Microorganisms as a Potential Source of Molecules to Control Trypanosomatid Diseases
Abstract
:1. Introduction
2. Trypanosomatid Diseases
3. Population Affected by these Diseases
4. Microbial Diversity as a Source of Antiprotozoal Metabolites
5. Bacterial Metabolites
5.1. Actinobacteria
5.2. Cyanobacteria
5.3. Firmicutes
5.4. Gammaproteobacteria
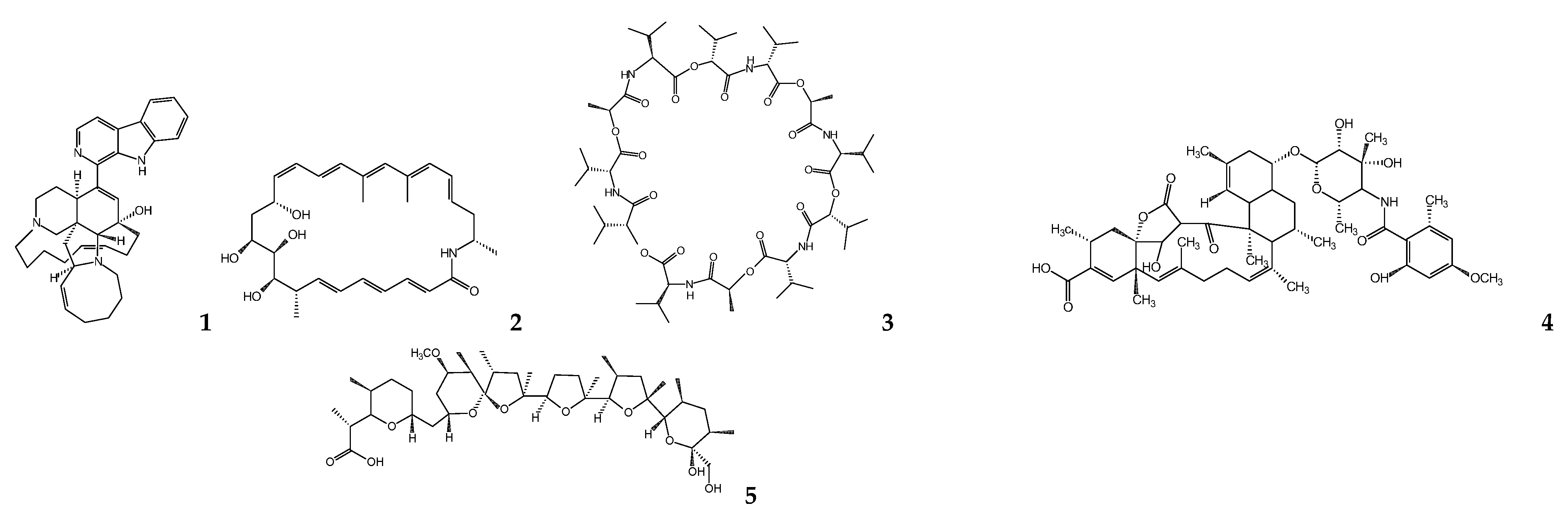
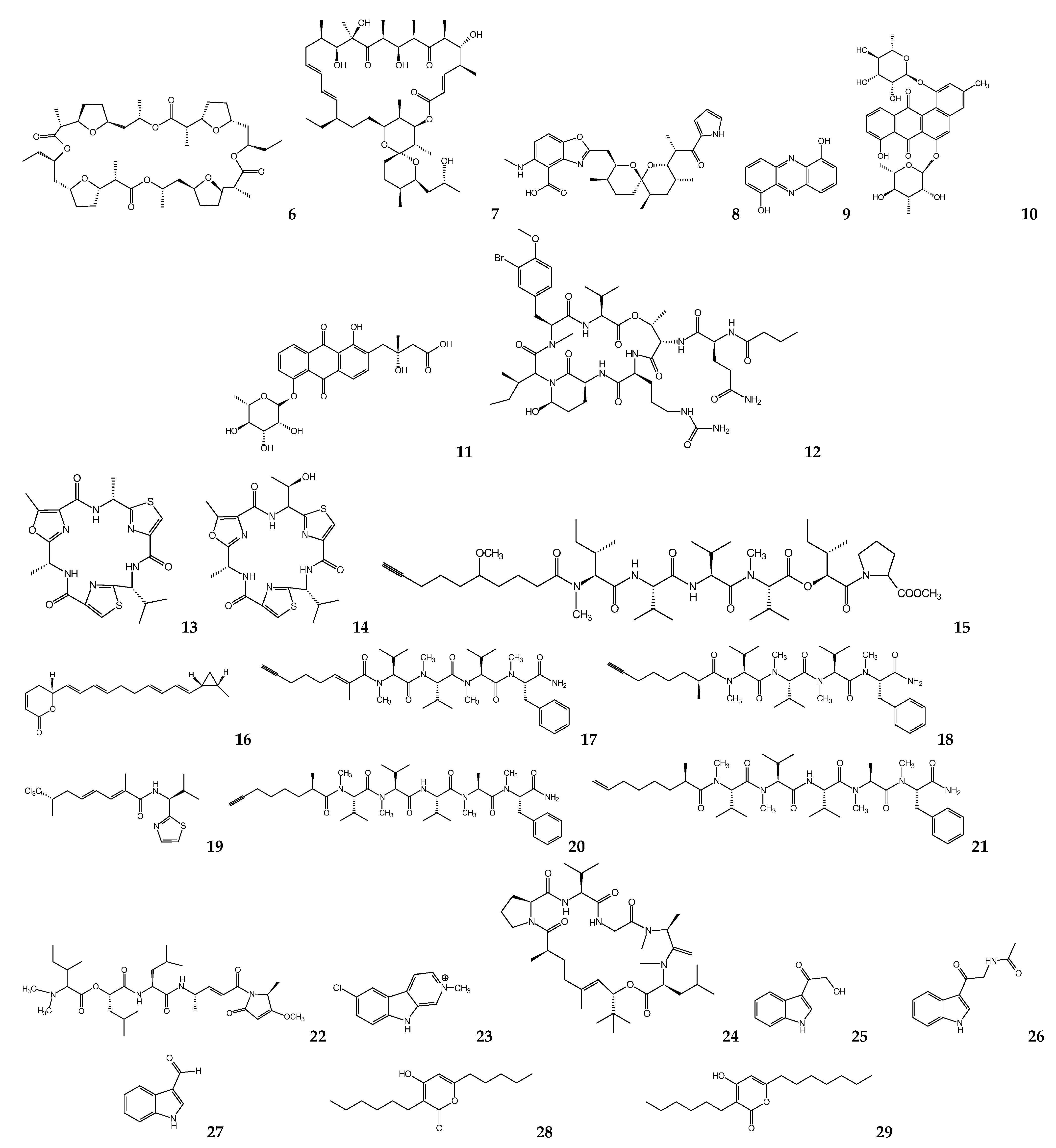
6. Fungal Metabolites
Ascomycetes
7. Future Microbial Metabolites
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Scotti, M.T.; Scotti, L.; Ishiki, H.; Ribeiro, F.F.; Cruz, R.M.; Oliveira, M.P.; Mendonça, F.J. Natural products as a source for antileishmanial and antitrypanosomal Agents. Comb. Chem. High Throughput Screen 2016, 19, 537–553. [Google Scholar] [CrossRef] [PubMed]
- Werbovetz, K.A. Target-based drug discovery for malaria, leishmaniasis, and trypanosomiasis. Curr. Med. Chem. 2000, 7, 835–860. [Google Scholar] [CrossRef] [PubMed]
- Verlinde, C.L.; Bressi, J.C.; Choe, J.; Suresh, S.; Buckner, F.S.; Van Voorhis, W.C.; Michels, P.A.M.; Gelb, M.H.; Hol, W.G.J. Protein structure-based design of anti-protozoal drugs. J. Braz. Chem. Soc. 2002, 3, 843–844. [Google Scholar] [CrossRef] [Green Version]
- Brennand, A.; Rico, E.; Michels, P.A. Autophagy in trypanosomatids. Cells 2012, 1, 346–371. [Google Scholar] [CrossRef]
- Biagiotti, M.; Dominguez, S.; Yamout, N.; Zufferey, R. Lipidomics and anti-trypanosomatid chemotherapy. Clin. Transl. Med. 2017, 6, 27. [Google Scholar] [CrossRef] [Green Version]
- Verlinde, C.L.; Hannaert, V.; Blonski, C.; Willson, M.; Périé, J.J.; Fothergill-Gilmore, L.A.; Opperdoes, F.R.; Gelb, M.H.; Hol, W.G.; Michels, P.A. Glycolysis as a target for the design of new anti-trypanosome drugs. Drug Resist. Updat. 2001, 4, 50–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyersoen, J.; Choe, J.; Fan, E.; Hol, W.G.; Michels, P.A. Biogenesis of peroxisomes and glycosomes: Trypanosomatid glycosome assembly is a promising new drug target. FEMS Microbiol. Rev. 2004, 28, 603–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varela, M.T.; Fernandes, J.P.S. Natural products: Key prototypes to drug discovery against neglected diseases caused by Trypanosomatids. Curr. Med. Chem. 2020, 27, 2133–2146. [Google Scholar] [CrossRef]
- Álvarez-Bardón, M.; Pérez-Pertejo, Y.; Ordóñez, C.; Sepúlveda-Crespo, D.; Carballeira, N.M.; Tekwani, B.L.; Murugesan, S.; Martinez-Valladares, M.; García-Estrada, C.; Reguera, R.M.; et al. Screening marine natural productsfor new drug leads against Trypanosomatids and Malaria. Mar. Drugs 2020, 18, 187. [Google Scholar] [CrossRef]
- Proksch, P.; Edrada, R.A.; Ebel, R. Drugs from the seas—Current status and microbiological implications. Appl. Microbiol. Biotechnol. 2002, 59, 125–134. [Google Scholar] [CrossRef]
- Shiomi, K.; Ōmura, M.J.A. Antiparasitic agents produced by microorganisms. Proc. Jpn. Acad. Ser. B 2004, 80, 245–258. [Google Scholar] [CrossRef] [Green Version]
- Domingues Passero, L.F.; Laurenti, M.D.; Santos-Gomes, G.; Soares Campos, B.L.; Sartoreli, P.; Lago, J.H.G. In vivo antileishmanial activity of plant-based secondary metabolites. In Fighting Multidrug Resistance with Herbal Extracts, Essential Oils and Their Components; Rai, M., Kon, K., Eds.; Academic Press: Cambridge, UK, 2013; Chapter 7; pp. 95–107. [Google Scholar] [CrossRef]
- Cruz, A.K.; de Toledo, J.S.; Falade, M.; Terrão, M.C.; Kamchonwongpaisan, S.; Kyle, D.E.; Uthaipibull, C. Current treatment and drug discovery against Leishmania spp. and Plasmodium spp.: A review. Curr. Drug Targets 2009, 10, 178–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Requena, J.M.; Iborra, S.; Carrión, J.; Alonso, C.; Soto, M. Recent advances in vaccines for leishmaniasis. Exp.Opin. Biol. Ther. 2004, 4, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Maurício, I.L.; Stothard, J.R.; Miles, M.A. The strange case of Leishmania chagasi. Parasitol. Today 2000, 16, 188–189. [Google Scholar] [CrossRef]
- Grant, K.M.; Dunion, M.H.; Yardley, V.; Skaltsounis, A.L.; Marko, D.; Eisenbrand, G.; Croft, S.L.; Meijer, L.; Mottram, J.C. Inhibitors of Leishmania mexicana CRK3 cyclin-dependent kinase: Chemical library screen and antileishmanial activity. Antimicrob. Agents Chemother. 2004, 48, 3033–3042. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Sivakumar, R. Challenges and new discoveries in the treatment of leishmaniasis. J. Infect. Chemother. 2004, 10, 307–315. [Google Scholar] [CrossRef]
- Liñares, G.E.; Ravaschino, E.L.; Rodriguez, J.B. Progresses in the field of drug design to combat tropical protozoan parasitic diseases. Curr. Med. Chem. 2006, 13, 335–360. [Google Scholar] [CrossRef]
- Caldas, I.S.; Santos, E.G.; Novaes, R.D. An evaluation of benznidazole as a Chagas disease therapeutic. Exp. Opin. Pharmacother. 2019, 20, 1797–1807. [Google Scholar] [CrossRef]
- Álvarez, M.G.; Vigliano, C.; Lococo, B.; Bertocchi, G.; Viotti, R. Prevention of congenital Chagas disease by benznidazole treatment in reproductive-age women. An observational study. Acta Trop. 2017, 174, 149–152. [Google Scholar] [CrossRef]
- Bermudez, J.; Davies, C.; Simonazzia, A.; Real, J.P.; Palma, S. Current drug therapy and pharmaceutical challenges for Chagas disease. Acta Trop. 2016, 156, 1–16. [Google Scholar] [CrossRef]
- Sales, P.A., Jr.; Molina, I.; Fonseca Murta, S.M.; Sánchez-Montalvá, A.; Salvador, F.; Corrêa-Oliveira, R.; Martins Carneiro, C. Experimental and clinical treatment of Chagas disease: A review. Am. J. Trop. Med. Hyg. 2017, 97, 1289–1303. [Google Scholar] [CrossRef]
- Annang, F.; Pérez-Moreno, G.; García-Hernández, R.; Cordon-Obras, C.; Martín, J.; Tormo, J.R.; Rodríguez, L.; de Pedro, N.; Gómez-Pérez, V.; Valente, M.; et al. High-throughput screening platform for natural product-based drug discovery against 3 neglected tropical diseases: Human African trypanosomiasis, leishmaniasis, and Chagas disease. J. Biomol. Screen. 2015, 20, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Priotto, G.; Kasparian, S.; Mutombo, W.; Ngouama, D.; Ghorashian, S.; Arnold, U.; Ghabri, S.; Baudin, E.; Buard, V.; Kazadi-Kyanza, S.; et al. Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: A multicentre, randomised, phase III, non-inferiority trial. Lancet 2009, 374, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Deeks, E.D. Fexinidazole: First global approval. Drugs 2019, 79, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Mesu, V.K.B.K.; Kalonji, W.M.; Bardonneau, C.; Mordt, O.V.; Blesson, S.; Simon, F.; Delhomme, S.; Bernhard, S.; Kuziena, W.; Lubaki, J.F.; et al. Oral fexinidazole for late-stage African Trypanosoma brucei gambiense trypanosomiasis: A pivotal multicentre, randomised, non-inferiority trial. Lancet 2018, 39, 144–154. [Google Scholar] [CrossRef]
- Lindner, A.K.; Lejon, V.; Chappuis, F.; Seixas, J.; Kazumba, L.; Barrett, M.P.; Mwamba, E.; Erphas, O.; Akl, E.A.; Villanueva, G.; et al. New WHO guidelines for treatment of gambiense human African trypanosomiasis including fexinidazole: Substantial changes for clinical practice. Lancet Infect. Dis. 2020, 20, e38–e46. [Google Scholar] [CrossRef]
- Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 29 November 2020).
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.J.; Kedzierski, L. Recent advances in antileishmanial drug development. Curr. Opin. Investig. Drugs 2005, 6, 163–169. [Google Scholar]
- Chagas in the Americas. Available online: https://www.paho.org/hq/index.php?option=com_content&view=article&id=13566:chagas-in-americas&Itemid=40721&lang=en (accessed on 7 December 2020).
- Trypanosomiasis, Human African (Sleeping Sickness). Available online: https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness) (accessed on 10 February 2021).
- Pagmadulam, B.; Tserendulam, D.; Rentsenkhand, T.; Igarashi, M.; Sawa, R.; Nihei, C.I.; Nishikawa, Y. Isolation and characterization of antiprotozoal compound-producing Streptomyces species from Mongolian soils. Parasitol. Int. 2020, 74, 101961. [Google Scholar] [CrossRef]
- Pham, J.V.; Yilma, M.A.; Feliz, A.; Majid, M.T.; Maffetone, N.; Walker, J.R.; Kim, E.; Cho, H.J.; Reynolds, J.M.; Song, M.C.; et al. A review of the microbial production of bioactive natural products and biologics. Front. Microbiol. 2019, 10, 1404. [Google Scholar] [CrossRef] [Green Version]
- Tempone, A.G.; Martins de Oliveira, C.; Berlinck, R.G. Current approaches to discover marine antileishmanial natural products. Planta Med. 2011, 77, 572–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, L.G.; Almeida, J.R.; Macêdo, R.O.; Barbosa-Filho, J.M. A review of natural products with antileishmanial activity. Phytomedicine 2005, 12, 514–535. [Google Scholar] [CrossRef]
- Fatima, N.; Muhammad, S.A.; Mumtaz, A.; Tariq, H.; Shahzadi, I.; Said, M.S.; Dawood, M. Fungal metabolites and leishmaniasis: A review. Br. J. Pharm. Res. 2016, 12, 1–12. [Google Scholar] [CrossRef]
- Uddin, G.M.S.; Nejum, M.R.; Islam, M.R.; Islam, M.M. Production of novel antiprotozoal and antihelmintic compounds from marine surface associated bacteria. J. Marine Sci. Res. Dev. 2019, 9, 1000266. [Google Scholar] [CrossRef]
- Sayed, K.A.; Khalil, A.A.; Yousaf, M.; Labadie, G.; Kumar, G.M.; Franzblau, S.G.; Mayer, A.M.; Avery, M.A.; Hamann, M.T. Semisynthetic studies on the manzamine alkaloids. J. Nat. Prod. 2008, 71, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Peraud, O. Isolation and Characterization of a Sponge-Associated Actinomycete that Produces Manzamines. Ph.D. Thesis, Faculty of the Graduate School of the University of Maryland, College Park, MD, USA, 2006. Available online: http://hdl.handle.net/1903/4114 (accessed on 20 November 2020).
- Rao, K.V.; Santarsiero, B.D.; Mesecar, A.D.; Schinazi, R.F.; Tekwani, B.L.; Hamann, M.T. New manzamine alkaloids with activity against infectious and tropical parasitic diseases from an Indonesian sponge. J. Nat. Prod. 2003, 66, 823–828. [Google Scholar] [CrossRef] [Green Version]
- Rao, K.V.; Kasanah, N.; Wahyuono, S.; Tekwani, B.L.; Schinazi, R.F.; Hamann, M.T. Three new manzamine alkaloids from a common Indonesian sponge and their activity against infectious and tropical parasitic diseases. J. Nat. Prod. 2004, 67, 1314–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Indraningrat, A.A.; Smidt, H.; Sipkema, D. Bioprospecting sponge-associated microbes for antimicrobial compounds. Mar. Drugs 2016, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Schulze, C.J.; Donia, M.S.; Siqueira-Neto, J.L.; Ray, D.; Raskatov, J.A.; Green, R.E.; McKerrow, J.H.; Fischbach, M.A.; Linington, R.G. Genome-directed lead discovery: Biosynthesis, structure elucidation, and biological evaluation of two families of polyene macrolactams against Trypanosoma brucei. ACS Chem. Biol. 2015, 10, 2373–2381. [Google Scholar] [CrossRef]
- Pimentel-Elardo, S.M.; Kozytska, S.; Bugni, T.S.; Ireland, C.M.; Moll, H.; Hentschel, U. Anti-parasitic compounds from Streptomyces sp. strains isolated from Mediterranean sponges. Mar. Drugs 2010, 8, 373–380. [Google Scholar] [CrossRef]
- Pimentel-Elardo, S.M.; Buback, V.; Gulder, T.A.; Bugni, T.S.; Reppart, J.; Bringmann, G.; Ireland, C.M.; Schirmeister, T.; Hentschel, U. New tetromycin derivatives with anti-trypanosomal and protease inhibitory activities. Mar. Drugs 2011, 9, 1682–1697. [Google Scholar] [CrossRef]
- Graven, S.N.; Estrada-O, S.; Lardy, H.A. Alkali metal cation release and respiratory inhibition induced by nigericin in rat liver mitochondria. Proc. Natl. Acad. Sci. USA 1966, 56, 654–658. [Google Scholar] [CrossRef] [Green Version]
- Ortega, H.E.; Ferreira, L.L.G.; Melo, W.G.P.; Oliveira, A.L.L.; Ramos Alvarenga, R.F.; Lopes, N.P.; Bugni, T.S.; Andricopulo, A.D.; Pupo, M.T. Antifungal compounds from Streptomyces associated with attine ants also inhibit Leishmania donovani. PLoS Negl. Trop. Dis. 2019, 13, e0007643. [Google Scholar] [CrossRef] [Green Version]
- Riddell, F.G.; Arumugam, S.; Brophy, P.J.; Cox, B.G.; Payne, M.C.H.; Southon, T.E. The nigericin-mediated transport of sodium and potassium ions through phospholipid bilayers studied by sodium-23 and potassium-39 NMR spectroscopy. J. Am. Chem. Soc. 1988, 110, 734–738. [Google Scholar] [CrossRef]
- Miller, P.G.; Klein, R.A. Effects of oligomycin on glucose utilization and calcium transport in African trypanosomes. J. Gen Microbiol. 1980, 116, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Nakata, M.; Ishiyama, T.; Akamatsu, S.; Hirose, Y.; Maruoka, H.; Suzuki, R.; Tatsuta, K. Synthetic studies on oligomycins. Synthesis of the oligomycin B spiroketal and polypropionate portions. Bull. Chem. Soc. Jpn. 1995, 66, 967–989. [Google Scholar] [CrossRef]
- Lee, H.J.; Moon, J.; Chung, I.; Chung, J.H.; Park, C.; Lee, J.O.; Han, J.A.; Kang, M.J.; Yoo, E.H.; Kwak, S.Y.; et al. ATP synthase inhibitory factor 1 (IF1), a novel myokine, regulates glucose metabolism by AMPK and Akt dual pathways. FASEB J. 2019, 33, 14825–14840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Peinado, N.; Martori, C.; Cortes-Serra, N.; Sherman, J.; Rodriguez, A.; Gascon, J.; Alberola, J.; Pinazo, M.J.; Rodriguez-Cortes, A.; Alonso-Padilla, J. Anti-Trypanosoma cruzi activity of metabolism modifier compounds. Int. J. Mol. Sci. 2021, 22, 688. [Google Scholar] [CrossRef] [PubMed]
- Reed, P.W.; Lardy, H.A. A23187: A divalent cation ionophore. J. Biol. Chem. 1972, 247, 6970–6977. [Google Scholar] [CrossRef]
- Pressman, B.C. Biological applications of ionophores. Annu. Rev. Biochem. 1976, 45, 501–530. [Google Scholar] [CrossRef]
- Buchmüller-Rouiller, Y.; Mauël, J. Macrophage activation for intracellular killing as induced by calcium ionophore. Correlation with biologic and biochemical events. J. Immunol. 1991, 146, 217–223. [Google Scholar] [PubMed]
- Lanza, H.; Afonso-Cardoso, S.R.; Silva, A.G.; Napolitano, D.R.; Espíndola, F.S.; Pena, J.D.; Souza, M.A. Comparative effect of ion calcium and magnesium in the activation and infection of the murine macrophage by Leishmania major. Biol. Res. 2004, 37, 385–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grekov, I.; Pombinho, A.R.; Kobets, T.; Bartůněk, P.; Lipoldová, M. Calcium ionophore, calcimycin, kills Leishmania promastigotes by activating parasite nitric oxide synthase. Biomed. Res. Int. 2017, 2017, 1309485. [Google Scholar] [CrossRef] [Green Version]
- Dashti, Y.; Grkovic, T.; Abdelmohsen, U.R.; Hentschel, U.; Quinn, R.J. Production of induced secondary metabolites by a co-culture of sponge-associated actinomycetes, Actinokineospora sp. EG49 and Nocardiopsis sp. RV163. Mar. Drugs 2014, 12, 3046–3059. [Google Scholar] [CrossRef]
- Abdelmohsen, U.R.; Cheng, C.; Viegelmann, C.; Zhang, T.; Grkovic, T.; Ahmed, S.; Quinn, R.J.; Hentschel, U.; Edrada-Ebel, R. Dereplication strategies for targeted isolation of new antitrypanosomalactinosporins A and B from a marine sponge associated-Actinokineospora sp. EG49. Mar. Drugs 2014, 12, 1220–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawfike, A.; Attia, E.Z.; Desoukey, S.Y.; Hajjar, D.; Makki, A.A.; Schupp, P.J.; Edrada-Ebel, R.; Abdelmohsen, U.R. New bioactive metabolites from the elicited marine sponge-derived bacterium Actinokineospora spheciospongiae sp. nov. AMB Express 2019, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Linington, R.G.; Edwards, D.J.; Shuman, C.F.; McPhail, K.L.; Matainaho, T.; Gerwick, W.H. Symplocamide A, a potent cytotoxin and chymotrypsin inhibitor from the marine cyanobacterium Symploca sp. J. Nat. Prod. 2008, 71, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Linington, R.G.; Gonzalez, J.; Ureña, L.D.; Romero, L.I.; Ortega-Barría, E.; Gerwick, W.H. Venturamides A and B: Antimalarial constituents of the panamanian marine cyanobacterium Oscillatoria sp. J. Nat. Prod. 2007, 70, 397–401. [Google Scholar] [CrossRef]
- Simmons, T.L.; Engene, N.; Ureña, L.D.; Romero, L.I.; Ortega-Barría, E.; Gerwick, L.; Gerwick, W.H. Viridamides A and B, lipodepsipeptides with antiprotozoal activity from the marine cyanobacterium Oscillatoria nigro-viridis. J. Nat. Prod. 2008, 71, 1544–1550. [Google Scholar] [CrossRef] [Green Version]
- Balunas, M.J.; Grosso, M.F.; Villa, F.A.; Engene, N.; McPhail, K.L.; Tidgewell, K.; Pineda, L.M.; Gerwick, L.; Spadafora, C.; Kyle, D.E.; et al. Coibacins A-D, antileishmanial marine cyanobacterial polyketides with intriguing biosynthetic origins. Org. Lett. 2012, 14, 3878–3881. [Google Scholar] [CrossRef]
- Balunas, M.J.; Linington, R.G.; Tidgewell, K.; Fenner, A.M.; Ureña, L.D.; Togna, G.D.; Kyle, D.E.; Gerwick, W.H. Dragonamide E, a modified linear lipopeptide from Lyngbya majuscula with antileishmanial activity. J. Nat. Prod. 2010, 73, 60–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Rein, K.S. New peptides isolated from Lyngbya species: A review. Mar. Drugs 2010, 8, 1817–1837. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, L.M.; Lopez, D.; Vesely, B.A.; Della Togna, G.; Gerwick, W.H.; Kyle, D.E.; Linington, R.G. Almiramides A-C: Discovery and development of a new class of leishmaniasis lead compounds. J. Med. Chem. 2010, 53, 4187–4197. [Google Scholar] [CrossRef] [Green Version]
- Linington, R.G.; Clark, B.R.; Trimble, E.E.; Almanza, A.; Ureña, L.D.; Kyle, D.E.; Gerwick, W.H. Antimalarial peptides from marine cyanobacteria: Isolation and structural elucidation of gallinamide A. J. Nat. Prod. 2009, 72, 14–17. [Google Scholar] [CrossRef] [Green Version]
- França, P.H.B.; da Silva-Júnior, E.F.; Santos, B.V.O.; Alexander-Moreira, M.S.; Quintans-Junior, L.S.; de Aquino, T.M.; Araújo-Júnior, J.X. Antileishmanial marine compounds: A review. Rec. Nat. Prod. 2017, 11, 92–113. [Google Scholar]
- Ogawa, H.; Iwasaki, A.; Sumimoto, S.; Kanamori, Y.; Ohno, O.; Iwatsuki, M.; Ishiyama, A.; Hokari, R.; Otoguro, K.; Ōmura, S.; et al. Janadolide, a cyclicpolyketide-peptide hybrid possessing a tert-butyl group from an Okeania sp. marine cyanobacterium. J. Nat. Prod. 2016, 79, 1862–1866. [Google Scholar] [CrossRef]
- Athawale, P.R.; Jachak, G.R.; Shukla, A.; Shanmugam, D.; Reddy, D.S. Efforts To access the potent antitrypanosomal marine natural product janadolide: Synthesis of des-tert-butyl janadolide and its biological evaluation. ACS Omega 2018, 3, 2383–2389. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.H.; Tang, A.H.; Geraghty, K.; Corcilius, L.; Kaiser, M.; Payne, R.J. Total synthesis and antitrypanosomal activity of janadolide and simplified analogues. Org. Lett. 2020, 22, 3089–3093. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Luis, S.; Gómez, J.F.; Spadafora, C.; Guzmán, H.M.; Gutiérrez, M. Antitrypanosomal alkaloids from the marine bacterium Bacillus pumilus. Molecules 2012, 17, 11146–11155. [Google Scholar] [CrossRef]
- Giddens, A.C.; Nielsen, L.; Boshoff, H.I.; Tasdemir, D.; Perozzo, R.; Kaiser, M.; Wang, F.; Sacchettini, J.C.; Copp, B.R. Natural product inhibitors of fatty acid biosynthesis: Synthesis of the marine microbial metabolites pseudopyronines A and B and evaluation of their anti-infective activities. Tetrahedron 2008, 64, 1242–1249. [Google Scholar] [CrossRef]
- Mutomba, M.C.; Wang, C.C. Effects of aphidicolin and hydroxyurea on the cell cycle and differentiation of Trypanosoma brucei bloodstream forms. Mol. Biochem. Parasitol. 1996, 80, 89–102. [Google Scholar] [CrossRef]
- Kayser, O.; Kiderlen, A.F.; Bertels, S.; Siems, K. Antileishmanial activities of aphidicolin and its semisynthetic derivatives. Antimicrob. Agents Chemother. 2001, 45, 288–292. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.; Khan, S.I.; Jacob, M.R.; Tekwani, B.L.; Li, Z.; Pasco, D.S.; Walker, L.A.; Khan, I.A. Antimicrobial and antileishmanial activities of hypocrellins A and B. Antimicrob. Agents Chemother. 2004, 48, 4450–4452. [Google Scholar] [CrossRef] [Green Version]
- Molinar, E.; Rios, N.; Spadafora, C.; Arnold, A.E.; Coley, P.D.; Kursar, T.A.; Gerwick, W.H.; Cubilla-Rios, L. Coibanoles, a new class of meroterpenoids produced by Pycnoporus sanguineus. Tetrahedron Lett. 2012, 53, 919–922. [Google Scholar] [CrossRef] [Green Version]
- do RosárioMarinho, A.M.; Rodrigues-Filho, E.; Moitinho, M.L.R.; Santos, L.S. Biologically active polyketides produced by Penicillium janthinellum isolated as an endophytic fungus from fruits of Melia azedarach. J. Braz. Chem. Soc. 2005, 16, 280–283. [Google Scholar]
- Schmidt, A.; Krauth-Siegel, R.L. Enzymes of the trypanothione metabolism as targets for antitrypanosomal drug development. Curr. Top. Med. Chem. 2002, 2, 1239–1259. [Google Scholar] [CrossRef]
- Cota, B.B.; Rosa, L.H.; Caligiorne, R.B.; Rabello, A.L.; Almeida Alves, T.M.; Rosa, C.A.; Zani, C.L. Altenusin, a biphenyl isolated from the endophytic fungus Alternaria sp., inhibits trypanothione reductase from Trypanosoma cruzi. FEMS Microbiol. Lett. 2008, 285, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idris, A.; Tantry, M.A.; Ganai, B.A.; Kamili, A.N.; Williamson, J.S. Reduced perylenequinone derivatives from an endophytic Alternaria sp. isolated from Pinus ponderosa. Phytochem. Lett. 2015, 11, 264–269. [Google Scholar] [CrossRef]
- Campos, F.F.; Rosa, L.H.; Cota, B.B.; Caligiorne, R.B.; Rabello, A.L.; Alves, T.M.; Rosa, C.A.; Zani, C.L. Leishmanicidal metabolites from Cochliobolus sp., an endophytic fungus isolated from Piptadenia adiantoides (Fabaceae). PLoS Negl. Trop. Dis. 2008, 2, e348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Luis, S.; Della-Togna, G.; Coley, P.D.; Kursar, T.A.; Gerwick, W.H.; Cubilla-Rios, L. Antileishmanial constituents of the Panamanian endophytic fungus Edenia sp. J. Nat. Prod. 2008, 71, 2011–2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, H.E.; Teixeira, E.M.; Rabello, A.; Higginbotham, S.; Cubilla-Ríos, L. Anti-L. donovani activity in macrophage/amastigote model of palmarumycin CP18 and its large scale production. Nat. Prod. Commun. 2014, 9, 95–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osterhage, C.; Kaminsky, R.; König, G.M.; Wright, A.D. Ascosalipyrrolidinone A, an antimicrobial alkaloid, from the obligate marine fungus Ascochyta salicorniae. J. Org. Chem. 2000, 65, 6412–6417. [Google Scholar] [CrossRef] [PubMed]
- Brissow, E.R.; da Silva, I.P.; de Siqueira, K.A.; Senabio, J.A.; Pimenta, L.P.; Januário, A.H.; Magalhães, L.G.; Furtado, R.A.; Tavares, D.C.; Sales Junior, P.A.; et al. 18-des-hydroxy cytochalasin: An antiparasitic compound of Diaporthe phaseolorum-92C, an endophytic fungus isolated from Combretum lanceolatum Pohl ex Eichler. Parasitol. Res. 2017, 116, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.F.; Sales Junior, P.A.; Romanha, A.J.; Araújo, M.S.; Siqueira, E.P.; Resende, J.M.; Alves, T.M.; Martins-Filho, O.A.; Santos, V.L.; Rosa, C.A.; et al. Bioactive endophytic fungi isolated from Caesalpinia echinata Lam. (Brazilwood) and identification of beauvericin as a trypanocidal metabolite from Fusarium sp. Mem. Inst. Oswaldo Cruz 2015, 110, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Cota, B.B.; Tunes, L.G.; Maia, D.N.B.; Ramos, J.P.; Oliveira, D.M.; Kohlhoff, M.; Alves, T.M.A.; Souza-Fagundes, E.M.; Campos, F.F.; Zani, C.L. Leishmanicidal compounds of Nectria pseudotrichia, an endophytic fungus isolated from the plant Caesalpinia echinata (Brazilwood). Mem. Inst. Oswaldo Cruz. 2018, 113, 102–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- do Nascimento, J.S.; Silva, F.M.; Magallanes-Noguera, C.A.; Kurina-Sanz, M.; Dos Santos, E.G.; Caldas, I.S.; Luiz, J.H.H.; Silva, E.O. Natural trypanocidalproducto produced by endophytic fungi through co-culturing. Folia Microbiol. 2020, 65, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Araki, Y.; Awakawa, T.; Matsuzaki Cho, R.; Matsuda, Y.; Hoshino, S.; Shinohara, Y.; Yamamoto, M.; Kido, Y.; Inaoka, D.K.; Nagamune, K.; et al. Complete biosynthetic pathways of ascofuranone and ascochlorin in Acremonium egyptiacum. Proc. Natl. Acad. Sci. USA 2019, 116, 8269–8274. [Google Scholar] [CrossRef] [Green Version]
- Minagawa, N.; Yabu, Y.; Kita, K.; Nagai, K.; Ohta, N.; Meguro, K.; Sakajo, S.; Yoshimoto, A. An antibiotic, ascofuranone, specifically inhibits respiration and in vitro growth of long slender blood stream forms of Trypanosoma brucei brucei. Mol. Biochem. Parasitol. 1997, 84, 271–280. [Google Scholar] [CrossRef]
- Saimoto, H.; Kido, Y.; Haga, Y.; Sakamoto, K.; Kita, K. Pharmacophore identification of ascofuranone, potent inhibitor of cyanide-insensitive alternative oxidase of Trypanosoma brucei. J. Biochem. 2013, 153, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Yabu, Y.; Yoshida, A.; Suzuki, T.; Nihei, C.; Kawai, K.; Minagawa, N.; Hosokawa, T.; Nagai, K.; Kita, K.; Ohta, N. The efficacy of ascofuranone in a consecutive treatment on Trypanosoma brucei brucei in mice. Parasitol. Int. 2003, 52, 155–164. [Google Scholar] [CrossRef]
- Nihei, C.; Fukai, Y.; Kita, K. Trypanosome alternative oxidase as a target of chemotherapy. Biochim. Biophys. Acta 2002, 1587, 234–239. [Google Scholar] [CrossRef] [Green Version]
- Haga, Y.; Tonoi, T.; Anbiru, Y.; Takahashi, Y.; Tamura, S.; Yamamoto, M.; Ifuku, S.; Morimoto, M.; Saimoto, H. A short and efficient total synthesis of (±)-ascofuranone. Chem. Lett. 2011, 39, 622–623. [Google Scholar] [CrossRef]
- Bessho, T.; Morii, S.; Kusumoto, T.; Shinohara, T.; Noda, M.; Uchiyama, S.; Shuto, S.; Nishimura, S.; Djikeng, A.; Duszenko, M.; et al. Characterization of the novel Trypanosoma brucei inosine 5’-monophosphate dehydrogenase. Parasitology 2013, 140, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, K.; Sarwono, A.E.; Mitsuhashi, S.; Jąkalski, M.; Okada, T.; Nthatisi, M.; Yamagishi, J.; Ubukata, M.; Inoue, N. Mycophenolic acid and its derivatives as potential chemotherapeutic agents targeting inosine monophosphate dehydrogenase in Trypanosoma congolense. Antimicrob. Agents Chemother. 2016, 60, 4391–4393. [Google Scholar] [CrossRef] [Green Version]
- Zulfiqar, B.; Jones, A.J.; Sykes, M.L.; Shelper, T.B.; Davis, R.A.; Avery, V.M. Screening a natural product-based library against kinetoplastid parasites. Molecules 2017, 22, 1715. [Google Scholar] [CrossRef] [Green Version]
- Endo, A.; Kuroda, M.; Tsujita, Y. ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium. J. Antibiot. 1976, 29, 1346–1348. [Google Scholar] [CrossRef] [Green Version]
- Dinesh, N.; Soumya, N.; Singh, S. Antileishmanial effect of mevastatin is due to interference with sterol metabolism. Parasitol. Res. 2015, 114, 3873–3883. [Google Scholar] [CrossRef]
- Pontius, A.; Krick, A.; Kehraus, S.; Brun, R.; König, G.M. Antiprotozoal activities of heterocyclic-substituted xanthones from the marine-derived fungus Chaetomium sp. J. Nat. Prod. 2008, 71, 1579–1584. [Google Scholar] [CrossRef]
- Rodrigues, A.G. Secondary metabolism and antimicrobial metabolites of Aspergillus. In New and Future Developments in Microbial Technology and Bioengineering: Aspergillus System Properties and Applications; Gupta, V.K., Ed.; Elsevier BV: Amsterdam, The Netherlands, 2016; Chapter 6; pp. 81–93. [Google Scholar] [CrossRef]
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed. Pharmacother. 2019, 110, 582–593. [Google Scholar] [CrossRef]
- Rodrigues, A.P.; Farias, L.H.; Carvalho, A.S.; Santos, A.S.; do Nascimento, J.L.; Silva, E.O. A novel function for kojic acid, a secondary metabolite from Aspergillus fungi, as antileishmanial agent. PLoS ONE 2014, 9, e91259. [Google Scholar] [CrossRef]
- Da Costa, J.P.; Rodrigues, A.P.D.; Farias, L.H.S.; Frade, P.C.R.; Da Silva, B.J.M.; Do Nascimento, J.L.M.; Silva, E.O. Biological effects of kojic acid on human monocytes in vitro. Biomed. Pharmacother. 2018, 101, 100–106. [Google Scholar] [CrossRef]
- Malak, L.G.; Ibrahim, M.A.; Bishay, D.W.; Abdel-Baky, A.M.; Moharram, A.M.; Tekwani, B.; Cutler, S.J.; Ross, S.A. Antileishmanial metabolites from Geosmithia langdonii. J. Nat. Prod. 2014, 77, 1987–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malak, L.G.; Ibrahim, M.A.; Moharram, A.M.; Pandey, P.; Tekwani, B.; Doerksen, R.J.; Ferreira, D.; Ross, S.A. Antileishmanial carbasugars from Geosmithia langdonii. J. Nat. Prod. 2018, 81, 2222–2227. [Google Scholar] [CrossRef]
- Atencio, N.; Miller, K.; Cheney, A.; Benally, B.A. The anti-leishmanial effects of compounds derived from the fungus Geosmithia langdonii on visceral leishmaniasis. FASEB J. 2020, 34. [Google Scholar] [CrossRef]
- Gao, J.; Radwan, M.M.; León, F.; Wang, X.; Jacob, M.R.; Tekwani, B.L.; Khan, S.I.; Lupien, S.; Hill, R.A.; Dugan, F.M.; et al. Antimicrobial and antiprotozoal activities of secondary metabolites from the fungus Eurotium repens. Med. Chem. Res. 2012, 21, 3080–3086. [Google Scholar] [CrossRef] [PubMed]
- Luque-Ortega, J.R.; Cruz, L.J.; Albericio, F.; Rivas, L. The antitumoral depsipeptide IB-01212 kills Leishmania through an apoptosis-like process involving intracellular targets. Mol. Pharm. 2010, 7, 1608–1617. [Google Scholar] [CrossRef]
- Hashida, J.; Niitsuma, M.; Iwatsuki, M.; Mori, M.; Ishiyama, A.; Namatame, M.; Nishihara-Tsukashima, A.; Nonaka, K.; Ui, H.; Masuma, R.; et al. Pyrenocine I, a new pyrenocine analog produced by Paecilomyces sp. FKI-3573. J. Antibiot. 2010, 63, 559–561. [Google Scholar] [CrossRef] [Green Version]
- Braun, G.H.; Ramos, H.P.; Candido, A.C.B.B.; Pedroso, R.C.N.; Siqueira, K.A.; Soares, M.A.; Dias, G.M.; Magalhães, L.G.; Ambrósio, S.R.; Januário, A.H.; et al. Evaluation of antileishmanial activity of harzialactone A isolated from the marine-derived fungus Paecilomyces sp. Nat. Prod. Res. 2019, 29, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Vivero Gómez, R.J.; Cadavid Restrepo, G.E.; Moreno Herrera, C.X.; Ospina, V.; Uribe, S.I.; Robledo, S.M. Antagonistic effect of bacteria isolated from the digestive tract of Lutzomyia evansi against promastigotes of Leishmania infantum, antimicrobial activities and susceptibility to antibiotics. Adv. Microbiol. 2016, 6, 760–775. [Google Scholar] [CrossRef] [Green Version]
- Vivero, R.J.; Mesa, G.B.; Robledo, S.M.; Herrera, C.X.M.; Cadavid-Restrepo, G. Enzymatic, antimicrobial, and leishmanicidal bioactivity of gram-negative bacteria strains from the midgut of Lutzomyia evansi, an insect vector of leishmaniasis in Colombia. Biotechnol. Rep. 2019, 24, e00379. [Google Scholar] [CrossRef]
- Li, T.; Ding, T.; Li, J. Medicinal purposes: Bioactive metabolites from marine-derived organisms. Mini Rev. Med. Chem. 2019, 19, 138–164. [Google Scholar] [CrossRef]
- Nweze, J.A.; Mbaoji, F.N.; Li, Y.M.; Yang, L.Y.; Huang, S.S.; Chigor, V.N.; Eze, E.A.; Pan, L.X.; Zhang, T.; Yang, D.F. Potentials of marine natural products against malaria, leishmaniasis, and trypanosomiasis parasites: A review of recent articles. Infect. Dis. Poverty 2021, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- de Menezes, G.C.A.; Porto, B.A.; Amorim, S.S.; Zani, C.L.; de Almeida Alves, T.M.; Junior, P.A.S.; Murta, S.M.F.; Simões, J.C.; Cota, B.B.; Rosa, C.A.; et al. Fungi in glacial ice of Antarctica: Diversity, distribution and bioprospecting of bioactive compounds. Extremophiles 2020, 24, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Ogaki, M.B.; Coelho, L.C.; Vieira, R.; Neto, A.A.; Zani, C.L.; Alves, T.M.A.; Junior, P.A.S.; Murta, S.M.F.; Barbosa, E.C.; Oliveira, J.G.; et al. Cultivable fungi present in deep-sea sediments of Antarctica: Taxonomy, diversity, and bioprospecting of bioactive compounds. Extremophiles 2020, 24, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Toghueo, R.M.K. Anti-leishmanial and anti-inflammatory agents from endophytes: A review. Nat. Prod.Bioprospect. 2019, 9, 311–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, L.; Shinwari, Z.K.; Iqrar, I.; Rahman, L.; Tanveer, F. An assessment on the role of endophytic microbes in the therapeutic potential of Fagonia indica. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Ungogo, M.A.; Ebiloma, G.U.; Ichoron, N.; Igoli, J.O.; de Koning, H.P.; Balogun, E.O. A review of the antimalarial, antitrypanosomal, and antileishmanial activities of natural compounds isolated from Nigerian flora. Front. Chem. 2020, 8, 617448. [Google Scholar] [CrossRef] [PubMed]
- Field, M.C.; Horn, D.; Fairlamb, A.H.; Ferguson, M.A.; Gray, D.W.; Read, K.D.; De Rycker, M.; Torrie, L.S.; Wyatt, P.G.; Wyllie, S.; et al. Anti-trypanosomatid drug discovery: An ongoing challenge and a continuing need. Nat. Rev. Microbiol. 2017, 15, 217–231. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, A.; Corbeil, A.; do Monte-Neto, R.L.; Fernandez-Prada, C. Of drugs and trypanosomatids: New tools and knowledge to reduce bottlenecks in drug discovery. Genes 2020, 11, 722. [Google Scholar] [CrossRef] [PubMed]
- Baell, J.B.; Holloway, G.A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 2010, 53, 2719–2740. [Google Scholar] [CrossRef] [Green Version]
- Baell, J.B. Feeling nature’s PAINS: Natural products, natural product drugs, and pan assay interference compounds (PAINS). J. Nat. Prod. 2016, 79, 616–628. [Google Scholar] [CrossRef]
- Baell, J.B.; Nissink, J.W.M. Seven year itch: Pan-assay interference compounds (PAINS) in 2017—Utility and limitations. ACS Chem. Biol. 2018, 13, 36–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsuno, K.; Burrows, J.N.; Duncan, K.; Hooft van Huijsduijnen, R.; Kaneko, T.; Kita, K.; Mowbray, C.E.; Schmatz, D.; Warner, P.; Slingsby, B.T. Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nat. Rev. Drug. Discov. 2015, 14, 751–758. [Google Scholar] [CrossRef] [PubMed]
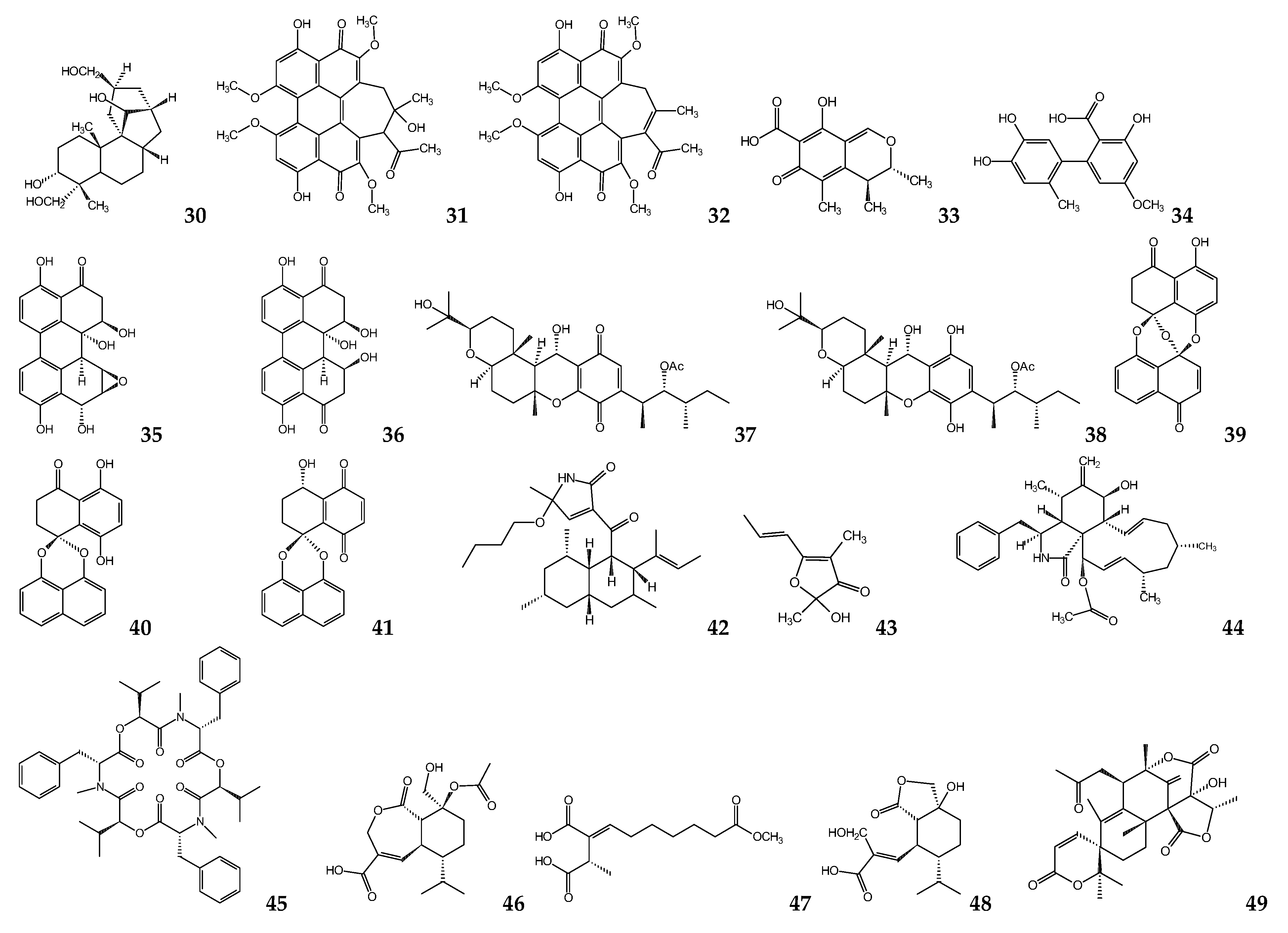
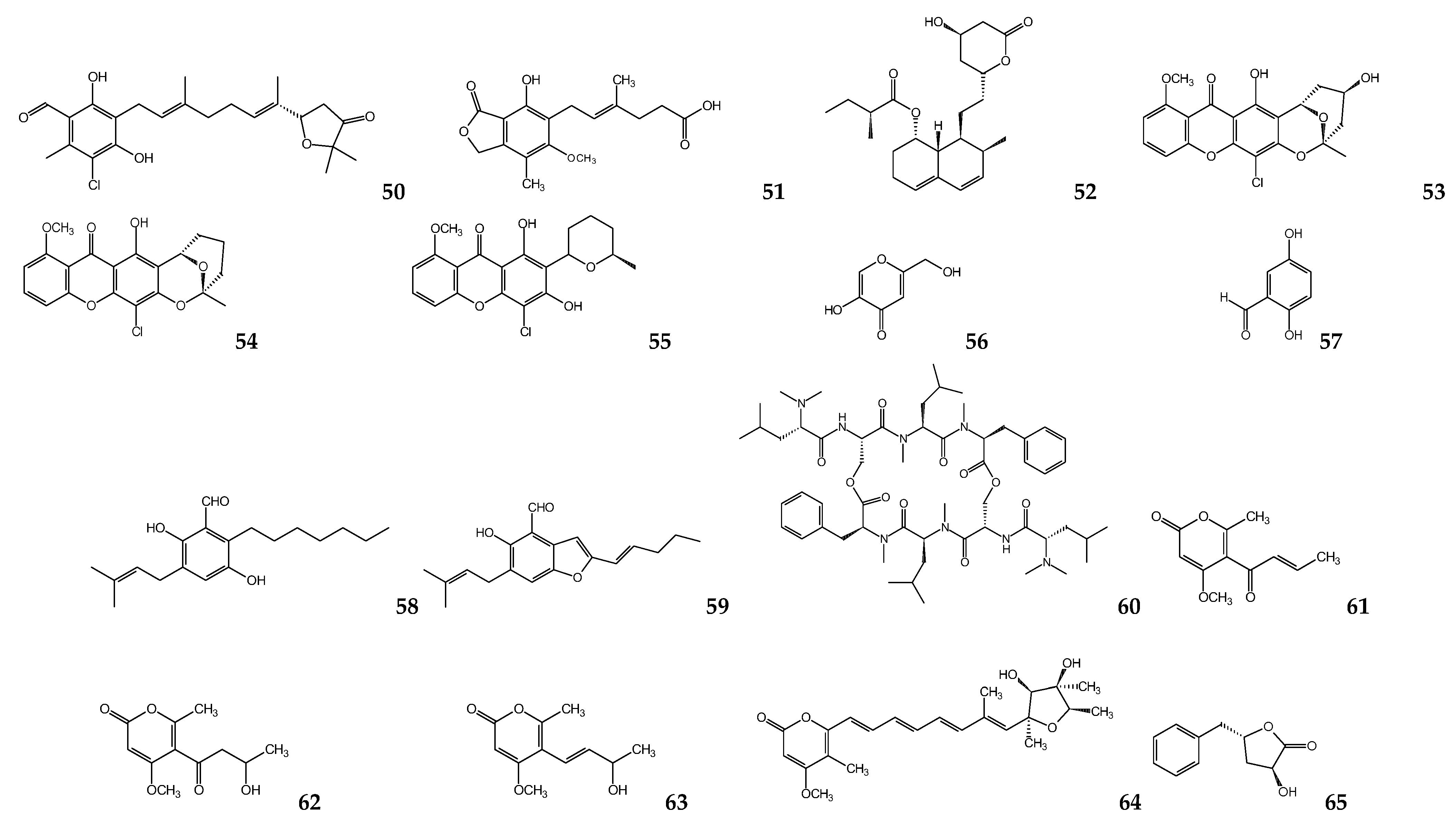
| Microbial Species | Metabolite(s) | Target Parasite | IC50 (μM) | Ref. |
|---|---|---|---|---|
| Bacteria | ||||
| Actinobacteria | ||||
| Micromonospora sp. | Manzamine A | L. donovania | 1.63 | [41,42] |
| Micromonospora sp. | Lobosamide A | T. b. bruceib | 0.80 | [44] |
| Streptomyces sp. | Valinomycin | L. majora T. b. bruceib | <0.11 0.0032 | [45] |
| Streptomyces axinellae | Tetromycin 1 | T. b. bruceib | 31.7 | [46] |
| Streptomyces sp. ICBG292 | Nigericin | L. donovania,c | 0.28 a 0.13 c | [48] |
| Streptomyces sp. ICBG233 | Dinactin | L. donovania,c | 0.03 a 0.02 c | [48] |
| Streptomyces diastatochromogenes | Oligomycin | T. bruceib T. cruzic | 3.8 0.52 | [50,53] |
| Streptomyces chartreusensis | Calcimycin | L.enriettiic L. majora | 0.01–0.25 0.16 | [56,58] |
| Actinokineospora sp. EG49 and Nocardiopsis sp. RV163 | 1,6-dihydroxyphe-nazine | T. bruceib | 19 | [59] |
| Actinokineospora sp. EG49 | Actinosporin A | T. b. bruceib | 15 | [60] |
| Actinokineosporaspheciospongiae | Fridamycin H | T. bruceib | 7.18 e 3.35 f | [61] |
| Cyanobateria | ||||
| Symploca sp. | Symplocamide A | L. donovanid T. cruzic | >9.5 >9.5 | [62] |
| Oscillatoria sp. | Venturamide A Venturamide B | T. cruzic | 14.6 15.8 | [63] |
| Oscillatoria nigro-viridis | Viridamine A | L. mexicanad T. cruzic | 1.5 1.1 | [64] |
| Oscillatoria sp. | Coibacin A | L. donovanid | 2.4 | [65] |
| Lyngbya majuscula | Dragonamide A Dragonamide E Herbamide B Almiramide B Almiramide C | L. donovanid | 6.5 5.1 6 2.4 2 | [66] [67,68] |
| Schizothrix sp. | Gallinamide A | L. donovanid | 9.3 | [69] |
| Nostoc78-12A | Nostocarboline | L. donovanid | 34.3 | [70] |
| Okenia sp. | Janadolide | T. bruceib T. b. rhodesienseb T. cruzic | 0.047 91.3 69.3 | [71,73] |
| Firmicutes | ||||
| Bacillus pumilus | 3-hydroxyacetyl-indole N-acetyl-β-oxotrypta-mine 3-formylindole | T. cruzic | 20.6 19.4 27 | [74] |
| Gammaproteobacteria | ||||
| Pseudomonas sp. | Pseudopyronine A Pseudopyronine B | L. donovanid | 9.8 4.65 | [75] |
| Fungi | ||||
| Ascomycetes | ||||
| Nigrospora sphaerica | Aphidicolin | L. donovania L. infantuma L. enriettiia L. majora L. donovanid | 157 40.8 90.4 55.4 35 | [76,77] |
| Hypocrella bambusae | Hypocrellin A Hypocrellin B | L. donovania | 0.5 24 | [78] |
| Penicillium janthinellum | Citrinin | L. mexicanaa | 160 e,l | [80] |
| Alternaria sp. | Altenusin | T. cruzig | 4.3 | [82] |
| Alternaria sp. (DC401) | 3,6a,9,10-pentahydroxy-7,8-epoxy-4-oxo-4,5,6,6a,6b,7,8,9-octahydro-perylene 3,6,6a,7,10-pentahdroxy-4,9-dioxo-4,5,6,6a,6b,7,8,9-octahydro-perylene | L. donovania | 7 12 | [83] |
| Cochliobolus sp. | Cochlioquinone A Isocochlioquinone A | L. amazonensisd | 1.7 4.1 | [84] |
| Edenia sp. | Preussomerin EG1 Palmarumycin CP17 Palmarumycin CP18 | L. donovanic,d | 0.12 d 1.34 d 0.62 d 23.5 c | [85,86] |
| Ascochyta salicorniae | Ascosalipyrrolidinone A 2,3-dihydro-2-hydroxy-2,4-dimethyl-5-trans-propenylfuran-3-one | T. cruzia T. b. rhodesienseb | 2.6 h 70.1 h 535 h 178 h | [87] |
| Diaporthe phaseolorum-92C | 18-des-hydroxy cytochalasin H | L. amazonensisa | 19.2 | [88] |
| Fusarium sp. KF611679 | Beauvericin | T. cruzi c | 2.43 | [89] |
| Nectria pseudotrichia | 10-acetyl trichoderonic acid A 6′-acetoxy-piliformic acid Hydroheptelidic acid | L. braziliensisc | 21.4 28.3 24.8 | [90] |
| Talaromycespurpurogenus H4 | Austin | T. cruzii | 73.1 | [91] |
| Acremonium egyptiacum | Ascofuranone | T. b. bruceij | 0.00013 | [93] |
| Penicillium sp. | Mycophenolic acid | T. bruceik T. bruceib T. cruzic | 0.021 0.51 1.6 | [99,100] |
| Penicillum citrinum | Mevastatin | L. donovania,c | 23.8 a 7.5 c | [102] |
| Chaetomium sp. | Chaetoxanthone A Chaetoxanthone B Chaetoxanthone C | T. b. rhodesienseb L. donovanic T. cruzic L. donovanic | 12.6 9.6 3.83 8 | [103] |
| Aspergillus oryzae | Kojic acid | L. amazonensisa,c | 239 a 193 c | [106] |
| Geosmithia langdonii | 2,5-dihydroxybenzaldehyde | L. donovania | 3.3 | [108] |
| Eurotium repens | Auroglaucin 2-(20,3-epoxy-10,30-heptadienyl)-6-hydroxy-5-(3-methyl-2-butenyl)benzaldehyde | L. donovania | 25 20.7 | [111] |
| Clonostachys sp. | IB-01212 | L. donovania L. pifanoic | 10.5 7.1 | [112] |
| Paecilomyces sp. FKI-3573 | Pyrenocines A Pyrenocine B Pyrenocine I Citreoviridin | T. b. bruceib | 0.57 3.35 8.56 1.2 | [113] |
| Paecilomyces sp. 7A22 | Harzialactone A | L. amazonensisa,c | 27.3 a | [114] |
| 94.6 c | ||||
| Basidiomycetes | ||||
| Phanerochaete sp. H2 | Austin | T. cruzii | 73.1 | [91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan-Bacab, M.J.; Reyes-Estebanez, M.M.; Camacho-Chab, J.C.; Ortega-Morales, B.O. Microorganisms as a Potential Source of Molecules to Control Trypanosomatid Diseases. Molecules 2021, 26, 1388. https://doi.org/10.3390/molecules26051388
Chan-Bacab MJ, Reyes-Estebanez MM, Camacho-Chab JC, Ortega-Morales BO. Microorganisms as a Potential Source of Molecules to Control Trypanosomatid Diseases. Molecules. 2021; 26(5):1388. https://doi.org/10.3390/molecules26051388
Chicago/Turabian StyleChan-Bacab, Manuel Jesús, María Manuela Reyes-Estebanez, Juan Carlos Camacho-Chab, and Benjamín Otto Ortega-Morales. 2021. "Microorganisms as a Potential Source of Molecules to Control Trypanosomatid Diseases" Molecules 26, no. 5: 1388. https://doi.org/10.3390/molecules26051388
APA StyleChan-Bacab, M. J., Reyes-Estebanez, M. M., Camacho-Chab, J. C., & Ortega-Morales, B. O. (2021). Microorganisms as a Potential Source of Molecules to Control Trypanosomatid Diseases. Molecules, 26(5), 1388. https://doi.org/10.3390/molecules26051388






