On the Use of Mechano-Chemically Modified Ground Tire Rubber (GTR) as Recycled and Sustainable Filler in Styrene-Butadiene Rubber (SBR) Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Grinding Protocols
2.1.2. Surface Modification of GTR
2.1.3. Composites Preparation
2.2. Characterization
2.2.1. Particle Size Distribution
2.2.2. Hydrophilicity Test
2.2.3. Fourier-Transform Infrared Spectroscopy
2.2.4. X-ray Photoelectron Spectroscopy
2.2.5. Scanning Electron Microscopy
2.2.6. Energy Dispersive X-ray Spectroscopy
2.2.7. Tensile Measurements
2.2.8. Cross-Link Density
2.2.9. Dynamic-Mechanical Analysis
3. Results and Discussion
3.1. Mechano-Chemical Modification of GTR
3.1.1. Particle Size Distribution
3.1.2. Surface Modification of GTR
3.2. Characterization of SBR–GTR Composites
3.2.1. Physico-Mechanical Properties
3.2.2. Dynamic Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ellen MacArthur Foundation. Available online: https://www.ellenmacarthurfoundation.org/explore/the-circular-economy-in-detail (accessed on 27 October 2020).
- Zefeng, W.; Yong, K.; Zhao, W.; Yi, C. Recycling waste tire rubber by water jet pulverization: Powder characteristics and reinforcing performance in natural rubber composites. J. Polym. Eng. 2018, 38, 51–62. [Google Scholar] [CrossRef]
- Hoyer, S.; Kroll, L.; Sykutera, D. Technology comparison for the production of fine rubber powder from end of life tyres. Procedia Manuf. 2020, 43, 193–200. [Google Scholar] [CrossRef]
- Petrella, A.; Notarnicola, M. Lightweight Cement Conglomerates Based on End-of-Life Tire Rubber: Effect of the Grain Size, Dosage and Addition of Perlite on the Physical and Mechanical Properties. Materials 2021, 14, 225. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Sun, C.; Yao, Z.; Jiang, H.; Zhang, J.; Ren, S. Utilization of wax residue as compatibilizer for asphalt with ground tire rubber/recycled polyethylene blends. Constr. Build. Mater. 2020, 230, 116966. [Google Scholar] [CrossRef]
- Ameli, A.; Maher, J.; Mosavi, A.; Nabipour, N.; Babagoli, R.; Norouzi, N. Performance evaluation of binders and Stone Matrix Asphalt (SMA) mixtures modified by Ground Tire Rubber (GTR), waste Polyethylene Terephthalate (PET) and Anti Stripping Agents (ASAs). Constr. Build. Mater. 2020, 251, 118932. [Google Scholar] [CrossRef]
- Yagneswaran, S.; Storer, W.J.; Tomar, N.; Chaur, M.N.; Echegoyen, L.; Smith, D.W. Surface-grafting of ground rubber tire by poly acrylic acid via self-initiated free radical polymerization and composites with epoxy thereof. Polym. Compos. 2013, 34, 769–777. [Google Scholar] [CrossRef]
- Formela, K.; Hejna, A.; Zedler, Ł.; Przybysz, M.; Ryl, J.; Saeb, M.R.; Piszczyk, Ł. Structural, thermal and physico-mechanical properties of polyurethane/brewers’ spent grain composite foams modified with ground tire rubber. Ind. Crop. Prod. 2017, 108, 844–852. [Google Scholar] [CrossRef]
- Aoudia, K.; Azem, S.; Hocine, N.A.; Gratton, M.; Pettarin, V.; Seghar, S. Recycling of waste tire rubber: Microwave devulcanization and incorporation in a thermoset resin. Waste Manag. 2017, 60, 471–481. [Google Scholar] [CrossRef]
- Hejna, A.; Korol, J.; Przybysz-Romatowska, M.; Zedler, Ł.; Chmielnicki, B.; Formela, K. Waste tire rubber as low-cost and environmentally-friendly modifier in thermoset polymers—A review. Waste Manag. 2020, 108, 106–118. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, F.D.B.; Gouveia, J.R.; De Camargo Filho, P.M.F.; Vidotti, S.E.; Scuracchio, C.H.; Amurin, L.G.; Valera, T.S. Blends of ground tire rubber devulcanized by microwaves/HDPE—Part A: Influence of devulcanization process. Polimeros 2015, 25, 256–264. [Google Scholar] [CrossRef]
- de Sousa, F.D.B.; Gouveia, J.R.; De Camargo Filho, P.M.F.; Vidotti, S.E.; Scuracchio, C.H.; Amurin, L.G.; Valera, T.S. Blends of ground tire rubber devulcanized by microwaves/HDPE-Part B: Influence of clay addition. Polimeros 2015, 25, 382–391. [Google Scholar] [CrossRef]
- Rosas, R.M.M.; Genescá, M.M.; Ballart-Prunell, J. Dielectric properties of various polymers (PVC, EVA, HDPE, and PP) reinforced with ground tire rubber (GTR). Sci. Eng. Compos. Mater. 2015, 22, 231–243. [Google Scholar] [CrossRef]
- Yehia, A.A.; Ismail, M.N.; Hefny, Y.A.; Abdel-Bary, E.M.; Mull, M.A. Mechano-chemical reclamation of waste rubber powder and its effect on the performance of NR and SBR vulcanizates. J. Elastomers Plast. 2004, 36, 109–123. [Google Scholar] [CrossRef]
- Carli, L.N.; Bianchi, O.; Mauler, R.S.; Crespo, J.S. Crosslinking kinetics of SBR composites containing vulcanized ground scraps as filler. Polym. Bull. 2011, 67, 1621–1631. [Google Scholar] [CrossRef]
- Formela, K.; Formela, M.; Thomas, S.; Haponiuk, J. Styrene-Butadiene Rubber/Modified Ground Tire Rubber Blends Co-Vulcanization: Effect of Accelerator Type. Macromol. Symp. 2016, 361, 64–72. [Google Scholar] [CrossRef]
- Santana, M.H.; Huete, M.; Lameda, P.; Araujo, J.; Verdejo, R.; López-Manchado, M.A. Design of a new generation of sustainable SBR compounds with good trade-off between mechanical properties and self-healing ability. Eur. Polym. J. 2018, 106, 273–283. [Google Scholar] [CrossRef]
- Araujo-Morera, J.; Santana, M.H.; Verdejo, R.; López-Manchado, M.A. Giving a Second Opportunity to Tire Waste: An Alternative Path for the Development of Sustainable Self-Healing Styrene–Butadiene Rubber Compounds Overcoming the Magic Triangle of Tires. Polymers 2019, 11, 2122. [Google Scholar] [CrossRef]
- Zedler, Ł.; Colom, X.; Cañavate, J.; Saeb, M.R.; Haponiuk, J.T.; Formela, K. Investigating the Impact of Curing System on Structure-Property Relationship of Natural Rubber Modified with Brewery By-Product and Ground Tire Rubber. Polymers 2020, 12, 545. [Google Scholar] [CrossRef]
- Fazli, A.; Rodrigue, D. Recycling Waste Tires into Ground Tire Rubber (GTR)/Rubber Compounds: A Review. J. Compos. Sci. 2020, 4, 103. [Google Scholar] [CrossRef]
- Majewska-Laks, K.; Sykutera, D.; Ościak, A. Reuse of ground tire rubber (GTR) as a filler of TPE matrix*. MATEC Web Conf. 2021, 332, 01003. [Google Scholar] [CrossRef]
- Zedler, Ł.; Przybysz-Romatowska, M.; Haponiuk, J.; Wang, S.; Formela, K. Modification of Ground Tire Rubber—Promising Approach for Development of Green Composites. J. Compos. Sci. 2019, 4, 2. [Google Scholar] [CrossRef]
- Maridass, B.; Gupta, B.R. Effect of Carbon Black on Devulcanized Ground Rubber Tire—Natural Rubber Vulcanizates: Cure Characteristics and Mechanical Properties. J. Elastomers Plast. 2006, 38, 211–229. [Google Scholar] [CrossRef]
- Cheng, X.; Long, D.; Huang, S.; Li, Z.; Guo, X. Time effectiveness of the low-temperature plasma surface modification of ground tire rubber powder. J. Adhes. Sci. Technol. 2015, 29, 1330–1340. [Google Scholar] [CrossRef]
- Li, J.; Yao, S.; Xiao, F.; Amirkhanian, S.N. Surface modification of ground tire rubber particles by cold plasma to improve compatibility in rubberised asphalt. Int. J. Pavement Eng. 2020, 1–12. [Google Scholar] [CrossRef]
- Cataldo, F.; Ursini, O.; Angelini, G. Surface oxidation of rubber crumb with ozone. Polym. Degrad. Stab. 2010, 95, 803–810. [Google Scholar] [CrossRef]
- Cao, X.-W.; Luo, J.; Cao, Y.; Yin, X.-C.; He, G.-J.; Peng, X.-F.; Xu, B.-P. Structure and properties of deeply oxidized waster rubber crumb through long time ozonization. Polym. Degrad. Stab. 2014, 109, 1–6. [Google Scholar] [CrossRef]
- Herrera-Sosa, E.S.; Martínez-Barrera, G.; Barrera-Díaz, C.E.; Cruz-Zaragoza, E.; Martí Nez-Barrera, G.; Dí, B.; Az, C. Waste Tire Particles and Gamma Radiation as Modifiers of the Mechanical Properties of Concrete. Adv. Mater. Sci. Eng. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Shanmugharaj, A.; Kim, J.K.; Ryu, S.H. UV surface modification of waste tire powder: Characterization and its influence on the properties of polypropylene/waste powder composites. Polym. Test. 2005, 24, 739–745. [Google Scholar] [CrossRef]
- Yehia, A.A.; Mull, M.A.; Ismail, M.N.; Hefny, Y.A.; Abdel-Bary, E.M. Effect of chemically modified waste rubber powder as a filler in natural rubber vulcanizates. J. Appl. Polym. Sci. 2004, 93, 30–36. [Google Scholar] [CrossRef]
- Colom, X.; Carrillo, F.; Cañavate, J. Composites reinforced with reused tyres: Surface oxidant treatment to improve the interfacial compatibility. Compos. Part A Appl. Sci. Manuf. 2007, 38, 44–50. [Google Scholar] [CrossRef]
- Colom, X.; Cañavate, J.; Carrillo, F.; Suñol, J.J. Effect of the particle size and acid pretreatments on compatibility and properties of recycled HDPE plastic bottles filled with ground tyre powder. J. Appl. Polym. Sci. 2009, 112, 1882–1890. [Google Scholar] [CrossRef]
- Gámez, J.F.H.; Hernández, E.H.; Céspedes, R.I.N.; Velázquez, M.G.N.; Rosales, S.G.S.; Corral, F.S.; Morones, P.G.; Tavizón, S.F.; De León, R.D.; Cepeda, L.F. Mechanical reinforcement of thermoplastic vulcanizates using ground tyre rubber modified with sulfuric acid. Polym. Compos. 2016, 39, 229–237. [Google Scholar] [CrossRef]
- Hernández, E.H.; Gámez, J.F.H.; Cepeda, L.F.; Muñoz, E.J.C.; Corral, F.S.; Rosales, S.G.S.; Velázquez, G.N.; Morones, P.G.; Martínez, D.I.S. Sulfuric acid treatment of ground tire rubber and its effect on the mechanical and thermal properties of polypropylene composites. J. Appl. Polym. Sci. 2017, 134, 1–7. [Google Scholar] [CrossRef]
- Naskar, A.K.; De, S.K.; Bhowmick, A.K. Thermoplastic elastomeric composition based on maleic anhydride-grafted ground rubber tire. J. Appl. Polym. Sci. 2002, 84, 370–378. [Google Scholar] [CrossRef]
- Aggour, Y.A.; Al-Shihri, A.S.; Bazzt, M.R. Surface Modification of Waste Tire by Grafting with Styrene and Maleic Anhydride. Open J. Polym. Chem. 2012, 2, 70–76. [Google Scholar] [CrossRef]
- Rungrodnimitchai, S.; Kotatha, D. Chemically modified ground tire rubber as fluoride ions adsorbents. Chem. Eng. J. 2015, 282, 161–169. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J., Jr. Statistical mechanics of cross-linked polymer networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Midhun Dominic, C.D.; Joseph, R.; Begum, P.M.S.; Joseph, M.; Padmanabhan, D.; Morris, L.A.; Kumar, A.S.; Formela, K. Cellulose nanofibers isolated from the cuscuta reflexa plant as a green reinforcement of natural rubber. Polymers 2020, 12, 814. [Google Scholar] [CrossRef]
- Liang, H.; Gagné, J.D.; Faye, A.; Rodrigue, D.; Brisson, J. Ground tire rubber (GTR) surface modification using thiol-ene click reaction: Polystyrene grafting to modify a GTR/polystyrene (PS) blend. Prog. Rubber Plast. Recycl. Technol. 2019, 36, 81–101. [Google Scholar] [CrossRef]
- Xinxing, Z.; Xiaoqing, Z.; Mei, L.; Canhui, L. Improvement of the properties of ground tire rubber (GTR)-filled nitrile rubber vulcanizates through plasma surface modification of GTR powder. J. Appl. Polym. Sci. 2009, 114, 1118–1125. [Google Scholar]
- Gibala, D.; Thomas, D.; Hamed, G.R. Cure and Mechanical Behavior of Rubber Compounds Containing Ground Vulcanizates: Part III. Tensile and Tear Strength. Rubber Chem. Technol. 1999, 72, 357–360. [Google Scholar] [CrossRef]
- Choi, S.-S.; Park, B.-H.; Song, H. Influence of filler type and content on properties of styrene-butadiene rubber(SBR) compound reinforced with carbon black or silica. Polym. Adv. Technol. 2004, 15, 122–127. [Google Scholar] [CrossRef]
- Nelson, P.; Kutty, S. Cure Characteristics and Mechanical Properties of Butadiene Rubber/Whole Tyre Reclaimed Rubber Blends. Prog. Rubber, Plast. Recycl. Technol. 2002, 18, 85–97. [Google Scholar] [CrossRef]
- Kang, M.-J.; Heo, Y.-J.; Jin, F.-L.; Park, S.-J. A review: Role of interfacial adhesion between carbon blacks and elastomeric materials. Carbon Lett. 2016, 18, 1–10. [Google Scholar] [CrossRef]
- Dai, S.-Y.; Ao, G.-Y.; Kim, M.-S. Properties of Carbon Black/SBR Rubber Composites Filled by Surface Modified Carbon Blacks. Carbon Lett. 2007, 8, 115–119. [Google Scholar] [CrossRef]
- Formela, K.; Hejna, A.; Piszczyk, Ł.; Saeb, M.R.; Colom, X. Processing and structure–property relationships of natural rubber/wheat bran biocomposites. Cellulose 2016, 23, 3157–3175. [Google Scholar] [CrossRef]
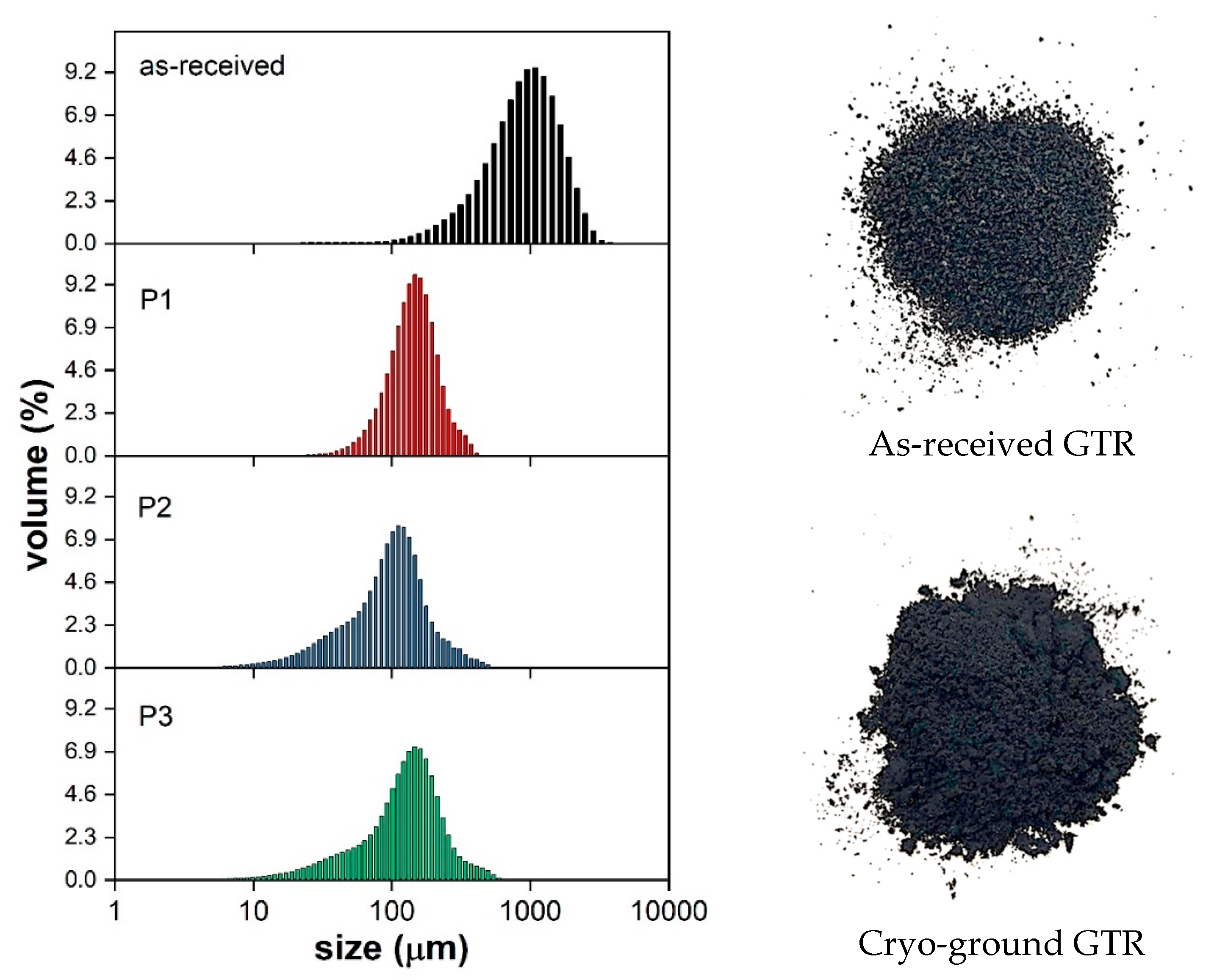

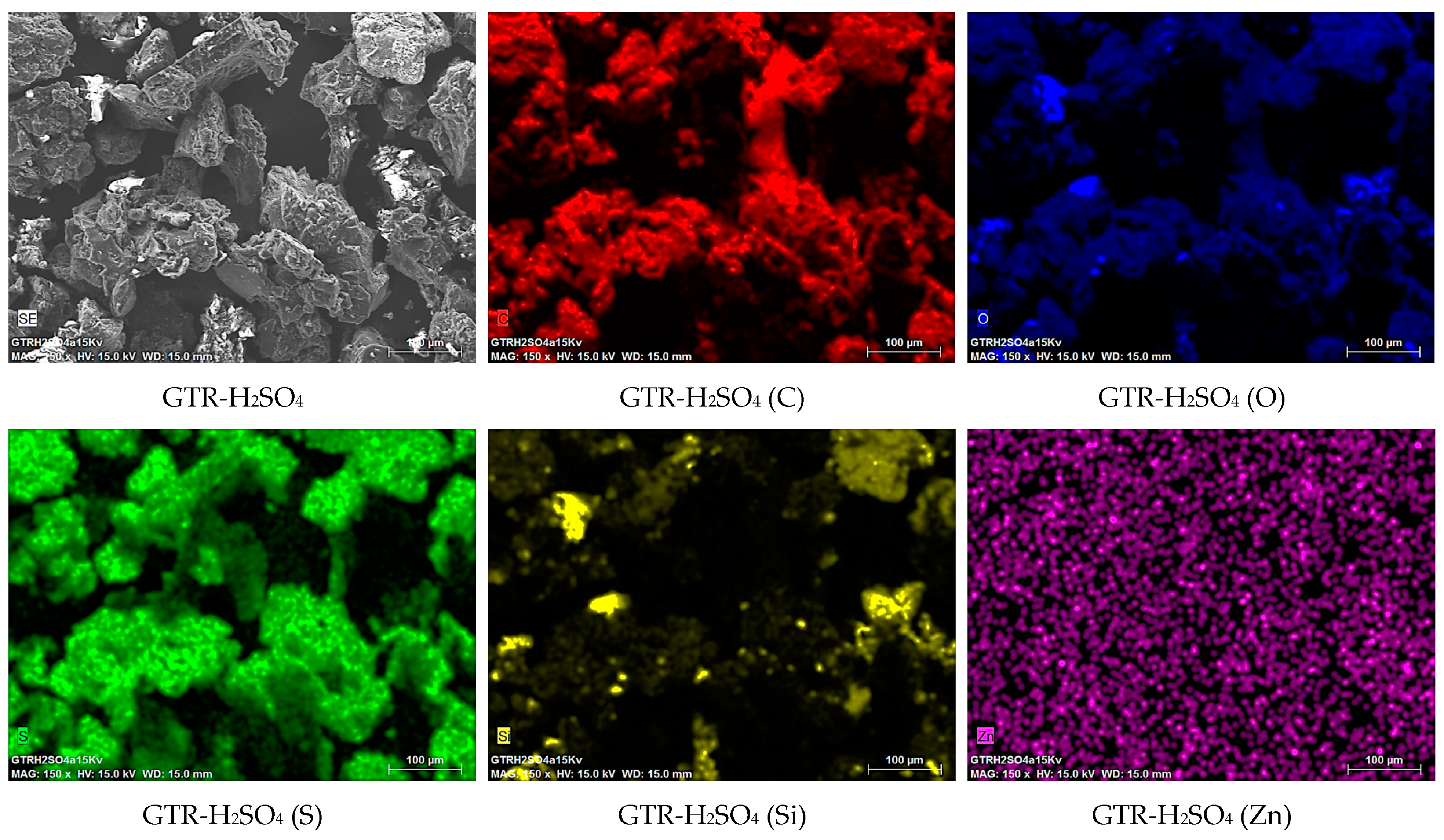





| Grinding Protocol | Number of Cycles | Grinding Time (min) | Intermediate Cooling (min) | Total Time (min) |
|---|---|---|---|---|
| P1 | 18 | 2 | 1 | 54 |
| P2 | 27 | 1 | 1 | 54 |
| P3 | 36 | 0.5 | 1 | 54 |
| Composite | Ingredient (phr) | ||||||
|---|---|---|---|---|---|---|---|
| SBR 1502 | ZnO | SA | CBS | S | GTR | m-GTR | |
| Unfilled | 100 | 5 | 1 | 1 | 1 | - | - |
| 10 GTR | 100 | 5 | 1 | 1 | 1 | 10 | - |
| 20 GTR | 100 | 5 | 1 | 1 | 1 | 20 | - |
| 30 GTR | 100 | 5 | 1 | 1 | 1 | 30 | - |
| 10 m-GTR | 100 | 5 | 1 | 1 | 1 | - | 10 |
| 20 m-GTR | 100 | 5 | 1 | 1 | 1 | - | 20 |
| 30 m-GTR | 100 | 5 | 1 | 1 | 1 | - | 30 |
| Grinding Protocol | Average Particle Size (μm) | Diameter on Cumulative Percentage (μm) | ||
|---|---|---|---|---|
| 10 | 50 | 90 | ||
| As-received | 1043 | 288 | 744 | 1439 |
| P1 | 153.8 | 81 | 145 | 236 |
| P2 | 115.0 | 33 | 99 | 171 |
| P3 | 147.1 | 42 | 135 | 254 |
| As-received GTR | GTR Cryo | GTR-H2O2 | GTR-H2SO4 | GTR-HNO3 | GTR-H2SO4/HNO3 | |
|---|---|---|---|---|---|---|
| XPS Analysis | ||||||
| Element | Normalized Content (%) | |||||
| C | 91.12 | 90.19 | 86.13 | 73.02 | 79.61 | 78.31 |
| O | 6.95 | 7.93 | 11.46 | 23.01 | 18.52 | 19.69 |
| S | 0.67 | 0.47 | - | 2.70 | 0.35 | 2.97 |
| Si | 1.25 | 1.41 | 2.40 | 1.28 | 1.51 | 1.43 |
| O/C | 0.076 | 0.088 | 0.133 | 0.315 | 0.233 | 0.251 |
| EDX Analysis | ||||||
| Element | Normalized Content (wt%) | |||||
| C | 82.97 | 81.58 | 82.71 | 68.88 | 79.39 | 85.58 |
| O | 8.71 | 10.08 | 10.64 | 21.82 | 16.30 | 11.56 |
| S | 1.95 | 1.68 | 1.56 | 7.26 | 0.75 | 0.67 |
| Si | 1.21 | 0.73 | 1.08 | 1.62 | 3.23 | 1.94 |
| Mg | 0.06 | 0.10 | 0.05 | 0.02 | 0.03 | 0.06 |
| Al | 0.18 | 0.12 | 0.14 | 0.10 | 0.12 | 0.18 |
| Ca | 0.55 | 0.25 | 0.06 | 0.04 | 0.01 | 0 |
| Fe | 1.59 | 2.60 | 1.63 | 0.09 | 0.15 | 0.01 |
| Zn | 2.78 | 2.86 | 2.13 | 0.17 | 0.02 | 0 |
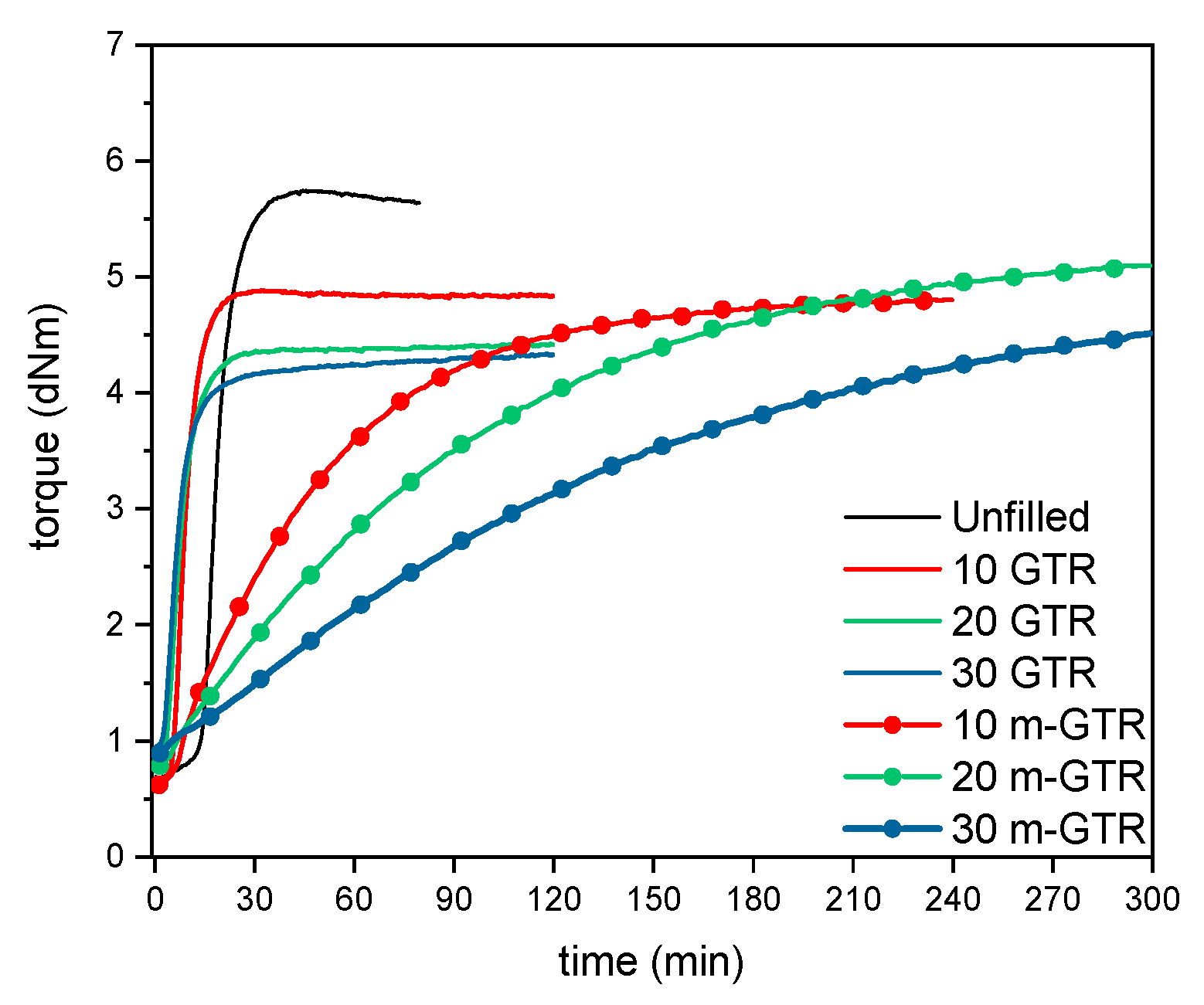
| Composite | ML (dNm) | MH (dNm) | ∆M (dNm) | ts2 (min) | t90 (min) |
|---|---|---|---|---|---|
| Unfilled | 0.7 | 5.8 | 5.1 | 17 | 27 |
| 10 GTR | 0.6 | 4.9 | 4.3 | 9 | 15 |
| 20 GTR | 0.8 | 4.4 | 3.6 | 8 | 16 |
| 30 GTR | 1 | 4.4 | 3.4 | 8 | 18 |
| 10 m-GTR | 0.6 | 3.6 | 3 | 34 | 52 |
| 20 m-GTR | 0.8 | 3.8 | 3 | 57 | 82 |
| 30 m-GTR | 0.9 | 3.9 | 3 | 100 | 136 |
| Composite | Unfilled (SBR) | 10 GTR | 20 GTR | 30 GTR | 10 m-GTR | 20 m-GTR | 30 m-GTR |
|---|---|---|---|---|---|---|---|
| M50 (MPa) | 0.52 ± 0.02 | 0.57 ± 0.04 | 0.5 ± 0.1 | 0.63 ± 0.03 | 0.52 ± 0.03 | 0.59 ± 0.03 | 0.58 ± 0.04 |
| M100 (MPa) | 0.67 ± 0.02 | 0.69 ± 0.01 | 0.6 ± 0.1 | 0.66 ± 0.03 | 0.62 ± 0.03 | 0.60 ± 0.03 | 0.6 ± 0.1 |
| M300 (MPa) | 0.97 ± 0.02 | 0.97 ± 0.01 | 0.8 ± 0.1 | 0.93 ± 0.02 | 0.75 ± 0.03 | 0.80 ± 0.02 | 0.81 ± 0.04 |
| M500 (MPa) | 1.33 ± 0.4 | 1.37 ± 0.02 | 1.3 ± 0.1 | 1.26 ± 0.02 | 0.94 ± 0.03 | 1.05 ± 0.02 | 1.08 ± 0.01 |
| σb (MPa) P | 1.4 ± 0.1 | 2.0 ± 0.1 | 2.5 ± 0.2 | 2.0 ± 0.1 | 2.8 ± 0.2 | 2.49 ± 0.02 | 2.0 ± 0.1 |
| εb (%) | 521 ± 18 | 732 ± 23 | 1016 ± 112 | 1017 ± 84 | 1187 ± 146 | 1305 ± 94 | 1388 ± 15 |
| ν × 10−6 (mol/cm3) | 22.7 ± 0.3 | 14.3 ± 0.2 | 6.4 ± 0.2 | 3.8 ± 0.1 | 6.5 ± 0.1 | 3.8 ± 0.1 | 2.4 ±0.1 |
| Hardness (Shore A) | 34 | 31 | 31 | 31 | 27 | 27 | 31 |
| Resilience (%) | 65 | 61 | 59 | 56 | 62 | 62 | 61 |
| Composite | Unfilled (SBR) | 10 GTR | 20 GTR | 30 GTR | 10 m-GTR | 20 m-GTR | 30 m-GTR |
|---|---|---|---|---|---|---|---|
| Tg (tanδ max) (°C) | −35.4 | −35.8 | −36.7 | −36.9 | −35.5 | −36.3 | −36.6 |
| tanδ at 25 °C | 0.1404 | 0.1653 | 0.1750 | 0.1871 | 0.1628 | 0.1941 | 0.2103 |
| E’ at 25 °C (MPa) | 2.68 | 3.16 | 3.59 | 3.91 | 3.22 | 3.98 | 4.73 |
| Adhesion factor, A | - | 0.2696 | 0.4421 | 0.6582 | 0.2503 | 0.5948 | 0.8498 |
| tanδ at −10 °C | 0.1663 | 0.1741 | 0.1676 | 0.1829 | 0.1665 | 0.1398 | 0.1428 |
| tanδ at 60 °C | 0.1241 | 0.1710 | 0.1950 | 0.2195 | 0.1721 | 0.2416 | 0.2742 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araujo-Morera, J.; Verdugo-Manzanares, R.; González, S.; Verdejo, R.; Lopez-Manchado, M.A.; Hernández Santana, M. On the Use of Mechano-Chemically Modified Ground Tire Rubber (GTR) as Recycled and Sustainable Filler in Styrene-Butadiene Rubber (SBR) Composites. J. Compos. Sci. 2021, 5, 68. https://doi.org/10.3390/jcs5030068
Araujo-Morera J, Verdugo-Manzanares R, González S, Verdejo R, Lopez-Manchado MA, Hernández Santana M. On the Use of Mechano-Chemically Modified Ground Tire Rubber (GTR) as Recycled and Sustainable Filler in Styrene-Butadiene Rubber (SBR) Composites. Journal of Composites Science. 2021; 5(3):68. https://doi.org/10.3390/jcs5030068
Chicago/Turabian StyleAraujo-Morera, Javier, Reyes Verdugo-Manzanares, Sergio González, Raquel Verdejo, Miguel Angel Lopez-Manchado, and Marianella Hernández Santana. 2021. "On the Use of Mechano-Chemically Modified Ground Tire Rubber (GTR) as Recycled and Sustainable Filler in Styrene-Butadiene Rubber (SBR) Composites" Journal of Composites Science 5, no. 3: 68. https://doi.org/10.3390/jcs5030068
APA StyleAraujo-Morera, J., Verdugo-Manzanares, R., González, S., Verdejo, R., Lopez-Manchado, M. A., & Hernández Santana, M. (2021). On the Use of Mechano-Chemically Modified Ground Tire Rubber (GTR) as Recycled and Sustainable Filler in Styrene-Butadiene Rubber (SBR) Composites. Journal of Composites Science, 5(3), 68. https://doi.org/10.3390/jcs5030068










