In Vitro Analysis of the Antioxidant and Antiviral Activity of Embelin against Herpes Simplex Virus-1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Preparation of Virus
2.3. Embelin
2.4. Cell Proliferation Assays
2.5. Antiviral Assays
2.5.1. Cell Proliferation Assay
2.5.2. Cell Viability Assay
2.6. Quantitative Polymerase Chain Reaction (qPCR)
2.7. Microscopic Observations
2.7.1. Inverted Microscopic Observation
2.7.2. Fluorescent Microscopy
2.8. Study of the Inhibitory Mechanism of Embelin on HSV-1 Infective Cycle
2.8.1. Binding Assay
2.8.2. Penetration and Post Penetration Assay
2.9. Study of Antioxidant and Antiviral Activity
2.10. Statistical Analyses
3. Results
3.1. Cytotoxicity Study of Treatment of Vero cells with Embelin
3.2. Antiviral Effects of Embelin on HSV-1
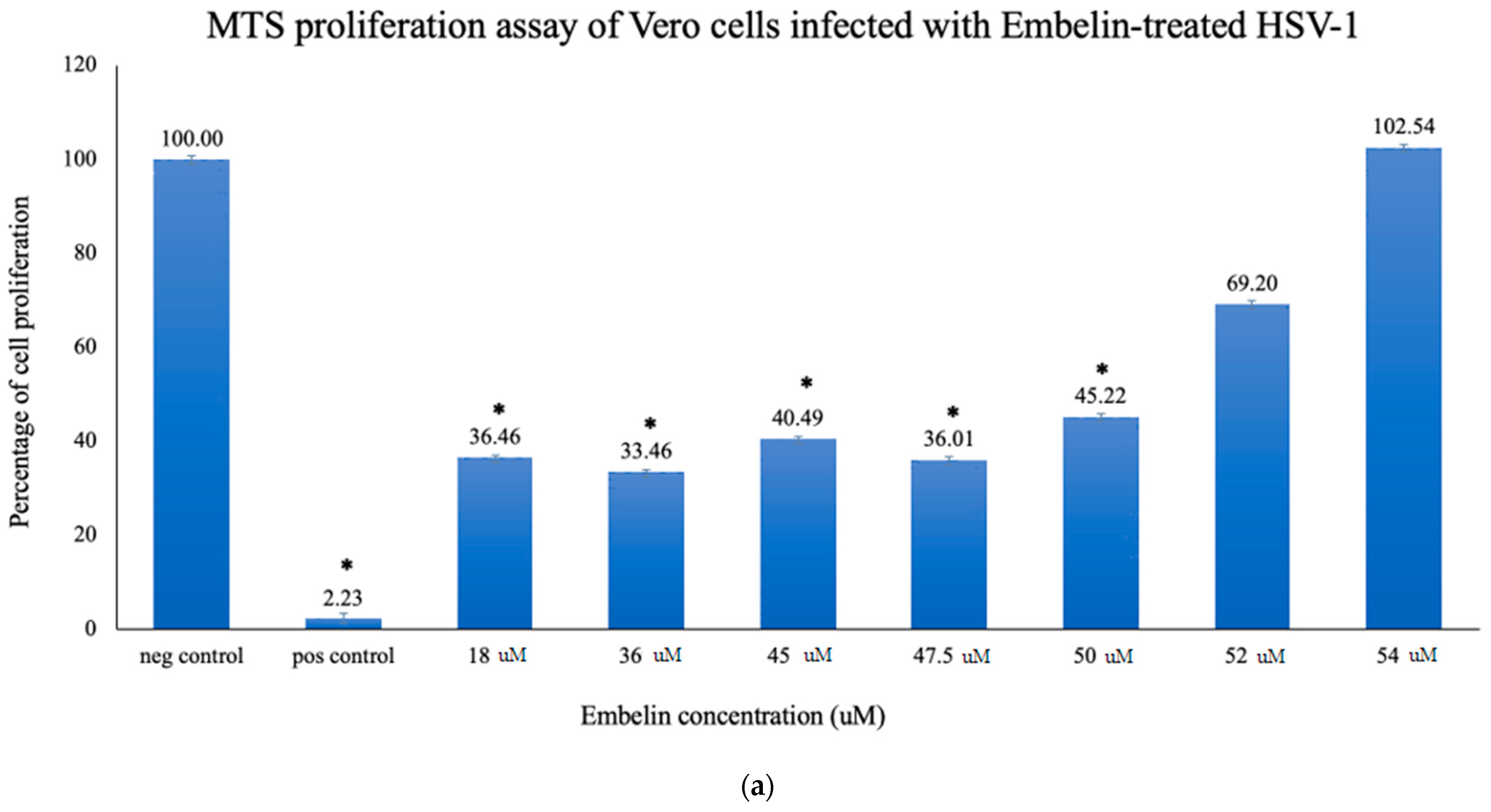
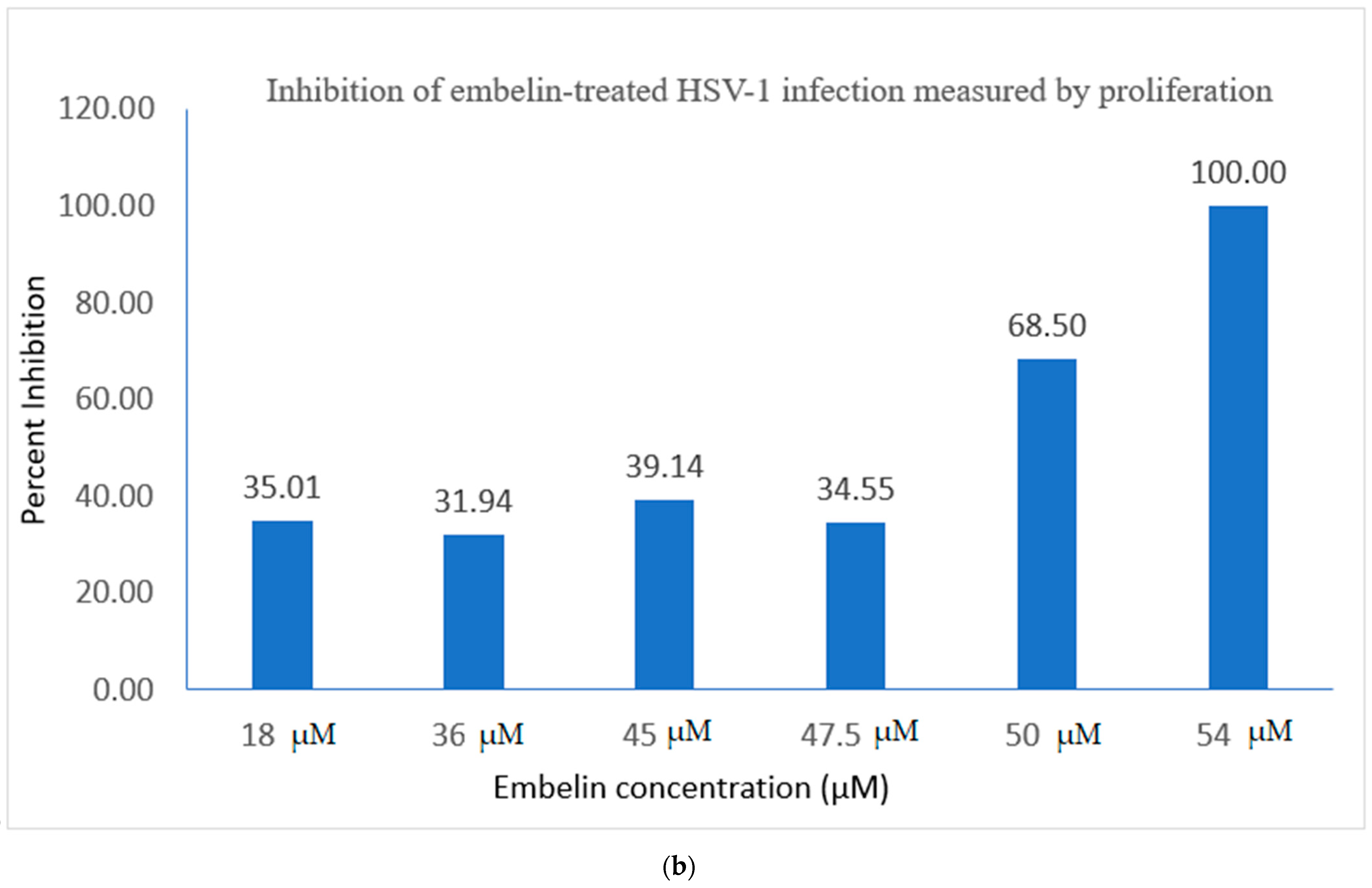
3.2.1. Proliferation Assay of Vero Cells Infected with HSV-1 and Embelin Treated HSV-1
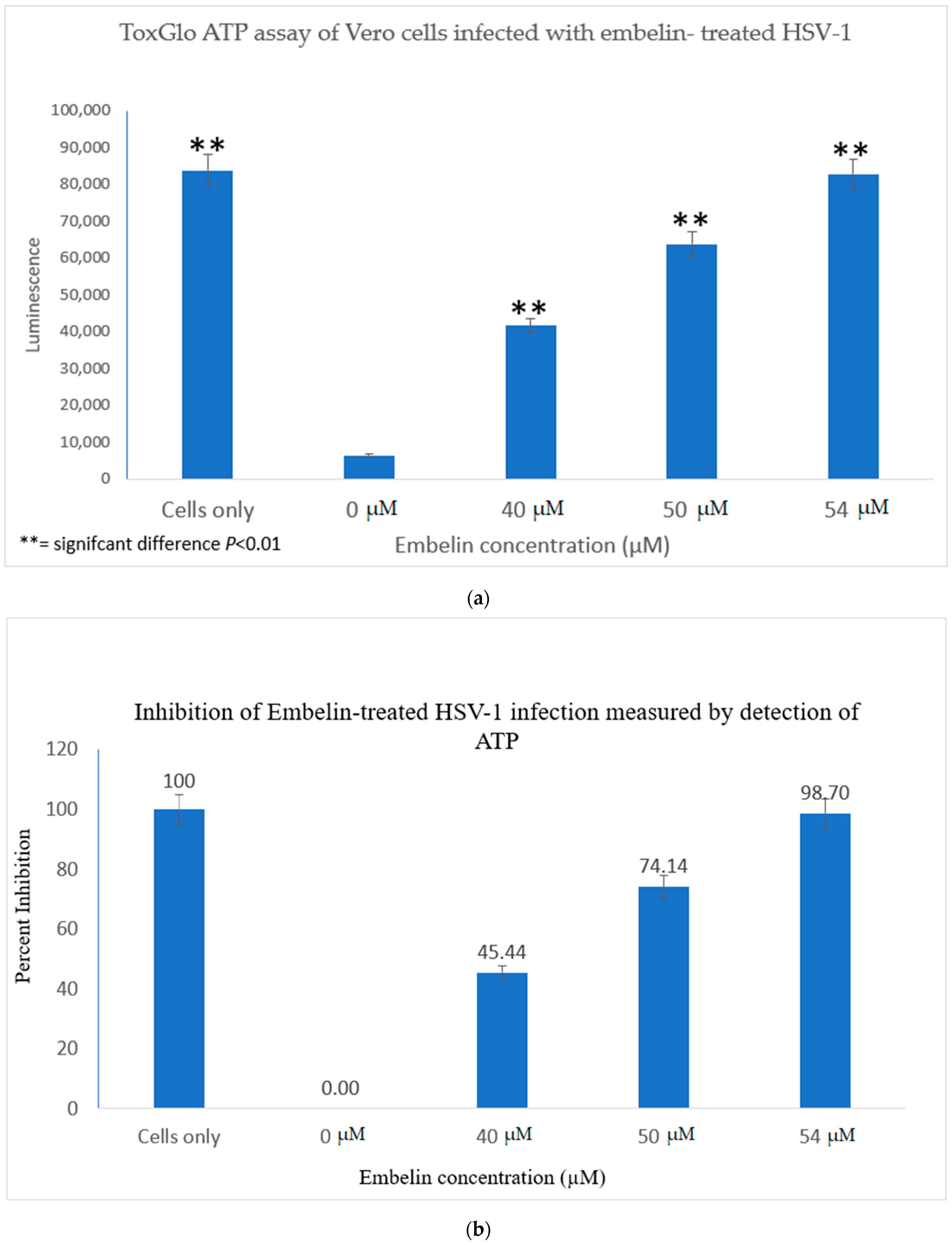
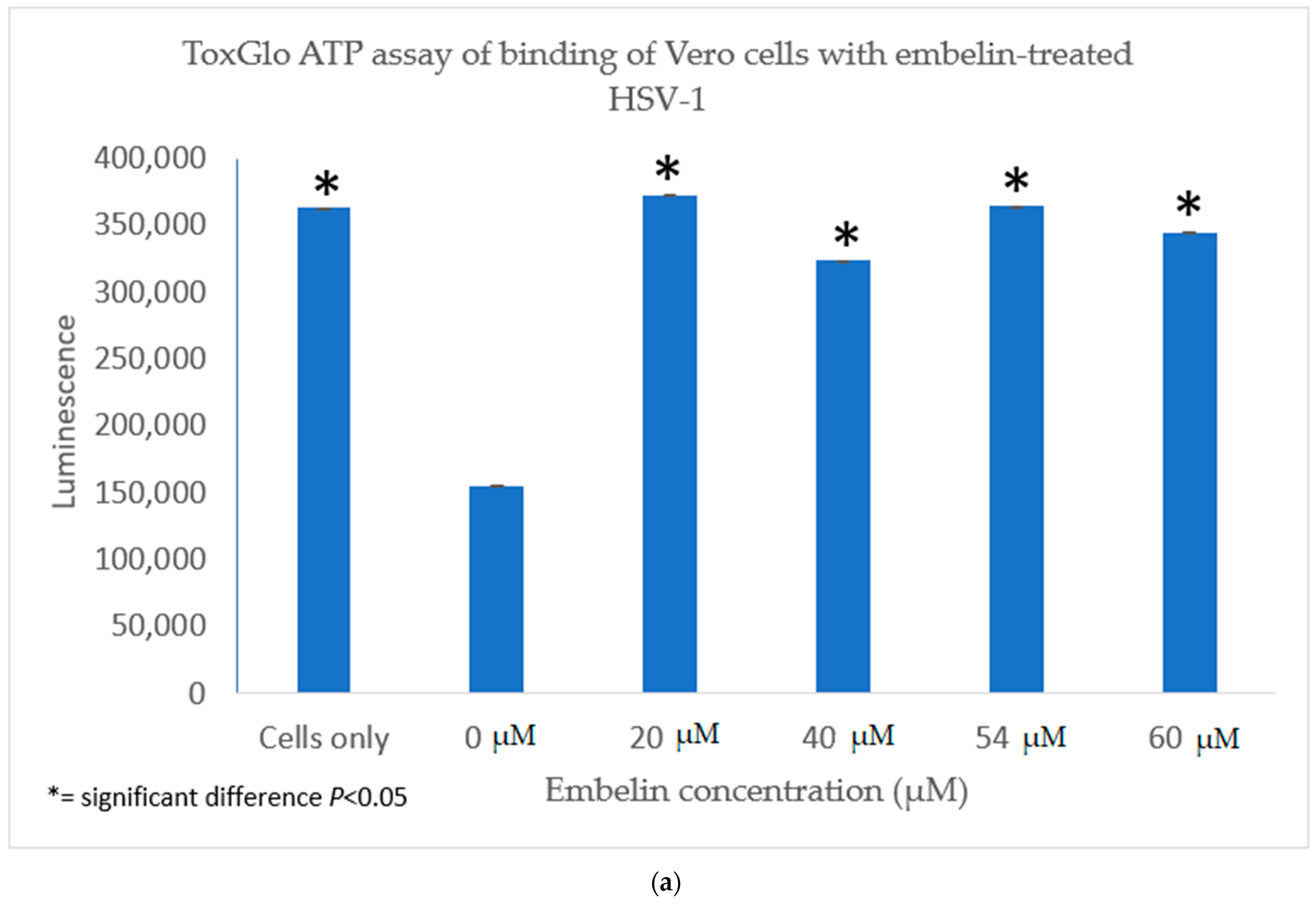
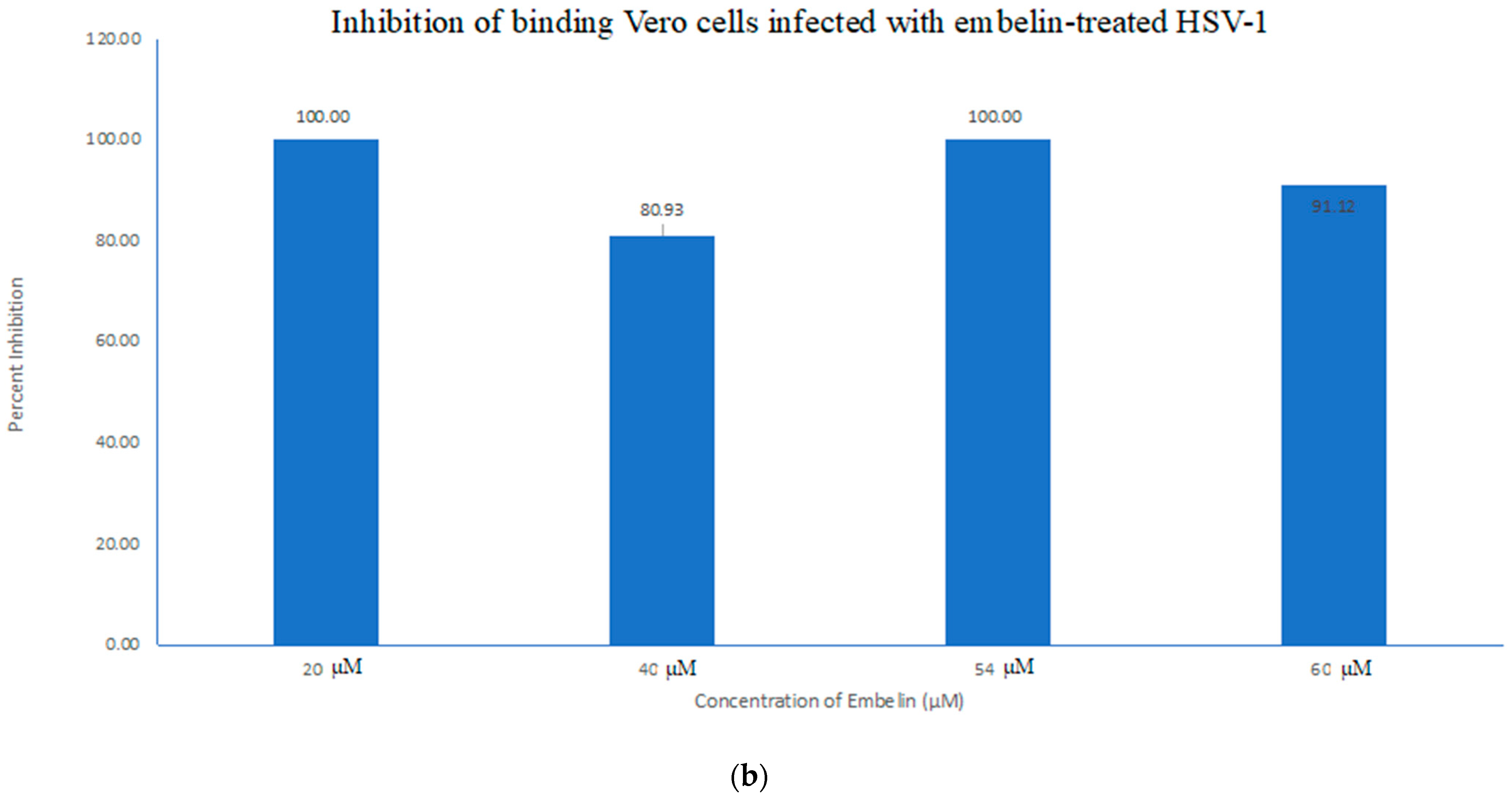
3.2.2. Viral ToxGlo ATP Detection Assay
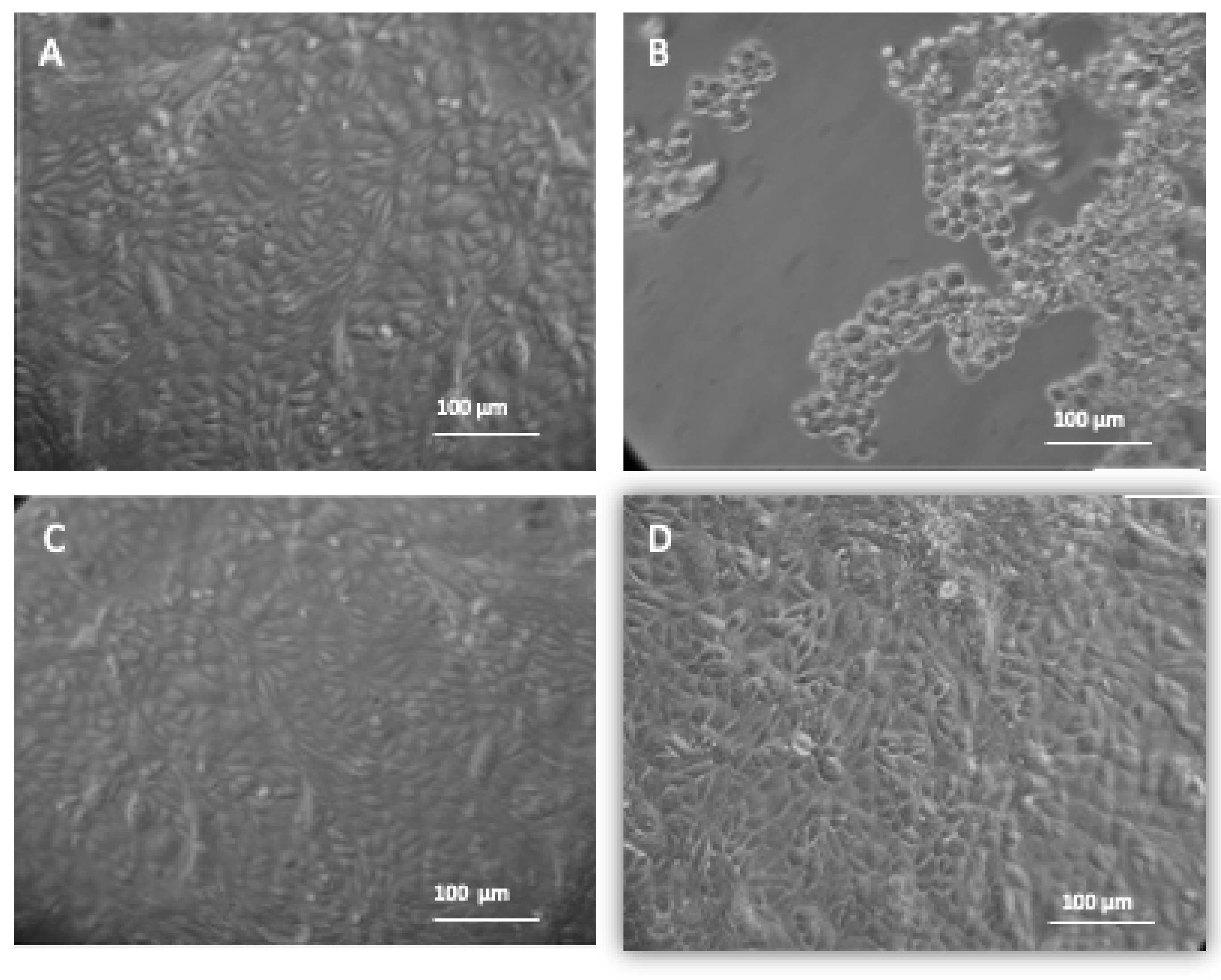
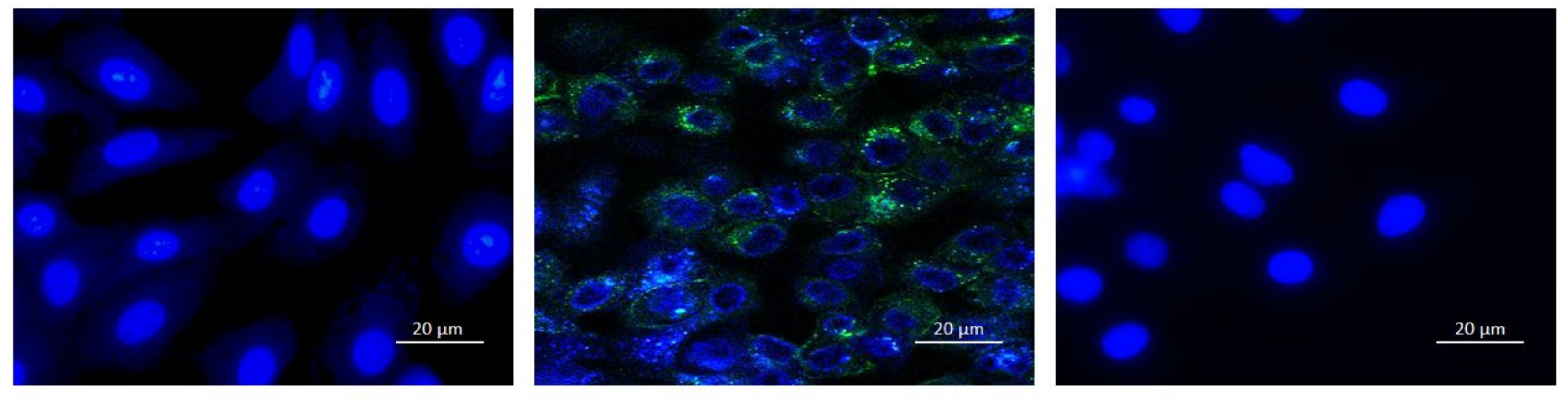
3.3. Microscopic Observation of Vero Cells, HSV-1 Infected Vero Cells and Embelin Treated HSV-1 Infected Vero Cells
3.3.1. Inverted Microscopic Observation
3.3.2. Fluorescent Microscopy
3.4. Study of the Mechanisms of Embelin on HSV-1 Infective Cycle
3.4.1. Inhibition of Binding by Embelin Treated HSV-1
3.4.2. Inhibition of Penetration by Embelin Treated HSV-1
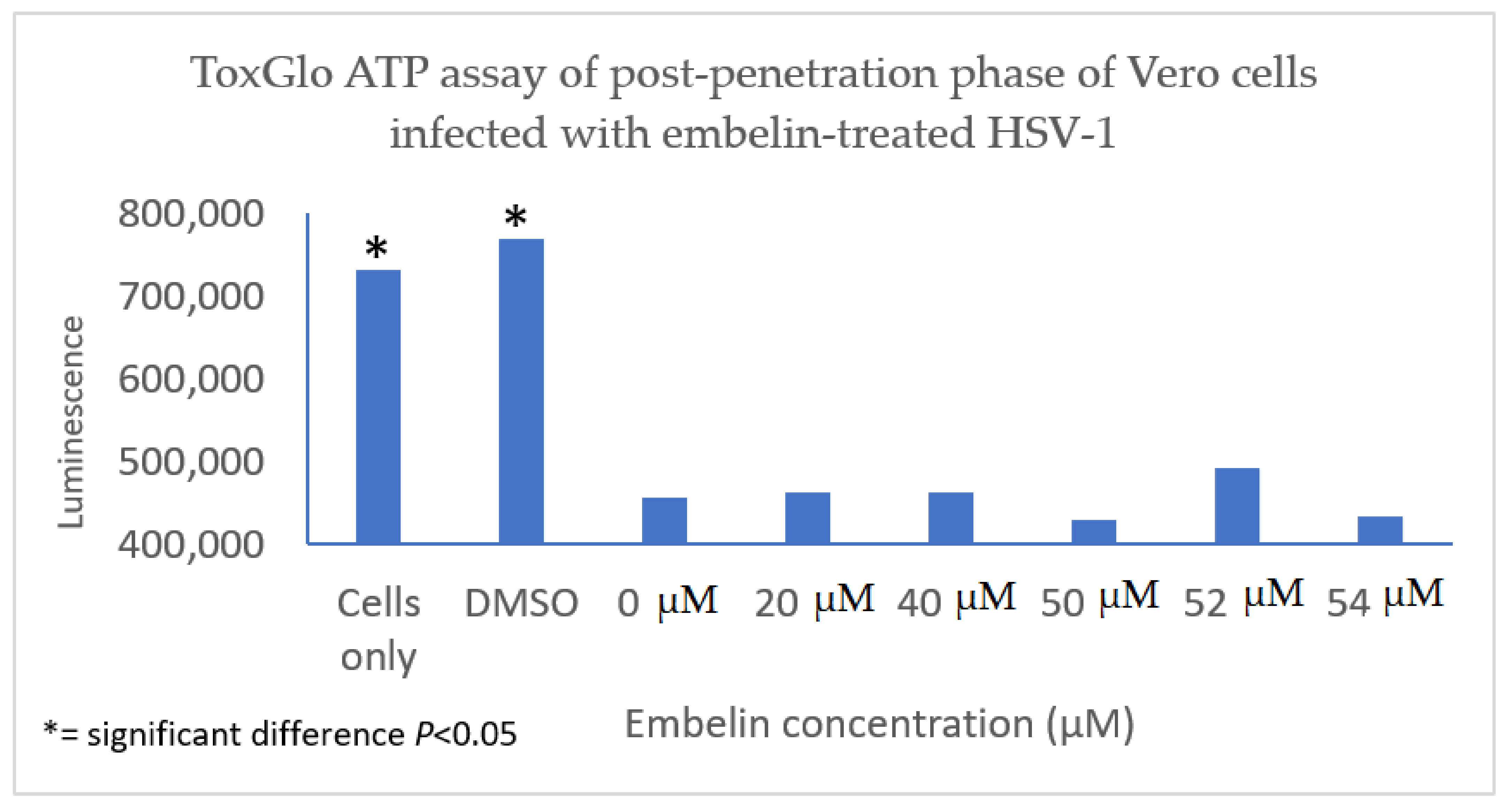
3.4.3. Inhibition of Post Penetration by Embelin Treated HSV-1
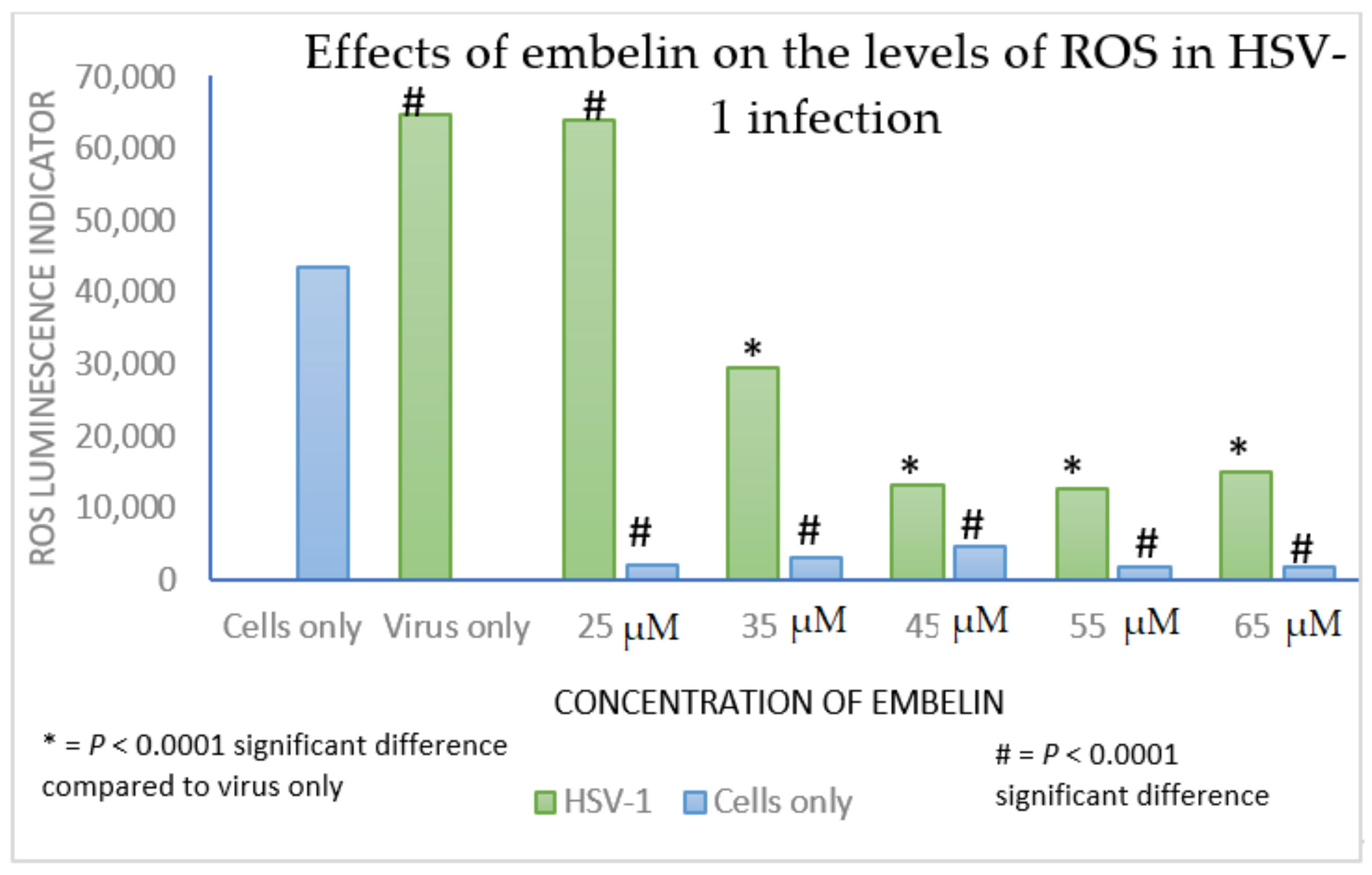
3.5. Effect of Antiviral Treatment on Circulating Redox Balance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whitley, R.J.; Roizman, B. Herpes simplex virus infections. Lancet 2001, 357, 1513–1518. [Google Scholar] [CrossRef]
- Roizman, B.; Pellett, P.E. The family Herpesviridae: A brief introduction. In Fields Virology, 4th ed.; Knipe, D.M., Howley, P.M., Griffin, D.E., Lamb, R.A., Martin, M.A., Roizman, B., Straus, S.E., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; Volume 2, pp. 66–73. ISBN -100781718325. [Google Scholar]
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.E.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS ONE 2015, 10, e0140765. [Google Scholar] [CrossRef] [Green Version]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.E.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalenceand incidence estimates. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar]
- Clarke, R.W. Forces and Structures of the Herpes Simplex Virus (HSV) Entry Mechanism. ACS Infect. Dis. 2015, 1, 403–415. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, R.P.; Geraghty, R.J. Herpes simplex virus type 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins D, H, L, and B. Proc. Natl. Acad. Sci. USA 2007, 104, 2903–2908. [Google Scholar] [CrossRef] [Green Version]
- Heldwein, E.E.; Lou, H.; Bender, F.C.; Cohen, G.H.; Eisenberg, R.J.; Harrison, S.C. Crystal Structure of Glycoprotein B from Herpes Simplex Virus 1. Science 2006, 313, 217–220. [Google Scholar] [CrossRef] [Green Version]
- Agelidis, A.M.; Shukla, D. Cell entry mechanisms of HSV: What we have learned in recent years. Future Virol. 2015, 10, 1145–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spear, P.G. Herpes simplex virus: Receptors and ligands for cell entry. Cell. Microbiol. 2004, 6, 401–410. [Google Scholar] [CrossRef]
- Nicola, A.V.; Straus, S.E.; Bubić, I.; Wagner, M.; Krmpotić, A.; Saulig, T.; Kim, S.; Yokoyama, W.M.; Jonjić, S.; Koszinowski, U.H. Cellular and Viral Requirements for Rapid Endocytic Entry of Herpes Simplex Virus. J. Virol. 2004, 78, 7536–7544. [Google Scholar] [CrossRef] [Green Version]
- Newcomb, W.W.; Booy, F.P.; Brown, J.C. Uncoating the Herpes Simplex Virus Genome. J. Mol. Biol. 2007, 370, 633–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mettenleiter, T.C.; Klupp, B.G.; Granzow, H. Herpesvirus assembly: An update. Virus Res. 2009, 143, 222–234. [Google Scholar] [CrossRef]
- McQuillan, G.; Kruszon-Moran, D.; Flagg, E.W.; Paulose-Ram, R. Prevalence of Herpes Simplex Virus Type 1 and Type 2 in Persons Aged 14–49: United States, 2015–2016. NCHS Data Brief 2018, 1–8. [Google Scholar]
- Tyring, S.K.; Beutner, K.R.; Tucker, B.A.; Anderson, W.C.; Crooks, R.J. Antiviral therapy for herpes zoster: Ran-domized, controlled clinical trial of valacyclovir and famciclovir therapy in immunocompetent patients 50 years and older. Arch. Fam. Med. 2000, 9, 863. [Google Scholar] [CrossRef]
- Reardon, J.E.; Spector, T. Herpes simplex virus type 1 DNA polymerase. Mechanism of inhibition by acyclovir triphosphate. J. Biol. Chem. 1989, 264, 2540193. [Google Scholar]
- Liang, H.; Gao, D.; Yu, W.; Chen, C.; Deng, Y.; Lei, Y.; Lu, J.; Xie, Q.; Xiong, S. Antiviral Activity and its Mecha-nism of LCVN Against HSV-1 Acyclovir-resistant Strains. J. Pharm. Biomed. Sci. 2018, 8, 41–47. [Google Scholar]
- Royer, D.J.; Cohen, A.W.; Carr, D.J. The current state of vaccine development for ocular HSV-1 infection. Expert Rev. Ophthalmol. 2015, 10, 113–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chentoufi, A.A.; Kritzer, E.; Yu, D.M.; Nesburn, A.B.; Benmohamed, L. Towards a Rational Design of an Asymptomatic Clinical Herpes Vaccine: The Old, the New, and the Unknown. Clin. Dev. Immunol. 2012, 2012, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Zandi, K.; Ramedani, E.; Mohammadi, K.; Tajbakhsh, S.; Deilami, I.; Rastian, Z.; Fouladvand, M.; Yousefi, F.; Farshadpour, F. Evaluation of Antiviral Activities of Curcumin Derivatives against HSV-1 in Vero Cell Line. Nat. Prod. Commun. 2010, 5, 1935–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.C.; Novellino, E. Resveratrol as a Novel Anti-Herpes Simplex Virus Nutraceutical Agent: An Overview. Viruses 2018, 10, 473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Docherty, J.; Fu, M.; Hah, J.; Sweet, T.; Faith, S.; Booth, T. Effect of resveratrol on herpes simplex virus vaginal infection in the mouse. Antivir. Res. 2005, 67, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Docherty, J.J.; Smith, J.S.; Fu, M.M.; Stoner, T.; Booth, T. Effect of topically applied resveratrol on cutaneous herpes simplex virus infections in hairless mice. Antivir. Res. 2004, 61, 19–26. [Google Scholar] [CrossRef]
- Flores, D.J.; Lee, L.H.; Adams, S.D. Inhibition of curcumin-treated Herpes Simplex Virus 1 and 2 in Vero cells. Adv Microbiol. 2016, 6, 276–287. [Google Scholar] [CrossRef] [Green Version]
- Houston, D.M.J.; Bugert, J.J.; Denyer, S.P.; Heard, C.M. Potentiated virucidal activity of pomegranate rind extract (PRE) and punicalagin against Herpes simplex virus (HSV) when co-administered with zinc (II) ions, and antiviral activity of PRE against HSV and aciclovir-resistant HSV. PLoS ONE 2017, 12, e0179291. [Google Scholar] [CrossRef] [Green Version]
- Lavoie, S.; Côté, I.; Pichette, A.; Gauthier, C.; Ouellet, M.; Nagau-Lavoie, F.; Mshvildadze, V.; Legault, J. Chemical composition and anti-herpes simplex virus type 1 (HSV-1) activity of extracts from Cornus canadensis. BMC Complement. Altern. Med. 2017, 17, 1–12. [Google Scholar] [CrossRef]
- De Oliveira, A.; Prince, D.; Lo, C.-Y.; Lee, L.H.; Chu, T.-C. Antiviral activity of theaflavin digallate against herpes simplex virus type 1. Antivir. Res. 2015, 118, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.N.; Adams, S.D.; Lee, L.H. Inhibition of Herpes Simplex Virus-1 by the Modified Green Tea Polyphenol EGCG-Stearate. Adv. Biosci. Biotechnol. 2018, 9, 679–690. [Google Scholar] [CrossRef] [Green Version]
- Cantatore, A.; Randall, S.D.; Traum, D.; Adams, S.D. Effect of black tea extract on herpes simplex virus-1 infection of cultured cells. BMC Complement. Altern. Med. 2013, 13, 1–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, A.; Adams, S.D.; Lee, L.H.; Murray, S.R.; Hsu, S.D.; Hammond, J.R.; Dickinson, D.; Chen, P.; Chu, T.-C. Inhibition of herpes simplex virus type 1 with the modified green tea polyphenol palmitoyl-epigallocatechin gallate. Food Chem. Toxicol. 2013, 52, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shatrov, V.A.; Ratter, F.; Gruber, A.; Dröoge, W.; Lehmann, V. HIV Type 1 Glycoprotein 120 Amplifies Tumor Necrosis Factor-Induced NF-kB Activation in Jurkat Cells. AIDS Res. Hum. Retrovir. 1996, 12, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wu, R.F.; Xu, Y.C.; Flores, S.C.; Terada, L.S. HIV Tat Activates c-Jun Amino-terminal Kinase through an Oxidant-Dependent Mechanism. Virology 2001, 286, 62–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, K.I.; Ko, D.-H.; Shin, N.; Pyo, C.W.; Choi, S.-Y. Endoplasmic reticulum-associated degradation potentiates the infectivity of influenza A virus by regulating the host redox state. Free. Radic. Biol. Med. 2019, 135, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Amatore, D.; Sgarbanti, R.; Aquilano, K.; Baldelli, S.; Limongi, D.; Civitelli, L.; Nencioni, L.; Garaci, E.; Ciriolo, M.R.; Palamara, A.T. Influenza virus replication in lung epithelial cells depends on redox-sensitive pathways acti-vated by NOX4-derived ROS. Cell Microbiol. 2015, 17, 131–145. [Google Scholar] [CrossRef] [Green Version]
- Nencioni, L.; Iuvara, A.; Aquilano, K.; Ciriolo, M.R.; Cozzolino, F.; Rotilio, G.; Garaci, E.; Palamara, A.T. Influen-za A virus replication is dependent on an antioxidant pathway that involves GSH and Bcl-2. FASEB J. 2003, 17, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Inn, K.-S.; Kim, H.; Lee, S.-A.; Kim, B.-J.; Kim, H.-I.; Won, Y.-S. Upregulation of endoplasmic reticulum stress and reactive oxygen species by naturally occurring mutations in hepatitis B virus core antigen. J. Gen. Virol. 2015, 96, 1850–1854. [Google Scholar] [CrossRef]
- Lee, I.-K.; Lee, S.-A.; Kim, H.; Won, Y.-S.; Kim, B.-J. Induction of endoplasmic reticulum-derived oxidative stress by an occult infection related S surface antigen variant. World J. Gastroenterol. 2015, 21, 6872–6883. [Google Scholar] [CrossRef]
- Bapat, A.; Glass, L.S.; Luo, M.; Fishel, M.L.; Long, E.C.; Georgiadis, M.M.; Kelley, M.R. Novel Small-Molecule Inhibitor of Apurinic/Apyrimidinic Endonuclease 1 Blocks Proliferation and Reduces Viability of Glioblastoma Cells. J. Pharmacol. Exp. Ther. 2010, 334, 988–998. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhong, C.; Wang, Q.; Chen, W.; Yuan, Y. Curcumin is an APE1 redox inhibitor and exhibits an antiviral activity against KSHV replication and pathogenesis. Antivir. Res. 2019, 167, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Rosanna, V.; Francesco, B.; Francesco, C.; Giorgia, D.B.; Rosanna, T.; Naik, B.V.; Archana, M.; Gaetano, S. Direct-acting antivirals restore systemic redox homeostasis in chronic HCV patients. Free. Radic. Biol. Med. 2020, 156, 200–206. [Google Scholar] [CrossRef]
- Caruso, F.; Paumier, S.; Rossi, M. X-ray Crystal Structure of Embelin and Its DFT Scavenging of Superoxide Radical. J. Comput. Chem. 2017, 39, 1143–1148. [Google Scholar] [CrossRef]
- Caruso, F.; Rossi, M.; Kaur, S.; Garcia-Villar, E.; Molasky, N.; Belli, S.; Sitek, J.D.; Gionfra, F.; Pedersen, J.Z.; Incerpi, S. Antioxidant Properties of Embelin in Cell Culture. Electrochemistry and Theoretical Mechanism of Scavenging. Potential Scavenging of Superoxide Radical through the Cell Membrane. Antioxidants 2020, 9, 382. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Qian, H.; Chen, Y.; Li, X.; Gong, W.; Jiang, G.; Zhang, W.; Cheng, S.; Li, X.; Li, P. X-linked inhibitor of apoptosis protein inhibitor Embelin induces apoptosis via PI3K/Akt pathway and inhibits invasion in osteosarcoma cells. J. Cancer Res. Ther. 2018, 14, 648. [Google Scholar] [CrossRef]
- Kumar, G.K.; Dhamotharan, R.; Kulkarni, N.M.; Mahat, M.Y.A.; Gunasekaran, J.; Ashfaque, M. Embelin reduces cutaneous TNF-α level and ameliorates skin edema in acute and chronic model of skin inflammation in mice. Eur J Pharmacol. 2011, 662, 63–69. [Google Scholar] [CrossRef]
- Xu, M.; Cui, J.; Fu, H.; Proksch, P.; Lin, W.; Li, M. Embelin Derivatives and Their Anticancer Activity through Microtubule Disassembly. Planta Medica 2005, 71, 944–948. [Google Scholar] [CrossRef]
- Joy, B.; Sivadasan, R.; Emilia, A.T.; John, M.; Sobhan, P.K.; Seervi, M. Santhoshkumar, T.R. Lysosomal destabilization and cathepsin B contributes for cytochrome c release and caspase activation in Embelin-induced apoptosis. Mol. Carcinog. 2010, 49, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Singh, R.; Singh, P.; Gupta, R.S. Effects of Embelin on Lipid Peroxidation and Free Radical Scavenging Activity against Liver Damage in Rats. Basic Clin. Pharmacol. Toxicol. 2009, 105, 243–248. [Google Scholar] [CrossRef]
- Chitra, M.; Devi, C.S.; Sukumar, E. Antibacterial activity of embelin. Fitoterapia 2003, 74, 401–403. [Google Scholar] [CrossRef]
- Chitra, M.; Sukumar, E.; Suja, V.; Devi, S. Antitumor, Anti-Inflammatory and Analgesic Property of Embelin, a Plant Product. Chemotherapy 1994, 40, 109–113. [Google Scholar] [CrossRef]
- Radhakrishnan, N.; Gnanamani, A. 2,5-DIihydroxy-3-undecyl-1,4-benzoquinone (Embelin)-a second solid gold of India- a review. Int. J. Pharm. Pharm. Sci. 2014, 6, 23–30. [Google Scholar]
- Hussein, G.; Miyashiro, H.; Nakamura, N.; Hattori, M.; Kakiuchi, N.; Shimotohno, K. Inhibitory effects of Sudanese medicinal plant extracts on hepatitis C virus (HCV) protease. Phytother Res. 2000, 14, 510–516. [Google Scholar] [CrossRef]
- Hossan, S.; Fatima, A.; Rahmatullah, M.; Khoo, T.J.; Nissapatorn, V.; Galochkina, A.V.; Slita, A.V.; Shtro, A.A.; Nikolaeva, Y.; Zarubaev, V.V.; et al. Antiviral activity of Embelia ribes Burm. f. against influenza virus in vitro. Arch. Virol. 2018, 163, 2121–2131. [Google Scholar] [CrossRef]
- Parvez, M.K.; Rehman, T.; Alam, P.; Al-Dosari, M.S.; Alqasoumi, S.I.; Alajmi, M.F. Plant-derived antiviral drugs as novel hepatitis B virus inhibitors: Cell culture and molecular docking study. Saudi Pharm. J. 2019, 27, 389–400. [Google Scholar] [CrossRef]
- Caruso, F.; Rossi, M.; Pedersen, J.Z.; Incerpi, S. Computational studies reveal mechanism by which quinone derivatives can inhibit SARS-CoV-2. Study of embelin and two therapeutic compounds of interest, methyl prednisolone and dexamethasone. J. Infect. Public Health 2020, 13, 1868–1877. [Google Scholar] [CrossRef]
- Willard, M. Rapid Directional Translocations in Virus Replication. J. Virol. 2002, 76, 5220–5232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harden, E.A.; Falshaw, R.; Carnachan, S.M.; Kern, E.R.; Prichard, M.N. Virucidal activity of polysaccharide extracts from four algal species against herpes simplex virus. Antivir. Res. 2009, 83, 282–289. [Google Scholar] [CrossRef] [Green Version]
- Coyle, C.H.; Kader, K.N. Mechanisms of H2O2-Induced Oxidative Stress in Endothelial Cells Exposed to Physiologic Shear Stress. ASAIO J. 2007, 53, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, S.; Badami, S.; Maithili, V. Evaluation of antidiabetic effect of Embelin from Embelia ribes in alloxan induced diabetes in rats. Biomed. Prev. Nutr. 2011, 1, 25–31. [Google Scholar]
- Sies, H.; Chance, B. The steady state level of catalase compound I in isolated hemoglobin-free perfused rat liver. FEBS Lett. 1970, 11, 172–176. [Google Scholar] [CrossRef] [Green Version]
- Kavouras, J.H.; Prandovszky, E.; Valyi-Nagy, K.; Kovacs, S.K.; Tiwari, V.; Kovács, M.; Shukla, D.; Valyi-Nagy, T. Herpes simplex virus type 1 infection induces oxidative stress and the release of bioactive lipid peroxidation by-products in mouse P19N neural cell cultures. J. NeuroVirol. 2007, 13, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Sethi, G.; Aggarwal, B.B. Embelin, an Inhibitor of X Chromosome-Linked Inhibitor-of-Apoptosis Protein, Blocks Nuclear Factor-κB (NF-κB) Signaling Pathway Leading to Suppression of NF-κB-Regulated Antiapoptotic and Metastatic Gene Products. Mol. Pharmacol. 2006, 71, 209–219. [Google Scholar] [CrossRef]
- Sreeharsha, N. Embelin impact on paraquat-induced lung injury through suppressing oxidative stress, inflammatory cascade, and MAPK/NF-κB signaling pathway. J. Biochem. Mol. Toxicol. 2020, 34, e22456. [Google Scholar] [CrossRef]
- Heo, J.Y.; Kim, H.J.; Kim, S.-M.; Park, K.-R.; Park, S.-Y.; Kim, S.W.; Nam, D.; Jang, H.-J.; Lee, S.-G.; Ahn, K.S.; et al. Embelin suppresses STAT3 signaling, proliferation, and survival of multiple myeloma via the protein tyrosine phosphatase PTEN. Cancer Lett. 2011, 308, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Roche, L.; Mesta, F. Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection. Arch. Med Res. 2020, 51, 384–387. [Google Scholar] [CrossRef]
- Kristen, H.; Sastre, I.; Muñoz-Galdeano, T.; Recuero, M.; Aldudo, J.; Bullido, M.J. The lysosome system is severely impaired in a cellular model of neurodegeneration induced by HSV-1 and oxidative stress. Neurobiol. Aging 2018, 68, 5–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gravier-Hernández, R.; Valle, L.G.-D.; Valdes-Alonso, L.; Hernández-Ayala, N.; Bermúdez-Alfonso, Y.; Hernández-Requejo, D.; Rosell-Guerra, T.; Hernández-González-Abreu, M.C. Oxidative stress in hepatitis C virus–human immunodeficiency virus co-infected patients. Ann. Hepatol. 2020, 19, 92–98. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elias, T.; Lee, L.H.; Rossi, M.; Caruso, F.; Adams, S.D. In Vitro Analysis of the Antioxidant and Antiviral Activity of Embelin against Herpes Simplex Virus-1. Microorganisms 2021, 9, 434. https://doi.org/10.3390/microorganisms9020434
Elias T, Lee LH, Rossi M, Caruso F, Adams SD. In Vitro Analysis of the Antioxidant and Antiviral Activity of Embelin against Herpes Simplex Virus-1. Microorganisms. 2021; 9(2):434. https://doi.org/10.3390/microorganisms9020434
Chicago/Turabian StyleElias, Tony, Lee H. Lee, Miriam Rossi, Francesco Caruso, and Sandra D. Adams. 2021. "In Vitro Analysis of the Antioxidant and Antiviral Activity of Embelin against Herpes Simplex Virus-1" Microorganisms 9, no. 2: 434. https://doi.org/10.3390/microorganisms9020434
APA StyleElias, T., Lee, L. H., Rossi, M., Caruso, F., & Adams, S. D. (2021). In Vitro Analysis of the Antioxidant and Antiviral Activity of Embelin against Herpes Simplex Virus-1. Microorganisms, 9(2), 434. https://doi.org/10.3390/microorganisms9020434







