The Composition, Physicochemical Properties, Antioxidant Activity, and Sensory Properties of Estonian Honeys
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Samples
2.2. Melissopalynological Analysis
2.3. Physicochemical Parameters
2.4. Amino Acids
2.5. Bioactive Compounds
2.5.1. Total Polyphenol Content and Identification
2.5.2. Total Flavonoid Content
2.5.3. Antioxidant Activity
2.6. Sensory Analysis
2.6.1. Sample Preparation
2.6.2. Training of Assessors
2.6.3. Sensory Evaluation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Melissopalynological Analysis
3.2. Physicochemical Parameters
3.3. Amino Acids
3.4. Bioactive Compounds
3.4.1. Polyphenols and Flavonoids
3.4.2. Antioxidant Activity
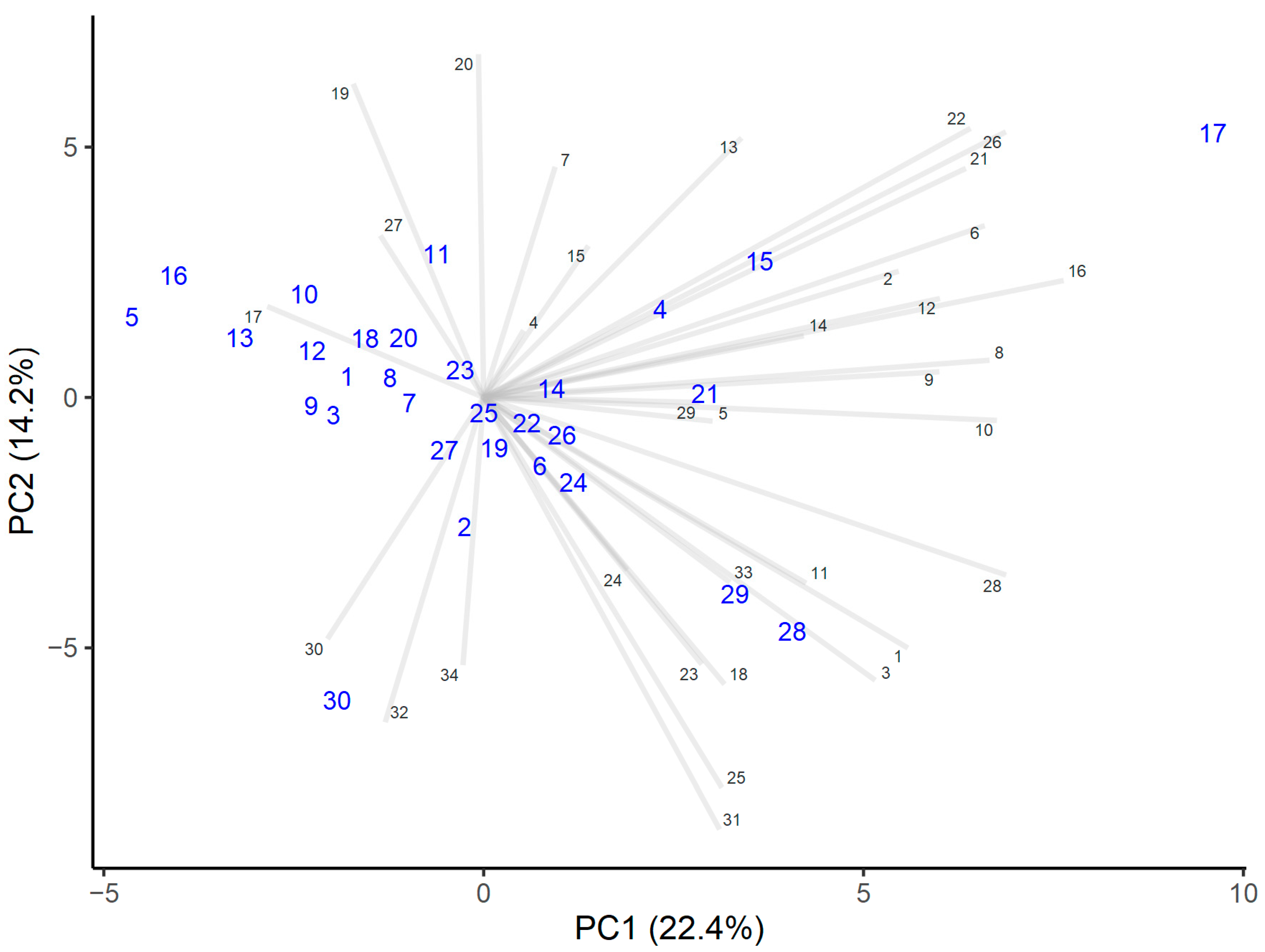
3.5. Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Bertoncelj, J.; Dobersek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Ferreira, I.C.; Aires, E.; Barreira, J.C.; Estevinho, L.M. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Lachman, J.; Hejtmánková, A.; Sykora, J.; Karban, J.; Orsák, M.; Rygerová, B. Contents of major phenolic and flavonoid antioxidants in selected Czech honey. Czech J. Food Sci. 2010, 28, 412–426. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Lamas, L.B.; Flórez, S.M.; Toyos, P.A.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef]
- Afrin, S.; Haneefa, S.M.; Fernandez-Cabezudo, M.J.; Giampieri, F.; Al-Ramadi, B.K.; Battino, M. Therapeutic and preventive properties of honey and its bioactive compounds in cancer: An evidence-based review. Nutr. Res. Rev. 2020, 33, 50–76. [Google Scholar] [CrossRef] [PubMed]
- Wilczyńska, A. Effect of filtration on colour, antioxidant activity and total phenolics of honey. LWT 2014, 57, 767–774. [Google Scholar] [CrossRef]
- Gašić, U.M.; Milojković-Opsenica, D.M.; Tešić, Ž.L. Polyphenols as Possible Markers of Botanical Origin of Honey. J. AOAC Int. 2017, 100, 852–861. [Google Scholar] [CrossRef]
- Blasa, M.; Candiracci, M.; Accorsi, A.; Piacentini, M.P.; Albertini, M.C.; Piatti, E. Raw Millefiori honey is packed full of antioxidants. Food Chem. 2006, 97, 217–222. [Google Scholar] [CrossRef]
- Kuś, P.M.; Congiu, F.; Teper, D.; Sroka, Z.; Jerković, I.; Tuberoso, C.I.G. Antioxidant activity, color characteristics, total phenol content and general HPLC fingerprints of six Polish unifloral honey types. LWT 2014, 55, 124–130. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Venskutonis, P.R. Floral Markers in Honey of Various Botanical and Geographic Origins: A Review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 620–634. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Jerković, I.; Sarais, G.; Congiu, F.; Marijanović, Z.; Kuś, P.M. Color evaluation of seventeen European unifloral honey types by means of spectrophotometrically determined CIE L∗Cab∗hab∘ chromaticity coordinates. Food Chem. 2014, 145, 284–291. [Google Scholar] [CrossRef]
- Piana, M.L.; Oddo, L.P.; Bentabol, A.; Bruneau, E.; Bogdanov, S.; Declerck, C.G. Sensory analysis applied to honey: State of the art. Apidologie 2004, 35, S26–S37. [Google Scholar] [CrossRef]
- Kortesniemi, M.; Rosenvald, S.; Laaksonen, O.; Vanag, A.; Ollikka, T.; Vene, K.; Yang, B. Sensory and chemical profiles of Finnish honeys of different botanical origins and consumer preferences. Food Chem. 2018, 246, 351–359. [Google Scholar] [CrossRef]
- Kütt, L.; Aasa, I. Teraviljasaak oli möödunud aastal tunamullusest suurem. Q. Bull. Stat. Estonia 2018, 1, 1–3. [Google Scholar]
- Kivima, E.; Seiman, A.; Pall, R.; Sarapuu, E.; Martverk, K.; Laos, K. Characterization of Estonian honeys by botanical origin. Proc. Estonian Acad. Sci. 2014, 63, 183. [Google Scholar] [CrossRef]
- Karise, R.; Raimets, R.; Bartkevics, V.; Pugajeva, I.; Pihlik, P.; Keres, I.; Williams, I.H.; Viinalass, H.; Mänd, M. Are pesticide residues in honey related to oilseed rape treatments? Chemosphere 2017, 188, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Laaniste, A.; Leito, I.; Rebane, R.; Lõhmus, R.; Lõhmus, A.; Punga, F.; Kruve, A.; Lohmus, R. Determination of neonicotinoids in Estonian honey by liquid chromatography–electrospray mass spectrometry. J. Environ. Sci. Health Part B 2016, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Puusepp, L.; Koff, T. Pollen analysis of honey from the Baltic region, Estonia. Grana 2014, 53, 54–61. [Google Scholar] [CrossRef]
- Rebane, R.; Herodes, K. Evaluation of the Botanical Origin of Estonian Uni- and Polyfloral Honeys by Amino Acid Content. J. Agric. Food Chem. 2008, 56, 10716–10720. [Google Scholar] [CrossRef]
- Kirs, E.; Pall, R.; Martverk, K.; Laos, K. Physicochemical and melissopalynological characterization of Estonian summer honeys. Procedia Food Sci. 2011, 1, 616–624. [Google Scholar] [CrossRef]
- Laos, K.; Kirs, E.; Pall, R.; Martverk, K. The crystallization behaviour of Estonian honeys. Agron. Res. 2011, 9, 427–432. [Google Scholar]
- Von Der Ohe, W.; Oddo, L.P.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized methods of melissopalynology. Apidologie 2004, 35, S18–S25. [Google Scholar] [CrossRef]
- International Honey Commission (IHC). Harmonized Methods of the International Honey Commission. 2009, pp. 1–63. Available online: https://www.ihc-platform.net/ihcmethods2009.pdf (accessed on 3 January 2021).
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Michalkiewicz, A.; Biesaga, M.; Pyrzynska, K. Solid-phase extraction procedure for determination of phenolic acids and some flavonols in honey. J. Chromatogr. A 2008, 1187, 18–24. [Google Scholar] [CrossRef]
- Bueno-Costa, F.M.; Zambiazi, R.C.; Bohmer, B.W.; Chaves, F.C.; da Silva, W.P.; Zanusso, J.T.; Dutra, I. Antibacterial and antioxidant activity of honeys from the state of Rio Grande do Sul, Brazil. LWT 2016, 65, 333–340. [Google Scholar] [CrossRef]
- Wesołowska, M.; Dżugan, M. The Use of the PHOTOCHEM Device in Evaluation of Antioxidant Activity of Polish Honey. Food Anal. Methods 2016, 10, 1568–1574. [Google Scholar] [CrossRef]
- European Committee for Standardization. Sensory Analysis—General Guidance for the Design of Test Rooms; European Standard: Brussels, Belgium, 2010. [Google Scholar]
- International Organization of Standardization. Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors; International Organization of Standardization: Genéve, Switzerland, 2012. [Google Scholar]
- International Organization of Standardization. Sensory Analysis—Methodology—General Guidance for Establishing a Sensory Profile; International Organization of Standardization: Genéve, Switzerland, 2016. [Google Scholar]
- The Council of The European Union. Council Directive 2001/110/EC of 20 December 2001 Relating to Honey. OJEC, 1 December 2002; 47–52. [Google Scholar]
- Liitmaa, M.; Sõukand, Ü. Meeproovide Kogumine Analüüsiks ja mee Kvaliteedi Määramine ja Jääkained Meeproovides; Estonian Environmental Research Centre: Tallinn, Estonia, 2009. [Google Scholar]
- Gleiter, R.; Horn, H.; Isengard, H.-D. Influence of type and state of crystallisation on the water activity of honey. Food Chem. 2006, 96, 441–445. [Google Scholar] [CrossRef]
- Oddo, L.P.; Piro, R.; Bruneau, É.; Guyot-Declerck, C.; Ivanov, T.; Piskulová, J.; Flamini, C.; Lheritier, J.; Morlot, M.; Russmann, H.; et al. Main European unifloral honeys: Descriptive sheets. Apidologie 2004, 35, S38–S81. [Google Scholar] [CrossRef]
- Salonen, A.; Virjamo, V.; Tammela, P.; Fauch, L.; Julkunen-Tiitto, R. Screening bioactivity and bioactive constituents of Nordic unifloral honeys. Food Chem. 2017, 237, 214–224. [Google Scholar] [CrossRef]
- Piotraszewska-Pająk, A.; Gliszczyńska-Świgło, A.; Piotraszewska-Pająk, A. Directions of Colour Changes of Nectar Honeys Depending on Honey Type and Storage Conditions. J. Apic. Sci. 2015, 59, 51–61. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.X. Chemical Composition, Characterization, and Differentiation of Honey Botanical and Geographical Origins. In Advances in Food and Nutrition Research; Elsevier Inc.: San Diego, CA, USA, 2011; Volume 62, pp. 89–137. [Google Scholar]
- Crane, E. A Book of Honey; International Bee Research Association: Cardiff, UK; Oxford University Press: Oxford, UK, 1980. [Google Scholar]
- Bogdanov, S.; Ruoff, K.; Oddo, L.P. Physico-chemical methods for the characterisation of unifloral honeys: A review. Apidologie 2004, 35, S4–S17. [Google Scholar] [CrossRef]
- Chen, H.; Jin, L.; Chang, Q.; Peng, T.; Hu, X.; Fan, C.; Pang, G.; Lu, M.; Wang, W. Discrimination of botanical origins for Chinese honey according to free amino acids content by high-performance liquid chromatography with fluorescence detection with chemometric approaches. J. Sci. Food Agric. 2017, 97, 2042–2049. [Google Scholar] [CrossRef] [PubMed]
- Escuredo, O.; Míguez, M.; Fernández-González, M.; Seijo, M.C. Nutritional value and antioxidant activity of honeys produced in a European Atlantic area. Food Chem. 2013, 138, 851–856. [Google Scholar] [CrossRef] [PubMed]
- A-Rahaman, N.L.; Chua, L.S.; Sarmidi, M.R.; Aziz, R. Physicochemical and radical scavenging activities of honey samples from Malaysia. Agric. Sci. 2013, 4, 46–51. [Google Scholar] [CrossRef]
- Khalil, I.; Moniruzzaman, M.; Boukraâ, L.; Benhanifia, M.; Islam, A.; Islam, N.; Sulaiman, S.A.; Gan, S.H. Physicochemical and Antioxidant Properties of Algerian Honey. Molecules 2012, 17, 11199–11215. [Google Scholar] [CrossRef]
- Trautvetter, S.; Koelling-Speer, I.; Speer, K. Confirmation of phenolic acids and flavonoids in honeys by UPLC-MS. Apidologie 2009, 40, 140–150. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Martos, I.; Ferreres, F.; Radovic, B.S.; Anklam, E. HPLC flavonoid profiles as markers for the botanical origin of European unifloral honeys. J. Sci. Food Agric. 2001, 81, 485–496. [Google Scholar] [CrossRef]
- Anupama, D.; Bhat, K.; Sapna, V. Sensory and physico-chemical properties of commercial samples of honey. Food Res. Int. 2003, 36, 183–191. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Xiaowei, H.; Jiyong, S.; Mariod, A.A. Discrimination of honeys using colorimetric sensor arrays, sensory analysis and gas chromatography techniques. Food Chem. 2016, 206, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Stolzenbach, S.; Byrne, D.V.; Bredie, W.L. Sensory local uniqueness of Danish honeys. Food Res. Int. 2011, 44, 2766–2774. [Google Scholar] [CrossRef]
- Aparna, A.; Rajalakshmi, D. Honey—Its characteristics, sensory aspects, and applications. Food Rev. Int. 1999, 15, 455–471. [Google Scholar] [CrossRef]

| Number | Polyphenol | (M-H)- | Number | Polyphenol | (M-H)- |
|---|---|---|---|---|---|
| 1 | Shikimic acid | 173.05 | 18 | Salicylic acid | 137.02 |
| 2 | Gallic acid | 169.01 | 19 | Abscisic acid | 263.13 |
| 3 | Protocatechuic acid | 153.02 | 20 | Abscisic acid D1 | 263.13 |
| 4 | Protocatechuic and gentisic acid D1 | 153.02 | 21 | Abscisic acid D2 | 263.13 |
| 5 | Chlorogenic acid | 353.09 | 22 | Abscisic acid D3 | 263.13 |
| 6 | Chlorogenic acid D1 | 353.09 | 23 | Luteolin | 285.05 |
| 7 | Catechin | 289.08 | 24 | Luteolin and kaempferol D1 | 285.05 |
| 8 | 4-hydroxybenzoic acid | 137.02 | 25 | Quercetin | 301.03 |
| 9 | Gentisic acid | 153.02 | 26 | Cinnamic acid D1 | 147.05 |
| 10 | Caffeic acid | 179.03 | 27 | Cinnamic acid D2 | 147.05 |
| 11 | Caffeic acid D1 | 179.03 | 28 | Apigenin | 269.05 |
| 12 | Coumaric acid | 163.04 | 29 | Naringenin | 271.07 |
| 13 | Coumaric acid D1 | 163.04 | 30 | Naringenin D1 | 271.07 |
| 14 | Ferulic acid | 193.05 | 31 | Kaempferol | 285.04 |
| 15 | Ferulic acid D1 | 193.05 | 32 | Chrysin | 253.05 |
| 16 | Myricetin | 317.03 | 33 | Chrysin D1 | 253.05 |
| 17 | Morin | 301.05 | 34 | Galangin | 269.05 |
| Sample | Electrical Conductivity (mS cm−1) | Moisture (%) | Invertase Activity (U kg−1) | Free Acidity (mmol kg−1) | Diastase (Schade Unit) | HMF (mg kg−1) | Fructose (g 100 g−1) | Glucose (g 100 g−1) | F/G | TPC (mg GAE 100 g−1) | TFC (mg QE 100 g−1) | ACW (mg AAE 100 g−1) | ACL (mg TE 100 g−1) | L* | a* | b* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.5 | 18.2 | 188.0 | 17.0 | 32.2 | 3.7 | 38.0 | 35.7 | 1.1 | 37.5 | 3.0 | 84.5 | 23.9 | 85.4 | −0.3 | 43.4 |
| 2 | 0.4 | 19.8 | 126.0 | 40.0 | 33.8 | 6.8 | 36.5 | 34.8 | 1.1 | 53.6 | 3.4 | 118.4 | 31.2 | 72.8 | 5.8 | 44.0 |
| 3 | 0.3 | 16.8 | 133.0 | 20.0 | 29.2 | 8.4 | 38.1 | 33.1 | 1.2 | 38.3 | 3.2 | 65.1 | 20.1 | 84.9 | 1.3 | 38.6 |
| 4 | 0.6 | 20.0 | 206.0 | 35.0 | 48.6 | 6.1 | 37.8 | 32.1 | 1.2 | 53.9 | 3.5 | 55.5 | 17.3 | 82.3 | 2.3 | 42.3 |
| 5 | 0.3 | 20.0 | 124.0 | 18.0 | 22.0 | 5.0 | 37.3 | 37.8 | 1.0 | 28.3 | 2.5 | 37.8 | 18.4 | 88.1 | −1.7 | 26.7 |
| 6 | 0.4 | 15.6 | 132.0 | 21.0 | 26.0 | 7.2 | 39.2 | 34.0 | 1.2 | 41.1 | 3.1 | 56.0 | 20.1 | 84.3 | 0.3 | 39.2 |
| 7 | 0.4 | 19.1 | 167.0 | 26.0 | 34.4 | 8.2 | 38.5 | 35.4 | 1.1 | 50.4 | 4.3 | 200.4 | 40.7 | 78.4 | 6.2 | 48.9 |
| 8 | 0.2 | 18.0 | 199.0 | 25.0 | 40.8 | 6.9 | 39.8 | 36.2 | 1.1 | 28.8 | 1.9 | 81.4 | 16.5 | 88.4 | −1.1 | 29.1 |
| 9 | 0.3 | 19.5 | 50.6 | 38.0 | 19.6 | 19.5 | 36.8 | 35.6 | 1.0 | 34.8 | 3.3 | 66.5 | 16.9 | 85.6 | −0.7 | 31.6 |
| 10 | 0.2 | 20.3 | 118.0 | 16.0 | 21.6 | 6.0 | 38.7 | 36.4 | 1.1 | 27.9 | 3.1 | 77.0 | 17.2 | 88.6 | −1.6 | 27.5 |
| 11 | 0.2 | 19.8 | 114.0 | 22.0 | 26.3 | 11.5 | 36.5 | 35.3 | 1.0 | 30.9 | 3.1 | 96.8 | 20.5 | 85.4 | −0.1 | 33.5 |
| 12 | 0.3 | 18.3 | 93.5 | 20.0 | 22.5 | 8.9 | 39.2 | 36.9 | 1.1 | 35.2 | 2.8 | 84.8 | 18.2 | 86.6 | −0.6 | 36.2 |
| 13 | 0.3 | 16.4 | 63.9 | 18.0 | 21.1 | 11.8 | 38.3 | 35.5 | 1.1 | 29.8 | 2.1 | 78.4 | 18.1 | 88.0 | −1.4 | 30.9 |
| 14 | 0.5 | 20.4 | 168.0 | 25.0 | 24.6 | 5.0 | 38.4 | 33.0 | 1.2 | 49.0 | 3.9 | 140.6 | 31.5 | 83.5 | 1.6 | 51.8 |
| 15 | 0.4 | 19.1 | 153.0 | 27.0 | 30.9 | 6.5 | 37.8 | 32.9 | 1.1 | 48.4 | 3.2 | 135.3 | 27.0 | 83.8 | 2.7 | 43.5 |
| 16 | 0.2 | 17.4 | 82.8 | 12.0 | 17.7 | 9.1 | 38.8 | 35.4 | 1.1 | 26.2 | 2.4 | 69.2 | 19.4 | 90.4 | −1.1 | 29.6 |
| 17 | 0.7 | 20.4 | 114.0 | 39.0 | 58.8 | 7.8 | 39.7 | 31.6 | 1.3 | 88.7 | 6.4 | 245.3 | 60.7 | 65.3 | 9.1 | 37.8 |
| 18 | 0.2 | 18.5 | 74.1 | 21.0 | 25.6 | 9.2 | 39.5 | 37.0 | 1.1 | 33.7 | 3.5 | 87.0 | 20.7 | 84.4 | 0.3 | 35.8 |
| 19 | 0.3 | 19.3 | 119.0 | 23.0 | 24.9 | 7.2 | 37.5 | 36.8 | 1.0 | 40.3 | 3.3 | 113.9 | 21.4 | 85.4 | 2.0 | 44.4 |
| 20 | 0.4 | 17.6 | 184.0 | 16.0 | 28.1 | 5.6 | 38.4 | 34.2 | 1.1 | 38.7 | 3.0 | 87.5 | 17.8 | 86.3 | 0.4 | 40.6 |
| 21 | 0.7 | 17.8 | 182.0 | 23.0 | 22.5 | 9.4 | 38.5 | 30.2 | 1.3 | 52.6 | 4.3 | 105.9 | 22.8 | 81.2 | 3.0 | 46.5 |
| 22 | 0.5 | 18.2 | 102.0 | 20.0 | 15.4 | 5.1 | 41.1 | 29.2 | 1.4 | 40.2 | 3.8 | 85.7 | 14.4 | 85.5 | 0.6 | 40.8 |
| 23 | 0.8 | 15.6 | 168.0 | 14.0 | 21.1 | 6.5 | 41.5 | 29.9 | 1.4 | 46.4 | 4.2 | 82.0 | 16.6 | 83.5 | 1.9 | 46.6 |
| 24 | 0.7 | 19.9 | 172.0 | 31.0 | 39.1 | 9.2 | 44.5 | 37.4 | 1.2 | 50.7 | 5.7 | 176.9 | 31.9 | 82.8 | 3.8 | 47.6 |
| 25 | 0.3 | 20.7 | 145.0 | 23.0 | 26.4 | 8.4 | 46.4 | 37.9 | 1.2 | 35.6 | 2.8 | 105.4 | 25.2 | 87.1 | −0.2 | 36.9 |
| 26 | 0.3 | 20.9 | 124.0 | 17.0 | 16.0 | 5.9 | 44.9 | 39.7 | 1.1 | 30.8 | 2.4 | 138.3 | 27.7 | 86.2 | 0.6 | 34.9 |
| 27 | 0.3 | 20.0 | 189.0 | 21.0 | 25.4 | 7.8 | 45.9 | 36.9 | 1.2 | 34.7 | 2.7 | 96.6 | 22.8 | 86.7 | 0.1 | 36.1 |
| 28 | 0.5 | 18.3 | 50.4 | 43.0 | 35.5 | 5.9 | 37.6 | 32.6 | 1.2 | 68.6 | 5.3 | 299.3 | 32.5 | 76.8 | 12.5 | 60.3 |
| 29 | 0.5 | 18.9 | 231.0 | 35.0 | 36.2 | 10.0 | 37.4 | 32.6 | 1.1 | 56.5 | 4.9 | 311.2 | 37.0 | 76.7 | 12.3 | 58.7 |
| 30 | 0.2 | 19.2 | 228.0 | 23.0 | 37.1 | 3.5 | 40.7 | 38.8 | 1.0 | 26.8 | 2.6 | 73.7 | 17.1 | 88.3 | −1.2 | 25.6 |
| Average | 0.4 | 18.8 | 140.0 | 24.3 | 28.8 | 7.7 | 39.4 | 34.8 | 1.1 | 41.9 | 3.5 | 115.2 | 24.2 | 83.7 | 1.9 | 39.6 |
| SD | 0.2 | 1.4 | 49.8 | 8.2 | 9.7 | 3.0 | 2.7 | 2.7 | 0.1 | 13.8 | 1.0 | 67.9 | 9.6 | 5.3 | 3.8 | 8.8 |
| Min. | 0.2 | 15.6 | 50.4 | 12.0 | 15.4 | 3.5 | 36.5 | 29.2 | 1.0 | 26.2 | 1.9 | 37.8 | 14.4 | 65.3 | −1.7 | 25.6 |
| Max. | 0.8 | 20.9 | 231.0 | 43.0 | 58.8 | 19.5 | 46.4 | 39.7 | 1.4 | 88.7 | 6.4 | 311.2 | 60.7 | 90.4 | 12.5 | 60.3 |
| EC | M | IA | FA | D | HMF | F/G | TPC | TFC | ACW | ACL | AA | L* | a* | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | −0.08 | |||||||||||||
| IA | 0.28 | 0.09 | ||||||||||||
| FA | 0.34 | 0.36 | −0.04 | |||||||||||
| D | 0.41 | 0.23 | 0.40 | 0.64 | ||||||||||
| HMF | −0.18 | −0.05 | −0.43 | 0.25 | −0.17 | |||||||||
| F/G | 0.68 | −0.21 | 0.20 | −0.01 | 0.03 | −0.17 | ||||||||
| TPC | 0.77 | 0.12 | 0.07 | 0.72 | 0.66 | −0.06 | 0.41 | |||||||
| TFC | 0.78 | 0.13 | 0.04 | 0.60 | 0.51 | 0.03 | 0.43 | 0.88 | ||||||
| ACW | 0.42 | 0.24 | 0.06 | 0.62 | 0.46 | 0.01 | 0.17 | 0.73 | 0.75 | |||||
| ACL | 0.46 | 0.37 | 0.06 | 0.56 | 0.61 | −0.02 | 0.14 | 0.80 | 0.72 | 0.80 | ||||
| AA | 0.39 | 0.07 | 0.04 | 0.73 | 0.54 | −0.04 | 0.12 | 0.74 | 0.71 | 0.86 | 0.62 | |||
| L* | −0.62 | −0.18 | −0.02 | −0.74 | −0.65 | −0.01 | −0.24 | −0.93 | −0.80 | −0.71 | −0.85 | −0.66 | ||
| a* | 0.55 | 0.11 | 0.08 | 0.73 | 0.54 | −0.02 | 0.23 | 0.85 | 0.80 | 0.92 | 0.75 | 0.89 | −0.85 | |
| b* | 0.65 | −0.06 | 0.20 | 0.47 | 0.24 | −0.12 | 0.36 | 0.69 | 0.69 | 0.71 | 0.46 | 0.71 | −0.57 | 0.79 |
| Sample | Ala | Asp | GABA | Gln | Glu | Gly | Ile | Leu | Lys | Phe | Pro | Ser | Thr | Tyr | Val |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 9 | 7 | 4 | 18 | 19 | 2 | 4 | 6 | 13 | 27 | 334 | 8 | 5 | 8 | 8 |
| 2 | 11 | 11 | 5 | 26 | 20 | 3 | 7 | 13 | 22 | 73 | 552 | 11 | 5 | 9 | 9 |
| 3 | 12 | 15 | 6 | 48 | 27 | 3 | 9 | 13 | 19 | 98 | 512 | 15 | 8 | 9 | 13 |
| 4 | 10 | 14 | 2 | 23 | 18 | 3 | 3 | 4 | 20 | 17 | 622 | 12 | 7 | 7 | 8 |
| 5 | 4 | 6 | 2 | 28 | 9 | 0 | 2 | 1 | 10 | 26 | 257 | 5 | 2 | 8 | 4 |
| 6 | 9 | 7 | 4 | 30 | 13 | 2 | 6 | 8 | 18 | 283 | 426 | 10 | 5 | 33 | 8 |
| 7 | 11 | 10 | 1 | 42 | 23 | 2 | 18 | 28 | 21 | 92 | 643 | 11 | 6 | 22 | 12 |
| 8 | 8 | 6 | 5 | 24 | 15 | 2 | 4 | 6 | 20 | 19 | 543 | 9 | 5 | 11 | 7 |
| 9 | 10 | 13 | 6 | 53 | 22 | 3 | 8 | 8 | 20 | 36 | 399 | 10 | 5 | 8 | 6 |
| 10 | 7 | 10 | 4 | 31 | 15 | 2 | 4 | 5 | 19 | 15 | 290 | 9 | 5 | 4 | 7 |
| 11 | 8 | 8 | 5 | 29 | 10 | 3 | 4 | 4 | 21 | 16 | 389 | 10 | 5 | 6 | 6 |
| 12 | 8 | 9 | 5 | 35 | 15 | 3 | 6 | 11 | 18 | 83 | 367 | 12 | 6 | 9 | 8 |
| 13 | 8 | 11 | 4 | 44 | 20 | 2 | 7 | 8 | 15 | 20 | 350 | 12 | 7 | 7 | 10 |
| 14 | 11 | 9 | 4 | 35 | 18 | 3 | 11 | 27 | 15 | 230 | 447 | 10 | 8 | 18 | 12 |
| 15 | 12 | 10 | 7 | 42 | 20 | 4 | 25 | 43 | 20 | 27 | 480 | 12 | 10 | 38 | 15 |
| 16 | 6 | 7 | 4 | 24 | 12 | 2 | 4 | 0 | 15 | 19 | 307 | 9 | 5 | 6 | 7 |
| 17 | 24 | 19 | 14 | 28 | 32 | 7 | 9 | 11 | 31 | 33 | 956 | 20 | 14 | 13 | 16 |
| 18 | 8 | 8 | 6 | 35 | 15 | 4 | 5 | 9 | 23 | 36 | 430 | 11 | 5 | 8 | 7 |
| 19 | 12 | 11 | 6 | 44 | 24 | 4 | 7 | 8 | 36 | 49 | 638 | 13 | 7 | 10 | 10 |
| 20 | 9 | 13 | 2 | 39 | 15 | 2 | 4 | 5 | 15 | 22 | 492 | 11 | 5 | 5 | 8 |
| 21 | 13 | 19 | 3 | 13 | 22 | 2 | 3 | 5 | 11 | 22 | 661 | 12 | 5 | 4 | 7 |
| 22 | 10 | 12 | 3 | 9 | 16 | 3 | 3 | 5 | 8 | 18 | 471 | 11 | 4 | 6 | 6 |
| 23 | 10 | 9 | 3 | 3 | 21 | 2 | 2 | 5 | 2 | 16 | 375 | 8 | 3 | 8 | 4 |
| 24 | 18 | 46 | 4 | 41 | 49 | 7 | 9 | 10 | 26 | 31 | 757 | 31 | 13 | 13 | 15 |
| 25 | 8 | 13 | 3 | 33 | 14 | 2 | 4 | 5 | 21 | 30 | 475 | 11 | 4 | 6 | 7 |
| 26 | 9 | 15 | 6 | 37 | 18 | 1 | 4 | 3 | 23 | 13 | 525 | 12 | 5 | 4 | 8 |
| 27 | 7 | 7 | 4 | 24 | 12 | 2 | 4 | 4 | 14 | 36 | 320 | 8 | 4 | 9 | 6 |
| 28 | 26 | 26 | 9 | 56 | 37 | 7 | 16 | 32 | 33 | 294 | 1328 | 28 | 11 | 28 | 19 |
| 29 | 18 | 20 | 6 | 46 | 29 | 4 | 9 | 20 | 30 | 205 | 1023 | 18 | 7 | 18 | 13 |
| 30 | 9 | 16 | 5 | 36 | 22 | 3 | 15 | 22 | 33 | 42 | 589 | 15 | 6 | 19 | 11 |
| Amino Acids | Ala | Asp | GABA | Gln | Glu | Gly | Ile | Leu | Lys | Phe | Pro | Ser | Thr | Tyr | Val |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPC | 0.88 | 0.47 | 0.54 | 0.07 | 0.63 | 0.73 | 0.33 | 0.41 | 0.33 | 0.37 | 0.77 | 0.58 | 0.71 | 0.37 | 0.67 |
| TFC | 0.86 | 0.67 | 0.42 | 0.09 | 0.77 | 0.78 | 0.27 | 0.33 | 0.32 | 0.34 | 0.74 | 0.71 | 0.69 | 0.27 | 0.63 |
| ACW | 0.85 | 0.54 | 0.51 | 0.40 | 0.66 | 0.67 | 0.47 | 0.55 | 0.56 | 0.49 | 0.87 | 0.70 | 0.63 | 0.42 | 0.74 |
| ACL | 0.72 | 0.38 | 0.59 | 0.22 | 0.54 | 0.61 | 0.39 | 0.42 | 0.46 | 0.28 | 0.63 | 0.50 | 0.68 | 0.33 | 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kivima, E.; Tanilas, K.; Martverk, K.; Rosenvald, S.; Timberg, L.; Laos, K. The Composition, Physicochemical Properties, Antioxidant Activity, and Sensory Properties of Estonian Honeys. Foods 2021, 10, 511. https://doi.org/10.3390/foods10030511
Kivima E, Tanilas K, Martverk K, Rosenvald S, Timberg L, Laos K. The Composition, Physicochemical Properties, Antioxidant Activity, and Sensory Properties of Estonian Honeys. Foods. 2021; 10(3):511. https://doi.org/10.3390/foods10030511
Chicago/Turabian StyleKivima, Evelin, Kristel Tanilas, Kaie Martverk, Sirli Rosenvald, Loreida Timberg, and Katrin Laos. 2021. "The Composition, Physicochemical Properties, Antioxidant Activity, and Sensory Properties of Estonian Honeys" Foods 10, no. 3: 511. https://doi.org/10.3390/foods10030511
APA StyleKivima, E., Tanilas, K., Martverk, K., Rosenvald, S., Timberg, L., & Laos, K. (2021). The Composition, Physicochemical Properties, Antioxidant Activity, and Sensory Properties of Estonian Honeys. Foods, 10(3), 511. https://doi.org/10.3390/foods10030511







