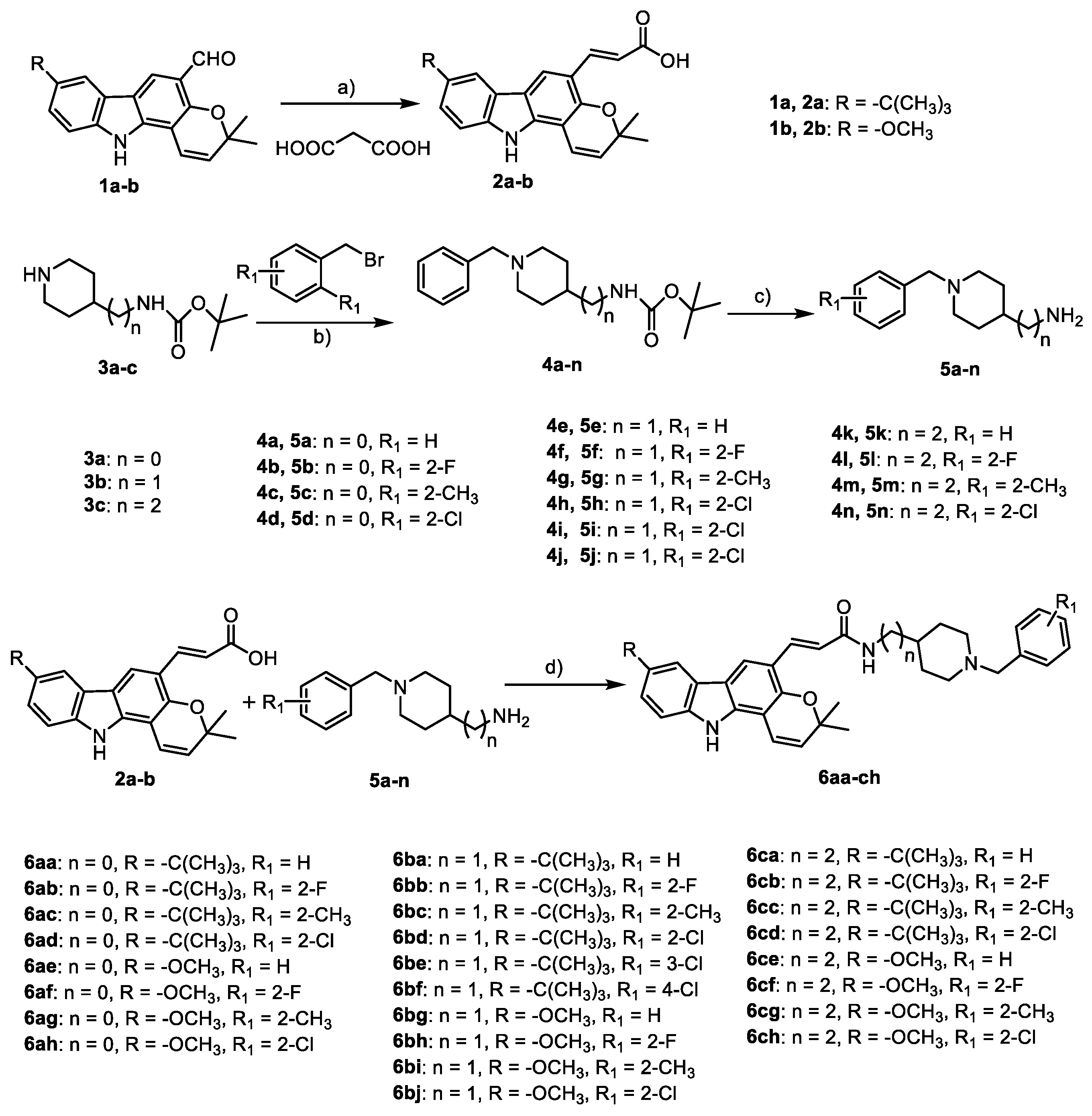
3.1.2. General Procedure for the Synthesis of 6aa–ch
To a solution of 2a,b (1 eq.) and 5a–n (2 eq.) in dry tetrahydrofuran (THF, 10 mL·mmol−1 2a,b), EDCI (2.0 eq.) and HOBt (2.0 eq.) were added by stirring at room temperature for 24 h. The solvent was removed by evaporation, and the residue was diluted with EtOAc. The solution was washed with water, dried over MgSO4, filtered, and concentrated in vacuo. Purification by column chromatography (petroleum ether/ethyl acetate, 3:1) gave compounds 6aa–ch.
(E)-N-(1-Benzylpiperidin-4-yl)-3-(8-(tert-butyl)-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)acrylamide(6aa). White solid, 58%; m.p.: 191–192 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.31 (s, 1H), 8.16 (s, 1H), 8.01 (s, 1H), 7.93 (d, J = 7.7 Hz, 1H), 7.74 (d, J = 15.7 Hz, 1H), 7.44–7.22 (m, 7H), 6.92 (d, J = 9.8 Hz, 1H), 6.72 (d, J = 15.8 Hz, 1H), 5.86 (d, J = 9.8 Hz, 1H), 3.76–3.61 (m, 1H), 3.48 (s, 2H), 2.84–2.74 (m, 2H), 2.17–2.01 (m, 2H), 1.83–1.75 (m, 2H), 1.48 (s, 6H), 1.46–1.43 (m, 2H), 1.38 (s, 9H). 13C-NMR (100 MHz, DMSO-d6) δ 165.0, 149.3, 141.8, 138.4, 138.4, 137.8, 134.5, 129.4, 128.8, 128.2, 126.9, 122.8, 122.6, 119.7, 118.7, 117.4, 117.4, 115.6, 115.4, 110.3, 104.4, 76.5, 62.1, 51.9, 45.9, 34.4, 31.8, 31.7, 27.4. HRESIMS m/z = 584.32721 [M + H]+ (calcd for C36H42O2N3, 548.32715).
(E)-3-(8-(tert-Butyl)-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)-N-(1-(2-fluorobenzyl)piperidin-4-yl)acrylamide(6ab). White solid, 56%; m.p.: 168–169 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.34 (s, 1H), 8.16 (s, 1H), 8.00 (s, 2H), 7.75 (d, J = 15.7 Hz, 1H), 7.54–7.30 (m, 4H), 7.28–7.13 (m, 2H), 6.93 (d, J = 9.8 Hz, 1H), 6.72 (d, J = 15.7 Hz, 1H), 5.85 (d, J = 9.8 Hz, 1H), 3.88–3.51 (m, 3H), 3.07–2.77 (m, 2H), 2.50 (s, 2H), 1.93–1.78 (m, 2H), 1.62–1.50 (m, 2H), 1.48 (s, 6H), 1.38 (s, 9H). 13C NMR (100 MHz, DMSO-d6) δ 165.1, 160.9 (d, JC-F = 245.8 Hz), 149.3, 141.8, 138.4, 137.8, 134.6, 132.0 (d, JC-F = 4.7 Hz), 129.4, 128.7 (d, JC-F = 8.2 Hz), 124.5 (d, JC-F = 14.6 Hz), 124.4 (d, JC-F = 3.4 Hz), 122.8, 122.6, 119.6, 118.6, 117.5, 117.4, 115.6, 115.3, 115.2 (d, JC-F = 22.1 Hz), 110.4, 104.4, 76.5, 59.7, 51.5, 45.0, 34.4, 31.8, 31.7, 27.4. HRESIMS m/z = 566.31696 [M + H]+ (calculated for C36H41O2N3F, 566.31773).
(E)-3-(8-(tert-Butyl)-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)-N-(1-(2-methylbenzyl)piperidin-4-yl)acrylamide(6ac). White solid, 52%; m.p.: 201–202 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.37 (s, 1H), 8.16 (s, 1H), 8.00 (d, J = 1.8 Hz, 1H), 7.92 (d, J = 7.8 Hz, 1H), 7.74 (d, J = 15.8 Hz, 1H), 7.40 (dd, J = 8.4, 1.8 Hz, 1H), 7.35 (d, J = 8.4 Hz, 1H), 7.23–7.20 (m, 1H), 7.16–7.11 (m, 3H), 6.93 (d, J = 9.8 Hz, 1H), 6.72 (d, J = 15.8 Hz, 1H), 5.85 (d, J = 9.8 Hz, 1H), 3.77–3.64 (m, 1H), 3.41 (s, 2H), 2.78–2.74 (m, 2H), 2.32 (s, 3H), 2.13–2.02 (m, 2H), 1.85–1.73 (m, 2H), 1.48 (s, 6H), 1.47–1.41 (m, 2H), 1.38 (s, 9H). 13C-NMR (100 MHz, DMSO-d6) δ 165.0, 149.3, 141.7, 138.4, 137.8, 137.0, 136.7, 134.5, 130.0, 129.5, 129.3, 126.8, 125.4, 122.8, 122.6, 119.8, 118.7, 117.5, 117.4, 115.6, 115.4, 110.4, 104.4, 76.5, 60.3, 52.1, 46.0, 34.4, 31.9, 31.8, 27.4, 18.8. HRESIMS m/z = 562.34235 [M + H]+ (calculated for C37H44O2N3, 562.34280).
(E)-3-(8-(tert-Butyl)-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)-N-(1-(2-chlorobenzyl)piperidin-4-yl)acrylamide(6ad). White solid, 62%; m.p.: 188–189 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.34 (s, 1H), 8.17 (s, 1H), 8.01 (d, J = 1.8 Hz, 1H), 7.94 (d, J = 7.7 Hz, 1H), 7.76 (d, J = 15.8 Hz, 1H), 7.48 (dd, J = 7.5, 1.9 Hz, 1H), 7.43–7.25 (m, 5H), 6.93 (d, J = 9.8 Hz, 1H), 6.74 (d, J = 15.8 Hz, 1H), 5.85 (d, J = 9.8 Hz, 1H), 3.79–3.66 (m, 1H), 3.55 (s, 2H), 2.82–2.78 (m, 2H), 2.21–2.10 (m, 2H), 1.85–1.75 (m, 2H), 1.55–1.43 (m, 8H), 1.38 (s, 9H). 13C-NMR (100 MHz, DMSO-d6) δ 165.1, 149.3, 141.8, 138.4, 137.8, 136.0, 134.5, 133.2, 130.7, 129.3, 129.2, 128.5, 127.0, 122.8, 122.6, 119.7, 118.7, 117.5, 117.4, 115.6, 115.4, 110.3, 104.4, 76.5, 58.7, 52.0, 45.8, 34.4, 31.8, 31.8, 27.4.HRESIMS m/z = 582.28790 [M + H]+ (calculated for C36H41O2N3Cl, 582.28818).
(E)-N-(1-Benzylpiperidin-4-yl)-3-(8-methoxy-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)acrylamide(6ae). White solid, 57%; m.p.: 212–213 °C. 1H-NMR (600 MHz, DMSO-d6) δ 11.26 (s, 1H), 8.13 (s, 1H), 7.94 (d, J = 7.4 Hz, 1H), 7.73 (d, J = 15.8 Hz, 1H), 7.58 (d, J = 2.5 Hz, 1H), 7.33 (d, J = 8.6 Hz, 1H), 7.33–7.27 (m, 4H), 7.27–7.21 (m, 1H), 6.96 (dd, J = 8.6, 2.5 Hz, 1H), 6.90 (d, J = 9.8 Hz, 1H), 6.70 (d, J = 15.8 Hz, 1H), 5.85 (d, J = 9.8 Hz, 1H), 3.83 (s, 3H), 3.73–3.65 (m, 1H), 3.35 (s, 2H), 2.81–2.74 (m, 2H), 2.11–2.01 (m, 2H), 1.83–1.72 (m, 2H), 1.48 (s, 6H), 1.47–1.41 (m, 2H). 13C-NMR (150 MHz, DMSO-d6) δ 165.1, 162.3, 153.5, 149.5, 138.6, 137.9, 134.8, 134.7, 129.3, 128.7, 128.2, 126.8, 123.4, 119.8, 119.2, 117.1, 115.4, 113.8, 111.5, 104.4, 102.8, 76.5, 62.2, 55.5, 52.0, 46.7, 31.8, 27.4. HRESIMS m/z = 522.27576 [M + H]+ (calculated for C33H36O3N3, 522.27512).
(E)-N-(1-(2-Fluorobenzyl)piperidin-4-yl)-3-(8-methoxy-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)acrylamide(6af). White solid, 65%; m.p.: 195–196 °C. 1H-NMR (600 MHz, DMSO-d6) δ 8.13 (s, 1H), 7.94 (d, J = 7.2 Hz, 1H), 7.74 (d, J = 15.7 Hz, 1H), 7.58 (d, J = 2.5 Hz, 1H), 7.42–7.38 (m, 1H), 7.33 (d, J = 8.7 Hz, 1H), 7.32–7.28 (m, 1H), 7.20–7.13 (m, 2H), 6.96 (dd, J = 8.7, 2.5 Hz, 1H), 6.90 (d, J = 9.7 Hz, 1H), 6.70 (d, J = 15.8 Hz, 1H), 5.84 (d, J = 9.8 Hz, 1H), 3.83 (s, 3H), 3.68 (d, J = 7.2 Hz, 1H), 3.52 (s, 2H), 2.79 (d, J = 11.2 Hz, 2H), 2.10 (t, J = 11.2 Hz, 2H), 1.79 (dd, J = 12.8, 4.0 Hz, 2H), 1.48 (s, 6H), 1.46–1.42 (m, 2H). 13C-NMR (150 MHz, DMSO-d6) δ 165.1, 160.8 (d, JC-F = 244.4 Hz), 153.5, 149.5, 137.9, 134.8, 134.7, 131.5 (d, JC-F = 4.9 Hz), 129.3, 129.0 (d, JC-F = 8.2 Hz), 124.9 (d, JC-F = 14.6 Hz), 124.2 (d, JC-F = 3.3 Hz), 123.4, 119.8, 119.2, 117.4, 117.2, 115.4, 115.1 (d, JC-F = 21.9 Hz), 113.8, 111.5, 104.4, 102.8, 76.5, 59.8, 55.5, 51.8, 45.9, 31.7, 27.4. HRESIMS m/z = 540.26569 [M + H]+ (calculated for C33H35O3N3F, 540.26570).
(E)-3-(8-Methoxy-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)-N-(1-(2-methylbenzyl)piperidin-4-yl)acrylamide(6ag). White solid, 58%; m.p.: 215–216 °C. 1H-NMR (600 MHz, DMSO-d6) δ 8.12 (s, 1H), 7.93 (d, J = 7.7 Hz, 1H), 7.72 (d, J = 15.8 Hz, 1H), 7.57 (d, J = 2.5 Hz, 1H), 7.33 (d, J = 8.7 Hz, 1H), 7.24–7.20 (m, 1H), 7.17–7.11 (m, 3H), 6.96 (dd, J = 8.7, 2.5 Hz, 1H), 6.90 (d, J = 9.8 Hz, 1H), 6.70 (d, J = 15.8 Hz, 1H), 5.85 (d, J = 9.8 Hz, 1H), 3.83 (s, 3H), 3.75–3.64 (m, 1H), 3.42 (s, 2H), 2.81–2.73 (m, 2H), 2.32 (s, 3H), 2.09 (s, 2H), 1.82–1.74 (m, 2H), 1.48 (s, 6H), 1.46–1.41 (m, 2H). 13C-NMR (150 MHz, DMSO-d6) δ 165.1, 153.5, 149.5, 137.9, 137.0, 136.7, 134.8, 134.7, 130.1, 129.5, 129.3, 126.9, 125.4, 123.4, 119.8, 119.3, 117.4, 117.1, 115.4, 113.8, 111.5, 104.4, 102.8, 76.5, 59.8, 55.5, 52.1, 46.0, 31.8, 27.4, 18.8. HRESIMS m/z = 536.29102 [M + H]+ (calculated for C34H38O3N3, 536.29007).
(E)-N-(1-(2-Chlorobenzyl)piperidin-4-yl)-3-(8-methoxy-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)acrylamide(6ah). White solid, 68%; m.p.: 197–198 °C. 1H-NMR (600 MHz, DMSO-d6) δ 11.25 (s, 1H), 8.13 (s, 1H), 7.95 (d, J = 7.7 Hz, 1H), 7.74 (d, J = 15.8 Hz, 1H), 7.58 (d, J = 2.5 Hz, 1H), 7.48 (d, J = 7.7 Hz, 1H), 7.42 (d, J = 7.7 Hz, 1H), 7.36–7.25 (m, 3H), 6.96 (dd, J = 8.7, 2.5 Hz, 1H), 6.90 (d, J = 9.8 Hz, 1H), 6.72 (d, J = 15.8 Hz, 1H), 5.85 (d, J = 9.8 Hz, 1H), 3.83 (s, 3H), 3.78–3.67 (m, 1H), 3.56 (s, 2H), 2.85–2.76 (m, 2H), 2.22–2.11 (m, 2H), 1.84–1.76 (m, 2H), 1.54–1.43 (m, 8H). 13C-NMR (150 MHz, DMSO-d6) δ 165.1, 153.5, 149.5, 137.9, 136.0, 134.8, 134.7, 133.3, 130.7, 129.3, 129.2, 128.5, 127.0, 123.4, 119.8, 119.2, 117.4, 117.1, 115.4, 113.8, 111.5, 104.4, 102.8, 76.5, 58.7, 55.5, 52.1, 45.9, 31.8, 27.4. HRESIMS m/z = 556.23633 [M + H]+ (calculated for C33H35O3N3Cl, 556.23615).
(E)-N-((1-Benzylpiperidin-4-yl)methyl)-3-(8-(tert-butyl)-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)acrylamide(6ba). White solid, 54%; m.p.: 163–164 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.30 (s, 1H), 8.16 (s, 1H), 8.01 (d, J = 1.8 Hz, 1H), 7.98 (d, J = 5.9 Hz, 1H), 7.74 (d, J = 15.8 Hz, 1H), 7.40 (dd, J = 8.6, 1.8 Hz, 1H), 7.35 (d, J = 8.6 Hz, 1H), 7.33–7.26 (m, 4H), 7.26–7.21 (m, 1H), 6.91 (d, J = 9.8 Hz, 1H), 6.75 (d, J = 15.8 Hz, 1H), 5.86 (d, J = 9.8 Hz, 1H), 3.45 (s, 2H), 3.12–3.06 (m, 2H), 2.86–2.77 (m, 2H), 1.91 (s, 2H), 1.69–1.59 (m, 2H), 1.48 (s, 6H), 1.40–1.36 (m, 10H), 1.28–1.12 (m, 2H). 13C-NMR (100 MHz, DMSO-d6) δ 165.9, 149.3, 141.8, 138.7, 138.4, 137.8, 134.4, 129.4, 128.8, 128.1, 126.9, 122.8, 122.6, 119.6, 118.6, 117.4, 117.4, 115.7, 115.4, 110.3, 104.4, 76.5, 62.4, 53.0, 44.3, 35.9, 34.4, 31.8, 29.7, 27.4. HRESIMS m/z = 562.34210 [M + H]+ (calculated for C37H44O2N3, 562.34280).
(E)-3-(8-(tert-Butyl)-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)-N-((1-(2-fluorobenzyl)piperidin-4-yl)methyl)acrylamide(6bb). White solid, 52%; m.p.: 142–143 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.32 (s, 1H), 8.16 (s, 1H), 8.05–7.94 (m, 2H), 7.74 (d, J = 15.7 Hz, 1H), 7.45–7.09 (m, 6H), 6.92 (d, J = 9.8 Hz, 1H), 6.75 (d, J = 15.7 Hz, 1H), 5.85 (d, J = 9.8 Hz, 1H), 3.49 (s, 2H), 3.12–3.03 (m, 2H), 2.81 (d, J = 11.3 Hz, 2H), 1.94 (t, J = 11.3 Hz, 2H), 1.69–1.58 (m, 2H), 1.48 (s, 6H), 1.40–1.35 (m, 10H), 1.26–1.11 (m, 2H). 13C-NMR (100 MHz, DMSO-d6) δ 165.9, 160.7 (d, JC-F = 244.1 Hz), 149.3, 141.8, 138.4, 137.8, 134.4, 131.4 (d, JC-F = 4.7 Hz), 129.4, 128.9(d, JC-F = 8.2 Hz), 125.0 (d, JC-F = 14.5 Hz), 124.1(d, JC-F = 3.4 Hz), 122.8, 122.6, 119.6, 118.6, 117.5, 117.3, 115.6, 115.4, 115.1 (d, JC-F = 22.0 Hz), 110.3, 104.4, 76.5, 54.9, 52.9, 44.2, 35.8, 34.4, 31.8, 29.7, 27.4. HRESIMS m/z = 580.33258 [M + H]+ (calculated for C37H43O2N3F, 580.233338).
(E)-3-(8-(tert-Butyl)-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)-N-((1-(2-methylbenzyl)piperidin-4-yl)methyl)acrylamide(6bc). White solid, 54%; m.p.: 168–169 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.33 (s, 1H), 8.16 (s, 1H), 8.05–7.96 (m, 2H), 7.74 (d, J = 15.8 Hz, 1H), 7.46–7.32 (m, 2H), 7.27–7.07 (m, 4H), 6.92 (d, J = 9.8 Hz, 1H), 6.75 (d, J = 15.8 Hz, 1H), 5.85 (d, J = 9.8 Hz, 1H), 3.38 (s, 2H), 3.13–3.05 (m, 2H), 2.79 (d, J = 11.4 Hz, 2H), 2.30 (s, 3H), 1.93 (t, J = 11.4 Hz, 2H), 1.70–1.59 (m, 2H), 1.48 (s, 6H), 1.48–1.41 (m, 1H), 1.38 (s, 9H), 1.25–1.06 (m, 2H). 13C-NMR (151 MHz, DMSO) δ 165.9, 153.5, 149.5, 137.9, 136.9, 136.7, 134.8, 134.6, 130.0, 129.4, 129.3, 126.7, 125.3, 123.4, 119.6, 119.2, 117.4, 117.1, 115.3, 113.8, 111.5, 104.4, 102.8, 76.5, 60.5, 55.5, 53.2, 44.3, 36.0, 29.9, 27.4, 18.8. HRESIMS m/z = 576.35785 [M + H]+ (calculated for C38H46O2N3, 576.35785).
(E)-3-(8-(tert-Butyl)-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)-N-((1-(2-chlorobenzyl)piperidin-4-yl)methyl)acrylamide(6bd). White solid, 66%; m.p.: 175–176 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.31 (s, 1H), 8.16 (s, 1H), 8.05–7.95 (m, 2H), 7.74 (d, J = 15.7 Hz, 1H), 7.51–7.23 (m, 6H), 6.92 (d, J = 9.8 Hz, 1H), 6.75 (d, J = 15.7 Hz, 1H), 5.86 (d, J = 9.8 Hz, 1H), 3.52 (s, 2H), 3.13–3.04 (m, 2H), 2.83 (d, J = 11.3 Hz, 2H), 2.01 (t, J = 11.3 Hz, 2H), 1.71–1.62 (m, 2H), 1.48 (s, 6H), 1.40–1.35 (m, 10H), 1.26–1.15 (m, 2H). 13C-NMR (101 MHz, DMSO) δ 165.9, 149.3, 141.8, 138.4, 137.8, 136.1, 134.4, 133.2, 130.6, 129.4, 129.2, 128.4, 127.0, 122.8, 122.6, 119.6, 118.6, 117.4, 117.4, 115.7, 115.4, 110.3, 104.4, 76.5, 58.9, 53.1, 44.3, 35.9, 34.4, 31.8, 29.8, 27.4. HRESIMS m/z = 596.30298 [M + H]+ (calculated for C37H43O2N3Cl, 596.30383).
(E)-3-(8-(tert-Butyl)-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)-N-((1-(3-chlorobenzyl)piperidin-4-yl)methyl)acrylamide(6be). White solid, 59%; m.p.: 171–172 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.30 (s, 1H), 8.16 (s, 1H), 8.07–7.93 (m, 2H), 7.74 (d, J = 15.7 Hz, 1H), 7.40 (dd, J = 8.5, 1.9 Hz, 1H), 7.37–7.32 (m, 3H), 7.32–7.26 (m, 2H), 6.91 (d, J = 9.8 Hz, 1H), 6.75 (d, J = 15.7 Hz, 1H), 5.86 (d, J = 9.8 Hz, 1H), 3.46 (s, 2H), 3.14–3.05 (m, 2H), 2.83–2.74 (m, 2H), 1.97–1.85 (m, 2H), 1.69–1.61 (m, 2H), 1.48 (s, 6H), 1.38 (s, 10H), 1.25–1.16 (m, 2H). 13C-NMR (100 MHz, DMSO-d6) δ 165.9, 149.3, 141.8, 138.4, 137.8, 136.1, 134.4, 132.9, 130.0, 129.4, 128.2, 127.3, 126.8, 122.8, 122.6, 119.6, 118.6, 117.4, 117.4, 115.7, 115.4, 110.3, 104.4, 76.5, 61.5, 53.0, 44.3, 35.9, 34.4, 31.8, 29.8, 27.4. HRESIMS m/z = 596.30365 [M + H]+ (calculated for C37H43O2N3Cl, 596.30383).
(E)-3-(8-(tert-Butyl)-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)-N-((1-(4-chlorobenzyl)piperidin-4-yl)methyl)acrylamide(6bf). White solid, 62%; m.p.: 158–159 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.30 (s, 1H), 8.16 (s, 1H), 8.01 (d, J = 1.8 Hz, 1H), 7.98 (d, J = 5.9 Hz, 1H), 7.74 (d, J = 15.8 Hz, 1H), 7.40 (dd, J = 8.5, 1.9 Hz, 1H), 7.40–7.32 (m, 3H), 7.31 (d, J = 8.5 Hz, 2H), 6.92 (d, J = 9.8 Hz, 1H), 6.75 (d, J = 15.8 Hz, 1H), 5.86 (d, J = 9.8 Hz, 1H), 3.42 (s, 2H), 3.13–3.04 (m, 2H), 2.82–2.72 (m, 2H), 1.96–1.84 (m, 2H), 1.68–1.58 (m, 2H), 1.48 (s, 6H), 1.38 (s, 9H), 1.26–1.11 (m, 3H). 13C-NMR (100 MHz, DMSO-d6) δ 165.9, 149.3, 141.8, 138.4, 137.8, 134.4, 131.2, 130.4, 129.4, 128.1, 122.8, 122.6, 119.6, 118.6, 117.4, 117.4, 115.7, 115.4, 110.3, 104.4, 76.5, 61.5, 52.9, 48.6, 44.3, 35.9, 34.4, 31.8, 29.8, 27.4. HRESIMS m/z = 596.30365 [M + H]+ (calculated for C37H43O2N3Cl, 596.30383).
(E)-N-((1-Benzylpiperidin-4-yl)methyl)-3-(8-methoxy-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)acrylamide(6bg). White solid, 64%; m.p.: 182–183 °C. 1H-NMR (600 MHz, DMSO-d6) δ 11.31 (s, 1H), 8.24 (s, 1H), 8.14 (s, 1H), 7.75 (d, J = 15.8 Hz, 1H), 7.58 (d, J = 2.5 Hz, 1H), 7.38–7.22 (m, 6H), 6.96 (dd, J = 8.6, 2.5 Hz, 1H), 6.91 (d, J = 9.8 Hz, 1H), 6.75 (d, J = 15.8 Hz, 1H), 5.84 (d, J = 9.8 Hz, 1H), 3.83 (s, 3H), 3.58 (s, 2H), 3.13–3.07 (m, 2H), 2.93–2.84 (m, 2H), 2.14–2.05 (m, 2H), 1.72–1.62 (m, 2H), 1.51–1.44 (m, 7H), 1.30–1.20 (m, 2H). 13C-NMR (150 MHz, DMSO-d6) δ 165.9, 153.5, 149.5, 138.0, 137.0, 134.9, 134.7, 129.2, 128.2, 127.3, 123.4, 123.4, 119.5, 119.2, 117.4, 117.2, 115.3, 113.8, 111.5, 104.4, 102.8, 76.5, 61.7, 55.5, 52.5, 44.0, 35.5, 29.1, 27.4. HRESIMS m/z = 536.29095 [M + H]+ (calculated for C34H38O3N3, 536.29077).
(E)-N-((1-(2-Fluorobenzyl)piperidin-4-yl)methyl)-3-(8-methoxy-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)acrylamide(6bh). White solid, 56%; m.p.: 162–163 °C. 1H-NMR (600 MHz, DMSO-d6) δ 11.25 (s, 1H), 8.13 (s, 1H), 8.01 (d, J = 6.1 Hz, 1H), 7.73 (d, J = 15.8 Hz, 1H), 7.58 (d, J = 2.5 Hz, 1H), 7.42–7.36 (m, 1H), 7.33 (d, J = 8.6 Hz, 1H), 7.33–7.26 (m, 1H), 7.19–7.11 (m, 2H), 6.95 (dd, J = 8.6, 2.5 Hz, 1H), 6.90 (d, J = 9.8 Hz, 1H), 6.73 (d, J = 15.8 Hz, 1H), 5.85 (d, J = 9.8 Hz, 1H), 3.83 (s, 3H), 3.48 (s, 2H), 3.12–3.04 (m, 2H), 2.84–2.75 (m, 2H), 2.00–1.89 (m, 2H), 1.67–1.59 (m, 2H), 1.50–1.45 (m, 7H), 1.24–1.13 (m, 2H). 13C-NMR (150 MHz, DMSO-d6) δ 165.9, 160.8(d, JC-F = 244.1 Hz), 153.5, 149.5, 137.9, 134.8, 134.6, 131.4 (d, JC-F = 4.6 Hz), 129.3, 128.9 (d, JC-F = 8.3 Hz), 125.0 (d, JC-F = 14.6 Hz), 124.1 (d, JC-F = 3.2 Hz), 123.4, 119.6, 119.2, 117.4, 117.1, 115.3, 115.1 (d, JC-F = 22.1 Hz), 113.8, 111.5, 104.4, 102.8, 76.5, 59.8, 55.5, 52.8, 44.3, 35.8, 29.7, 27.4. HRESIMS m/z = 554.28119 [M + H]+ (calculated for C34H37O3N3F, 554.28135).
(E)-3-(8-Methoxy-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)-N-((1-(2-methylbenzyl)piperidin-4-yl)methyl)acrylamide(6bi). White solid, 61%; m.p.: 181–182 °C. 1H-NMR (600 MHz, DMSO-d6) δ 8.14 (s, 1H), 8.02 (t, J = 5.8 Hz, 1H), 7.75 (d, J = 15.8 Hz, 1H), 7.58 (d, J = 2.4 Hz, 1H), 7.34 (d, J = 8.6 Hz, 1H), 7.22–7.18 (m, 1H), 7.16–7.07 (m, 3H), 6.96 (dd, J = 8.6, 2.4 Hz, 1H), 6.90 (d, J = 9.8 Hz, 1H), 6.74 (d, J = 15.8 Hz, 1H), 5.85 (d, J = 9.8 Hz, 1H), 3.83 (s, 3H), 3.37 (s, 2H), 3.12–3.06 (m, 2H), 2.82–2.71 (m, 2H), 2.29 (s, 3H), 1.97–1.88 (m, 2H), 1.68–1.60 (m, 2H), 1.52–1.42 (m, 7H), 1.22–1.10 (m, 2H). 13C-NMR (150 MHz, DMSO-d6) δ 165.9, 153.5, 149.5, 137.9, 136.9, 136.7, 134.8, 134.6, 130.0, 129.4, 129.3, 126.7, 125.3, 123.4, 119.6, 119.2, 117.4, 117.1, 115.3, 113.8, 111.5, 104.4, 102.8, 76.5, 60.5, 55.5, 53.2, 44.3, 36.0, 29.9, 27.4, 18.8. HRESIMS m/z = 550.30609 [M + H]+ (calculated for C35H40O3N3, 550.30642).
(E)-N-((1-(2-Chlorobenzyl)piperidin-4-yl)methyl)-3-(8-methoxy-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)acrylamide(6bj). White solid, 63%; m.p.: 165–166 °C. 1H-NMR (600 MHz, DMSO-d6) δ 8.13 (s, 1H), 8.03 (d, J = 6.0 Hz, 1H), 7.73 (d, J = 15.8 Hz, 1H), 7.58 (d, J = 2.5 Hz, 1H), 7.52–7.23 (m, 5H), 6.95 (dd, J = 8.7, 2.5 Hz, 1H), 6.90 (d, J = 9.8 Hz, 1H), 6.73 (d, J = 15.8 Hz, 1H), 5.85 (d, J = 9.8 Hz, 1H), 3.83 (s, 3H), 3.53 (s, 2H), 3.13–3.05 (m, 2H), 2.87–2.78 (m, 2H), 2.05–1.96 (m, 2H), 1.69–1.62 (m, 2H), 1.51–1.43 (m, 7H), 1.26–1.15 (m, 2H). 13C-NMR (150 MHz, DMSO-d6) δ 165.8, 153.5, 149.5, 137.9, 136.0, 134.8, 134.6, 133.2, 130.7, 129.3, 129.2, 128.5, 127.0, 123.4, 119.6, 119.2, 117.4, 117.1, 115.3, 113.8, 111.5, 104.4, 102.8, 76.5, 58.9, 55.5, 53.1, 44.1, 35.8, 29.8, 27.4. HRESIMS m/z = 570.25189 [M + H]+ (calculated for C34H37O3N3Cl, 570.25180).
(E)-N-(2-(1-Benzylpiperidin-4-yl)ethyl)-3-(8-(tert-butyl)-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)acrylamide(6ca). White solid, 68%. White solid, 65%; m.p.:144–145 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.34 (s, 1H), 8.17 (s, 1H), 8.01 (d, J = 1.8 Hz, 1H), 7.97 (t, J = 5.6 Hz, 1H), 7.74 (d, J = 15.8 Hz, 1H), 7.40 (dd, J = 8.5, 1.9 Hz, 1H), 7.37–7.31 (m, 5H), 7.30–7.26 (m, 1H), 6.93 (d, J = 9.8 Hz, 1H), 6.71 (d, J = 15.8 Hz, 1H), 5.85 (d, J = 9.8 Hz, 1H), 3.60 (s, 2H), 3.27–3.14 (m, 2H), 2.89 (s, 2H), 2.26–1.98 (m, 2H), 1.75–1.64 (m, 2H), 1.48 (s, 6H), 1.45–1.40 (m, 2H), 1.38 (s, 9H), 1.29–1.13 (m, 3H). 13C-NMR (100 MHz, DMSO-d6) δ 165.7, 149.3, 143.9, 141.7, 138.4, 137.8, 137.7, 134.4, 129.3, 128.9, 128.2, 122.8, 122.6, 119.6, 118.7, 117.5, 117.3, 115.7, 115.3, 110.3, 104.4, 76.5, 59.8, 52.8, 36.1, 35.9, 34.4, 32.3, 31.8, 31.2, 27.4. HRESIMS m/z = 576.35858 [M + H]+ (calculated for C38H46O2N3, 576.35845).
(E)-3-(8-(tert-Butyl)-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)-N-(2-(1-(2-fluorobenzyl)piperidin-4-yl)ethyl)acrylamide(6cb). White solid, 57%; m.p.: 122–123 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.35 (s, 1H), 8.17 (s, 1H), 8.01 (s, 1H), 7.96 (t, J = 5.7 Hz, 1H), 7.73 (d, J = 15.8 Hz, 1H), 7.40 (dd, J = 8.4, 1.8 Hz, 1H), 7.38–7.26 (m, 3H), 7.19–7.11 (m, 2H), 6.93 (d, J = 9.8 Hz, 1H), 6.71 (d, J = 15.8 Hz, 1H), 5.85 (d, J = 9.8 Hz, 1H), 3.49 (s, 2H), 3.27–3.15 (m, 2H), 2.83–2.76 (m, 2H), 2.02–1.88 (m, 2H), 1.71–1.59 (m, 2H), 1.48 (s, 6H), 1.45–1.39 (m, 2H), 1.38 (s, 10H), 1.21–1.08 (m, 2H).13C-NMR (100 MHz, DMSO-d6) δ 165.7, 160.8 (d, JC-F = 244.5 Hz), 149.3, 141.8, 138.4, 137.8, 134.3, 131.5 (d, JC-F = 5.0 Hz), 129.3, 128.9 (d, JC-F = 8.3 Hz), 124.9 (d, JC-F = 14.6 Hz), 124.1 (d, JC-F = 3.2 Hz), 122.8, 122.6, 119.6, 118.7, 117.5, 117.4, 115.7, 115.3, 115.1 (d, JC-F = 22.0 Hz), 110.3, 104.4, 76.5, 54.9, 53.1, 36.2, 36.0, 35.0, 34.4, 32.7, 31.8, 27.4. HRESIMS m/z = 594.34808 [M + H]+ (calculated for C38H45O2N3F, 594.34903).
(E)-3-(8-(tert-Butyl)-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)-N-(2-(1-(2-methylbenzyl)piperidin-4-yl)ethyl)acrylamide(6cc). White solid, 48%; m.p.: 158–159 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.34 (s, 1H), 8.17 (s, 1H), 8.02 (d, J = 1.8 Hz, 1H), 7.96 (t, J = 5.6 Hz, 1H), 7.75 (d, J = 15.7 Hz, 1H), 7.40 (dd, J = 8.6, 1.8 Hz, 1H), 7.35 (d, J = 8.6 Hz, 1H), 7.22–7.16 (m, 1H), 7.14–7.08 (m, 3H), 6.93 (d, J = 9.8 Hz, 1H), 6.72 (d, J = 15.7 Hz, 1H), 5.85 (d, J = 9.8 Hz, 1H), 3.36 (s, 2H), 3.26–3.17 (m, 2H), 2.81–2.71 (m, 2H), 2.29 (s, 3H), 1.96–1.86 (m, 2H), 1.69–1.60 (m, 2H), 1.48 (s, 6H), 1.45–1.39 (m, 2H), 1.39–1.37 (m, 10H), 1.19–1.05 (m, 2H). 13C-NMR (100 MHz, DMSO-d6) δ 165.7, 149.3, 141.8, 138.4, 137.8, 136.9, 136.8, 134.4, 130.0, 129.4, 129.3, 126.7, 125.3, 122.8, 122.6, 119.6, 118.7, 117.5, 117.4, 115.7, 115.4, 110.3, 104.4, 76.5, 60.6, 53.5, 36.3, 36.1, 34.4, 32.9, 32.0, 31.8, 27.4, 18.8. HRESIMS m/z = 590.37402 [M + H]+ (calculated for C39H48O2N3, 590.37410).
(E)-3-(8-(tert-Butyl)-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)-N-(2-(1-(2-chlorobenzyl)piperidin-4-yl)ethyl)acrylamide(6cd). White solid, 56%; m.p.: 160–161 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.31 (s, 1H), 8.17 (s, 1H), 8.02 (d, J = 1.8 Hz, 1H), 7.96 (t, J = 5.6 Hz, 1H), 7.75 (d, J = 15.8 Hz, 1H), 7.49–7.23 (m, 6H), 6.92 (d, J = 9.8 Hz, 1H), 6.72 (d, J = 15.8 Hz, 1H), 5.85 (d, J = 9.8 Hz, 1H), 3.51 (s, 2H), 3.26–3.17 (m, 2H), 2.86–2.71 (m, 2H), 2.08–1.92 (m, 2H), 1.72–1.58 (m, 2H), 1.48 (s, 6H), 1.45–1.40 (m, 2H), 1.38 (s, 10H), 1.27–1.05 (m, 2H). 13C-NMR (100 MHz, DMSO-d6) δ 165.7, 149.3, 141.8, 138.4, 137.8, 136.1, 134.4, 133.2, 130.7, 129.3, 129.2, 128.4, 126.9, 122.8, 122.6, 119.6, 118.7, 117.5, 117.4, 115.7, 115.4, 110.3, 104.4, 76.4, 59.0, 53.4, 36.9, 36.2, 34.9, 34.4, 32.7, 31.8, 27.4. HRESIMS m/z = 610.31873 [M + H]+ (calculated for C38H45O2N3Cl, 610.31948).
(E)-N-(2-(1-Benzylpiperidin-4-yl)ethyl)-3-(8-methoxy-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)acrylamide(6ce). White solid, 47%; m.p.:152–153 °C. 1H-NMR (600 MHz, DMSO-d6) δ 8.14 (s, 1H), 7.98 (t, J = 5.6 Hz, 1H), 7.73 (d, J = 15.8 Hz, 1H), 7.59 (d, J = 2.5 Hz, 1H), 7.38–7.18 (m, 6H), 6.96 (dd, J = 8.7, 2.5 Hz, 1H), 6.90 (d, J = 9.8 Hz, 1H), 6.69 (d, J = 15.8 Hz, 1H), 5.85 (d, J = 9.8 Hz, 1H), 3.83 (s, 3H), 3.44 (s, 2H), 3.25–3.16 (m, 2H), 2.86–2.73 (m, 2H), 1.98–1.84 (m, 2H), 1.71–1.58 (m, 2H), 1.48 (s, 6H), 1.44–1.37 (m, 2H), 1.36–1.25 (m, 1H), 1.22–1.09 (m, 2H). 13C-NMR (150 MHz, DMSO-d6) δ 165.7, 153.5, 149.5, 138.1, 137.9, 134.8, 134.6, 129.3, 128.8, 128.1, 126.8, 123.4, 119.7, 119.2, 117.4, 117.1, 115.3, 113.8, 111.5, 104.4, 102.8, 76.5, 62.4, 55.5, 53.2, 36.3, 36.0, 32.8, 31.8, 27.4. HRESIMS m/z = 550.30670 [M + H]+ (calculated for C35H40O3N3, 550.30642).
(E)-N-(2-(1-(2-Fluorobenzyl)piperidin-4-yl)ethyl)-3-(8-methoxy-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)acrylamide(6cf). White solid, 58%; m.p.: 141–142 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.26 (s, 1H), 8.13 (s, 1H), 7.96 (t, J = 5.6 Hz, 1H), 7.72 (d, J = 15.8 Hz, 1H), 7.58 (d, J = 2.5 Hz, 1H), 7.41–7.36 (m, 1H), 7.33 (d, J = 8.7 Hz, 1H), 7.31–7.26 (m, 1H), 7.19–7.11 (m, 2H), 6.95 (dd, J = 8.7, 2.5 Hz, 1H), 6.90 (d, J = 9.9 Hz, 1H), 6.68 (d, J = 15.8 Hz, 1H), 5.85 (d, J = 9.9 Hz, 1H), 3.83 (s, 3H), 3.48 (s, 2H), 3.24–3.15 (m, 2H), 2.84–2.72 (m, 2H), 1.98–1.88 (m, 2H), 1.70–1.59 (m, 2H), 1.48 (s, 6H), 1.44–1.35 (m, 2H), 1.25–1.21 (m, 1H), 1.20–1.08 (m, 2H). 13C-NMR (100 MHz, DMSO-d6) δ 165.7, 160.8 (d, JC-F = 244.2 Hz), 153.5, 149.4, 138.0, 134.9, 134.5, 131.5 (d, JC-F = 4.5 Hz), 129.3, 128.9 (d, JC-F = 8.1 Hz), 124.9 (d, JC-F = 14.7 Hz), 124.1(d, JC-F = 3.5 Hz), 123.4, 119.7, 119.2, 117.4, 117.2, 115.3, 115.1(d, JC-F = 22.0 Hz), 113.8, 111.5, 104.4, 102.8, 76.5, 55.5, 54.9, 53.1, 36.2, 36.0, 32.7, 31.8, 27.4. HRESIMS m/z = 568.29767 [M + H]+ (calculated for C35H39O3N3F, 568.29809).
(E)-3-(8-Methoxy-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)-N-(2-(1-(2-methylbenzyl)piperidin-4-yl)ethyl)acrylamide(6cg). White solid, 51%; m.p.: 161–162 °C. 1H-NMR (600 MHz, DMSO-d6) δ 8.14 (s, 1H), 7.98 (t, J = 5.7 Hz, 1H), 7.74 (d, J = 15.8 Hz, 1H), 7.59 (d, J = 2.5 Hz, 1H), 7.34 (d, J = 8.6 Hz, 1H), 7.22–7.07 (m, 4H), 6.96 (dd, J = 8.6, 2.5 Hz, 1H), 6.90 (d, J = 9.8 Hz, 1H), 6.70 (d, J = 15.8 Hz, 1H), 5.85 (d, J = 9.8 Hz, 1H), 3.83 (s, 3H), 3.37 (s, 2H), 3.26–3.18 (m, 2H), 2.80–2.71 (m, 2H), 2.29 (s, 3H), 1.95–1.85 (m, 2H), 1.68–1.60 (m, 2H), 1.48 (s, 6H), 1.42–1.37 (m, 2H), 1.35–1.27 (m, 1H), 1.20–1.06 (m, 2H). 13C-NMR (150 MHz, DMSO-d6) δ 165.7, 153.5, 149.5, 137.9, 136.9, 136.7, 134.8, 134.6, 130.0, 129.4, 129.3, 126.7, 125.3, 123.4, 119.7, 119.2, 117.4, 117.1, 115.3, 113.8, 111.5, 104.4, 102.8, 76.5, 60.5, 55.5, 53.5, 36.3, 36.0, 33.0, 32.0, 27.4, 18.8. HRESIMS m/z = 564.32147 [M + H]+ (calculated for C36H42O3N3,564.32207).
(E)-N-(2-(1-(2-Chlorobenzyl)piperidin-4-yl)ethyl)-3-(8-methoxy-3,3-dimethyl-3,11-dihydropyrano[3,2-a]carbazol-5-yl)acrylamide(6ch). White solid, 57%; m.p.: 145–146 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.24 (s, 1H), 8.13 (s, 1H), 7.97 (t, J = 5.6 Hz, 1H), 7.72 (d, J = 15.8 Hz, 1H), 7.59 (d, J = 2.5 Hz, 1H), 7.51–7.38 (m, 2H), 7.36–7.23 (m, 3H), 6.95 (dd, J = 8.7, 2.5 Hz, 1H), 6.90 (d, J = 9.8 Hz, 1H), 6.69 (d, J = 15.8 Hz, 1H), 5.85 (d, J = 9.8 Hz, 1H), 3.83 (s, 3H), 3.53 (s, 2H), 3.27–3.17 (m, 2H), 2.91–2.74 (m, 2H), 2.07–1.93 (m, 2H), 1.72–1.62 (m, 2H), 1.48 (s, 6H), 1.45–1.37 (m, 2H), 1.38–1.26 (m, 1H), 1.23–1.11 (m, 2H). 13C-NMR (100 MHz, DMSO-d6) δ 165.7, 153.5, 149.4, 138.0, 136.1, 134.9, 134.5, 133.2, 130.7, 129.3, 129.2, 128.5, 127.0, 123.4, 119.7, 119.2, 117.4, 117.2, 115.3, 113.8, 111.5, 104.4, 102.8, 76.5, 59.0, 55.5, 53.4, 36.2, 36.0, 32.7, 31.9, 27.4. HRESIMS m/z = 584.26843 [M + H]+ (calculated for C35H39O3N3Cl, 584.26745).