Abstract
Most urban greening interventions involve soil de-sealing and management to enhance fertility. Management typically requires translocating fertile topsoil to the site, which comes at great environmental costs. We hypothesized that de-sealed urban soils would undergo an increase of their fertility without exogenous topsoil application. We assessed experimental plots with de-sealed soil with topsoil, and de-sealed soil without topsoil. Both treatments were vegetated with two ornamental shrub species and irrigated. Soil fertility was analyzed by chemical (total and organic carbon) and biological indicators of soils (biological quality index and microbial activities). Since metal contamination is related to urban de-sealed soil, we also monitored the concentration of Zn, Cu and Pb in soil and detected it in plant leaves. The results demonstrate that de-sealed urban soils rapidly restore their biological quality and fertility. Restoration of de-sealing soils can contribute to the recent growing interest reclamation of urban soils for improving the urban environment quality through the restoration of soil functions and related ecosystem services. Overall, the results of this study demonstrate that de-sealed soils can improve their functionality and can contribute to the recent growing interest in reclamation of urban soils for improving the urban environment quality.
1. Introduction
The ever-growing urbanization with the related household and mobility infrastructure construction, jointly with past urban sprawl and industrialization, have consumed soil mainly by sealing, leading to soil loss and contamination [1]. Soil sealing, together with erosion, is a major cause of soil loss in the European Union [2] and is per se an indicator of land degradation [3]. Soil sealing can be defined as any physical separation of soil from the atmosphere and above-ground biosphere by impermeable layers [4]. Although the degree to which a soil surface can be sealed varies depending on land use [4,5], it has both direct and indirect adverse effects on soil properties and ecological functions, and on the overall quality of the surrounding environment. Main adverse effects of soil sealing include loss of vegetation, local alteration of the microclimate due to increased average air temperature [6], generating the urban heat island effect [7,8], reducing water infiltration [9] and increasing surface runoff [10]. Sealed soils can also be contaminated by heavy metals and organic pollutants [11].
Creation of new green space in cities can increase the urban resilience to the extreme climatic events and improve the citizens’ life quality. In sealed areas, urban greening interventions require as a preliminary step the soil de-sealing and the adoption of appropriate agro-environmental practices for restoring the quality of de-sealed soils, which generally require backfill soil for leveling and allowing the optimal plant rooting. The soil material is conventionally taken from the first layers (topsoil) of agricultural land, with high impacts on both soil consumption and transport environmental and economic costs. In this context, urban soil restoration and management can be hampered by the lack of available topsoil with suitable physicochemical properties and adequate fertility. This practice is generally adopted because data on physico-chemical and biological fertility of de-sealed soils are still scarce [12].
However, while knowledge is available on the impact of sealing on soil physical, chemical and biological fertility [2,12], the potential fertility of de-sealed soils is still poorly known. Furthermore, another problem related to the use of de-sealed soils in urban green areas is the possible chemical contamination by organic and inorganic compounds (heavy metals, PHAs), or soil compaction and drainage capacity.
We hypothesized that de-sealed urban soils, after shrubs planting, would undergo an increase in their fertility. We tested our hypothesis by monitoring the chemical and biological indicators of soil health from experimental plots to demonstrate the feasibility of de-sealing as a possible land take compensation measure. Since metal contamination is related to urban de-sealed soil, we also monitored the concentration of Zn, Cu and Pb in soil and detected it in plant leaves. In this case, the increase of fertility of de-sealed urban soils could avoid the movement of topsoil from peri-urban areas, increasing the sustainability of urban regeneration projects, in line with the EU Roadmap to a resource-efficient Europe [13].
2. Materials and Methods
2.1. Sites and De-Sealing Operations
The study was done in three municipalities of Italy, in Emilia Romagna Region (Northern Italy), Carpi, San Lazzaro di Savena and Forlì, which planned a de-sealing action in their urban fabric as a compensation measure for new building sites (Table 1, Figure 1). In these municipalities, urban sealed soils were de-sealed and for demonstrating the potential of land compensation in the ambit of the project “Save our Soils for LIFE” (SOS4LIFE, LIFE15ENV/IT/000225).

Table 1.
Location of the de-sealing sites.
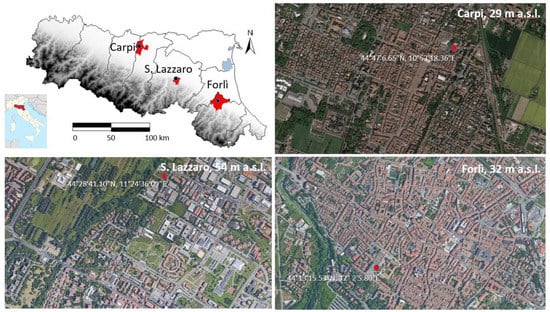
Figure 1.
Map of Emilia Romagna region (Northern Italy) with geo-localization of sites subject to greening intervention, respectively, in Carpi (site 1, top right), Forlì (site 2, bottom right) and San Lazzaro (site 3, bottom left).
The sealed soils were de-paved with a caterpillar in late spring of 2017 and the underneath techno-soils were observed and described before gathering the soil material to be used in the experiment. De-sealed soils were extracted from underneath the sealing cover of the site for the purposes of regeneration. In Site 1 and 2 at ca. 35 cm depth under the anthropogenic cover (asphalt, sand and pebbles) and in Site 3, de-sealed soil was likely to result from land filling of in situ soil material which had been weakly weathered afterwards [14].
2.2. Experimental Plots
In each experimental site, the same experimental scheme was applied with two treatments: de-sealed soil (De-Sealed) and agricultural topsoil (Topsoil). A plot having the size of 2 × 1 × 0.6 m (L × W × H = 1.2 m3) was created and was split equally between the two treatments.
The Topsoil plot was filled with 20 cm of de-sealed soil at the bottom, topped with 30 cm of topsoil for simulating the capping of the de-sealed surface at the regeneration site. The De-Sealed plots were filled with de-sealed soil only (Figure 2). In summer 2017, three 6-month-old plants of two ornamental shrubs, Eleagnus × ebbingei and Viburnum tinus L., widely used in urban green areas, were transplanted in each plot (Figure 2) and in each site [14]. The plots were irrigated with an automated irrigation system in the April–September period every day, for 10 minutes per day [14]. At each experimental site, the soil sampling was done for three consecutive years (2017–2019), identifying three different sampling dates: T0 at the beginning of the experiment, T1 in late spring 2018 and T2 in late spring 2019. Three sample replicates for undisturbed soil cores for chemical analysis and for soil biological quality (QBS) were sampled at each sampling date. Soil microbial biomass, respiration, enzymatic activities and trace elements analysis of plant leaves were performed at T1. The characteristics and the effects of topsoil and de-sealed soil used on plant growth have already been studied and published [14].
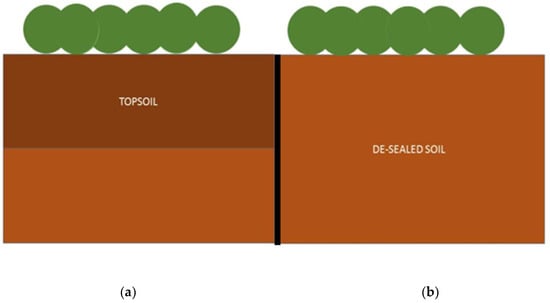
Figure 2.
Graphic representation of experimental plots: (a) Topsoil subplot (left); (b) De-sealed soil subplot (right).
The topsoil used in the plot experiment was sampled in the peri-urban areas of each municipality. In Site 1, the topsoil was taken from an agricultural field along a channel in a depression of the alluvial plain in the per urban area north of the city center (Hypocalcic Haplic Calcisols, [15]). In Site 2, the topsoil was collected from an agricultural field under a fallow on an ancient fluvial terrace (Haplic Luvisos, [15]). In Site 3, the topsoil was taken from a construction site in a former agricultural field on an alluvial terrace (Fluvic Cambisol, [15]).
2.3. Soil Analyses
Chemical analyses were done on air-dried soil samples from each site, sieved at 2 mm and milled. Total soil carbon (TC) and total nitrogen (TN) were determined by dry combustion elemental analyzer (CHN- S Flash E1112 Thermofinnigan, Mundelein, IL, USA). Total organic carbon (TOC) was determined with the same method of TC after acidification with 1% HCl to completely remove carbonates. The total concentration of three potential toxic metals of Zn, Pb and Cu used a high-performance microwave reaction with HNO3:H2O2, followed by determination at ICP (Perkin Elmer Optima 2000 OES DV Santa Clara, CA, USA) analysis. The soil biological quality index (QBS) was analyzed by microarthropod extraction using a Berlese-Tüllgren funnel typology from each undisturbed soil sample. The extracted specimens were observed under a stereomicroscope for identification at different taxonomical levels [16]. Soil biological quality was evaluated using the QBS index proposed by [16] based on the Ecomorphological Index (EMI) evaluation of morphology reflecting their adaptation to the edaphic environment. The sum of all EMI scores assigned to the groups was in turn used to calculate the QBS index [16]. The QBS is a sensitive indicator and its validity has been shown by application in different environmental contexts. Among several indices to detect soil quality, QBS is applied for easy and expeditious use to evaluate the state of suffering of soil in restored areas. Soil respiration was measured using the alkaline titration method [17], and soil microbial biomass by the ATP content, according to [18]. Enzyme activities were used as indicators of soil quality and changes in biogeochemical functions in response to management or perturbations [19]. Enzyme activities of phoshomonoesterase, arysulfatase, aryesterase urease, cellulose and glucosidase were determined according to [20,21,22,23,24,25]. p-nitrophenyl derivates were used to determine phosphomonoesterase, arylsulfatase, arylesterase, β-glucosidase and cellulase activities; therefore, the activities of these enzyme were measured by quantification of the p-nitrophenol (PNP) released during the incubation [26]. The urease activity was quantified by the ammonium (NH4+), released during the incubation. The CO2-C-to-ATP ratio was used to calculate the metabolic quotient (qCO2) value, as an indicator of microbial stress.
2.4. Plant Leaf Elemental Analysis
Six young plant leaves were sampled from both plant species, placed on ice and immediately shipped to the analytical laboratories. Leaves were carefully washed three times with deionized water, dried at 50 °C for 48 h, and 0.1 g of each dried leaf was digested in 10 mL of 69% HNO3 using a microwave digestion system (Mars 6, CEM Corporation, Matthews, NC, USA). Elemental concentrations in the digests were determined by atomic absorption spectroscopy (PinnAAcle 500, PerkinElmer, Inc., Waltham, MA, USA).
2.5. Data Analysis
All analytical results were reported as mean values ± standard deviation. Data were analyzed by analysis of variance followed by post-hoc comparison of means to identify the statistical differences between de-sealed and topsoil plots within each municipality and between the three sampling points by JMP 10 software (SAS Institute Inc., Cary, NC, USA).
3. Results
De-sealed and topsoil properties showed few significant differences after the setup of trial plots (T0) in all sites (Table 2). No significant differences were observed for the pH value and bulk density between de-sealed soils and topsoil in each site. Bulk density of de-sealed soil results was lower than the topsoil in each site. Soil texture revealed a higher sand percentage in the de-sealed soil of Site 2 and Site 3, of clay in the respective topsoil, and a high texture similarity between topsoil and de-sealed soils of Site 1.

Table 2.
Physical characteristics of topsoil and de-sealed soils from three sites at T0 n = 3.
During three years of experimentations, no differences for total carbon (C tot) were found between de-sealed soils and topsoil in each site, except for the C org, which resulted in higher de-sealed soils compared to topsoil (Figure 3). Site 3 showed a significant increase of C org in both de-sealed and topsoil plots at T2 (Figure 3). Concerning the total metal concentrations, Zn, Pb and Cu significantly changed during the times when all topsoil and de-sealed soil plots were recorded (Table 3).
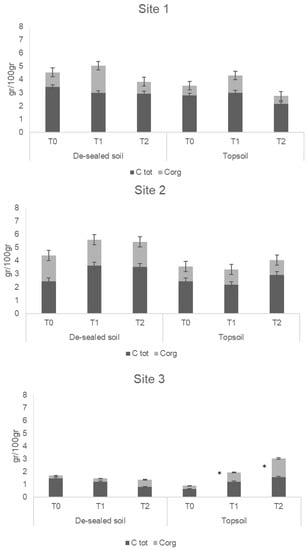
Figure 3.
Total concentration of total carbon (C tot) and organic carbon (C org) for Site 1, Site 2 and Site 3, respectively, during the three sampling points (T0, T1 and T2). Values are the average (n = 3) ± standard error of the means. Asterisk identifies statistical differences among topsoil and de-sealed soil within the same time identified by Tukey’s test at p < 0.05.

Table 3.
Total concentration of carbon Zn, Pb and Cu for Site 1, Site 2 and Site 3 during the three sampling points.
Concerning soil microarthropods analysis, the number of taxa (NT) analyzed in each site at T0 was <5. In Site 1 at T1 and T2, the NT found was 7 for topsoil and 8 for de-sealed soil; in Site 2 in both T1 and T2, the number of taxa was 10 for topsoil and 8 for de-sealed soils; in Site 3, the number of taxa was 8 for topsoil and 9 for de-sealed soil for both T1 and T2. Acari and Collembola were the most represented taxa (approximately 40%) of the total microarthropods extracted from all the soil samples analyzed. The calculation of soil biological quality index (QBS) showed a clear increase of values during the time, with significant differences between T0 compared with T1 and T2. No significant differences between de-sealed and topsoil plots were seen during the time, with the exception of T0 in Sites 1 and 2 (Figure 4). At intermediate time T1, in Site 1 QBS values ranged between 86 and 96 in de-sealed and topsoil plots; in Site 2, QBS results ranged between 127 and 107 in de-sealed and topsoil, while the site report showed a score of 120 for topsoil and 117 for de-sealed plots in Site 3. The values scored at T1 were not significantly different from T2 for each site (Figure 4).
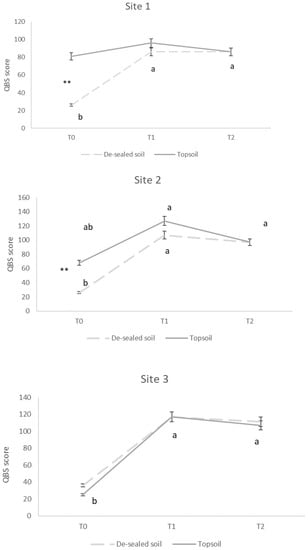
Figure 4.
Total score for Soil Biological Quality index (QBS-ar) in de-sealed and topsoil plots for each site at three sampling points (T0, T1 and T2). Significant differences between de-sealed and topsoil (at least p < 0.05) within the same time are shown by asterisks (** < 0.01); small letters denote a comparison across time for de-sealed soil and topsoil.
The results of the measured biochemical parameters at T1 are reported in Figure 5. Concerning soil enzyme activities, in Site 1 the only difference was parameter cellulase activity was higher in the de-sealed as compared to the topsoil; in Site 3 no differences in the biochemical parameters were observed, whereas in Site 2 the topsoil showed higher alkaline phosphomonoesterase (AlkP), acid phosphomonoesterase (Acid P), arylsulfatase and β-glucosidase activities compared to the de-sealed soil (Figure 5). In Site 2, the cellulase activity did not show significant differences, whereas arylestease activity was higher in de-sealed soil than topsoil (Figure 5). Soil microbial biomass was higher in the topsoil in Site 2 than in the de-sealed plot, whereas no significant difference was observed in soil respiration. No significant difference was observed for qCO2 between de-sealed and topsoil plots in each site (Figure 5). The enzyme activities, soil respiration, soil microbial biomass and metabolic quotient did not show significant differences between de-sealed soil in different sites.
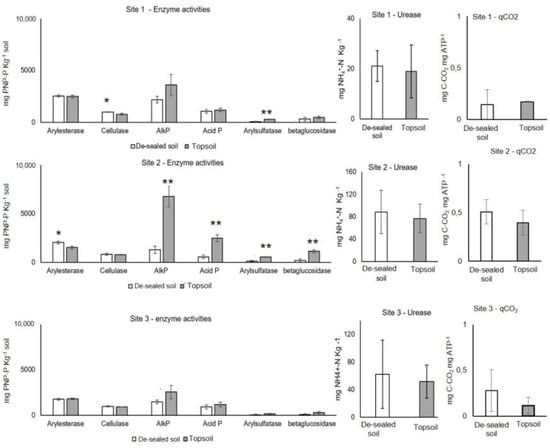
Figure 5.
Enzyme activities, microbial biomass, respiration and metabolic quotient of de-sealed and topsoil in Site 1, 2 and 3 at T1. AlkP = alkaline phosphomonoesterase, Acid P = acid phosphomonoesterase. Asterisks indicate significant statistics by ANOVA (p < 0.05) between de-sealed and topsoil in different sites.
Heavy metal concentrations in E. × ebbingei and V. tinus leaves after two years of growth are reported in Table 4. For Cu, no significant differences were observed between plants grown on de-sealed or topsoil except for E. × ebbingei on Site 2, which showed a lower leaf Cu concentration when grown on de-sealed soil (Table 4). The Zn concentrations showed no significant differences between de-sealed and topsoil, with the exception of V. tinus on Site 2, where plant leaves grown on de-sealed soil showed lower Zn concentrations compared to plants grown on topsoil (Table 4). Regarding Pb concentrations, the only significant difference between plants from de-sealed and topsoil was observed in Site 1, where V. tinus leaves of plants grown on de-sealed soil showed a higher concentration in comparison with plants grown on topsoil (Table 4).

Table 4.
Heavy metal concentrations (µg g−1 d.w.) in leaf samples after two years of plant cultivation in de-sealed and topsoil at T1. Significant differences (at least p < 0.05) are shown by different small letters for comparison between de-sealed and topsoil within the same species.
4. Discussion
Our results showed that de-sealed soils could restore their quality and fertility without the using exogenous topsoil. In all the experimental plots, the presence of ornamental shrubs enriched the soil organic C through leaf litter and rhizo-depositions in both de-sealed soils and topsoil. De-sealed soils showed high chemical fertility, in terms of bulk density, pH values and total and organic carbon. The metal concentrations of the topsoil and de-sealed soils lie in the background ranges of the soils of the Emilia Romagna region plain [27]. Heavy metal concentrations in soils of the Emilia-Romagna alluvial plain are chiefly controlled by texture and less by the parent material composition, and under such conditions, Cu, Pb and Zn are preferentially bound to the silt and clay fractions and characterized by low bioavailability [28]. The metal enrichment over time in all sites and plots in our experiment is due to the accumulation of pollutants derived by exposition on anthropogenic activities in urban areas, as already showed in previous studies [29,30,31]. Anthropogenic sources such as vehicle exhaust, household waste and construction activities could make heavy metal concentrations higher than their background values. Furthermore, many variables influence the concentrations of heavy metals in urban soils: hydrological characteristics, compaction, perturbations and parent material. Non-negligible is the historical aspect of past use of heavy metals in pigments in urban cites [31]. These variables play an important role in their accumulation in urban soils, making the sources of heavy metals in urban soils extremely heterogeneous.
An analysis of the QBS index showed a clear increase of values at T1 and T2 compared T0 in all sites for both de-sealed and topsoil. The low values of T0 are related to the soil disturbances (e.g., setup of experiment) since soil microarthropods are extremely sensitives to soil perturbation [32]. The QBS values at T1 and T2 in all sites resulted in comparable data to those of other studies, e.g., the values were typical of temporary pasture soils [16,32]. These relatively good values of the QBS could be related to the presence of ornamental shrubs, mainly due to the release of leaf litter to feed the primary decomposers [33]. The soil microbial biomass, soil respiration and soil enzyme activities showed more variation between topsoil than de-sealed soils in different sites. The small differences between the microbial and biochemical parameters between topsoil and de-sealed soils could be due to the fact that the successful plant rooting on both soils equally sustained the microbial activities in all the studied soils [14]. Plant rhizo-depositions account for a significant part of the plant-assimilated C [34,35]. These large inputs of labile C sustain the proliferation and activity of the plant rhizosphere microbial communities. For these reasons, our results were in contrast with those of [2] who reported significant lower values of soil respiration and urease activity in some sealed soil, especially compared to agricultural soils. This was probably related to differences in soil TOC content, which is the prime source of energy for soil microorganisms [36], and to the microbial stimulation effect of the plant’s presence. The metabolic quotient qCO2 showed comparable values between topsoil and de-sealed plots in each site, indicating the substrate quality and no stressful conditions of soils at T1 [37]. Low metal concentrations detected in the plant leaves were within the typical ranges reported by [38]. The results of soil microbial activities and heavy metals concentrations in soil and leaves suggest that de-sealed soils could sustain plant growth quality, as previous shown [14].
Overall, our results outline the possible rapid biological restoration of de-sealed soils for saving agricultural soils in urban expansion [39].
5. Conclusions
Our results demonstrate that de-sealed soils, after accurate management (shrub planting, irrigation), can improve their fertility reach in a short time, as well as functional and biological stability. De-sealing can contribute to the recent growing interest of reclamation of urban soils for improving the urban environment quality through the restoration of soil functions and related ecosystem services.
Author Contributions
Conceptualization A.M., C.C., F.U. (Fabrizio Ungaro); methodology, A.M., L.G., G.R.; formal analysis, A.M., L.G., I.C.; investigation, A.M., C.C., F.U. (Fabrizio Ungaro), F.U. (Francesca Ugolini), S.B.; data curation, A.M., L.G., C.G.; writing—original draft preparation, A.M., C.C., F.U. (Fabrizio Ungaro), C.G., G.R.; writing—review and editing, A.M.; project administration, C.C. and F.U. (Fabrizio Ungaro); funding acquisition, C.C. and F.U. (Fabrizio Ungaro). All authors have read and agreed to the published version of the manuscript.
Funding
This research was founded by the EU LIFE Program, within the framework of the project SOS4LIFE—Save Our Soil for Life (LIFE15 ENV/IT/000225).
Institutional Review Board Statement
The study did not involve humans or animals.
Informed Consent Statement
The study did not involve humans.
Data Availability Statement
The study report only original data.
Acknowledgments
The authors wish to thank Cristiana Caivano as MSc student of Biology at Florence University, Elena Bartoli of the Planning and Urban Development Office, Alfonso Paltrinieri of the Urban Greening Office of the Municipality of Carpi, Pasquale Ricciato and Stefano Bazzocchi of the Planning and Urban Development Office of the Municipality of Forlì, and Fernanda Canino, Cosetta Giovannini and Marco Grillini of the Planning and Urban Development Office of the Municipality of San Lazzaro di Savena for logistical support during the installation of the plots and for their continuous assistance over the three years.
Conflicts of Interest
We declare no conflict of interest.
References
- EEA. Land and Soil in Europe—Ever-Sprawling Urban Concrete? 2019. Available online: https://www.eea.europa.eu/signals/signals-2019-content-list/articles/land-and-soil-in-europe (accessed on 25 May 2020).
- Piotrowska-Długosz, A.; Charzyński, P. The impact of the soil sealing degree on microbial biomass, enzymatic activity, and physicochemical properties in the Ekranic Technosols of Toruń (Poland). J. Soils Sediments 2014, 15, 47–59. [Google Scholar] [CrossRef]
- Salvati, L.; Mancini, A.; Bajocco, S.; Gemmiti, R.; Carlucci, M. Socioeconomic development and vulnerability to land degradation in Italy. Reg. Environ. Chang. 2011, 11, 767–777. [Google Scholar] [CrossRef]
- Burghardt, I.; Hynes, J.T. Excited-State Charge Transfer at a Conical Intersection: Effects of an Environment. J. Phys. Chem. A 2006, 110, 11411–11423. [Google Scholar] [CrossRef] [PubMed]
- Wessolek, G.; Schwärzel, K.; Greiffenhagen, A.; Stoffregen, H. Percolation characteristics of a water-repellent sandy forest soil. Eur. J. Soil Sci. 2007, 59, 14–23. [Google Scholar] [CrossRef]
- Gábor, P.; Jombach, S.; Ongjerth, R. The relation between the biological activity and the land surface temperature in Budapest. Appl. Ecol. Environ. Res. 2009, 7, 241–251. [Google Scholar] [CrossRef]
- Fokaides, P.A.; Kylili, A.; Nicolaou, L.; Ioannou, B. The effect of soil sealing on the urban heat island phenomenon. Indoor Built Environ. 2016, 25, 1136–1147. [Google Scholar] [CrossRef]
- Murata, T.; Kawai, N. Degradation of the urban ecosystem function due to soil sealing: Involvement in the heat island phenomenon and hydrologic cycle in the Tokyo metropolitan area. Soil Sci. Plant Nutr. 2018, 64, 145–155. [Google Scholar] [CrossRef]
- Bhaduri, B.; Minner, M.; Tatalovich, S.; Harbor, J. Long-term hydrologic impact of urbanization: A tale of two mod-els. J. Water Resour. Plan. Manag. 2001, 127, 13–19. [Google Scholar] [CrossRef]
- Ungaro, F.; Calzolari, C.; Pistocchi, A.; Malucelli, F. Modelling the impact of increasing soil sealing on runoff coeffi-cients at regional scale: A hydropedological approach. J. Hydrol. Hydromech. 2014, 62, 33–42. [Google Scholar] [CrossRef]
- Kayhanian, M.; Suverkropp, C.; Ruby, A.; Tsay, K. Characterization and prediction of highway runoff constituent event mean concentration. J. Environ. Manag. 2007, 85, 279–295. [Google Scholar] [CrossRef]
- Cambou, A.; Shaw, R.K.; Huot, H.; Vidal-Beaudet, L.; Hunault, G.; Cannavo, P.; Nold, F.; Schwartz, C. Estimation of soil organic carbon stocks of two cities, New York City and Paris. Sci. Total Environ. 2018, 644, 452–464. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. Roadmap to a Resource Efficient Europe. COM (2011) 571 Final, 2011. Brussels, 20.9.2011. Available online: https://eurlex.europa.eu/legalcontent/EN/TXT/PDF/?uri=CELEX:52011DC0571&from=EN (accessed on 30 October 2020).
- Ugolini, F.; Baronti, S.; Lanini, G.M.; Maienza, A.; Ungaro, F.; Calzolari, C. Assessing the influence of topsoil and technosol characteristics on plant growth for the green regeneration of urban built sites. J. Environ. Manag. 2020, 273, 111168. [Google Scholar] [CrossRef] [PubMed]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Update 2015; Food and Agriculture Organization of The United Nations: Rome, Italy, 2015. [Google Scholar]
- Parisi, V.; Menta, C.; Gardi, C.; Jacomini, C.; Mozzanica, E. Microarthropod communities as a tool to assess soil quality and biodiversity: A new approach in Italy. Agric. Ecosyst. Environ. 2005, 105, 323–333. [Google Scholar] [CrossRef]
- Anderson, J.; Domsch, K. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Ciardi, C.; Nannipieri, P. A comparison of methods for measuring ATP in soil. Soil Biol. Biochem. 1990, 22, 725–727. [Google Scholar] [CrossRef]
- Browman, M.G.; Tabatabai, M.A. Phosphodiesterase Activity of Soils. Soil Sci. Soc. Am. J. 1978, 42, 284–290. [Google Scholar] [CrossRef]
- Tabatabai, M.; Bremner, J. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Arylsulfatase Activity of Soils. Soil Sci. Soc. Am. J. 1970, 34, 225–229. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ceccanti, B.; Cervelli, S.; Sequi, P. Use of 0·1 m pyrophosphate to extract urease from a podzol. Soil Biol. Biochem. 1974, 6, 359–362. [Google Scholar] [CrossRef]
- Zornoza, R.; Landi, L.; Nannipieri, P.; Renella, G. A protocol for the assay of arylesterase activity in soil. Soil Biol. Biochem. 2009, 41, 659–662. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy/Soil Science Society of America: Madison, WI, USA, 1982; pp. 903–947. [Google Scholar]
- Imparato, V.; Hansen, V.; Santos, S.S.; Nielsen, T.K.; Giagnoni, L.; Hauggaard-Nielsen, H.; Winding, A. Gasifica-tion biochar has limited effects on functional and structural diversity of soil microbial communities in a temperate agroeco-system. Soil Biol. Biochem. 2016, 99, 128–136. [Google Scholar] [CrossRef]
- Dick, R.; Kandeler, E. Enzymes in Soils. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Oxford, UK, 2005; pp. 448–456. [Google Scholar]
- Marchi, N.; Ungaro, F. Carta del Fondo Naturale—Antropico dei Metalli Pesanti. 2019. Available online: https://ambiente.regione.emilia-romagna.it/it/geologia/suoli/metalli-pesanti/carta-del-fondo-naturale-antropico-della-pianura-emiliano-romagnola-alla-scala1-250-000-2012 (accessed on 30 October 2020).
- Kim, R.-Y.; Yoon, J.-K.; Kim, T.-S.; Yang, J.E.; Owens, G.; Kim, K.-R. Bioavailability of heavy metals in soils: Definitions and practical implementation—A critical review. Environ. Geochem. Health 2015, 37, 1041–1061. [Google Scholar] [CrossRef]
- Imperato, M.; Adamo, P.; Naimo, D.; Arienzo, M.; Stanzione, D.; Violante, P. Spatial distribution of heavy metals in urban soils of Naples city (Italy). Environ. Pollut. 2003, 124, 247–256. [Google Scholar] [CrossRef]
- Charzyński, P.; Plak, A.; Hanaka, A. Influence of the soil sealing on the geoaccumulation index of heavy metals and various pollution factors. Environ. Sci. Pollut. Res. 2017, 24, 4801–4811. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.-Y.; Mo, C.-H.; Li, H.-Q.; Lü, H.; Zeng, Q.-Y.; Li, Y.-W.; Wu, X.-L. Heavy metal contamination of urban soils and dusts in Guangzhou, South China. Environ. Monit. Assess. 2012, 185, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Menta, C.; Conti, F.D.; Pinto, S.; Bodini, A. Soil Biological Quality index (QBS-ar): 15 years of application at global scale. Ecol. Indic. 2018, 85, 773–780. [Google Scholar] [CrossRef]
- Lynch, J.M.; Whipps, J.M. Substrate flow in the rhizosphere. Plant Soil 1990, 129, 1–10. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Domanski, G. Carbon input by plants into the soil. Review. J. Plant Nutr. Soil Sci. 2000, 163, 421–431. [Google Scholar] [CrossRef]
- Cardoso, E.J.B.N.; Vasconcellos, R.L.F.; Bini, D.; Miyauchi, M.Y.H.; Dos Santos, C.A.; Alves, P.R.L.; De Paula, A.M.; Nakatani, A.S.; Pereira, J.D.M.; Nogueira, M.A. Soil health: Looking for suitable indicators. What should be considered to assess the effects of use and management on soil health? Sci. Agric. 2013, 70, 274–289. [Google Scholar] [CrossRef]
- Brookes, P.C. The use of microbial parameters in monitoring soil pollution by heavy metals. Biol. Fertil. Soils 1995, 19, 269–279. [Google Scholar] [CrossRef]
- Eitminaviciute, I. Microarthropod communities in anthropogenic urban soils. 1. Structure of microarthropod complexes in soils of roadside lawns. Èntomol. Rev. 2006, 86, S128–S135. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Metals in Plants and Soils; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Salvati, L. Monitoring high-quality soil consumption driven by urban pressure in a growing city (Rome, Italy). Cities 2013, 31, 349–356. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).