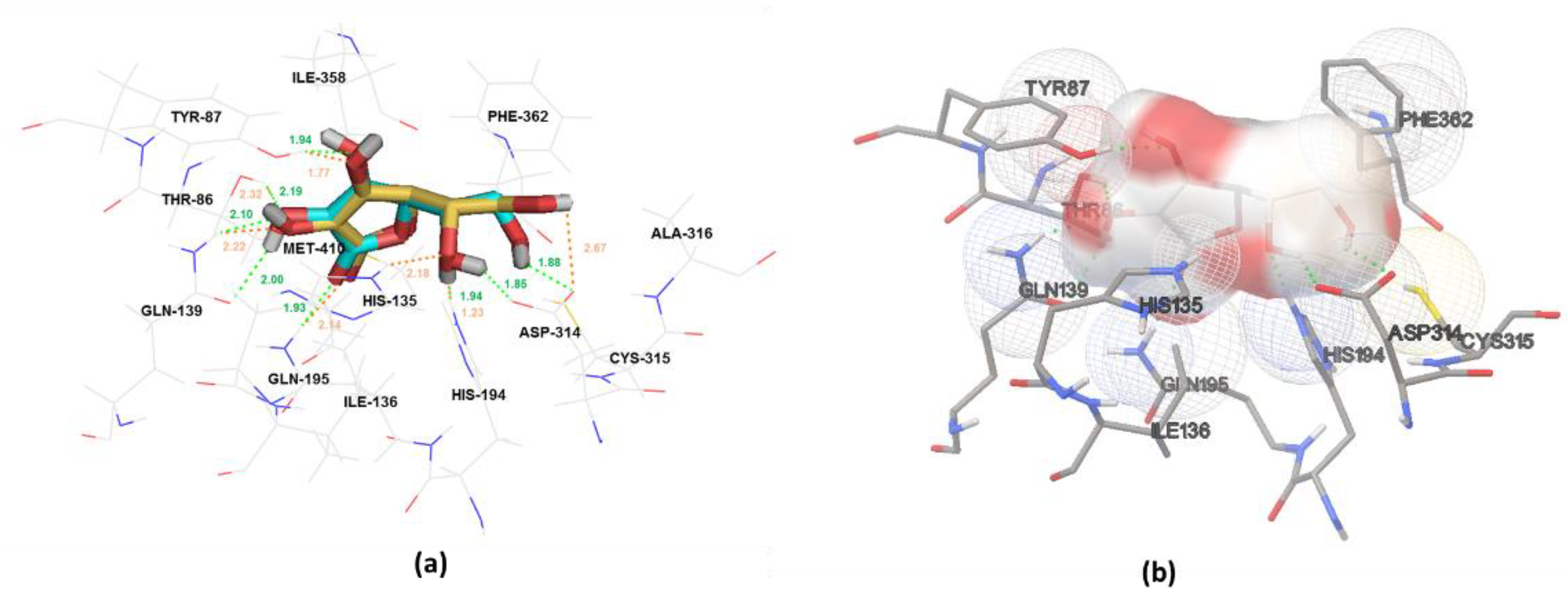
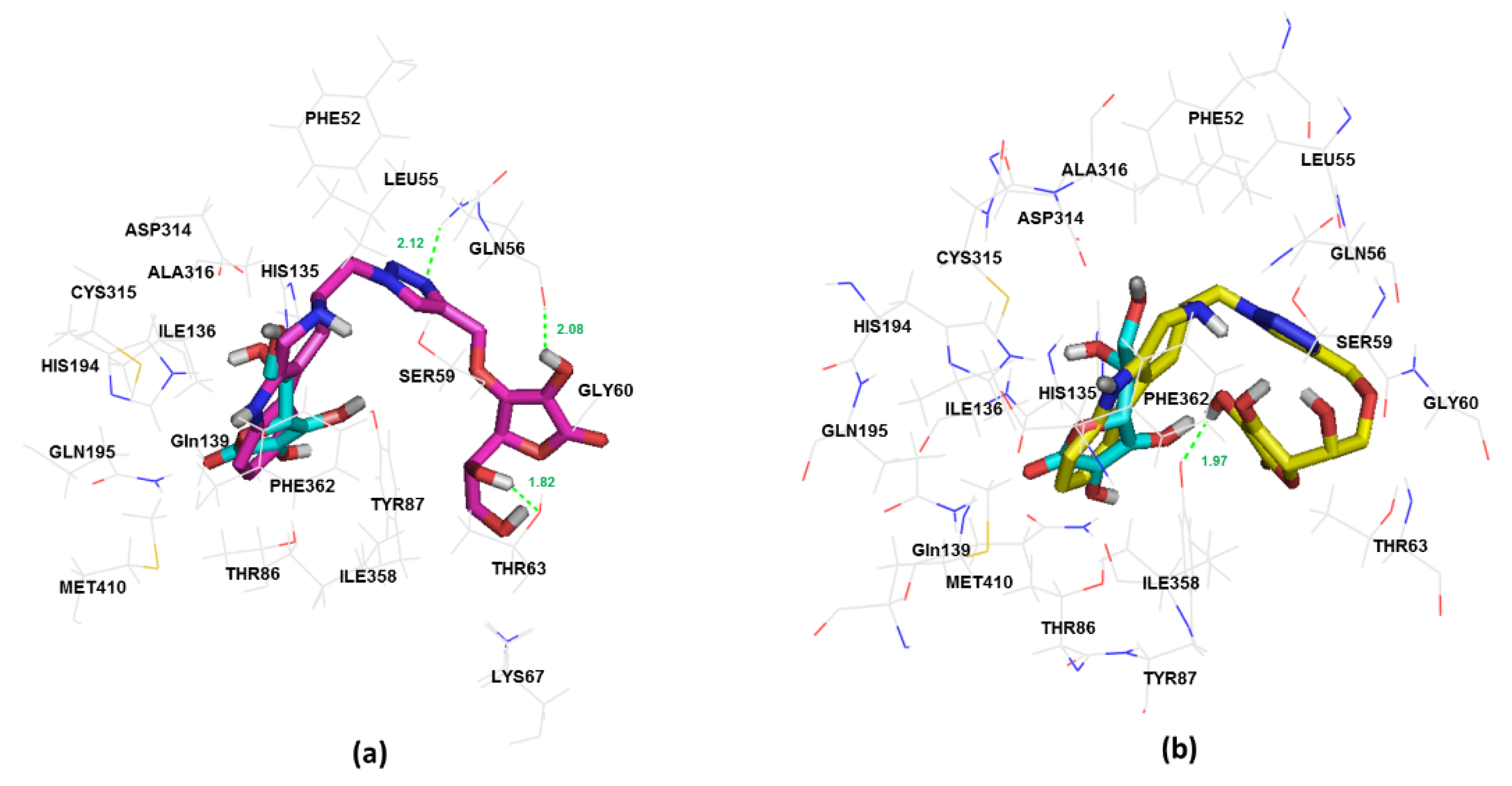
2.3. In Silico Study of Sodium-Dependent Vitamin C Transporter2 (SVCT2) Binding
3. Conclusions
4. Materials and Methods
4.1. General
4.2. Synthesis
4.2.1. (R)-3,4-Dihydroxy-5-((S)-1-hydroxy-2-(prop-2-yn-1-yloxy)ethyl)furan-2(5H)-one; 2
4.2.2. (R)-5-((S)-2,2-Dimethyl-1,3-dioxolan-4-yl)-3,4-dihydroxyfuran-2(5H)-one; 3
4.2.3. (R)-5-((S)-2,2-Dimethyl-1,3-dioxolan-4-yl)-3-hydroxy-4-(prop-2-yn-1-yloxy)furan-2(5H)-one; 4
4.2.4. (R)-5-((S)-1,2-Dihydroxyethyl)-3-hydroxy-4-(prop-2-ynyloxy)furan-2(5H)-one; 5
4.2.5. 4-(Azidomethyl)phenol; a
4.2.6. 4-(Azidomethyl)-2-methoxyphenol; b
4.2.7. 4-(Azidomethyl)-2-methoxyphenol; c
4.2.8. Methyl 2,3,4,9-tetrahydro-1H-pyrido [3,4-b]indole-3-carboxylate; In-c1
4.2.9. (2,3,4,9-Tetrahydro-1H-pyrido [3,4-b]indol-3-yl)methanol; In-c2
4.2.10. (S)-3-(Azidomethyl)-2-(4-nitrophenylsulfonyl)-2,3,4,9-tetrahydro-1H –pyrido[3,4-b]indole; In-c4
4.2.11. (S)-3-(Azidomethyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole; c
4.2.12. (R)-3,4-Dihydroxy-5-((S)-1-hydroxy-2-((1-(4-hydroxybenzyl)-1H-1,2,3-triazol-4-yl)metho-xy)ethyl)furan-2(5H)-one; 2a
4.2.13. (R)-3,4-Dihydroxy-5-((S)-1-hydroxy-2-((1-(4-hydroxy-3-methoxybenzyl)-1H-1,2,3-triazol-4-yl)methoxy)ethyl)furan-2(5H)-one; 2b
4.2.14. (R)-3,4-Dihydroxy-5-((S)-1-hydroxy-2-((1-((2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-3-yl)-methyl)-1H-1,2,3-triazol-4-yl)methoxy)ethyl)furan-2(5H)-one; 2c
4.2.15. (R)-5-((S)-1,2-Dihydroxyethyl)-3-hydroxy-4-((1-(4-hydroxybenzyl)-1H-1,2,3-triazol-4-yl)-methoxy)furan-2(5H)-one; 5a
4.2.16. (R)-5-((S)-1,2-Dihydroxyethyl)-3-hydroxy-4-((1-(4-hydroxy-3-methoxybenzyl)-1H-1,2,3-triazol-4-yl)methoxy)furan-2(5H)-one; 5b
4.2.17. (R)-5-((S)-1,2-Dihydroxyethyl)-3-hydroxy-4-((1-((2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-3-yl)methyl)-1H-1,2,3-triazol-4-yl)methoxy)furan-2(5H)-one; 5c
4.3. Biological Activity Assays
4.3.1. β-Secretase (BACE1) Inhibition Assay
4.3.2. Amyloid Aggregation Inhibition Assay
4.3.3. Antioxidant Activity Assay
4.3.4. Neuroprotective Activity Assay
Cell Culture Preparation
Differentiation of P19 Cells into P19-Derived Neurons
Viability Assay
Neuroprotective Assay
4.3.5. Anti-Inflammatory Activity Assay
Cell Culture Preparation
Determination of Cell Viability
Determination of mRNA Expression Levels of COX-2 and iNOS Genes
4.3.6. In Silico Study of Sodium-Dependent Vitamin C Transporter 2 (SVCT2) Binding
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Avalability
References
- Dementia Statistics. Alzheimer’s Disease International. Available online: https://www.alzint.org/about/dementia-facts-figures/dementia-statistics (accessed on 4 February 2021).
- Mattson, M.P. Pathways towards and away from Alzheimer’s disease. Nature 2004, 430, 631–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yatin, S.M.; Yatin, M.; Aulick, T.; Ain, K.B.; Butterfield, D.A. Alzheimer’s amyloid beta-peptide associated free radicals increase rat embryonic neuronal polyamine uptake and ornithine decarboxylase activity: Protective effect of vitamin E. Neurosci. Lett. 1999, 263, 17–20. [Google Scholar] [CrossRef]
- Heneka, M.T.; Golenbock, D.T.; Latz, E. Innate immunity in Alzheimer’s disease. Nat. Immunol. 2015, 16, 229–236. [Google Scholar] [CrossRef]
- Chen, G.-F.; Xu, T.-H.; Yan, Y.; Zhou, Y.-R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef] [PubMed]
- Pasinetti, G.M.; Aisen, P.S. Cyclooxygenase-2 expression is increased in frontal cortex of Alzheimer’s disease brain. Neuroscience 1998, 87, 319–324. [Google Scholar] [CrossRef]
- Nathan, C.; Calingasan, N.; Nezezon, J.; Ding, A.; Lucia, M.S.; La Perle, K.; Fuortes, M.; Lin, M.; Ehrt, S.; Kwon, N.S.; et al. Protection from Alzheimer’s-like disease in the mouse by genetic ablation of inducible nitric oxide synthase. J. Exp. Med. 2005, 202, 1163–1169. [Google Scholar] [CrossRef] [Green Version]
- Van Marum, R.J. Current and future therapy in Alzheimer’s disease. Fundam. Clin. Pharmacol. 2008, 22, 265–274. [Google Scholar] [CrossRef]
- Gabr, M.T.; Ibrahim, M.M. Multitarget therapeutic strategies for Alzheimer’s disease. Neural Regen. Res. 2019, 14, 437–440. [Google Scholar] [CrossRef]
- Harrison, F.E.; May, J.M. Vitamin C function in the brain: Vital role of the ascorbate transporter SVCT2. Free Radic. Biol. Med. 2009, 46, 719–730. [Google Scholar] [CrossRef] [Green Version]
- Kook, S.-Y.; Lee, K.-M.; Kim, Y.; Cha, M.-Y.; Kang, S.; Baik, S.H.; Lee, H.; Park, R.; Mook-Jung, I. High-dose of vitamin C supplementation reduces amyloid plaque burden and ameliorates pathological changes in the brain of 5XFAD mice. Cell Death Dis. 2014, 5, e1083. [Google Scholar] [CrossRef] [Green Version]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The Pharmacokinetics of Vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kübler, W.; Gehler, J. Kinetics of intestinal absorption of ascorbic acid. Calculation of non-dosage-dependent absorption processes. Int. Z. Vitam. 1970, 40, 442–453. [Google Scholar]
- Mayersohn, M. Ascorbic acid absorption in man—Pharmacokinetic implications. Eur. J. Pharmacol. 1972, 19, 140–142. [Google Scholar] [CrossRef]
- Hasselholt, S.; Tveden-Nyborg, P.; Lykkesfeldt, J. Distribution of vitamin C is tissue specific with early saturation of the brain and adrenal glands following differential oral dose regimens in guinea pigs. Br. J. Nutr. 2015, 113, 1539–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, M.; Rumsey, S.C.; Daruwala, R.; Park, J.B.; Wang, Y. Criteria and Recommendations for Vitamin C Intake. JAMA 1999, 281, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.; Padayatty, S.J.; Espey, M.G. Vitamin C: A Concentration-Function Approach Yields Pharmacology and Therapeutic Discoveries. Adv. Nutr. 2011, 2, 78–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Lee, J.-H.; Krishna, M.C.; Shacter, E.; Choyke, P.L.; Pooput, C.; Kirk, K.L.; Buettner, G.R.; et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 8749–8754. [Google Scholar] [CrossRef] [Green Version]
- Macan, A.M.; Kraljević, T.G.; Raić-Malić, S. Therapeutic Perspective of Vitamin C and Its Derivatives. Antioxidants 2019, 8, 247. [Google Scholar] [CrossRef] [Green Version]
- Timoshnikov, V.A.; Kobzeva, T.V.; Polyakov, N.E.; Kontoghiorghes, G.J. Redox Interactions of Vitamin C and Iron: Inhibition of the Pro-Oxidant Activity by Deferiprone. Int. J. Mol. Sci. 2020, 21, 3967. [Google Scholar] [CrossRef]
- Teponnou, G.A.K.; Joubert, J.; Malan, S.F. Tacrine, Trolox and Tryptoline as Lead Compounds for the Design and Synthesis of Multi-target Agents for Alzheimer’s Disease Therapy. Open Med. Chem. J. 2017, 11, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Herraiz, T.; González, D.; Ancín-Azpilicueta, C.; Arán, V.J.; Guillén, H. β-Carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO). Food Chem. Toxicol. 2010, 48, 839–845. [Google Scholar] [CrossRef]
- Frost, D.; Meechoovet, B.; Wang, T.; Gately, S.; Giorgetti, M.; Shcherbakova, I.; Dunckley, T. β-carboline compounds, including harmine, inhibit DYRK1A and tau phosphorylation at multiple Alzheimer’s disease-related sites. PLoS ONE 2011, 6, e19264. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Wang, S.Y. Antioxidant Activity and Phenolic Compounds in Selected Herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Reinke, A.A.; Gestwicki, J.E. Structure-Activity Relationships of Amyloid Beta-Aggregation Inhibitors Based on Curcumin: Influence of Linker Length and Flexibility. Chem. Biol. Drug Des. 2007, 70, 206–215. [Google Scholar] [CrossRef] [Green Version]
- Jiaranaikulwanitch, J.; Govitrapong, P.; Fokin, V.V.; Vajragupta, O. From BACE1 Inhibitor to Multifunctionality of Tryptoline and Tryptamine Triazole Derivatives for Alzheimer’s Disease. Molecules 2012, 17, 8312–8333. [Google Scholar] [CrossRef] [PubMed]
- Jones-Villeneuve, E.M.V.; McBurney, M.W.; Rogers, K.A.; Kalnins, V.I. Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. J. Cell Biol. 1982, 94, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Jones-Villeneuve, E.M.; Rudnicki, M.A.; Harris, J.F.; McBurney, M.W. Retinoic acid-induced neural differentiation of embryonal carcinoma cells. Mol. Cell. Biol. 1983, 3, 2271–2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacPherson, P.A.; McBurney, M.W. P19 Embryonal Carcinoma Cells: A Source of Cultured Neurons Amenable to Genetic Manipulation. Methods 1995, 7, 238–252. [Google Scholar] [CrossRef]
- Tadtong, S.; Meksuriyen, D.; Tanasupawat, S.; Isobe, M.; Suwanborirux, K. Geldanamycin derivatives and neuroprotective effect on cultured P19-derived neurons. Bioorg. Med. Chem. Lett. 2007, 17, 2939–2943. [Google Scholar] [CrossRef]
- Tangsaengvit, N.; Kitphati, W.; Tadtong, S.; Bunyapraphatsara, N.; Nukoolkarn, V. Neurite Outgrowth and Neuroprotective Effects of Quercetin from Caesalpinia mimosoides Lamk. on Cultured P19-Derived Neurons. Evid. Based Complement. Alternat. Med. 2013, 2013, 838051. [Google Scholar] [CrossRef] [Green Version]
- Tadtong, S.; Kanlayavattanakul, M.; Lourith, N. Neuritogenic and Neuroprotective Activities of Fruit Residues. Nat. Prod. Commun. 2013, 8, 1583–1586. [Google Scholar] [CrossRef] [Green Version]
- Panyatip, P.; Tadtong, S.; Sousa, E.; Puthongking, P. BACE1 Inhibitor, Neuroprotective, and Neuritogenic Activities of Melatonin Derivatives. Sci. Pharm. 2020, 88, 58. [Google Scholar] [CrossRef]
- Su, X.; Shen, Z.; Yang, Q.; Sui, F.; Pu, J.; Ma, J.; Ma, S.; Yao, D.; Ji, M.; Hou, P. Vitamin C kills thyroid cancer cells through ROS-dependent inhibition of MAPK/ERK and PI3K/AKT pathways via distinct mechanisms. Theranostics 2019, 9, 4461–4473. [Google Scholar] [CrossRef]
- Clément, M.-V.; Ramalingam, J.; Long, L.H.; Halliwell, B. The In Vitro Cytotoxicity of Ascorbate Depends on the Culture Medium Used to Perform the Assay and Involves Hydrogen Peroxide. Antioxid. Redox Signal. 2001, 3, 157–163. [Google Scholar] [CrossRef]
- Liu, P.-Y.; Jiang, N.; Zhang, J.; Wei, X.; Lin, H.-H.; Yu, X.-Q. The Oxidative Damage of Plasmid DNA by Ascorbic Acid Derivatives In Vitro: The First Research on the Relationship between the Structure of Ascorbic Acid and the Oxidative Damage of Plasmid DNA. Chem. Biodivers. 2006, 3, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.; Li, J. Comparison of Vitamin C and Its Derivative Antioxidant Activity: Evaluated by Using Density Functional Theory. ACS Omega 2020, 5, 25467–25475. [Google Scholar] [CrossRef] [PubMed]
- Cesarini, E.; Cerioni, L.; Canonico, B.; Di Sario, G.; Guidarelli, A.; Lattanzi, D.; Savelli, D.; Guescini, M.; Nasoni, M.G.; Bigini, N.; et al. Melatonin protects hippocampal HT22 cells from the effects of serum deprivation specifically targeting mitochondria. PLoS ONE 2018, 13, e0203001. [Google Scholar] [CrossRef]
- Lee, J.-S.; Lee, M.-O.; Moon, B.-H.; Shim, S.H.; Fornace, A.J.; Cha, H.-J. Senescent Growth Arrest in Mesenchymal Stem Cells Is Bypassed by Wip1-Mediated Downregulation of Intrinsic Stress Signaling Pathways. Stem Cells 2009, 27, 1963–1975. [Google Scholar] [CrossRef] [PubMed]
- Biteghe, F.N.; Davids, L.M. A combination of photodynamic therapy and chemotherapy displays a differential cytotoxic effect on human metastatic melanoma cells. J. Photochem. Photobiol. B Biol. 2017, 166, 18–27. [Google Scholar] [CrossRef]
- Sun, M.; Wang, M.; Chen, M.; Dagnaes-Hansen, F.; Le, D.Q.S.; Baatrup, A.; Horsman, M.R.; Kjems, J.; Bünger, C.E. A tissue-engineered therapeutic device inhibits tumor growth in vitro and in vivo. Acta Biomater. 2015, 18, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Sarkar, N.; Vahabzadeh, S. Sustained release of vitamin C from PCL coated TCP induces proliferation and differentiation of osteoblast cells and suppresses osteosarcoma cell growth. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110096. [Google Scholar] [CrossRef]
- Sommer, F.; Kobuch, K.; Brandl, F.; Wild, B.; Framme, C.; Weiser, B.; Tessmar, J.; Gabel, V.-P.; Blunk, T.; Goepferich, A. Ascorbic Acid Modulates Proliferation and Extracellular Matrix Accumulation of Hyalocytes. Tissue Eng. 2007, 13, 1281–1289. [Google Scholar] [CrossRef]
- Garciá-Martínez, O.; De Luna-Bertos, E.; Ramos-Torrecillas, J.; Ruiz, C.; Milia, E.; Lorenzo, M.L.; Jiménez, B.; Sánchez-Ortiz, A.; Rivas, A. Phenolic Compounds in Extra Virgin Olive Oil Stimulate Human Osteoblastic Cell Proliferation. PLoS ONE 2016, 11, e0150045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zadeh, K.M.; Luyt, A.S.; Zarif, L.; Augustine, R.; Hasan, A.; Messori, M.; Hassan, M.K.; Yalcin, H.C. Electrospun polylactic acid/date palm polyphenol extract nanofibres for tissue engineering applications. Emergent Mater. 2019, 2, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Fiorillo, M.; Tóth, F.; Sotgia, F.; Lisanti, M.P. Doxycycline, Azithromycin and Vitamin C (DAV): A potent combination therapy for targeting mitochondria and eradicating cancer stem cells (CSCs). Aging 2019, 11, 2202–2216. [Google Scholar] [CrossRef] [PubMed]
- Jiaranaikulwanitch, J.; Tadtong, S.; Govitrapong, P.; Fokin, V.V.; Vajragupta, O. Neuritogenic activity of bi-functional bis-tryptoline triazole. Bioorg. Med. Chem. 2017, 25, 1195–1201. [Google Scholar] [CrossRef]
- Trigo, D.; Goncalves, M.B.; Corcoran, J.P.T. The regulation of mitochondrial dynamics in neurite outgrowth by retinoic acid receptor β signaling. FASEB J. 2019, 33, 7225–7235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdollahzad, H.; Eghtesadi, S.; Nourmohammadi, I.; Khadem-Ansari, M.; Nejad-Gashti, H.; Esmaillzadeh, A. Effect of Vitamin C Supplementation on Oxidative Stress and Lipid Profiles in Hemodialysis Patients. Int. J. Vitam. Nutr. Res. 2009, 79, 281–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yimcharoen, M.; Kittikunnathum, S.; Suknikorn, C.; Nak-On, W.; Yeethong, P.; Anthony, T.G.; Bunpo, P. Effects of ascorbic acid supplementation on oxidative stress markers in healthy women following a single bout of exercise. J. Int. Soc. Sports Nutr. 2019, 16, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawashima, A.; Sekizawa, A.; Koide, K.; Hasegawa, J.; Satoh, K.; Arakaki, T.; Takenaka, S.; Matsuoka, R. Vitamin C Induces the Reduction of Oxidative Stress and Paradoxically Stimulates the Apoptotic Gene Expression in Extravillous Trophoblasts Derived from First-Trimester Tissue. Reprod. Sci. 2015, 22, 783–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, L.; Purohit, D.; Haroutunian, V.; Luterman, J.D.; Willis, F.; Naslund, J.; Buxbaum, J.D.; Mohs, R.C.; Aisen, P.S.; Pasinetti, G.M. Neuronal Cyclooxygenase 2 Expression in the Hippocampal Formation as a Function of the Clinical Progression of Alzheimer Disease. Arch. Neurol. 2001, 58, 487–492. [Google Scholar] [CrossRef]
- Han, S.S.; Kim, K.; Hahm, E.R.; Lee, S.J.; Surh, Y.J.; Park, H.K.; Kim, W.S.; Jung, C.W.; Lee, M.H.; Park, K.; et al. L-ascorbic acid represses constitutive activation of NF-κB and COX-2 expression in human acute myeloid leukemia, HL-60. J. Cell. Biochem. 2004, 93, 257–270. [Google Scholar] [CrossRef]
- Wu, F.; Wilson, J.X.; Tyml, K. Ascorbate inhibits iNOS expression and preserves vasoconstrictor responsiveness in skeletal muscle of septic mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R50–R56. [Google Scholar] [CrossRef] [Green Version]
- Caprile, T.; Salazar, K.; Astuya, A.; Cisternas, P.; Silva-Alvarez, C.; Montecinos, H.; Millán, C.; García, M.D.L.A.; Nualart, F. The Na+-dependent l-ascorbic acid transporter SVCT2 expressed in brainstem cells, neurons, and neuroblastoma cells is inhibited by flavonoids. J. Neurochem. 2009, 108, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, S.; Pavan, B.; Vertuani, S.; Scaglianti, M.; Compagnone, D.; Biondi, C.; Scatturin, A.; Tanganelli, S.; Ferraro, L.; Prasad, P.; et al. Design, synthesis and activity of ascorbic acid prodrugs of nipecotic, kynurenic and diclophenamic acids, liable to increase neurotropic activity. J. Med. Chem. 2002, 45, 559–562. [Google Scholar] [CrossRef]
- Yue, Q.; Peng, Y.; Zhao, Y.; Lu, R.; Fu, Q.; Chen, Y.; Yang, Y.; Hai, L.; Guo, L.; Wu, Y. Dual-targeting for brain-specific drug delivery: Synthesis and biological evaluation. Drug Deliv. 2018, 25, 426–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, P.; Yu, X.; Wang, W.; Fan, S.; Li, X.; Wang, J. Crystal structure of a phosphorylation-coupled vitamin C transporter. Nat. Struct. Mol. Biol. 2015, 22, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Olabisi, A.O.; Wimalasena, K. Rational Approach to Selective and Direct 2-O-Alkylation of 5,6-O-Isopropylidine-l-ascorbic Acid. J. Org. Chem. 2004, 69, 7026–7032. [Google Scholar] [CrossRef] [PubMed]
- Jiaranaikulwanitch, J.; Boonyarat, C.; Fokin, V.V.; Vajragupta, O. Triazolyl tryptoline derivatives as β-secretase inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 6572–6576. [Google Scholar] [CrossRef] [Green Version]
- Ermolieff, J.; Loy, J.A.; Koelsch, G.; Tang, J. Proteolytic Activation of Recombinant Pro-Memapsin 2 (Pro-β-Secretase) Studied with New Fluorogenic Substrates. Biochemistry 2000, 9, 12450–12456. [Google Scholar] [CrossRef]
- LeVine, H. Thioflavine T interaction with synthetic Alzheimer’s disease beta-amyloid peptides: Detection of amyloid aggregation in solution. Protein Sci. 1993, 2, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Iacovitti, L.; Stull, N.D.; Johnston, K. Melatonin rescues dopamine neurons from cell death in tissue culture models of oxidative stress. Brain Res. 1997, 768, 317–326. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
| Compound | BACE1 | Amyloid Aggregation | Antioxidant | |||
|---|---|---|---|---|---|---|
| %Inhibition (±SEM) | IC50 (μM) | %Inhibition (±SEM) | IC50 (μM) | %Inhibition (±SEM) | IC50 (μM) | |
| 2a | 6.80 ± 2.69 | - | 53.01 ± 0.60 | 318.60 | 66.54 ± 0.56 * | 206.50 |
| 2b | 6.29 ± 0.98 | - | 50.99 ± 2.12 * | 318.80 | 62.81 ± 2.62 * | 155.70 |
| 2c | 23.49 ± 1.66 * | 725.70 | 71.79 ± 1.42 | 92.33 | 94.67 ± 0.50 | 72.26 |
| 5a | ND | - | 34.53 ± 3.62 * | - | 78.42 ± 0.93 * | 91.15 |
| 5b | ND | - | 19.58 ± 1.83 * | - | 77.37 ± 1.60 * | 103.10 |
| 5c | 27.33 ± 1.41 * | 593.10 | 68.26 ± 0.67 | 136.00 | 85.84 ± 2.43 | 62.89 |
| Ascorbic acid | 7.84 ± 1.75 | - | 63.44 ± 0.87 | 146.10 | 94.85 ± 0.62 | 21.28 |
| BACE1 inhibitor I (100 μM) | 99.14 ± 0.11 * | - | - | - | - | - |
| Curcumin (100 μM) | - | - | 94.45 ± 1.15 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiaranaikulwanitch, J.; Pandith, H.; Tadtong, S.; Thammarat, P.; Jiranusornkul, S.; Chauthong, N.; Nilkosol, S.; Vajragupta, O. Novel Multifunctional Ascorbic Triazole Derivatives for Amyloidogenic Pathway Inhibition, Anti-Inflammation, and Neuroprotection. Molecules 2021, 26, 1562. https://doi.org/10.3390/molecules26061562
Jiaranaikulwanitch J, Pandith H, Tadtong S, Thammarat P, Jiranusornkul S, Chauthong N, Nilkosol S, Vajragupta O. Novel Multifunctional Ascorbic Triazole Derivatives for Amyloidogenic Pathway Inhibition, Anti-Inflammation, and Neuroprotection. Molecules. 2021; 26(6):1562. https://doi.org/10.3390/molecules26061562
Chicago/Turabian StyleJiaranaikulwanitch, Jutamas, Hataichanok Pandith, Sarin Tadtong, Phanit Thammarat, Supat Jiranusornkul, Nattapong Chauthong, Supitcha Nilkosol, and Opa Vajragupta. 2021. "Novel Multifunctional Ascorbic Triazole Derivatives for Amyloidogenic Pathway Inhibition, Anti-Inflammation, and Neuroprotection" Molecules 26, no. 6: 1562. https://doi.org/10.3390/molecules26061562







