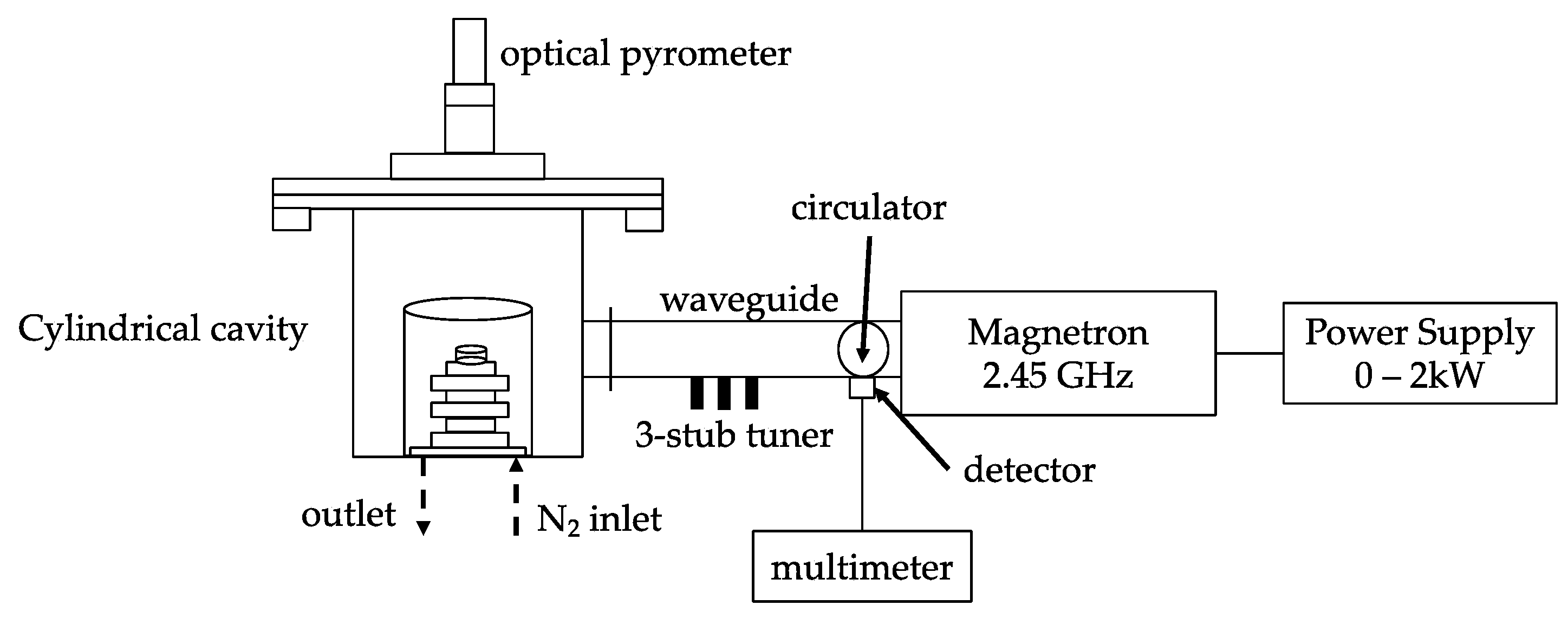
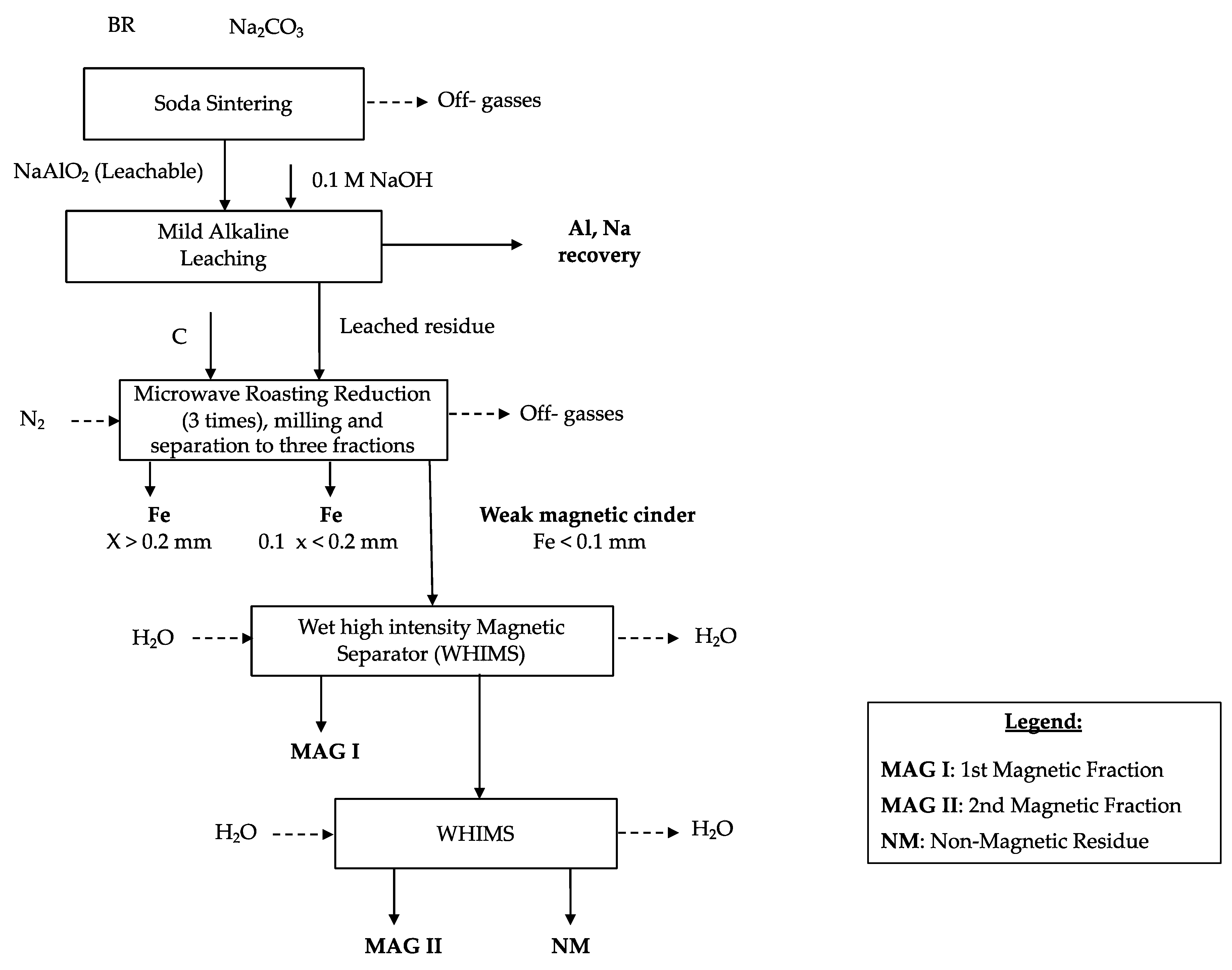
3.3. Microwave Roasting Process of Modified Bauxite Residue
The dried modified BR (MBR) was blended with metallurgical coke at a mass ratio C/MBR 0.225 and it was transformed in tablets using a manual hydraulic press. The sample was treated through microwave roasting process at optimum conditions (0.6 kW, 1 L/min N
2 flow constant and 300 s) and then was characterized via ED-XRF, XRD, and SEM analyses [
31].
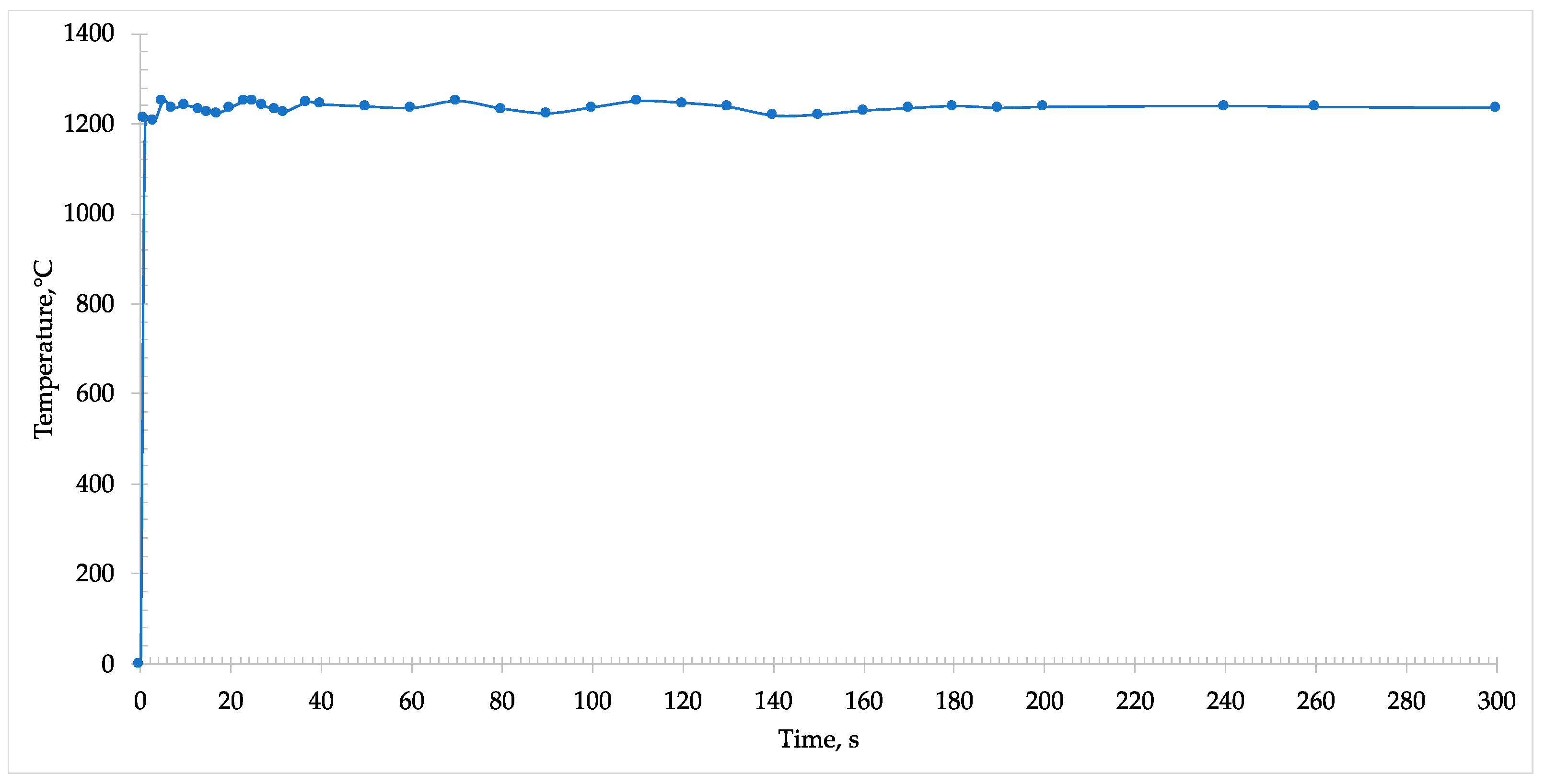
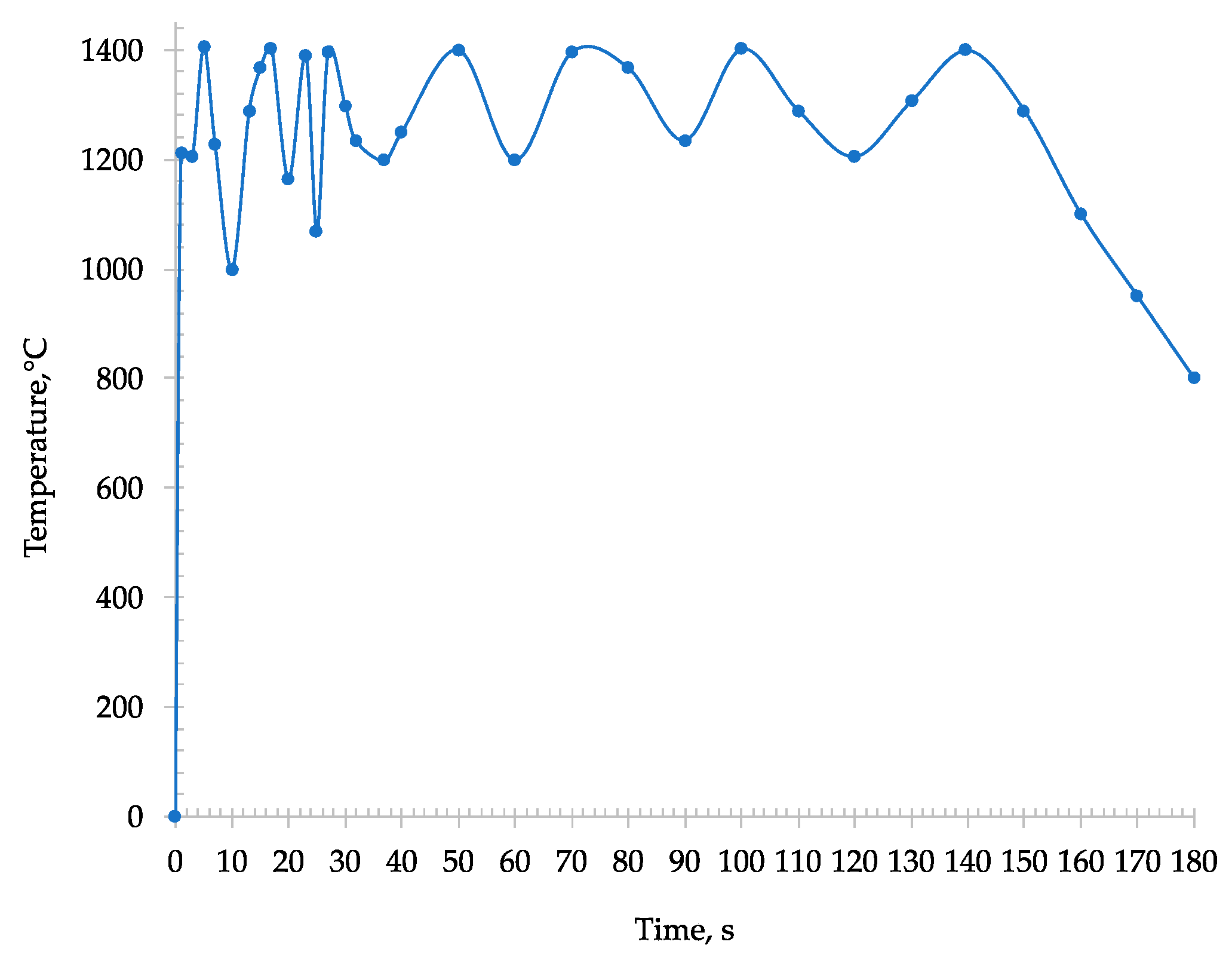
During the microwave heating of the sample, an immediate absorption was detected, and the temperature almost instantaneously rose within some seconds to 1250 °C (
Figure 5).
The formation of melt phases was observed through the window of the pyrometer and started from the center and propagated to the rim of the disk. In addition, at this temperature it was possible to note the reduction of iron oxide into metallic iron. In
Figure 6 the cinder after the microwave roasting reduction is presented, and the presence of metallic iron nuggets is evident.
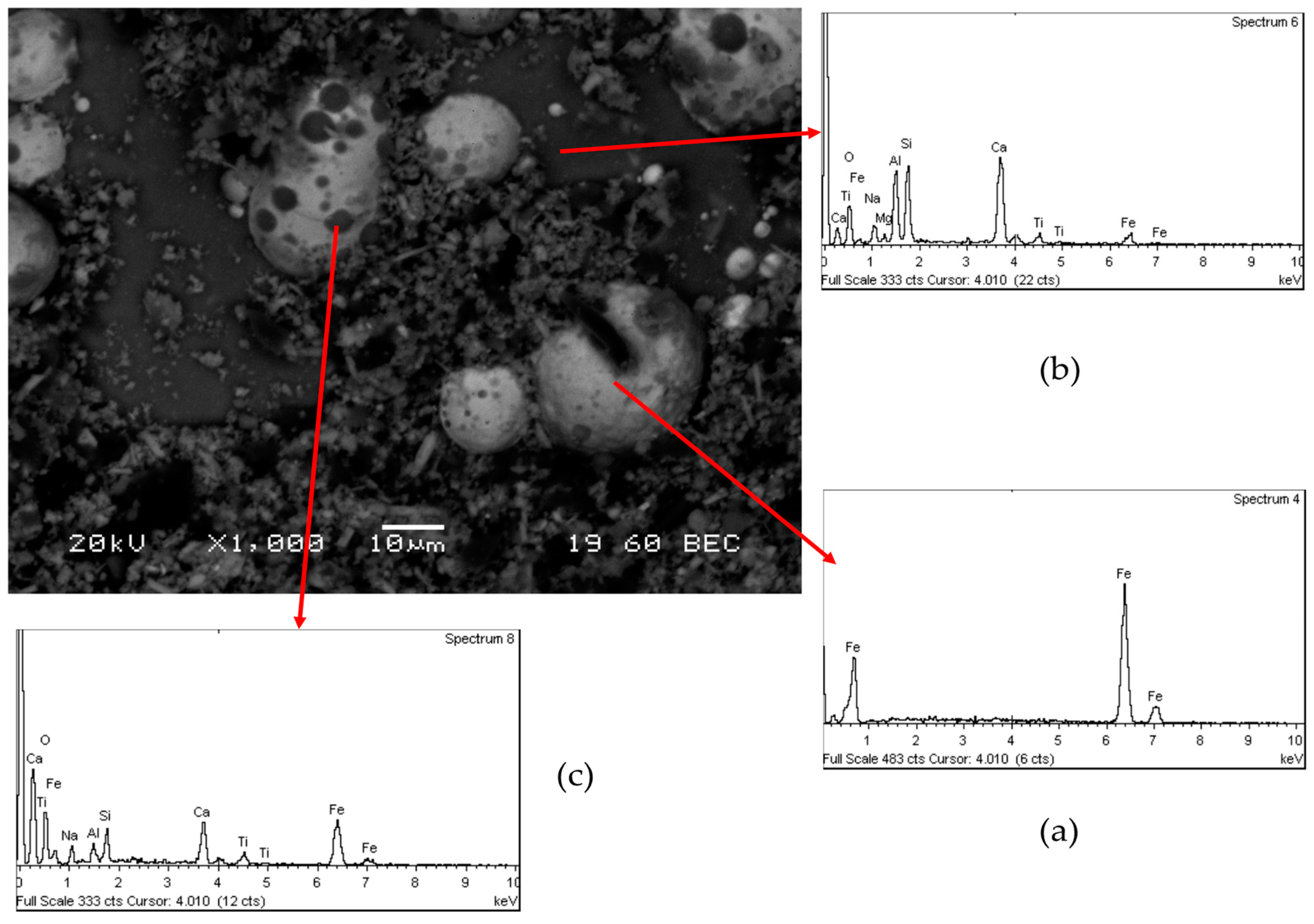
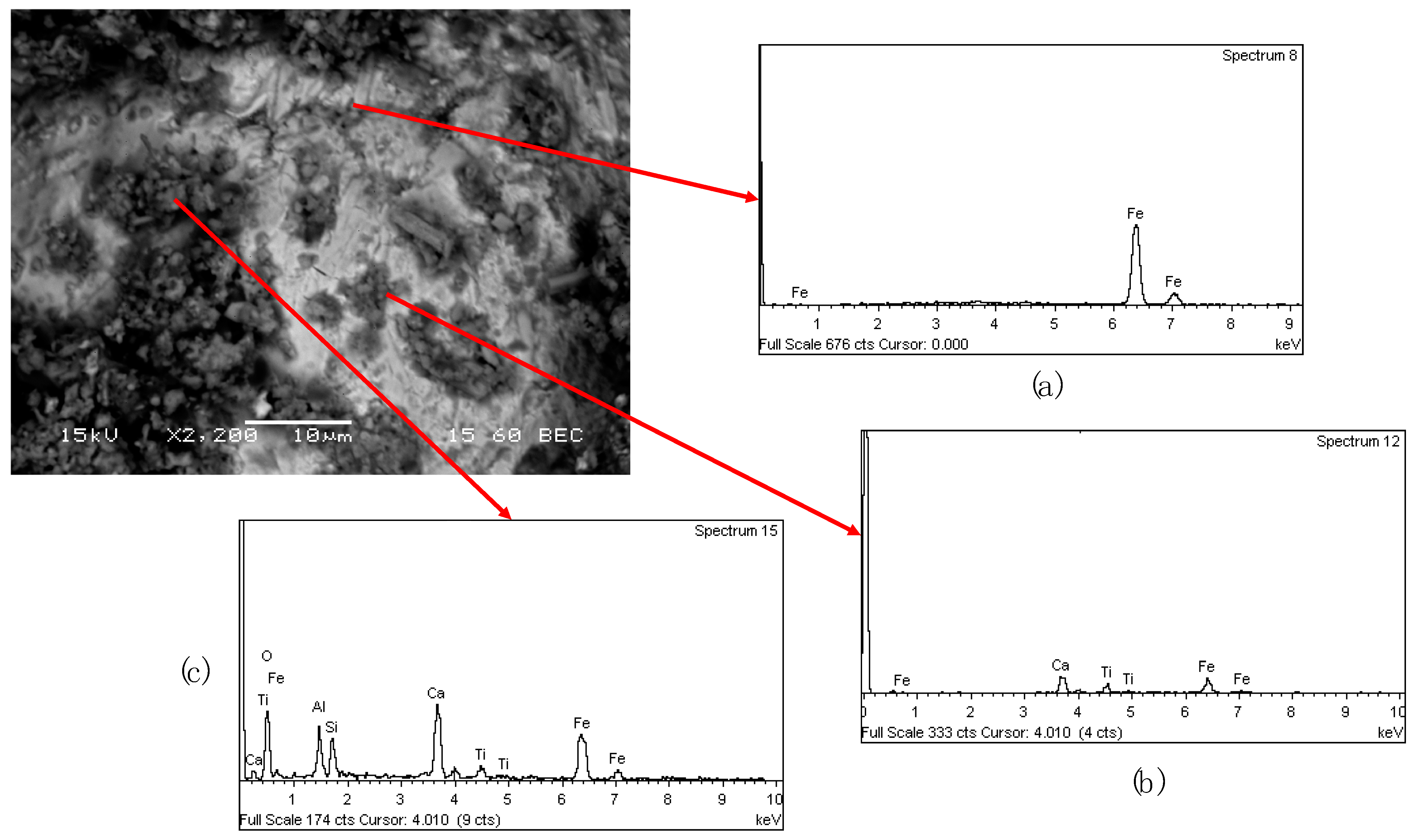
SEM analysis of the cinder confirmed the presence of the spherical metallic particles which are entrapped in the matrix of the solid samples (
Figure 7a). The nuggets are covered by a layer of powder (
Figure 7b), which has the same composition of the matrix (Ca, Al, Si, Na, Ti and Fe) as it is shown in
Figure 7c.
To release the metallic iron spheres, the sample was milled using a planetary ball mill (Planetary Ball Mill PM 100–RESTCH, Haan, Germany) with a grinding time of 5 min and a speed a 400 rpm (revolutions per minute). The sample was treated four times with the planetary ball mill to transform the cinder into fine powder. The metallic iron was then separated from the matrix by employing a manual sieve. The sample was, therefore, physically separated into two fractions: The first one with a particle size higher than 0.2 mm mostly composed by Fe nuggets (around 7 wt.% of the total solid sample) and the other one with a particle size lower than 0.2 mm (around 93 wt.% of the total solid sample).
Samples were analyzed via fusion method; chemical composition of the fraction with the particle size higher than 0.2 mm and the other fraction is shown in
Table 5.
Chemical analysis confirmed that the main component of the fraction with particle size higher than 0.2 mm is metallic iron, being more than 95 wt.% of the total weight of this fraction. In addition, the other fraction (<0.2 mm) still contains a considerable amount of iron.
Analyzing the Fe mass balance, from 35.53 g of Fe present in MBR, 29.59 g of Fe are present in the 1st MW cinder while 5.94 g in the > 0.2 mm fraction.
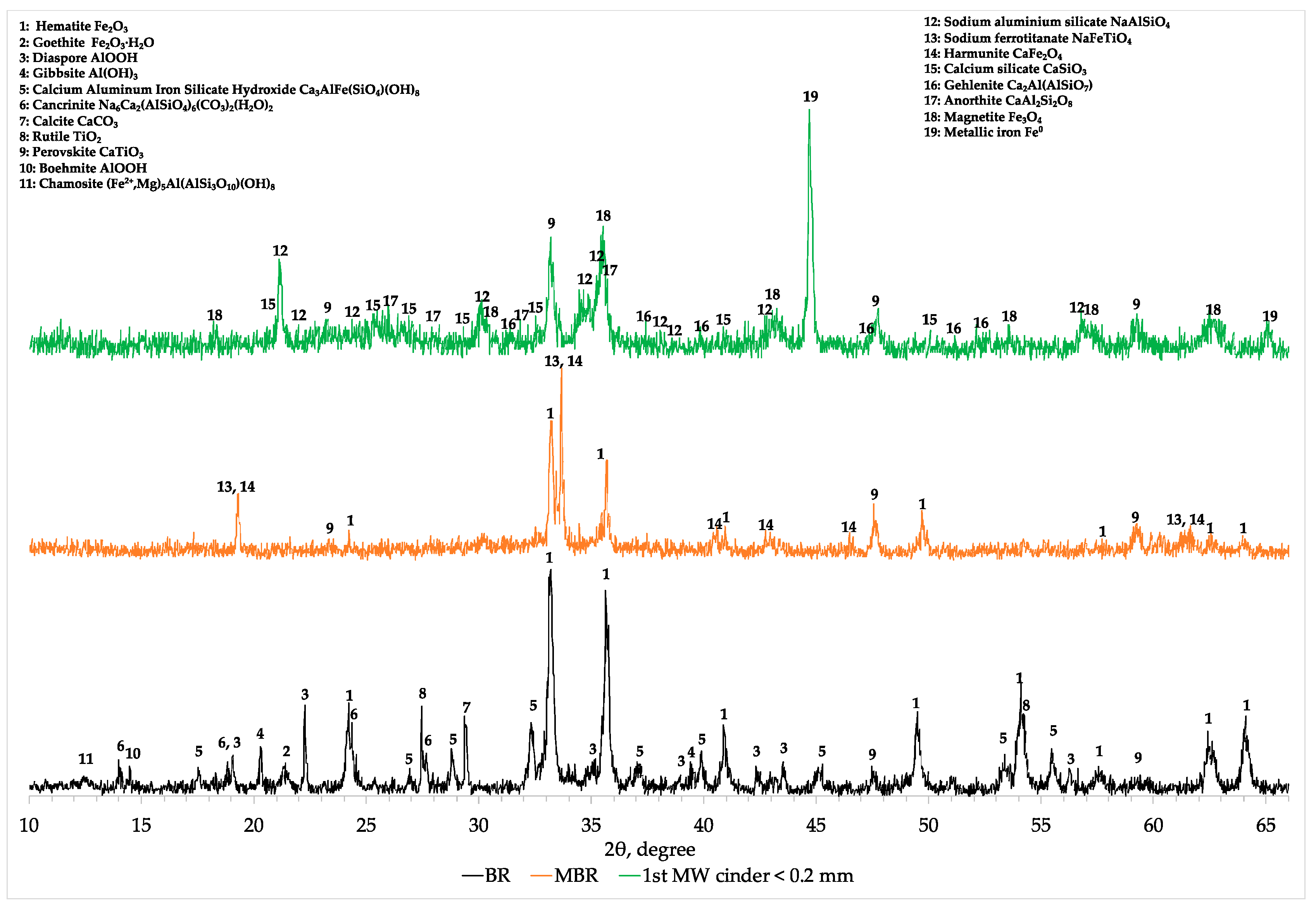
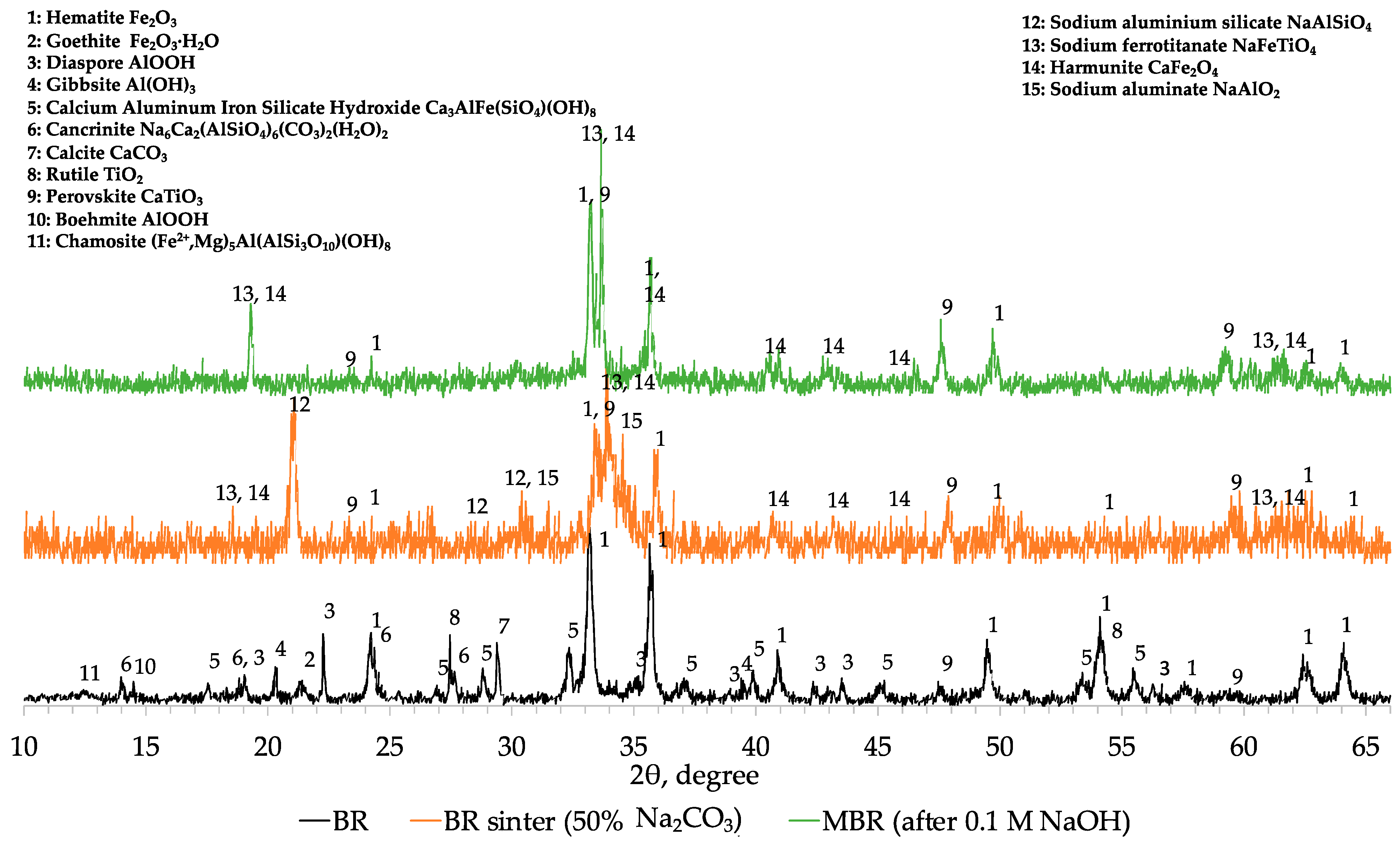
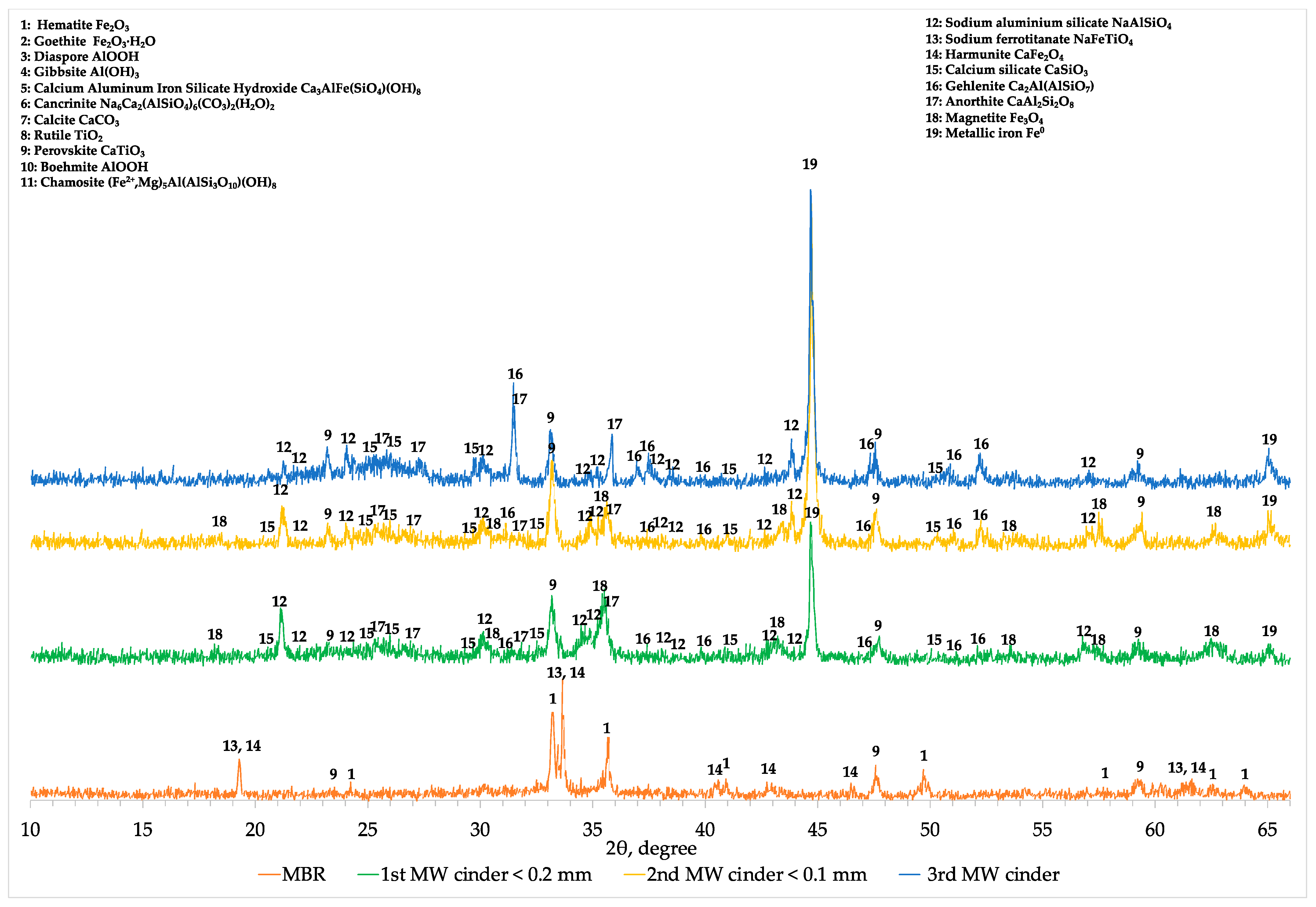
Comparing the XRD profiles of BR, modified BR and the 1st cinder fraction with a particle size lower than 0.2 mm (
Appendix A,
Figure A1), the results obtained with chemical and SEM analyses were confirmed. In fact, magnetite (Fe
3O
4) and metallic iron (Fe
0) are the main iron mineralogical phases after the microwave roasting reduction at the optimum conditions (C/MBR 0.225, 0.6 kW, 1 L/min N
2 flow constant and 300 s). Hercynite (FeAl
2O
4) is a mineralogical phase that is not formed during microwave reductive roasting of aluminum depleted MBR materials (
Appendix A,
Figure A1).
Decreasing the concentration of aluminum in the MBR sample, hematite and other iron-bearing phases are directly reduced into magnetite and then due to the high temperature into metallic iron. At the same time, under these conditions, small part of sodium aluminum silicate (NaAlSiO
4) existing initially in MBR is transformed to anorthite (CaAl
2SiO
3) as well as gehlenite (Ca
2Al(AlSiO
7)) but the major amount of aluminum in cinder remains in the form of sodium aluminum silicate (NaAlSiO
4) (
Appendix A,
Figure A1). In the 2-theta range of 22 to 28°, the mineralogical characterization revealed the formation of a glassy region indicated by the hump and consisting of calcium silicate (CaSiO
3) and anorthite. Titanium phases are converted into perovskite already from the soda roasting process and remain unchanged during the MW reductive roasting process having been beneficiated in the resulted cinder.
Since the cinder fraction with a particle size lower than 0.2 mm contained good microwave receptors (mineralogical phases such as (Fe3O4 and C), the sample was subjected for a second time to a microwave roasting process at the same optimum condition (0.6 kW, 1 L/min N2 flow constant and 300 s), following the already described procedure.
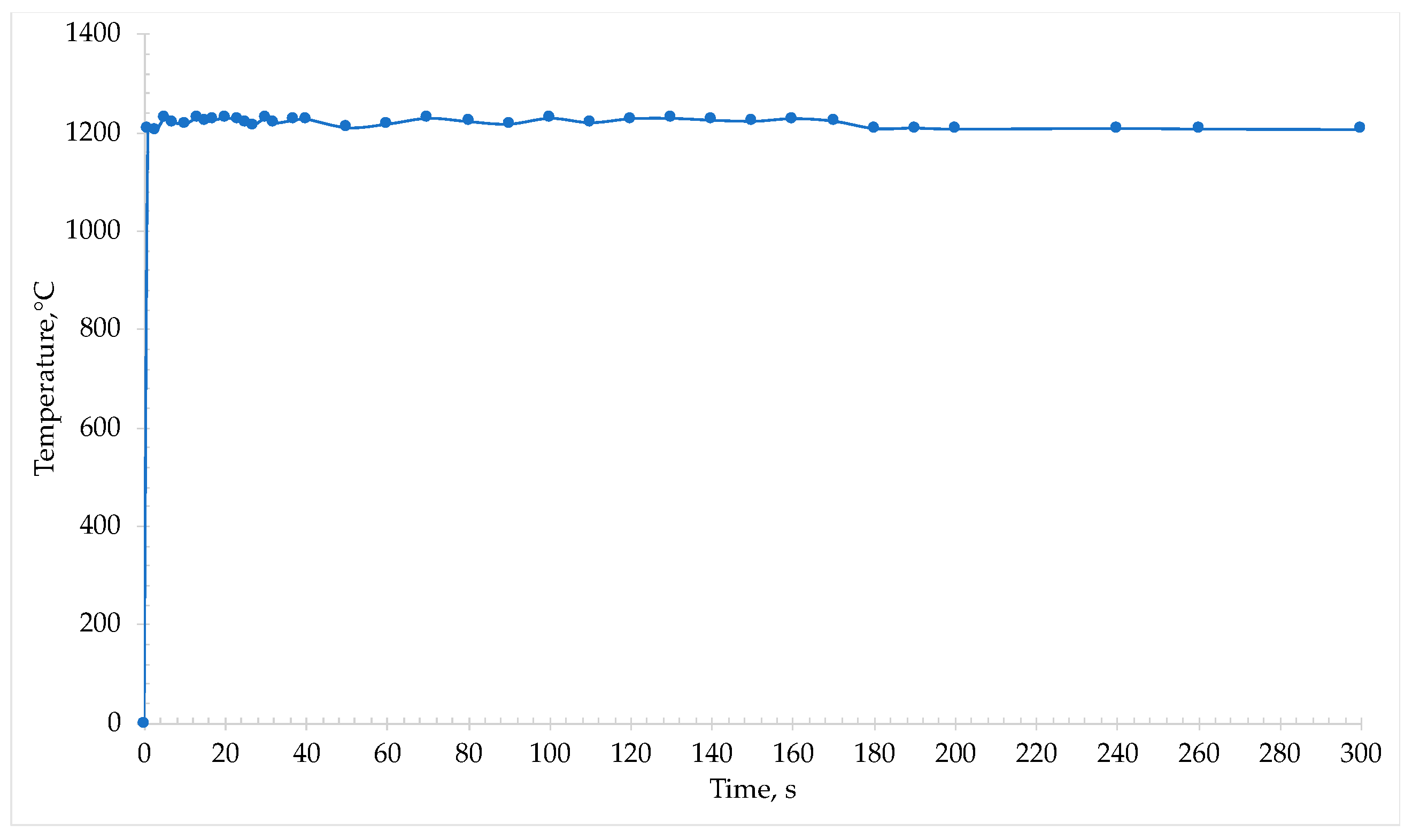
During irradiation with microwaves, an immediate absorption from the sample was detected and the temperature rose within some seconds to 1230 °C (
Figure 8).
At this temperature it was possible to note the reduction of iron oxide into metallic iron and the formation of metal phase. Macroscopically, the 2nd cinder appeared similar to the one from the first microwave treatment, but the iron nuggets were smaller in size.
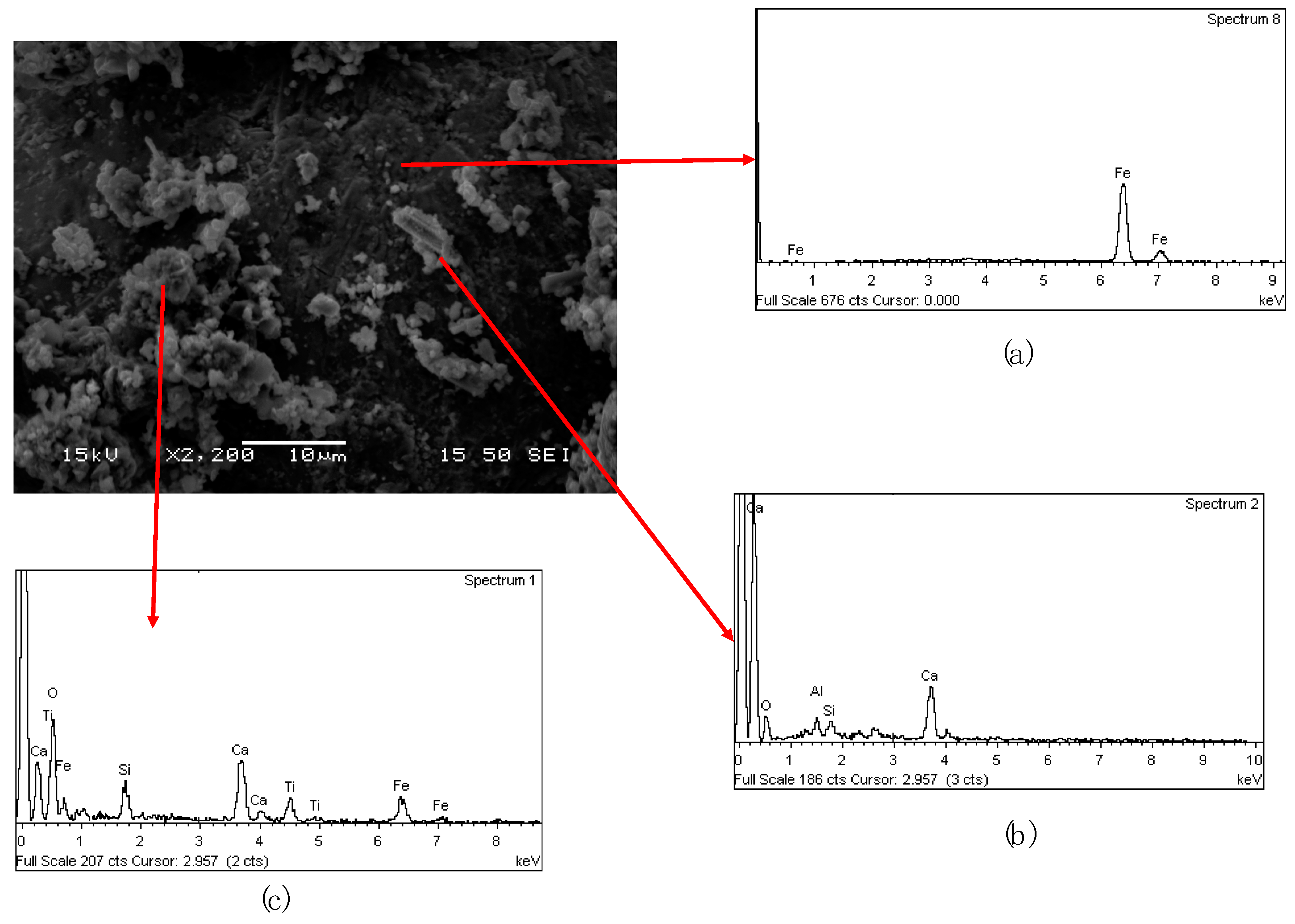
In
Figure 9a back-electron scattering (BEC) picture of the 2nd cinder is shown together with EDS chemical analysis on specific components. The light gray spherical particles are metallic iron particles which are entrapped in the matrix that is mainly composed from all the other elements (Ca, Al, Si, Na, Ti, and Fe) (
Figure 9b). On the top of the metallic spheres are present also some dark gray particles with the same composition of the matrix (
Figure 9c).
The 2nd cinder was ground to release the metallic iron spheres. In this case, for the physical separation, a sieve with openings size of 0.1 mm was used. The chemical analysis of the two fractions formed are presented in
Table 6.
Table 6 shows that the main component of the fraction with particle size ≥ 0.1 mm, which is 1 wt.% of the total cinder weight, is metallic iron.
The other fraction, which is the 99 wt.% of the total cinder weight, still contains a considerable amount of iron. Analyzing the Fe mass balance, from 29.59 g of Fe present in the 1st MW cinder, 28.87 g of Fe are present in the 2nd MW cinder while 0.71 g in the >0.1 mm fraction.
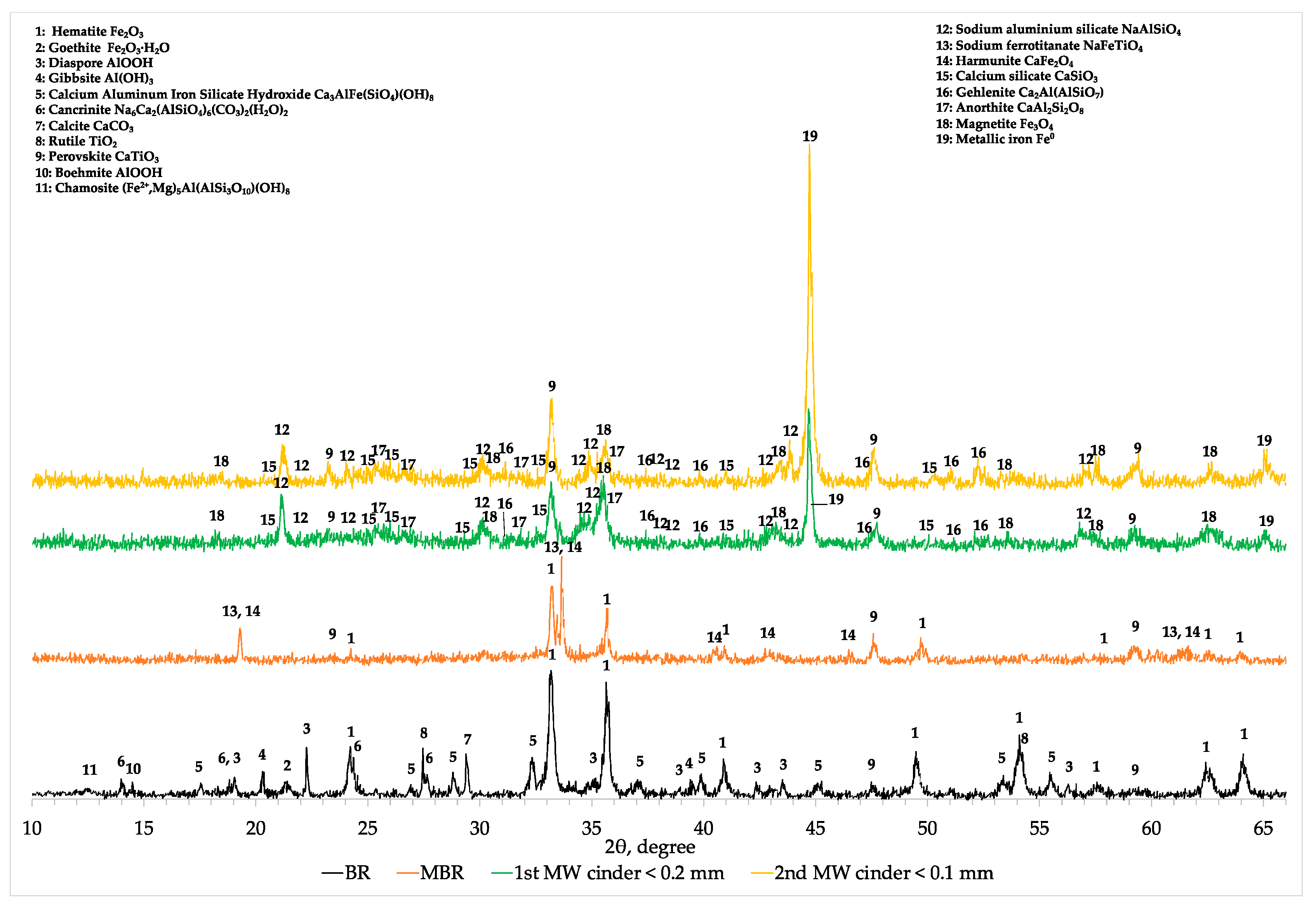
The XRD analysis (
Appendix A,
Figure A2) revealed a mineralogical conformation similar to the one presented in
Figure 9, where magnetite (Fe
3O
4) and metallic iron (Fe
0) were the main iron mineralogical phases after the second microwave roasting reduction. The other elements are present in form of anorthite (CaAl
2SiO
3) gehlenite (Ca
2Al(AlSiO
7)) sodium aluminum silicate (NaAlSiO
4), calcium silicate (CaSiO
3) and perovskite CaTiO
3 as it is shown in
Appendix A,
Figure A2.
The experimental results from MW reductive roasting showed that although 5 min are enough for the full transformation of hematite to a mixture of magnetite and metallic iron the full transformation to metallic iron necessitates more time.
The 2nd cinder was again treated at optimum condition (0.6 kW and 1 L/min N2 flow constant) in the microwave furnace due to the high absorption properties of Fe3O4 to allow the complete formation of metallic iron in the system.
The sample was transformed into tablets and placed inside the microwave furnace. As the previous microwave roasting process, an immediate absorption was detected causing an instantaneous incrementation of the temperature (reaching about 1250 °C). Furthermore, through the window of the pyrometer, light arcing phenomena that took place in some areas of the sample were observed. Due to these phenomena the recorded temperature locally reached around 1400 °C (
Figure 10). The presence of these hot spots in the tablet depends on the interaction of metal iron, already present in the sample, with the microwave which creates a micro-arcing process [
5,
9,
10,
55,
56].
After 180 s, a drastically reduction of T was detected from the pyrometer (
Figure 10) [
2,
10] and the experiments were stopped as the tablet reflected the microwave energy due to the increased concentration of conductive material (such as metallic iron).
From the comparison of the XRD profile of the cinder after the 1st microwave roasting reduction (1st MW), the cinder after the 2nd microwave roasting reduction (2nd MW) and the cinder after the 3rd microwave roasting reduction (3rd MW), it is possible to observe that metallic iron (Fe
0) is the main iron mineralogical phases in the last MW stage. In the 2-theta range of 22 to 28°, the mineralogical characterization revealed that the hump related to the formation of a glassy region consisting of calcium silicate (CaSiO
3) and anorthite (CaAl
2SiO
3), is more evident, due to the high temperature reached during the 3rd MW roasting reduction. The other elements are present in the mixed forms of gehlenite (Ca
2Al(AlSiO
7)), sodium aluminum silicate (NaAlSiO
4), and perovskite CaTiO
3 as it is shown in
Figure 11 [
57].
To confirm the incrementation of the metallic iron content in each sample of the whole microwave roasting reduction (1st, 2nd and 3rd MW process), the above mentioned Zhiyong Xu method was used [
54].
As it can be seen from the results shown in
Table 7, after each microwave roasting reduction the percentage of the metallic iron present in the sample increased, comparing with the total amount of Fe.
The 3rd MW cinder was collected due to its strong magnetic properties and was treated through a CarpcoTM wet high intensity magnetic separator (WHIMS).
3.4. Magnetic Separation Process
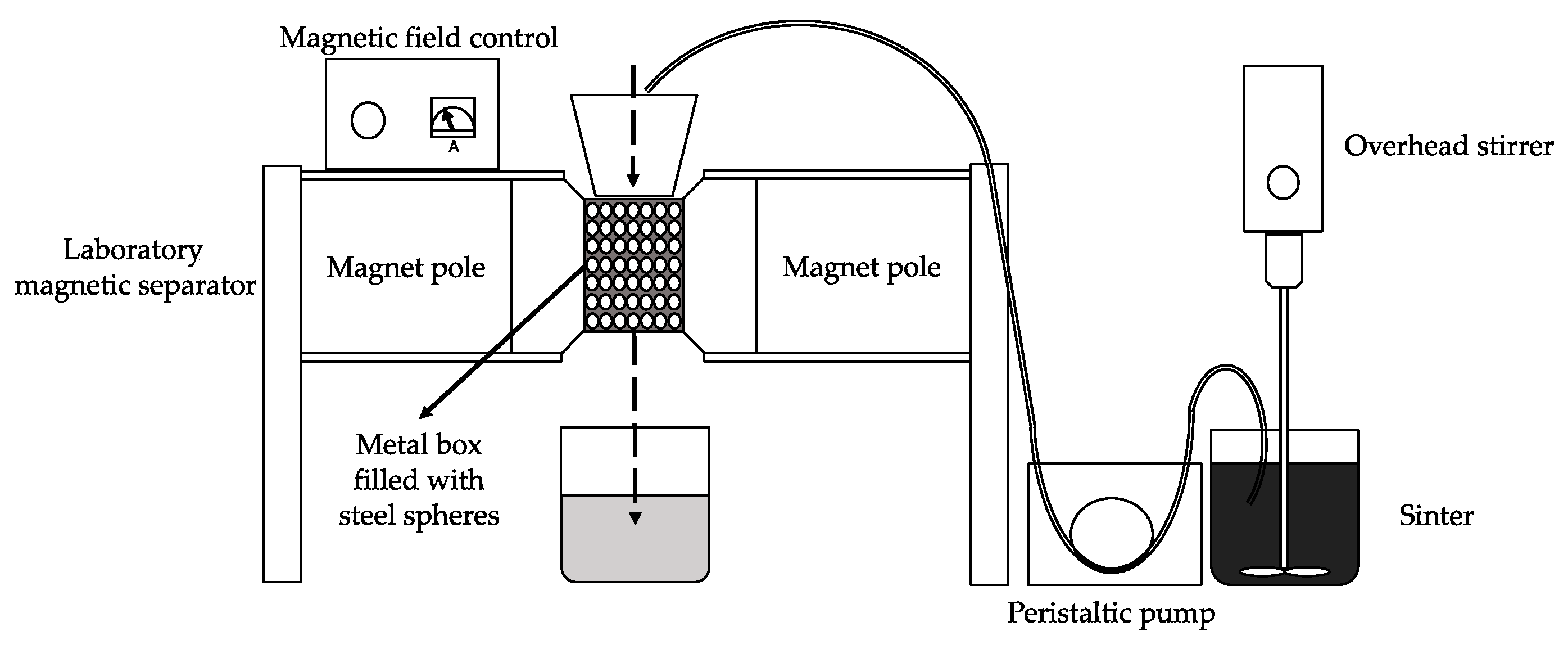
The purposes of the magnetic separation are to liberate from the cinder the iron based components from the matrix and to concentrate them in the magnetic product.
To achieve these aims, the cinder was dispersed in water and was treated through the WHIMS two times following the procedure described in paragraph Material and Method.
Three different products (MAG I, MAG II, and NM) were collected, and representative samples were analyzed. MAG I represented the 62 wt.% of the 3rd MW cinder whereas MAG II was the 17 wt.% and NM the 21 wt.%
The three fractions were treated with Zhiyong Xu method to investigate the percentage of metallic iron in the sample comparing with the total amount of Fe. The results showed that in all the magnetic fractions, the iron analyzed was almost completely in form of metallic iron (about 98%).
As it can be seen from chemical analysis shown in
Table 8, the MAG I is mainly concentrated in iron, while calcium, silicon, titanium are present in minor amount. Aluminum is around 2 wt.% and Na content is negligible. On the other hand, in the NM fraction metallic iron is still present (4 wt.%), whereas the concentration of the other elements that composed the matrix is substantially higher.
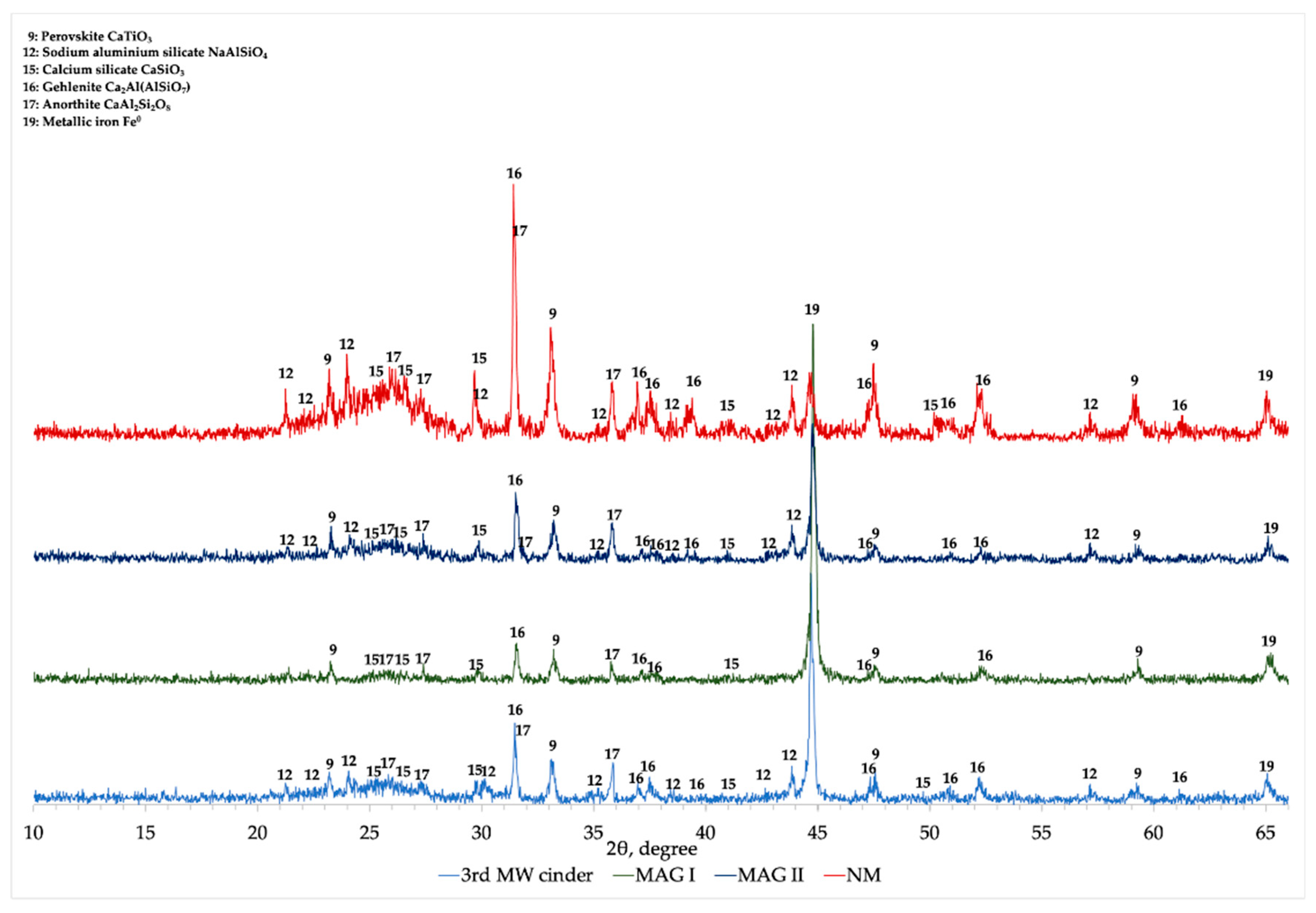
The XRD comparison of 3rd MW cinder, MAG I, MAG II, and NM is presented in
Figure 12. In MAG I the main peak is related to metallic iron, while the other mineralogical phases (gehlenite, calcium titanate, and anorthite) are detected with low intensity. Moreover, between 22 to 28° the characteristic hump associated to calcium silicate and anorthite is not visible and the peaks attributed to sodium aluminum silicate disappear.
XRD analysis of NM fraction showed a different profile comparing with the one of MAG I. As it can be seen, the peak related to metallic iron (about 45°) is drastically reduced in intensity, while the hump associated to calcium silicate and anorthite is noticeable. Gehlenite and sodium aluminum silicate are detected with high intensity peaks.
Regarding MAG II, it is possible to notice that its mineralogical characterization is comparable to the initial cinder (LR 3rd MW).
SEM-EDS analysis of the three fractions confirmed the results of chemical and XRD analyses.
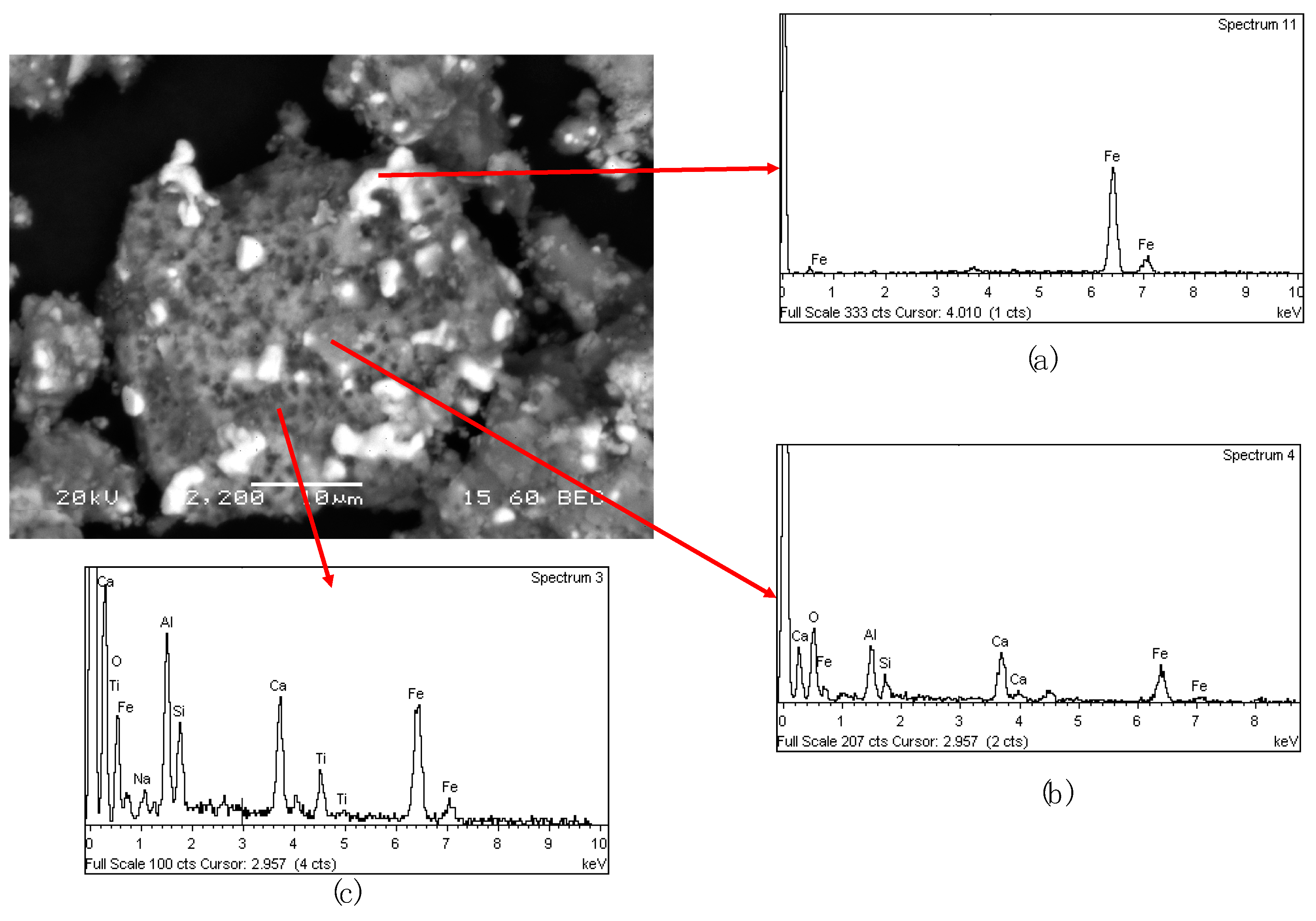
The back electron scattering macrograph of MAG I fraction (
Figure 13) showed that the sample is composed by a melted metallic iron (
Figure 13a) which has entrapped due to imperfect separation a solid matrix containing all the cinder constituents (
Figure 13c). In addition, small particles with calcium titanate were detected also entrapped inside the metallic iron phase (
Figure 13b).
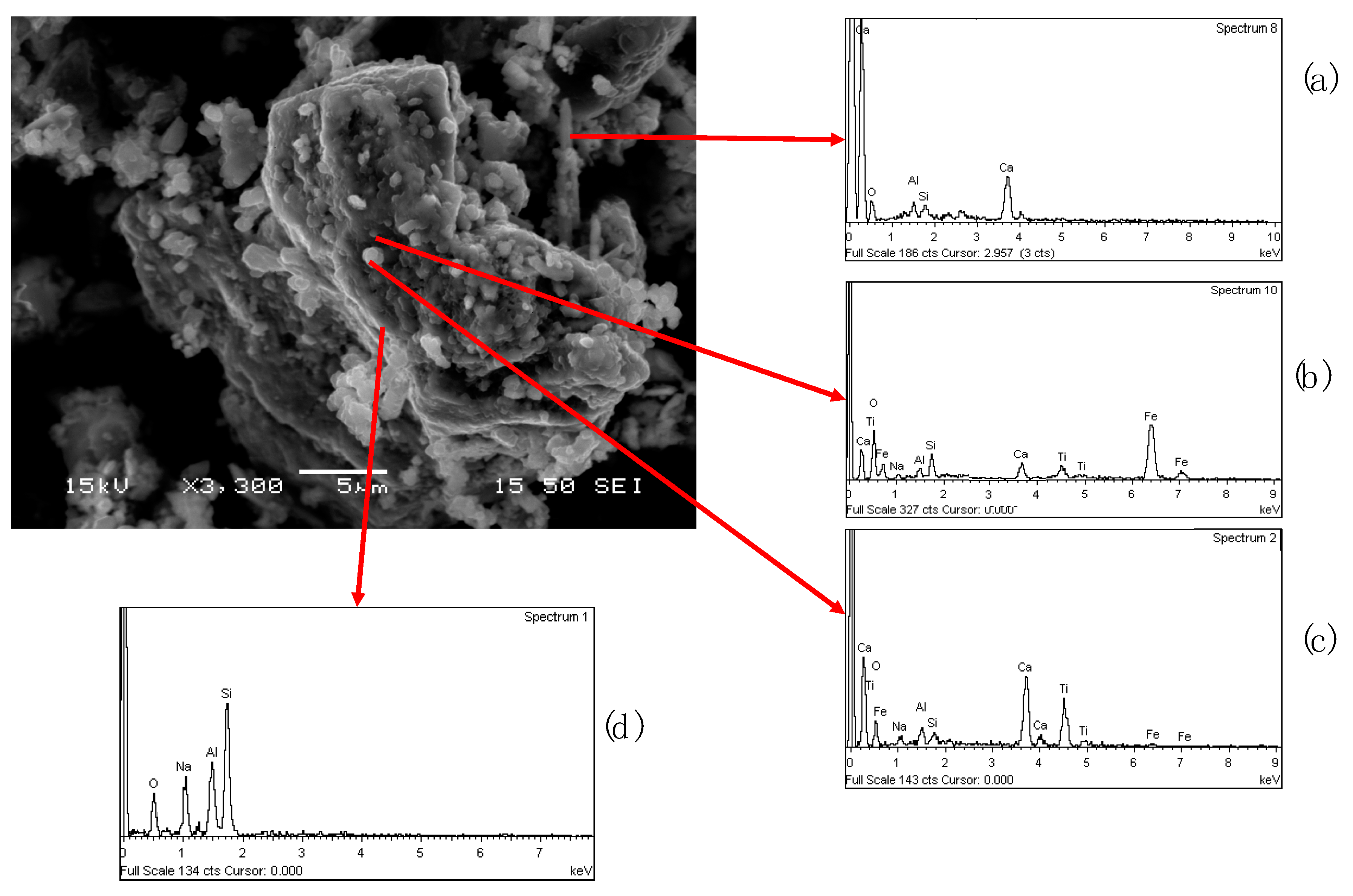
To understand the stratification of the different constituents of the sample, the area of sample shown in
Figure 13 was studied through second electron imaging (SEI) mode. As it is observed in
Figure 14, the particles containing the elements of the matrix are embedded on the melted metallic iron (dark substrate,
Figure 14a) forming aggregates. Some particles were identified as calcium aluminum silicon oxide (
Figure 14b), while some others were composed of Si, Ca, and Ti oxide mixture (
Figure 14c).
The presence of Fe in the spectrum 1 (
Figure 14c), is attributed to interferences from the metallic substrate, due the high magnification.
In
Figure 15 the SEM analysis of the NM fraction with back electron scattering mode is shown. The image shows the presence of small metallic iron (
Figure 15a) particles surrounded by light gray particles (calcium aluminum silicon oxide) (
Figure 15b) and entrapped into dark gray area which was mainly composed by all the cinder constituents (Na, Al, Ca, Si, Ti, and Fe) (
Figure 15c). Also, in this case, the presence of Fe in the spectra 3 and 4 (
Figure 15b,c) is due to the interference from the metallic particles close to the detected area.
Furthermore, the NM fraction was analyzed by employing a secondary electron imaging mode to comprehend the existing different mineralogical phases (
Figure 16).
Big particles composed with sodium aluminum silicate were identified (
Figure 16d), in this grain also a calcium titanium phase (
Figure 16c) and iron (
Figure 16b) were detected. The presence of the other elements in the EDS-spectra depends on the proximity interference of the other mineralogical phases. Calcium aluminum silicate were found in a specific area of the sample (
Figure 16a).
In conclusion, in terms of Fe partition within the different phases formed during the complex treatment of BR (
Figure 17), after the 1st and 2nd microwave roasting reduction, nuggets were produced with high purity in Fe, overall extracting about 16% in the metallic iron spheres fraction with a particle size >0.2 mm and 2% in the spheres with a particle size >0.1 mm of the total Fe content of BR. Moreover, Fe was recovered through a magnetic separation process. Three fractions (MAG I, MAG II, and NM) were produced and analyzed. The Fe recovery within the MAG I was 69%, while it was 6% in MAG II and 1% in NM. The losses between bauxite residue and the sinter, and between the sinter and the modified bauxite residue are attributed to within experimental error margin of 5–10%.