Clinical Approach to Cleft Lip and Palate with or Without Surgical Correction in Ten Brachycephalic Puppies
Simple Summary
Abstract
1. Introduction
1.1. Palate Morphogenesis
1.2. Etiology
1.3. Types of Palatoschisis
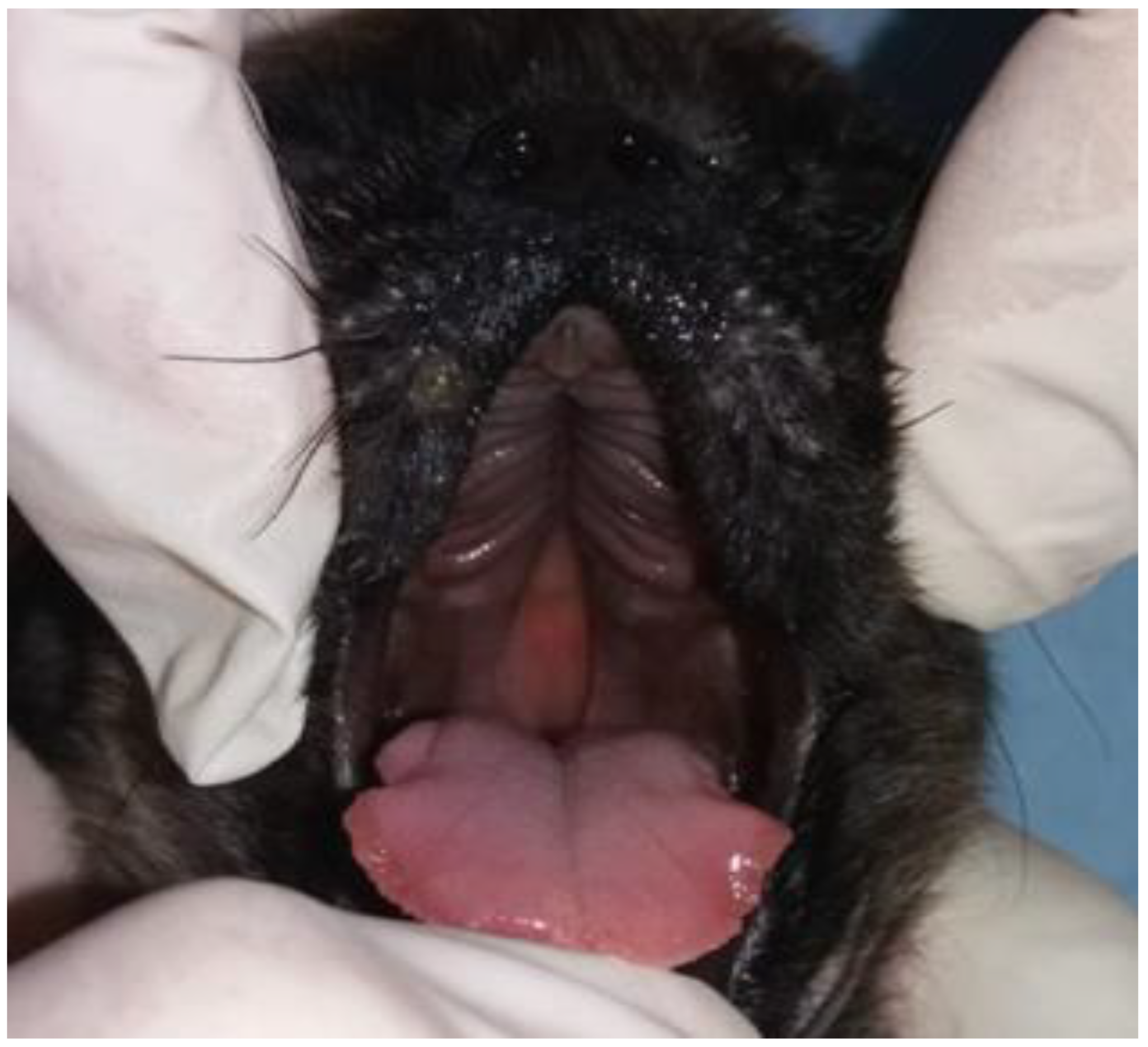
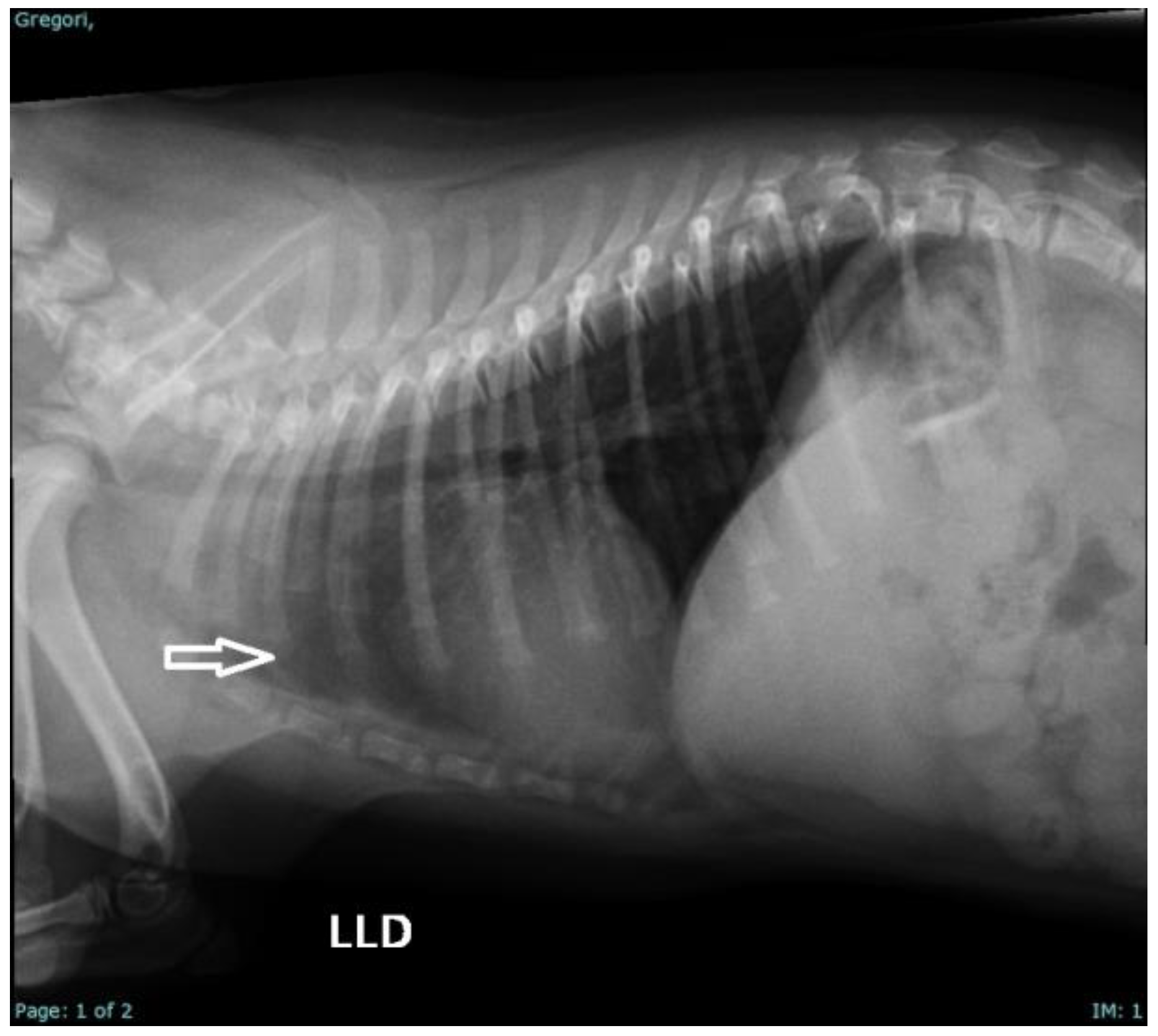
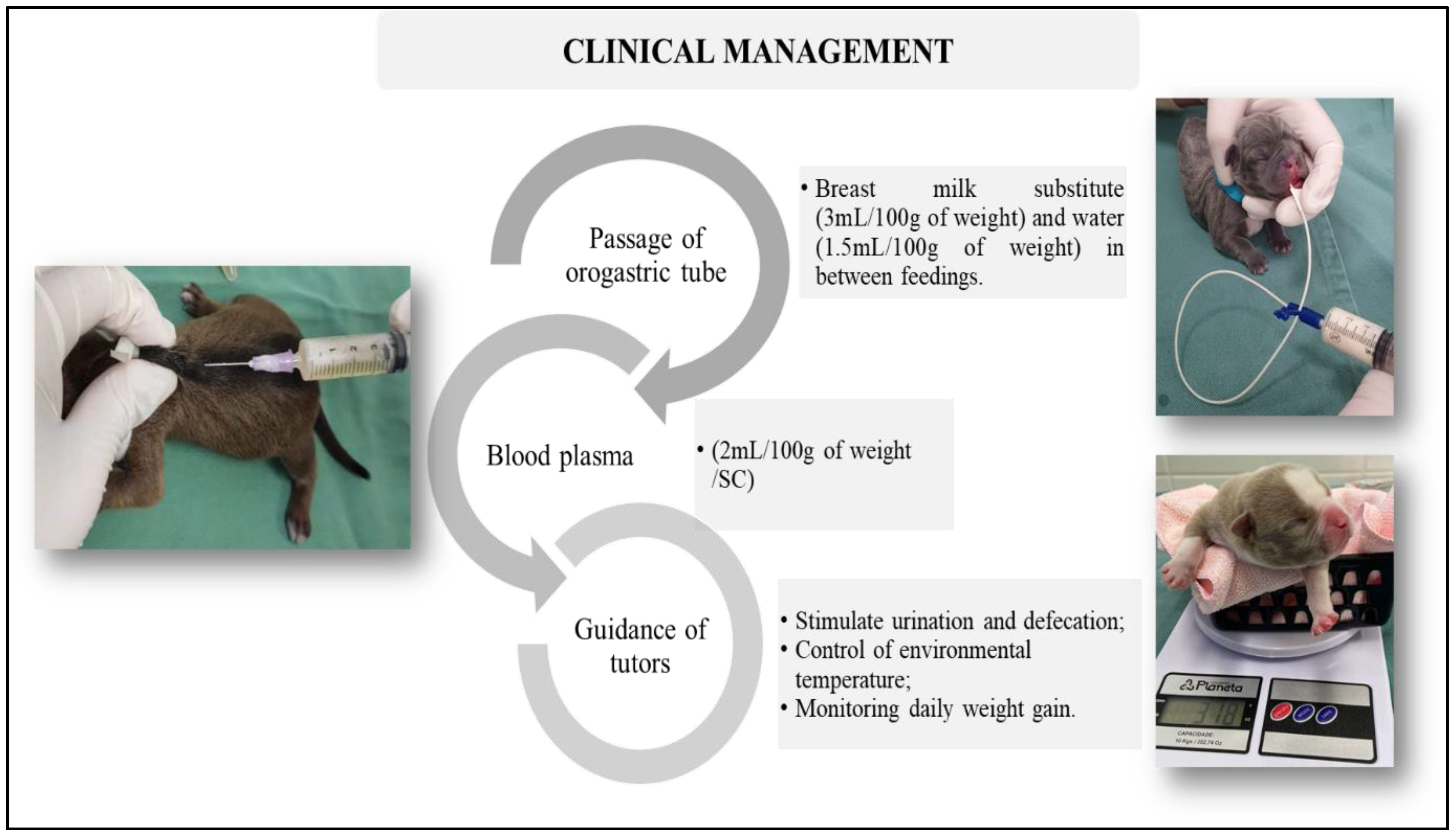
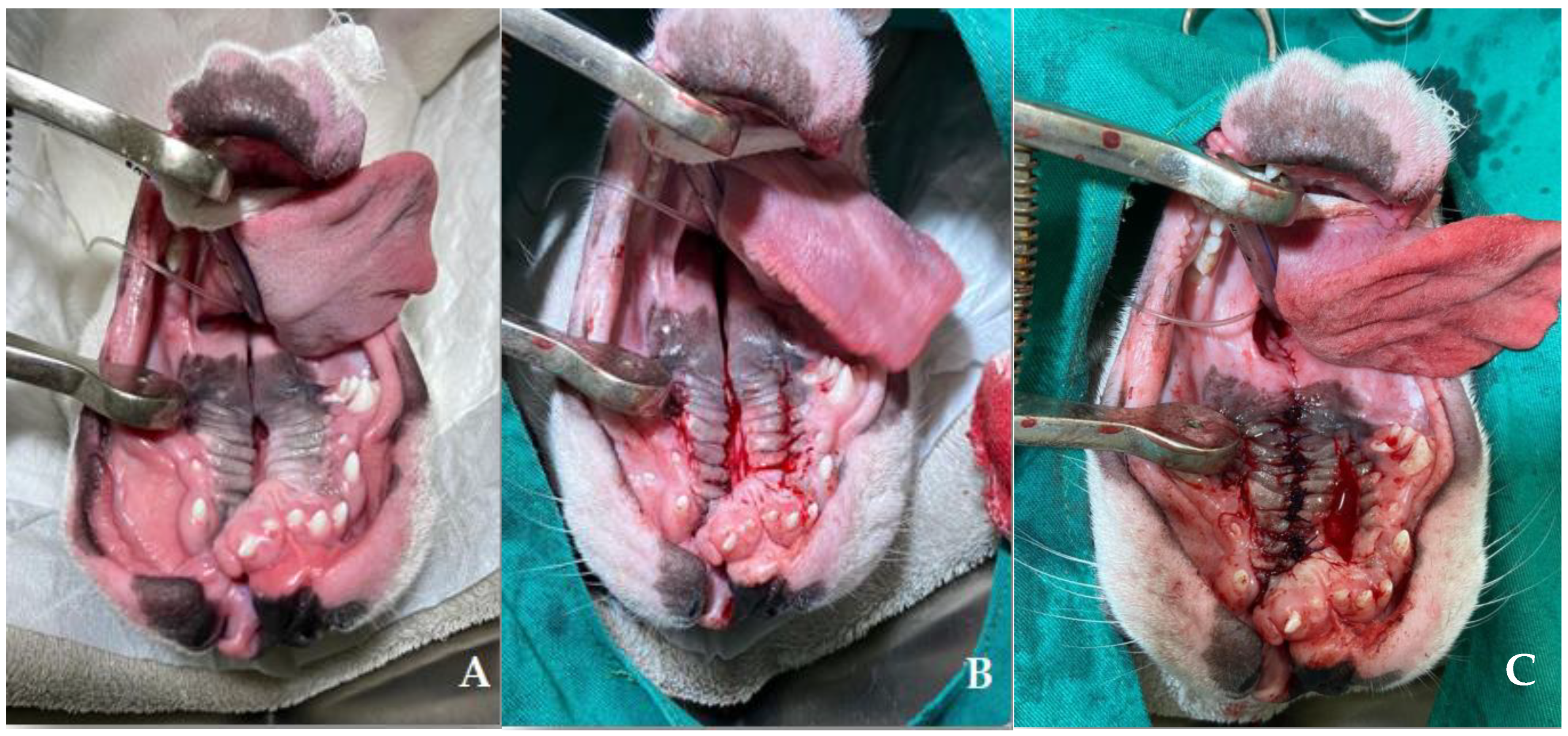
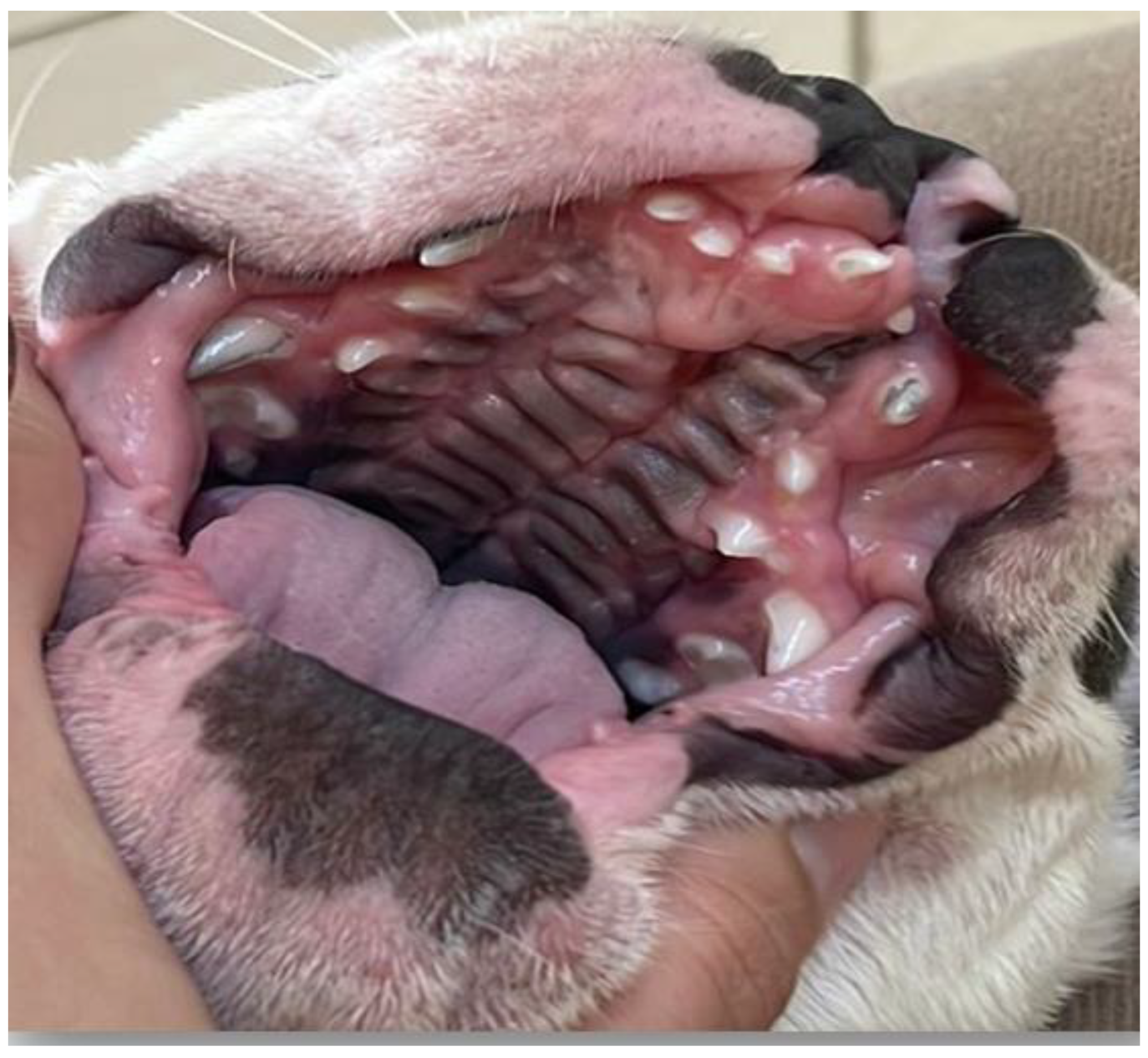
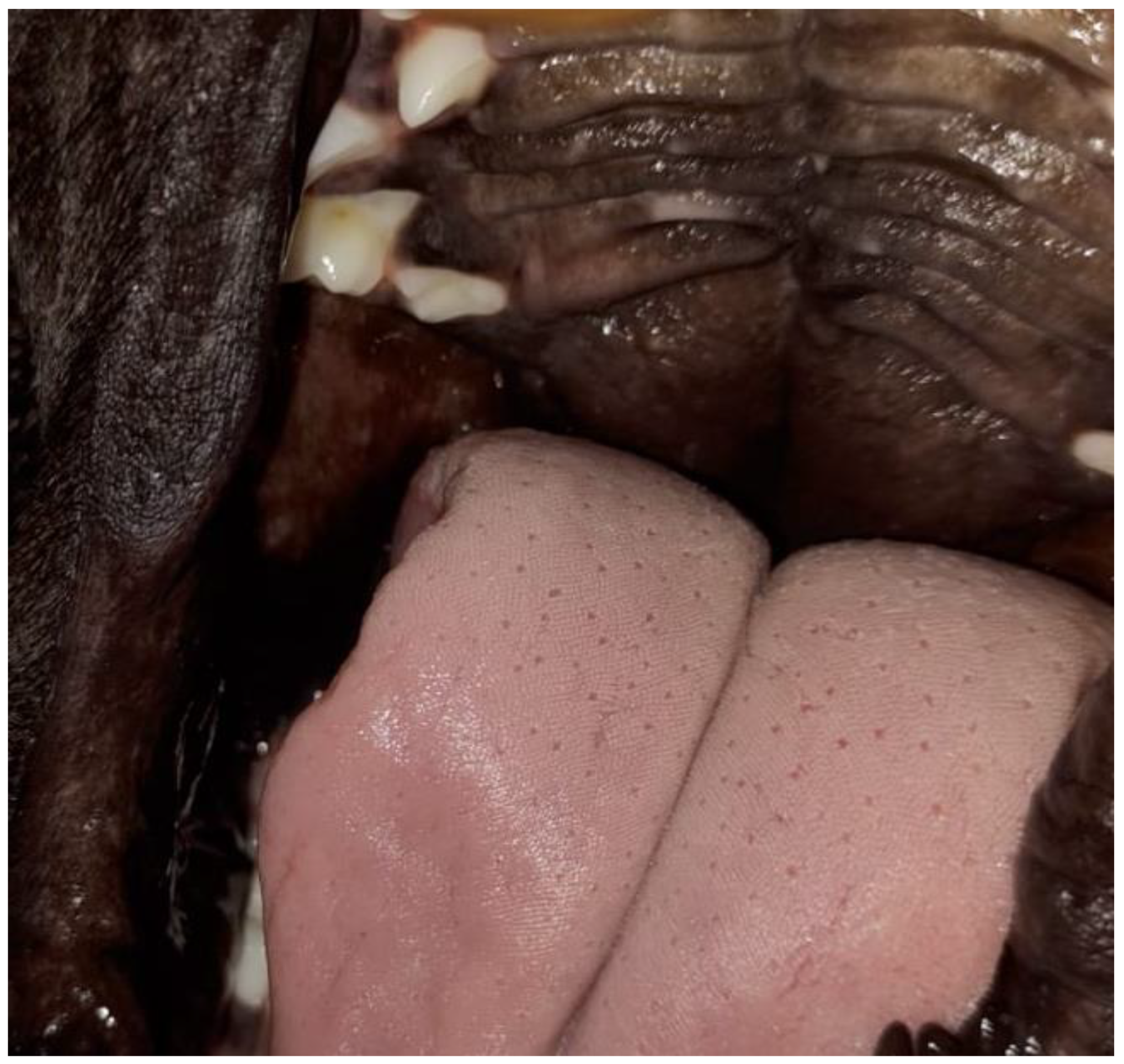
2. Case Series Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leite, I.C.G.; Paumgartten, F.J.R.; Koifman, S. Orofacial clefts in newborns and the use of medications and maternal health conditions: A case-control study in the city of Rio de Janeiro, Brazil. Braz. J. Matern. Child. Health 2005, 5, 35–43. [Google Scholar]
- Li, C.; Lan, Y.; Jiang, R. Molecular and cellular mechanisms of palate development. J. Dent. Res. 2017, 96, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Mcgeady, T.A.; Quinn, P.J.; Fitzpatrick, E.S.; Ryan, M.T.; Kilroy, D.; Lonergan, P. Veterinary Embryology, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; 379p. [Google Scholar]
- Freiberger, K.; Hemker, S.; McAnally, R.; King, R.; Meyers-Wallen, V.N.; Schutte, B.C.; Fyfe, J.C. Secondary palate development in the dog (Canis lupus familiaris). Cleft Palate Craniofacial J. 2021, 58, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Schoenebeck, J.J.; Ostrander, E.A. Insights into morphology and disease from the dog genome project. Annu. Rev. Cell Dev. Biol. 2014, 30, 535–560. [Google Scholar] [CrossRef]
- Maldonado, E.; Martínez-Sanz, E.; Partearroyo, T.; Varela-Moreiras, G.; Pérez-Miguelsanz, J. Maternal folic acid deficiency is associated to developing nasal and palate malformations in mice. Nutrients 2021, 13, 251. [Google Scholar] [CrossRef]
- Contesini, E.A.; Pippi, N.L.; Beck, C.A.D.C.; Brun, M.V.; Leme, M.D.C.; Raiser, A.G.; Pellegrini, L.C.; Bonfada, A.T.; Silva, T.F.; Costa, J.S.C.; et al. Clinical and macroscopic aspects of immediate palatoplasty with implantation of auricular pinna cartilage, preserved in 98% glycerin, after experimental induction of cleft palate in dogs. Rural Sci. 2003, 33, 103–108. [Google Scholar]
- Pereira, K.H.N.P.; Correia, L.E.C.S.; Oliveira, E.L.; Bernardo, R.B.; Nagib, J.M.L.; Mezzena, G.M.L. Incidence of congenital malformations and impact on the mortality of neonatal canines. Theriogenology 2019, 140, 52–57. [Google Scholar] [CrossRef]
- Sperber, G.H.; Sperber, S.M. Embryogenetics of Cleft Lip and Palate. In Cleft Lip and Palate: Diagnosis and Management; Springer: Berlin/Heidelberg, Germany, 2012; pp. 3–33. [Google Scholar]
- Ornoy, A. Craniofacial malformations and their association with brain development: The importance of a multidisciplinary approach for treatment. Odontology 2020, 108, 1–15. [Google Scholar] [CrossRef]
- Rahimov, F.; Jugessur, A.; Murray, J.C. Genética de fissuras orofaciais não sindrômicas. Cleft Palate Craniofacial J. 2012, 49, 73–91. [Google Scholar] [CrossRef]
- Moura, E.; Pimpão, C.T. Cleft lip and palate in the dog: Medical and genetic aspects. Intech. Sci. 2017, 8, 143–166. [Google Scholar]
- Dreyer, C.J.; Preston, C.B. Classification of cleft lip and palate in animals. Cleft Palate J. 1974, 11, 327–332. [Google Scholar] [PubMed]
- Evans, H.E.; Sack, O.W. Prenatal development of domestic and laboratory mammals: Growth curves, external features and selected references. Anat. Histol. Embryol. 1973, 2, 11–45. [Google Scholar] [CrossRef] [PubMed]
- Noden, D.M.; De Lahunta, A. Embryology of Domestic Animals: Developmental Mechanisms and Malformations; Williams and Wilkins: Philadelphia, PA, USA, 1985. [Google Scholar]
- Nelson, R.W.; Couto, G.C. Small Animal Internal Medicine-E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Van Den Berghe, F.; Cornillie, P.; Stegen, L.; Van Goethem, B.; Simoens, P. Palatoschisis in the dog: Developmental mechanisms and etiology. Vlaams Diergeneeskd. Tijdschr. 2010, 79, 117–123. [Google Scholar] [CrossRef]
- Dixon, M.J.; Marazita, M.L.; Beaty, T.H.; Murray, J.C. Cleft lip and palate: Understanding genetic and environmental influences. Nat. Rev. Genet. 2011, 12, 167–178. [Google Scholar] [CrossRef]
- Lobodzinska, A.; Gruszczynska, J.; Max, A.; Bartyzel, B.J.; Mikula, M.; Mikula Jr, I.; Grzegrzolka, B. Cleft palate in the domestic dog Canis lupus familiaris—Etiology, pathophysiology, diagnosis, prevention and treatment. Acta Sci. Pol. Zootech. 2014, 13, 5–28. [Google Scholar]
- Kang, P.; Svoboda, K.K.H. Epithelial-mesenchymal transformation during craniofacial development. J. Dent. Res. 2005, 84, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Okano, J.; Suzuki, S.; Shiota, K. Regional heterogeneity in the developing palate: Morphological and molecular evidence for normal and abnormal palatogenesis. Congenit. Anom. 2006, 46, 49–54. [Google Scholar] [CrossRef]
- Foley, C.W.; Lasley, J.F.; Osweiler, G.D. Companion Animal Abnormalities: Analysis of Heredity; The Iowa State University Press: Ames, IA, USA, 1979; 279p. [Google Scholar]
- Mulvihill, J.J.; Mulvihill, C.G.; Priester, W.A. Cleft palate in domestic animals: Epidemiologic features. Teratology 1980, 21, 109–112. [Google Scholar] [CrossRef]
- Kirk, R.A. Catalogue of Congenital and Hereditary Disorders of Dogs (by Breeds); Current Veterinary Therapy IX.; W. B. Saunders Company: Philadelphia, PA, USA, 1986; pp. 1281–1285. [Google Scholar]
- Padgett, G.A. Canine Genetic Disease Control; Howell Book House: New York, NY, USA, 1998; 264p. [Google Scholar]
- Kemp, C.; Thiele, H.; Dankof, A.; Schmidt, G.; Lauster, C.; Fernahl, G.; Lauster, R. Cleft lip and/or palate with monogenic autosomal recessive transmission in Pyrenean sheepdogs. Craniofacial J. 2009, 46, 81–88. [Google Scholar]
- Martínez-Sanz, E.; Casado-Gómez, I.; Martín, C.; López-Gordillo, Y.; González, P.; Rodríguez-Bobada, C.; Paradas, I.; González-Meli, B.; Maldonado, E.; Maestro, C.; et al. A new technique for feeding dogs with a congenital cleft palate for surgical research. Lab. Anim. 2011, 45, 70–80. [Google Scholar] [CrossRef]
- Ruszkowski, J.J.; Nowacka-Woszuk, J.; Nowak, T.; Rozynek, J.; Serwanska-Leja, K.; Gogulski, M.; Kolodziejski, P.; Switonski, M.; Zdun, M.; Szczerbal, I. Cleft Lip and Palate in Four Full-Sib Puppies from a Single Litter of Staffordshire Bull Terrier Dogs: An Anatomical and Genetic Study. Animals. 2023, 13, 2749. [Google Scholar] [CrossRef] [PubMed]
- Richtsmeier, J.T.; Flaherty, K.; Koller, R. Genetic and environmental factors contributing to craniofacial morphology in dogs. Dev. Dyn. 2006, 235, 1346–1361. [Google Scholar]
- Wilcox, A.J.; Lie, R.T.; Solvoll, K.; Taylor, J.; McConnaughey, D.R.; Åbyholm, F.; Drevon, C.A. Folic acid supplements and risk of facial clefts: National population based case-control study. BMJ 2007, 334, 464. [Google Scholar] [CrossRef]
- Mossey, P.A.; Little, J.; Munger, R.G.; Dixon, M.J.; Shaw, W.C. Cleft lip and palate. Lancet 2009, 374, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- López-Gordillo, Y.; Maldonado, E.; Nogales, L.; Del Río, A.; Barrio, M.C.; Murillo, J.; Martínez-Álvarez, C. Maternal folic acid supplementation reduces the severity of cleft palate in Tgf-β 3 null mutant mice. Pediatr. Res. 2019, 85, 566–573. [Google Scholar] [CrossRef]
- Morriss-Kay, G.M.; Wilkie, A.O.M. Growth of the normal skull vault and its alteration in craniosynostosis: Insights from human genetics and experimental studies. J. Anat. 2005, 207, 637–653. [Google Scholar] [CrossRef]
- Krapels, I.P.; Vermeij-Keers, C.; Müller, M.; de Klein, A.; Steegers-Theunissen, R.P. Nutrition and genes in the development of orofacial clefting. Nutr. Rev. 2006, 64, 280–288. [Google Scholar] [CrossRef]
- Lan, S.; Yang, X.; Li, T.; Yang, T.; Rong, L. Dexamethasone Induces Apoptosis of Embryonic Palatal Mesenchymal Cells Through the GATA-6/Bone Morphogenetic Protein-2/p38 MAPK Pathway. J. Craniofacial Surg. 2022, 33, 1335–1340. [Google Scholar] [CrossRef]
- Everson, J.L.; Fink, D.M.; Zohn, I.E. Environmental toxicants and craniofacial development. Birth Defects Res. Part. C Embryo Today Rev. 2009, 87, 101–111. [Google Scholar]
- Greene, C.E. Infectious Diseases of the Dog and Cat; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Fantuzzi, N.; Piro, M.; Peric Tet, a.l. Congenital hypothyroidism in a dog: Case report. Vet. Res. Commun. 2016, 40, 201–205. [Google Scholar]
- Webster, W.S.; Ritchie, H.E. Craniofacial malformations and hypoxia: An overview. Teratology 1991, 44, 3–14. [Google Scholar]
- Diewert, V.M.; Wahab, R.A.; Lozanoff, S. Fetal hypoxia and secondary palatal development. Anat. Rec. 2008, 291, 110–122. [Google Scholar]
- Warzee, C.C.; Bellah, J.R.; Richards, D. Congenital unilateral cleft of the soft palate in six dogs. J. Small Anim. Pract. 2001, 42, 338–340. [Google Scholar] [CrossRef]
- Sinibaldi, K.R. Cleft Palate. Vet. Clin. N. Am. Small Anim. Pract. 1979, 9, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Thornton, J.B.; Nimer, S.; Howard, P.S. The incidence, classification, etiology, and embryology of oral clefts. Semin. Orthod. 1996, 2, 162–168. [Google Scholar] [CrossRef]
- Lourenço, M.L.G. Care of Newborns and Puppies. In Treatise on Internal Medicine for Dogs and Cats; Jericó, M.M., Kogika, M.M., Andrade Neto, J.P., Eds.; Guanabara Koogan: Rio de Janeiro, RJ, USA, 2023. [Google Scholar]
- Davidson, A.P.; Gregory, C.; Dedrick, P. Successful management permitting delayed operative revision of cleft palate in a labrador retriever. Vet. Clin: Small Anim. 2014, 44, 325–329. [Google Scholar] [CrossRef]
- Fossum, T.W. Small Animal Surgery, 5th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Apparício, M. Neonatology. In Reproduction and Obstetrics in Dogs and Cats; Apparício, M., Vicente, W.R.R., Eds.; MedVet: São Paulo, Brazil, 2015; pp. 313–332. [Google Scholar]
- Kutritz, M.V.R. Clinical Canine and Feline Reproduction: Evidence-Based Answers; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Tobias, K.M.; Johnston, S.A. Veterinary Surgery: Small Animal; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Pereira, K.H.N.P.; Fuchs, K.M.; Mendonça, J.C.; Xavier, G.M.; Knupp, F.C.; Lourenço, M.L.G. Topics on maternal, fetal and neonatal immunology of dogs and cats. Vet. Immunol. Immunopathol. 2023, 266, 110678. [Google Scholar] [CrossRef]
- Ritto, F.G.; Sperber, G.H.; Smith, K.S. Spontaneous Palatal Cleft Closure. Cleft Palate Craniofacial J. 2021, 58, 662–664. [Google Scholar] [CrossRef]
- Friedl, R.M.; Raja, S.; Metzler, M.A.; Patel, N.D.; Brittian, K.R.; Jones, S.P.; Sandell, L.L. RDH10 function is necessary for spontaneous fetal mouth movement that facilitates palate shelf elevation. Dis. Models Mech. 2019, 12, dmm039073. [Google Scholar] [CrossRef]
- Walker, B.E. Induction of cleft palate in rats with antiinflammatory drugs. Teratology 1971, 4, 39–42. [Google Scholar] [CrossRef]
- Pereira, K.H.N.P.; Fuchs, K.D.M.; Terçariol, L.A.A.; Silva, R.C.; Camargo, G.D.A.; Mendonça, J.C.; Lourenço, M.L.G. Two Types of Management for the Noninvasive Treatment of Pectus Excavatum in Neonatal Puppies. Animals 2023, 13, 906. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.P.; Ahmad, Z.S.; Mittal, R.; Kukreja, S.; Jha, C.; Raheja, K. Nasogastric vs orogastric feeding in stable preterm (≤32 weeks) neonates: A randomized open-label controlled trial. Indian Pediatr. 2023, 60, 726–730. [Google Scholar] [CrossRef] [PubMed]




| Neonate | Breed | Gender | Diagnosis | Clinical Signs | Treatment |
|---|---|---|---|---|---|
| 1 | American Bully | Male | At birth | Weak sucking and pectus excavatum | Clinical management + box management |
| 2 | French Bulldog | Male | At birth | Weak sucking | Clinical management |
| 3 | French Bulldog | Male | At birth | Weak sucking | Clinical management |
| 4 | French Bulldog | Male | At birth | Weak sucking | Clinical management |
| 5 | French Bulldog | Male | At birth | Weak sucking * | Clinical management |
| 6 | French Bulldog | Female | At birth | Cleft lip and weak sucking | Clinical management |
| 7 | French Bulldog | Male | At birth | Weak sucking | Clinical management |
| 8 | Pug | Male | At 3 days of life | Gagging, milk coming out of the nose, weak sucking | Clinical management |
| 9 | Pug | Male | At 2 days of life | Sepsis, dyspnea, pulmonary crackles, neonatal triad, and weak sucking | Clinical management + Amoxicillin + clavulanate (20 mg/kg/BID/7 days); N-acetylcysteine (3 mg/kg/PO/TID, for 5 days) and Nebulization (TID) [44] |
| 10 | Pug | Male | At 14 days | Sepsis, dyspnea, pulmonary crackles, neonatal triad, weak sucking, and hydrocephalus | Clinical management; Amoxicillin + clavulanate (20 mg/kg/BID/7 days); N-acetylcysteine (3 mg/kg/PO/TID, for 5 days); Nebulization (TID); Prednisolone (0.5 mg/kg/PO/BID/7 days); Omeprazole (1 mg/kg/PO/SID); Furosemide (2 mg/kg/BID/2 days) [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xavier, G.M.; Pereira, K.H.N.P.; Fuchs, K.d.M.; Mendonça, J.C.; Abibe, R.B.; Brandão, C.V.S.; Apparício, M.; de Souza, F.F.; Crema, M.G.; Machado, V.M.d.V.; et al. Clinical Approach to Cleft Lip and Palate with or Without Surgical Correction in Ten Brachycephalic Puppies. Vet. Sci. 2025, 12, 474. https://doi.org/10.3390/vetsci12050474
Xavier GM, Pereira KHNP, Fuchs KdM, Mendonça JC, Abibe RB, Brandão CVS, Apparício M, de Souza FF, Crema MG, Machado VMdV, et al. Clinical Approach to Cleft Lip and Palate with or Without Surgical Correction in Ten Brachycephalic Puppies. Veterinary Sciences. 2025; 12(5):474. https://doi.org/10.3390/vetsci12050474
Chicago/Turabian StyleXavier, Gleice Mendes, Keylla Helena Nobre Pacífico Pereira, Kárita da Mata Fuchs, Júlia Consenza Mendonça, Rebeca Bastos Abibe, Claudia Valéria Seullner Brandão, Maricy Apparício, Fabiana Ferreira de Souza, Matheus Gabriel Crema, Vânia Maria de Vasconcelos Machado, and et al. 2025. "Clinical Approach to Cleft Lip and Palate with or Without Surgical Correction in Ten Brachycephalic Puppies" Veterinary Sciences 12, no. 5: 474. https://doi.org/10.3390/vetsci12050474
APA StyleXavier, G. M., Pereira, K. H. N. P., Fuchs, K. d. M., Mendonça, J. C., Abibe, R. B., Brandão, C. V. S., Apparício, M., de Souza, F. F., Crema, M. G., Machado, V. M. d. V., & Lourenço, M. L. G. (2025). Clinical Approach to Cleft Lip and Palate with or Without Surgical Correction in Ten Brachycephalic Puppies. Veterinary Sciences, 12(5), 474. https://doi.org/10.3390/vetsci12050474








