Investigation of Galectin-3 and Cardiotrophin-1 Concentrations as Biomarkers in Dogs with Neurological Distemper
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Groups
2.2. Clinical Examination
2.3. Rapid Diagnostic Test Applications
2.4. Collection of Blood Samples
2.5. Complete Blood Count Analysis
2.6. Serum Biochemical Analyses
2.7. Measurement of Serum Galectin-3 and Cardiotrophin-1 Concentrations
2.8. Statistical Analysis
3. Results
3.1. Clinical Examination Findings
3.2. Hematobiochemical Results
3.3. Biomarker Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lan, N.T.; Yamaguchı, R.; Inomata, A.; Furuya, Y.; Uchıda, K.; Sugano, S.; Tateyama, S. Comparative analyses of canine distemper viral isolates from clinical cases of canine distemper in vaccinated dogs. Vet. Microbiol. 2006, 115, 32–42. [Google Scholar] [CrossRef]
- Willi, B.; Spiri, A.M.; Meli, M.L.; Grimm, F.; Beatrice, L.; Riond, B.; Bley, T.; Jordi, R.; Dennler, M.; Hofmann-Lehmann, R. Clinical and molecular investigation of a canine distemper outbreak and vector-borne infections in a group of rescue dogs imported from Hungary to Switzerland. BMC Vet. Res. 2015, 11, 154. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Martínez, A.; Rodríguez-Alarcón, C.A.; Adame-Gallegos, J.R.; Laredo-Tiscareño, S.V.; de Luna-Santillana, E.D.J.; Hernández-Triana, L.M.; Garza-Hernández, J.A. Canine distemper virus: Origins, mutations, diagnosis, and epidemiology in Mexico. Life 2024, 14, 1002. [Google Scholar] [CrossRef] [PubMed]
- Tipold, A.; Vandevelde, M.; Jaggy, A. Neurological manifestations of canine distemper virus infection. J. Small Anim. Pract. 1992, 33, 466–470. [Google Scholar] [CrossRef]
- Carvalho, O.V.; Botelho, C.V.; Ferreira, C.G.T.; Scherer, P.O.; Soares-Martins, J.A.P.; Almeida, M.R.; Silva Júnior, A. Immunopathogenic and neurological mechanisms of canine distemper virus. Adv. Virol. 2012, 2012, 163860. [Google Scholar] [CrossRef]
- Verdes, J.M.; Larrañaga, C.; Godiño, G.; Varela, B.; Yozzi, V.; Iribarnegaray, V.; Delucchi, L.; Yamasaki, K. Pathological study of demyelination with cellular reactions in the cerebellum of dogs infected with canine distemper virus. Viruses 2024, 16, 1719. [Google Scholar] [CrossRef]
- Gastelum-Leyva, F.; Peña-Jasso, A.; Alvarado-Vera, M.; Plascencia-López, I.; Patrón-Romero, L.; Loera-Castañeda, V.; Gándara-Mireles, J.A.; Lares-Asseff, I.; Leal-Ávila, M.A.; Alvelais-Palacios, J.A.; et al. Evaluation of the efficacy and safety of silver nanoparticles in the treatment of non-neurological and neurological distemper in dogs: A randomized clinical trial. Viruses 2022, 14, 2329. [Google Scholar] [CrossRef]
- Duque-Valencia, J.; Sarute, N.; Olarte-Castillo, X.A.; Ruíz-Sáenz, J. Evolution and interspecies transmission of canine distemper virus an outlook of the diverse evolutionary landscapes of a multi-host virus. Viruses 2019, 11, 582. [Google Scholar] [CrossRef]
- Uhl, E.W.; Thomas, R. Uncovering tales of transmission: An integrated palaeopathological perspective on the evolution of shared human and animal pathogens. In Palaeopathology and Evolutionary Medicine: An Integrated Approach, 1st ed.; Plomp, K.A., Roberts, C.A., Elton, S., Bentley, G.R., Eds.; Oxford University Press: New York, NY, USA, 2022; pp. 317–349. [Google Scholar] [CrossRef]
- Blanda, V.; Bracale, U.M.; Di Taranto, M.D.; Fortunato, G. Galectin-3 in Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 9232. [Google Scholar] [CrossRef]
- Srejovic, I.; Selakovic, D.; Jovicic, N.; Jakovljević, V.; Lukic, M.L.; Rosic, G. Galectin-3: Roles in neurodevelopment, neuroinflammation, and behavior. Biomolecules 2020, 10, 798. [Google Scholar] [CrossRef]
- Soares, L.C.; Al-Dalahmah, O.; Hillis, J.; Young, C.C.; Asbed, I.; Sakaguchi, M.; O’Neill, E.; Szele, F.G. Novel galectin-3 roles in neurogenesis, inflammation and neurological diseases. Cells 2021, 10, 3047. [Google Scholar] [CrossRef] [PubMed]
- Levison, S.W.; Goldman, J.E. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron 1993, 10, 201–212. [Google Scholar] [CrossRef]
- Jaquenod De Giusti, C.; Alberdi, L.; Frik, J.; Ferrer, M.F.; Scharrig, E.; Schattner, M.; Gomez, R.M. Galectin-3 is upregulated in activated glia during Junin virus–induced murine encephalitis. Neurosci. Lett. 2011, 501, 163–166. [Google Scholar] [CrossRef] [PubMed]
- James, R.E.; Hillis, J.; Adorján, I.; Gration, B.; Mundim, M.V.; Iqbal, A.J.; Majumdar, M.M.; Yates, R.L.; Richards, M.M.; Goings, G.E.; et al. Loss of galectin–3 decreases the number of immune cells in the subventricular zone and restores proliferation in a viral model of multiple sclerosis. Glia 2016, 64, 105–121. [Google Scholar] [CrossRef]
- Arcuri, G.; Valente, C.; Romito, G.; Bonsembiante, F.; Mazzoldi, C.; Contiero, B.; Poser, H.; Guglielmini, C. Evaluation of Galectin-3 in dogs with atrial fibrillation. Animals 2024, 14, 2547. [Google Scholar] [CrossRef]
- López-Yoldi, M.; Moreno-Aliaga, M.J.; Bustos, M. Cardiotrophin-1: A multifaceted cytokine. Cytokine Growth Factor Rev. 2015, 26, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Konii, H.; Sato, K. Emerging roles of cardiotrophin-1 in the pathogenesis and biomarker of atherosclerosis. J. Multidiscip. Sci. J. 2018, 1, 94–105. [Google Scholar] [CrossRef]
- Pennica, D.; Arce, V.; Swanson, T.A.; Vejsada, R.; Pollock, R.A.; Armanini, M.; Dudley, K.; Phillips, H.S.; Rosenthal, A.; Kato, A.C.; et al. Cardiotrophin-1, a cytokine present in embryonic muscle, supports long-term survival of spinal motoneurons. Neuron 1996, 17, 63–74. [Google Scholar] [CrossRef]
- Bordet, T.; Schmalbruch, H.; Pettmann, B.; Hagege, A.; Castelnau-Ptakhine, L.; Kahn, A.; Haase, G. Adenoviral cardiotrophin-1 gene transfer protects pmn mice from progressive motor neuronopathy. J. Clin. Investig. 1999, 104, 1077–1085. [Google Scholar] [CrossRef]
- Pennica, D.; Shaw, K.J.; Swanson, T.A.; Moore, M.W.; Shelton, D.L.; Zioncheck, K.A.; Rosenthal, A.; Taga, T.; Paoni, N.F.; Wood, W.I. Cardiotrophin-1: Biological activities and binding to the leukemia inhibitory factor receptor/gp130 signaling complex. J. Biol. Chem. 1995, 270, 10915–10922. [Google Scholar] [CrossRef]
- Koutinas, A.F.; Polizopoulou, Z.S.; Baumgaertner, W.; Lekkas, S.; Kontos, V. Relation of clinical signs to pathological changes in 19 cases of canine distemper encephalomyelitis. J. Comp. Pathol. 2002, 126, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Gülersoy, E.; Balikçi, C.; Günal, İ.; Şahan, A.; Kismet, E.; Akdağ, F.; Ok, M. 1H-NMR analysis of amino acid metabolism in cerebrospinal fluid of dogs with neurological distemper. Maced. Vet. Rev. 2024, 47, 131–140. [Google Scholar] [CrossRef]
- Moritz, A.; Frisk, A.L.; Baumgärtner, W. The evaluation of diagnostic procedures for the detection of canine distemper virus infection. Eur. J. Comp. Anim. Pract. 2000, 10, 37–47. [Google Scholar]
- Gülersoy, E.; Kapar, M.M.; Durgut, M.K.; Naseri, A.; Ok, M. Evaluation of clinical, hematochemical and cerebrospinal fluid analysis findings in dogs naturally affected by the neurological form of canine distemper. Magy. Allatorvosok Lapja 2022, 144, 13–29. [Google Scholar]
- Caniglia, J.L.; Asuthkar, S.; Tsung, A.J.; Guda, M.R.; Velpula, K.K. Immunopathology of galectin-3: An increasingly promising target in COVID-19. F1000Res 2020, 9, 1078. [Google Scholar] [CrossRef]
- Stojanovic, B.S.; Stojanovic, B.; Milovanovic, J.; Arsenijević, A.; Dimitrijevic Stojanovic, M.; Arsenijevic, N.; Milovanovic, M. The pivotal role of galectin-3 in viral ınfection: A multifaceted player in host–pathogen ınteractions. Int. J. Mol. Sci. 2023, 24, 9617. [Google Scholar] [CrossRef]
- Díaz-Alvarez, L.; Ortega, E. The many roles of galectin-3, a multifaceted molecule, in innate immune responses against pathogens. Mediat. Inflamm. 2017, 2017, 9247574. [Google Scholar] [CrossRef]
- Rahimian, R.; Béland, L.C.; Kriz, J. Galectin-3: Mediator of microglia responses in injured brain. Drug Discov. Today 2018, 23, 375–381. [Google Scholar] [CrossRef]
- Hara, A.; Niwa, M.; Noguchi, K.; Kanayama, T.; Niwa, A.; Matsuo, M.; Hatano, Y.; Tomita, H. Galectin-3 as a next-generation biomarker for detecting early stage of various diseases. Biomolecules 2020, 10, 389. [Google Scholar] [CrossRef]
- Boziki, M.; Polyzos, S.A.; Deretzi, G.; Kazakos, E.; Katsinelos, P.; Doulberis, M.; Kotronis, G.; Giartza-Taxidou, E.; Laskaridis, L.; Tzivras, D.; et al. A potential impact of Helicobacter pylori-related galectin-3 in neurodegeneration. Neurochem. Int. 2018, 113, 137–151. [Google Scholar] [CrossRef]
- Doverhag, C.; Hedtjärn, M.; Poirier, F.; Mallard, C.; Hagberg, H.; Karlsson, A.; Sävman, K. Galectin-3 contributes to neonatal hypoxic–ischemic brain injury. Neurobiol. Dis. 2010, 38, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.R.; Al Rasebi, Z.; Mensah–Brown, E.; Shahin, A.; Xu, D.; Goodyear, C.S.; Fukada, S.Y.; Liu, F.T.; Liew, F.Y.; Lukic, M.L. Galectin 3 deficiency reduces the severity of experimental autoimmune encephalomyelitis. J. Immunol. 2009, 182, 1167–1173. [Google Scholar] [CrossRef]
- Lee, G.W.; Kang, M.H.; Ro, W.B.; Song, D.W.; Park, H.M. Circulating galectin-3 evaluation in dogs with cardiac and non-cardiac diseases. Front. Vet. Sci. 2021, 8, 741210. [Google Scholar] [CrossRef]
- Pasquini, L.A.; Millet, V.; Hoyos, H.C.; Giannoni, J.P.; Croci, D.O.; Marder, M.; Liu, F.T.; Rabinovich, G.A.; Pasquini, J.M. Galectin-3 drives oligodendrocyte differentiation to control myelin integrity and function. Cell Death Differ. 2011, 18, 1746–1756. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, W.; Zheng, Y.; Yang, J.; Liu, Y.; Qi, Z.; Wu, M.; Fan, Z.; Yin, K.; Chen, Y.; et al. Galectin-3 enhances platelet aggregation and thrombosis via Dectin-1 activation: A translational study. Eur. Heart J. 2022, 43, 3556–3574. [Google Scholar] [CrossRef]
- Arce, V.; Pollock, R.A.; Philippe, J.M.; Pennica, D.; Henderson, C.E.; deLapeyrière, O. Synergistic effects of Schwann- and muscle-derived factors on motoneuron survival involve GDNF and cardiotrophin-1 (CT-1). J. Neurosci. 1998, 18, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Pennica, D.; Wood, W.I.; Chien, K.R. Cardiotrophin-1: A multifunctional cytokine that signals via LIF receptor gp130 dependent pathways. Cytokine Growth Factor Rev. 1996, 7, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Sola, A.; Moore, J.; Wen, T. Caspase inhibition by cardiotrophin-1 prevents neuronal death in vivo and in vitro. J. Neurosci. Res. 2010, 88, 1041–1051. [Google Scholar] [CrossRef]
- Barnabe-Heider, F.; Wasylnka, J.A.; Fernandes, K.J.L.; Porsche, C.; Sendtner, M.; Kaplan, D.R.; Miller, F.D. Evidence that embryonic neurons regulate the onset of cortical gliogenesis via cardiotrophin-1. Neuron 2005, 48, 253–265. [Google Scholar] [CrossRef]
- Bordet, T.; Lesbordes, J.C.; Rouhani, S.; Castelnau-Ptakhine, L.; Schmalbruch, H.; Haase, G.; Kahn, A. Protective effects of cardiotrophin-1 adenoviral gene transfer on neuromuscular degeneration in transgenic ALS mice. Hum. Mol. Genet. 2001, 10, 1925–1933. [Google Scholar] [CrossRef]
- Lesbordes, J.C.; Cifuentes-Diaz, C.; Miroglio, A.; Joshi, V.; Bordet, T.; Kahn, A.; Melki, J. Therapeutic benefits of cardiotrophin-1 gene transfer in a mouse model of spinal muscular atrophy. Hum. Mol. Genet. 2003, 12, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Mitsumoto, H.; Klinkosz, B.; Pioro, E.P.; Tsuzaka, K.; Ishiyama, T.; O’Leary, R.M.; Pennica, D. Effects of cardiotrophin-1 (CT-1) in a mouse motor neuron disease. Muscle Nerve 2001, 24, 769–777. [Google Scholar] [CrossRef]
- Shu, X.; Du, S.; Chen, X.; Li, Z. Transplantation of neural stem cells overexpressing cardiotrophin-1 inhibits sprouting of hippocampal mossy fiber in a rat model of status epilepticus. Cell Biochem. Biophys. 2011, 61, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, X.; Gao, K.; Lu, D.; Zhang, X.; Ma, C.; Ye, F.; Zhang, L. Cardiotrophin-1 (CTF1) ameliorates glucose-uptake defects and improves memory and learning deficits in a transgenic mouse model of Alzheimer’s disease. Pharmacol. Biochem. Behav. 2013, 107, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Fritzenwanger, M.; Jung, C.; Franz, M.; Foerster, M.; Figulla, H.R. Immunomodulatory effects of cardiotrophin-1 on in vitro cytokine production of monocytes & CD4+ T-lymphocytes. Indian J. Med. Res. 2012, 136, 471–476. [Google Scholar]
- Adams, J.M.; Brown, W.J.; Snow, H.D.; Lincoln, S.D.; Sears, A.W.; Barenfus, M.; Holliday, T.A.; Cremer, N.E.; Lennette, E.H. Old Dog Encephalitis and Demyelinating Diseases in Man. Vet. Pathol. 1975, 12, 220–226. [Google Scholar] [CrossRef]
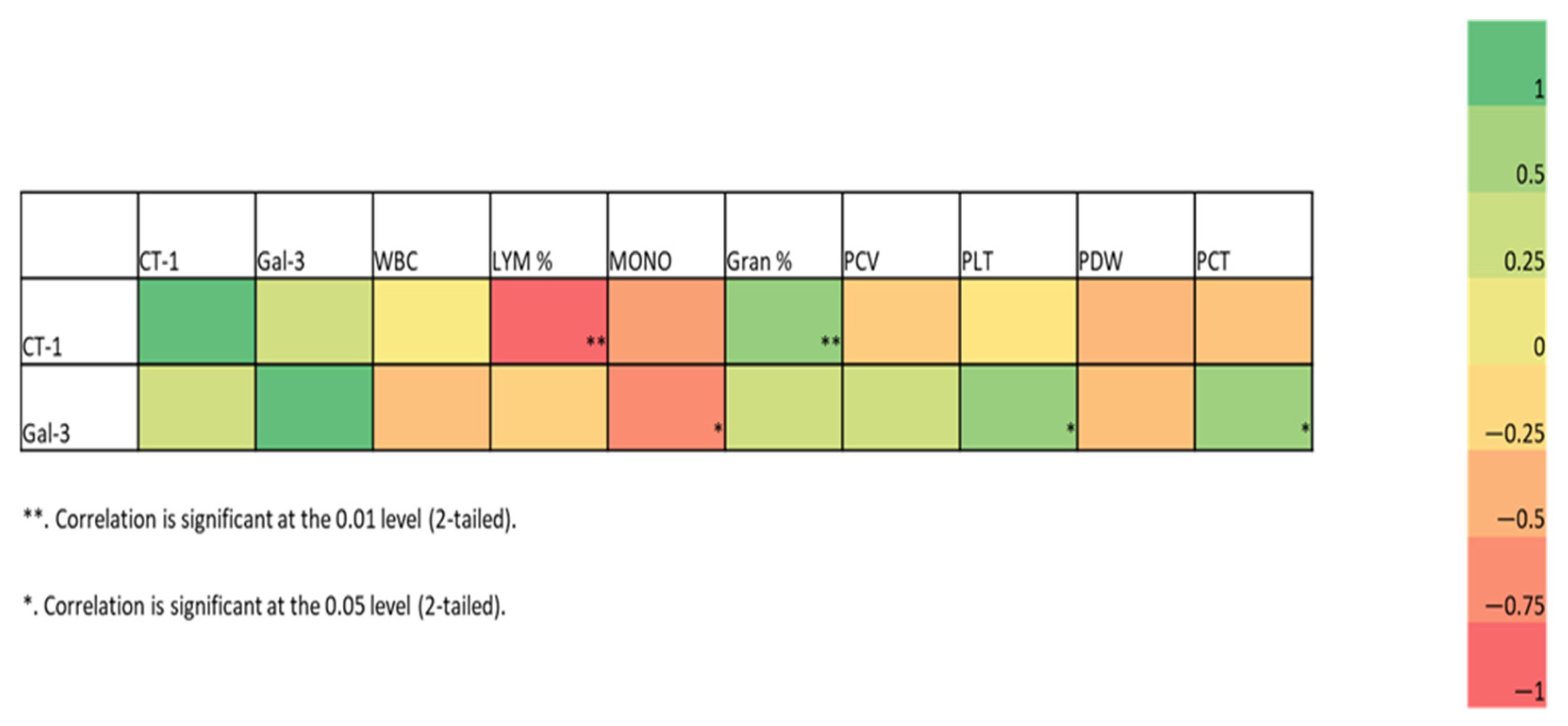
| Control Group (Mean ± SD) | Neurological Distemper (Mean ± SD) | p-Value | |
|---|---|---|---|
| WBC m/mm3 (5.0–19.0) * | 13.91 ± 2.52 | 12.32 ± 16.59 | 0.820 |
| Lymphocyte % (5.0–30.0) * | 29.82 ± 15.82 | 10.41 ± 3.0 | 0.003 * |
| Granulocyte % (40.0–80.0) * | 60.64 ± 12.30 | 86.94 ± 3.29 | 0.000 * |
| Monocyte cells/mL (0.1–1.1) * | 0.72 ± 0.25 | 0.32 ± 0.42 | 0.047 * |
| PLT m/mm3 (211–621) * | 334.00 ± 171.76 | 536.44 ± 313.99 | 0.176 |
| MPV fl | 8.75 ± 0.33 | 8.68 ± 1.11 | 0.881 |
| PDW | 15.22 ± 3.08 | 15.20 ± 2.73 | 0.991 |
| PCT % | 0.29 ± 0.15 | 0.40 ± 0.21 | 0.298 |
| Creatine mg/dL (0.3–1.4) * | 0.64 ± 0.25 | 0.59 ± 0.42 | 0.776 |
| BUN mg/dL (14–36) * | 16.33 ± 5.24 | 15.23 ± 10.72 | 0.816 |
| Total protein g/dL (4.0–5.8) * | 4.93 ± 0.8 | 5.38 ± 1.28 | 0.438 |
| Albumin g/dL (2.5–3.9) * | 3.14 ± 0.4 | 2.99 ± 0.5 | 0.594 |
| Total bilirubin mg/dL (0.1–1.0) * | 0.6 ± 0.2 | 0.4 ± 0.2 | 0.285 |
| ALT U/L (10–100) * | 24.67 ± 9.93 | 21.85 ± 7.47 | 0.499 |
| LDH U/L (20–500) * | 160.83 ± 189.85 | 247.92 ± 214.67 | 0.407 |
| ALP U/L (75–450) * | 136.83 ± 83.60 | 125.46 ± 100.30 | 0.813 |
| CK U/L (50–450) * | 247.58 ± 206.36 | 308.39 ± 152.42 | 0.479 |
| Control Group (Mean ± SD) | Neurological Distemper (Mean ± SD) | p-Value | |
|---|---|---|---|
| Galectin-3 (ng/mL) | 9.56 ± 1.32 | 12.61 ± 1.90 | 0.009 * |
| Cardiotrophin-1 (ng/L) | 29.96 ± 13.41 | 44.64 ± 10.46 | 0.024 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erturk, A.; Ozturk, A.S.; Ozturk, A. Investigation of Galectin-3 and Cardiotrophin-1 Concentrations as Biomarkers in Dogs with Neurological Distemper. Vet. Sci. 2025, 12, 499. https://doi.org/10.3390/vetsci12050499
Erturk A, Ozturk AS, Ozturk A. Investigation of Galectin-3 and Cardiotrophin-1 Concentrations as Biomarkers in Dogs with Neurological Distemper. Veterinary Sciences. 2025; 12(5):499. https://doi.org/10.3390/vetsci12050499
Chicago/Turabian StyleErturk, Alper, Aliye Sagkan Ozturk, and Atakan Ozturk. 2025. "Investigation of Galectin-3 and Cardiotrophin-1 Concentrations as Biomarkers in Dogs with Neurological Distemper" Veterinary Sciences 12, no. 5: 499. https://doi.org/10.3390/vetsci12050499
APA StyleErturk, A., Ozturk, A. S., & Ozturk, A. (2025). Investigation of Galectin-3 and Cardiotrophin-1 Concentrations as Biomarkers in Dogs with Neurological Distemper. Veterinary Sciences, 12(5), 499. https://doi.org/10.3390/vetsci12050499







