Parity-Associated Differences in the Antioxidants and Fecal Microbiota of Bactrian Camels
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Animals, and Sample Collection
2.2. Antioxidant Analysis in Milk and Serum
2.3. Microbial DNA Extraction and 16S rRNA Sequencing
2.4. Libraries Generated, Illumina NovaSeq Sequencing, and Bioinformatics
2.5. Statistical Analysis
3. Results
3.1. Dynamics of Antioxidants in the Lactating Camel
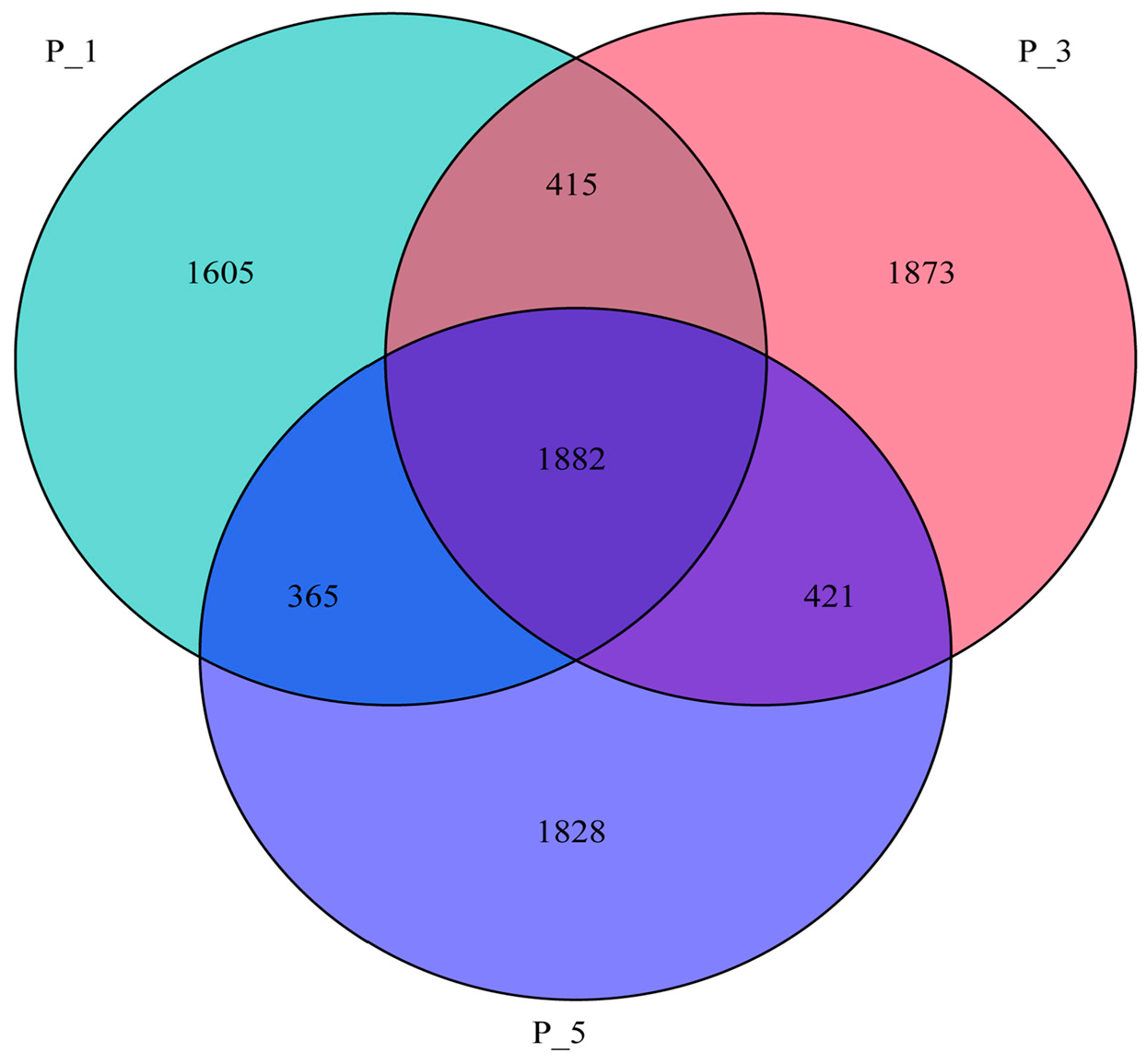
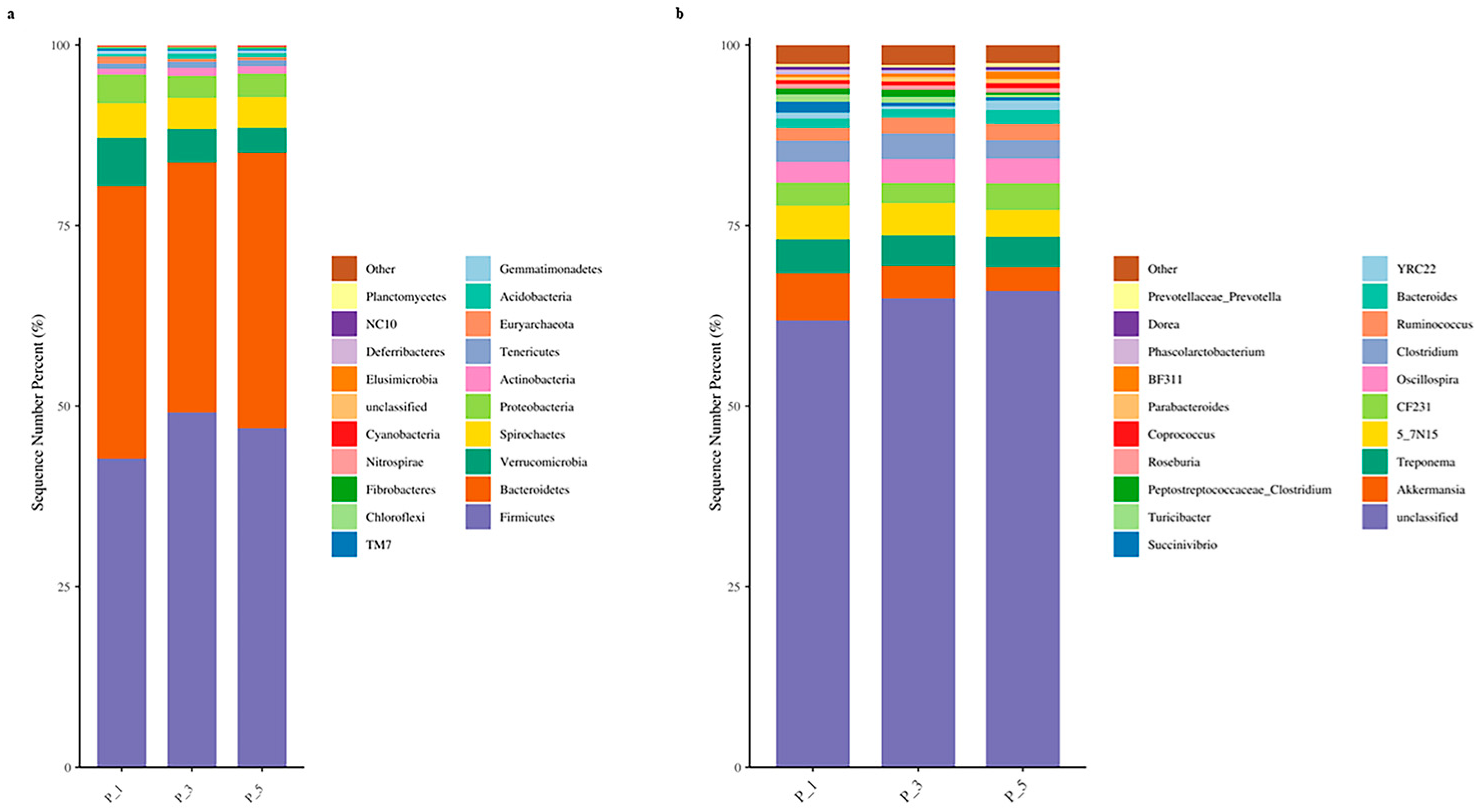
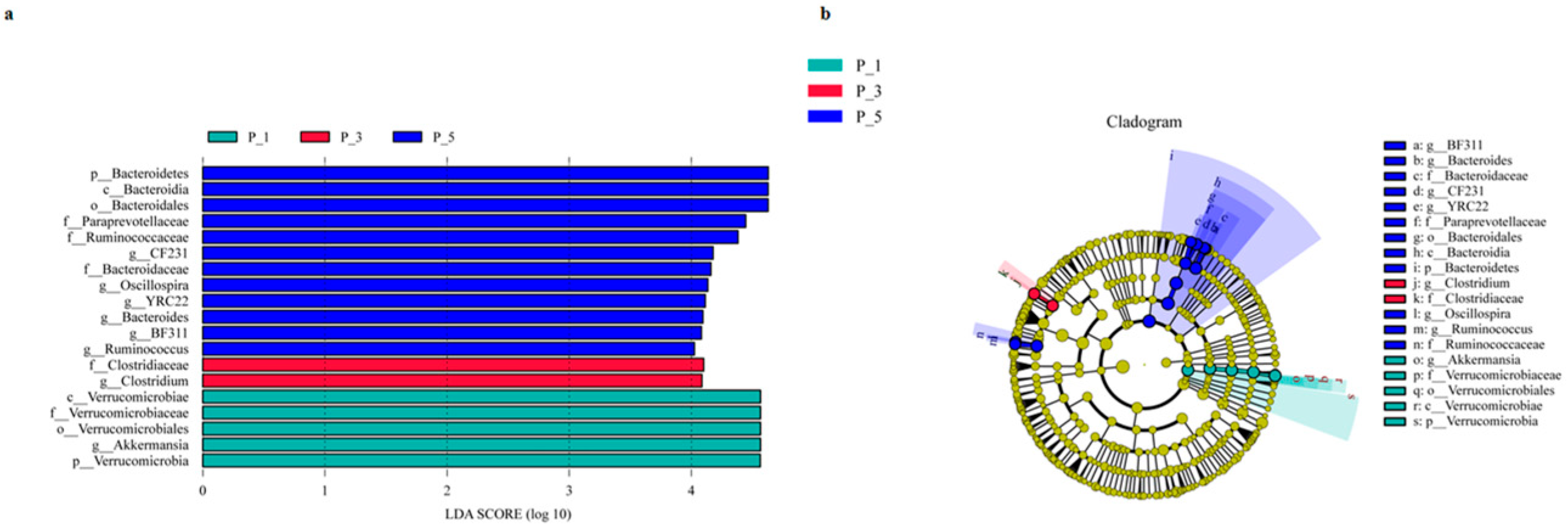
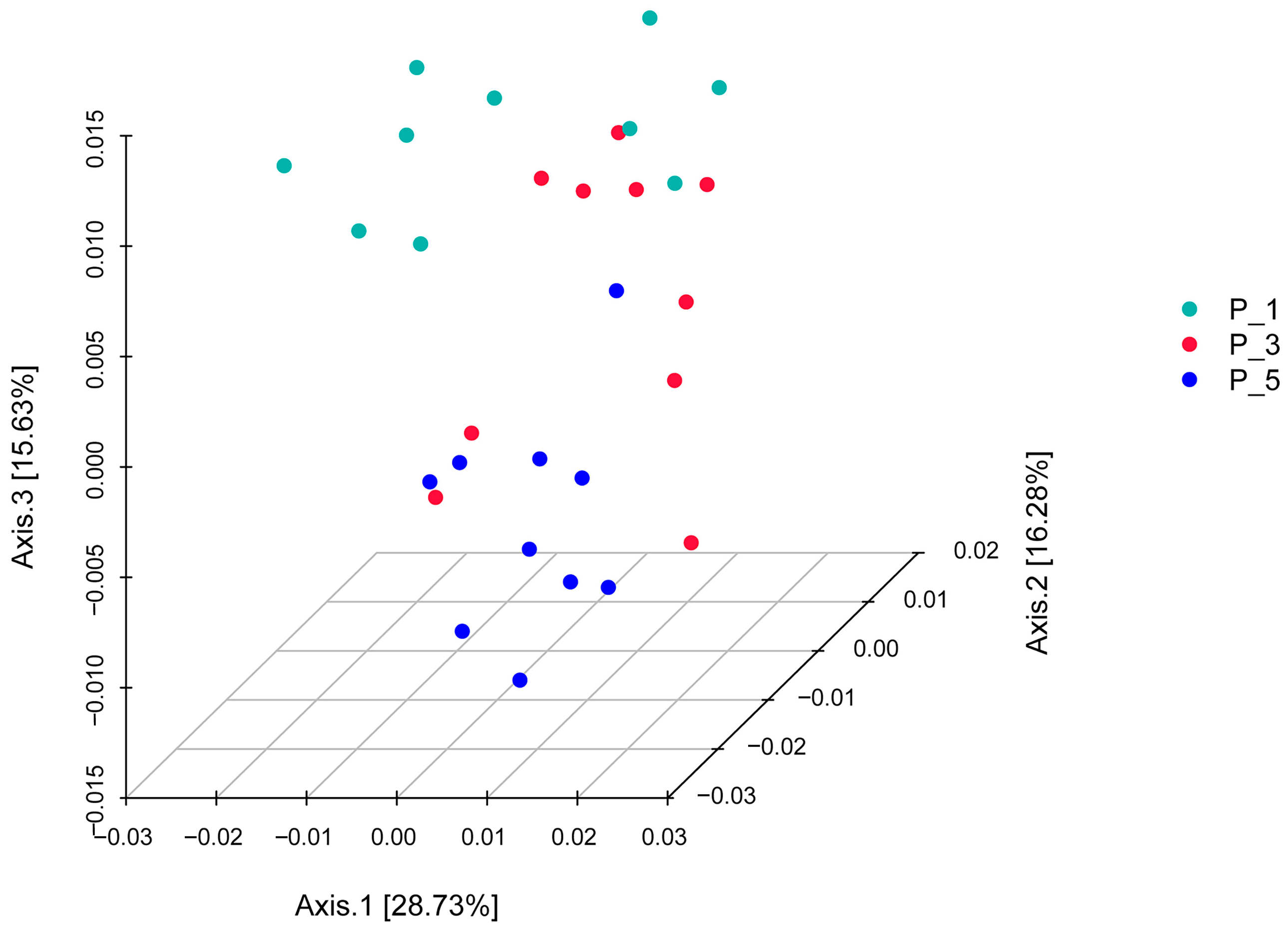
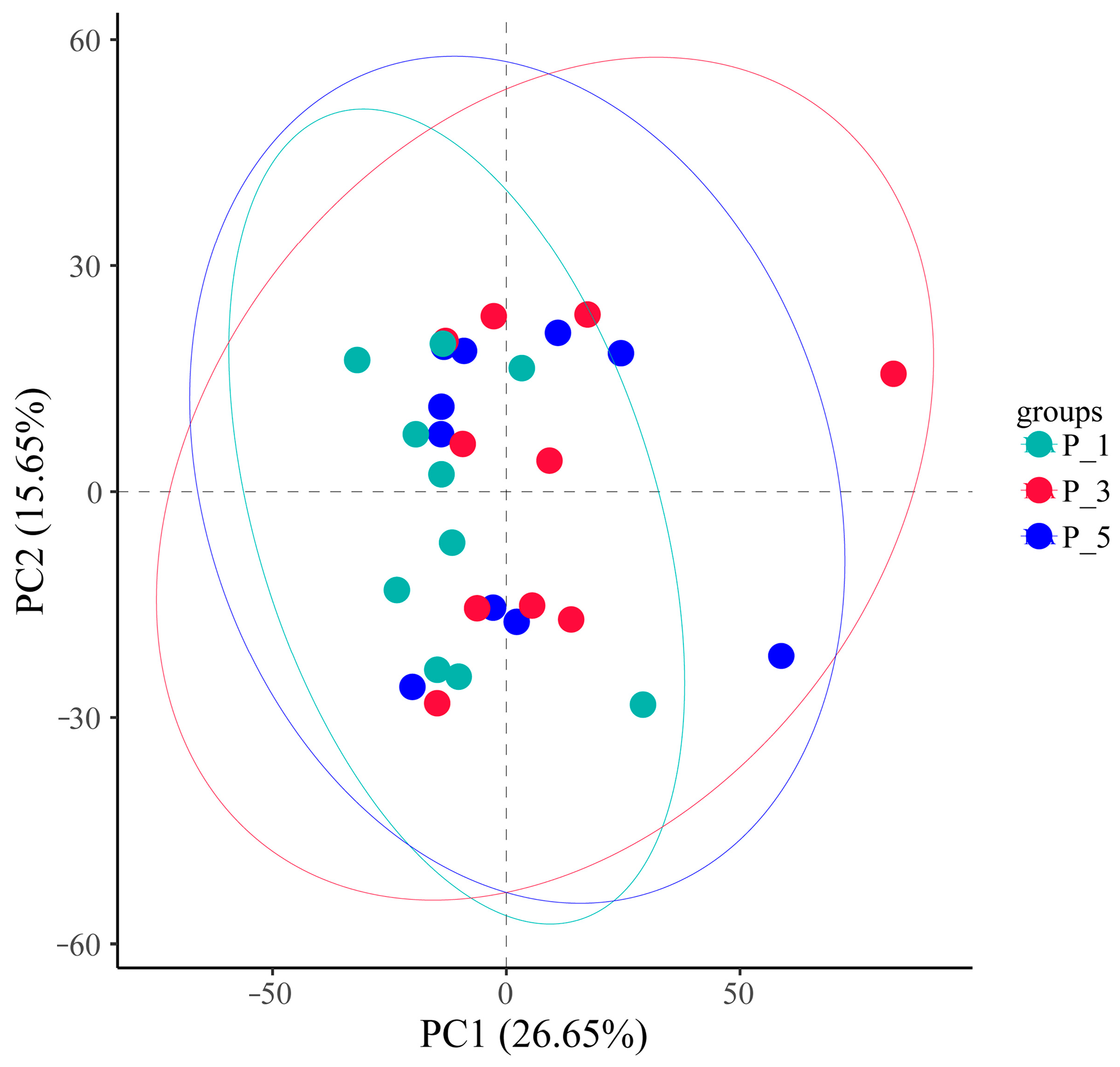
3.2. Dynamics of the Fecal Microbial Community in the Lactating Camel
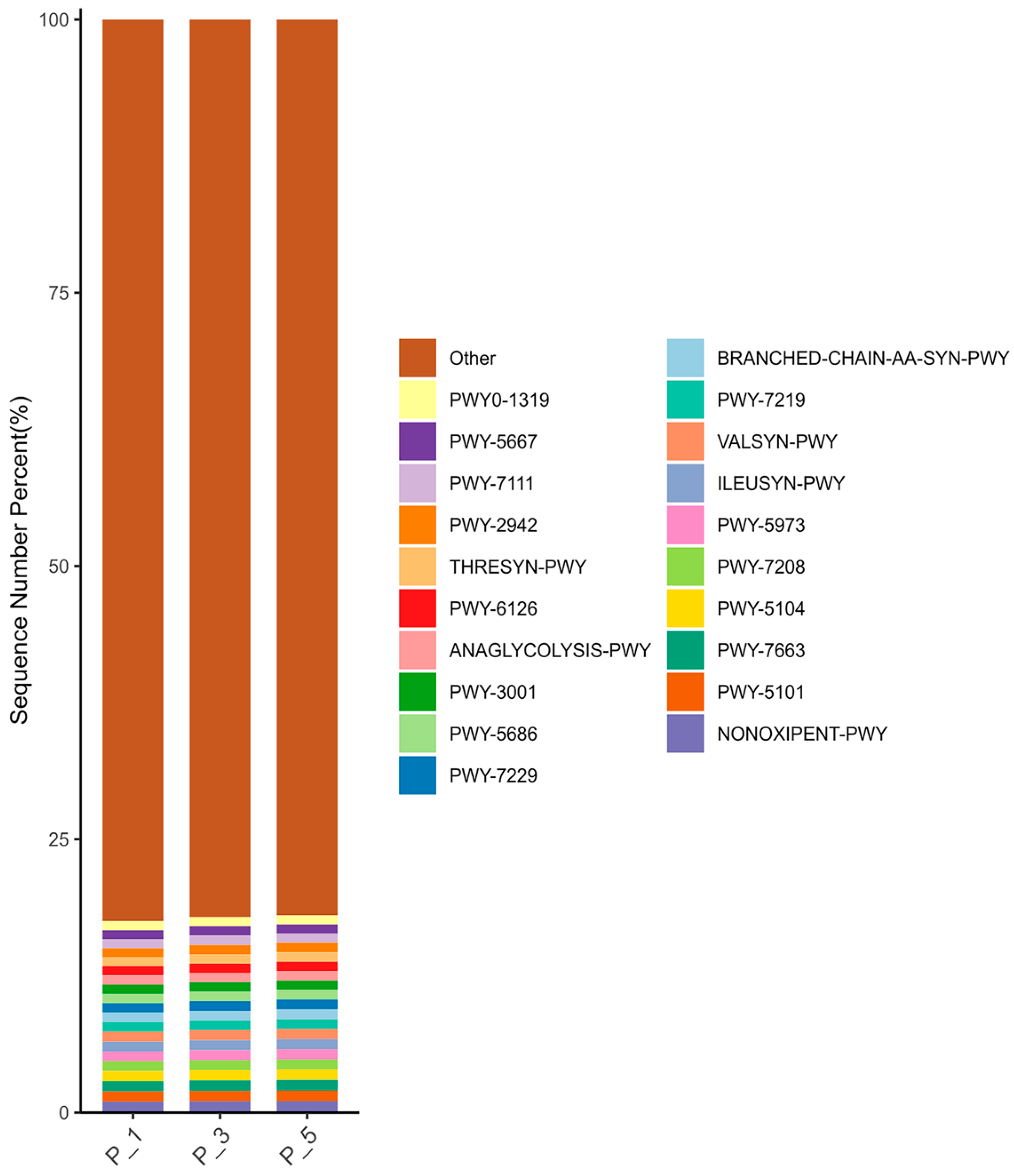
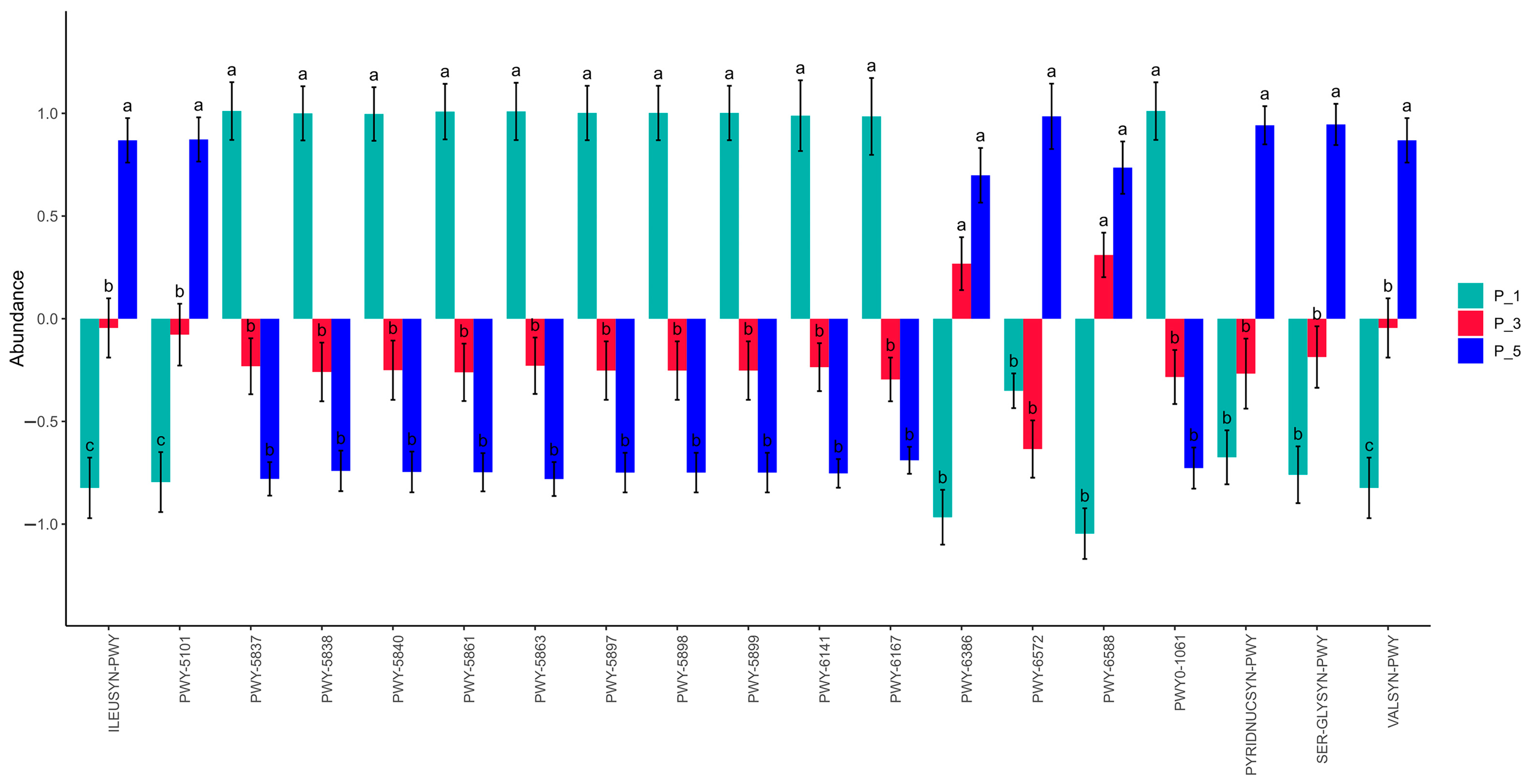
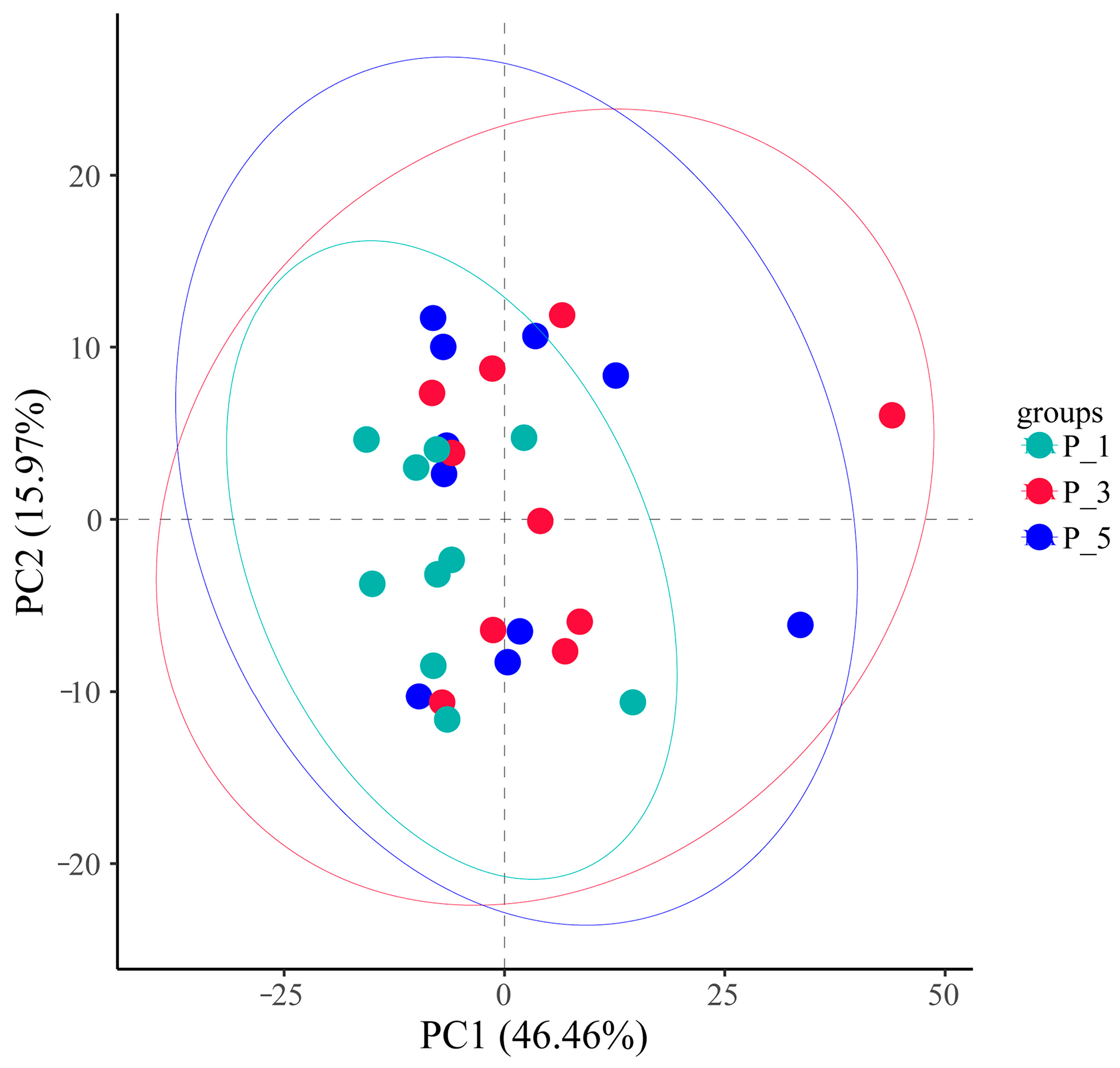
3.3. Dynamics of MetaCyc Pathways in Lactating Camel Feces
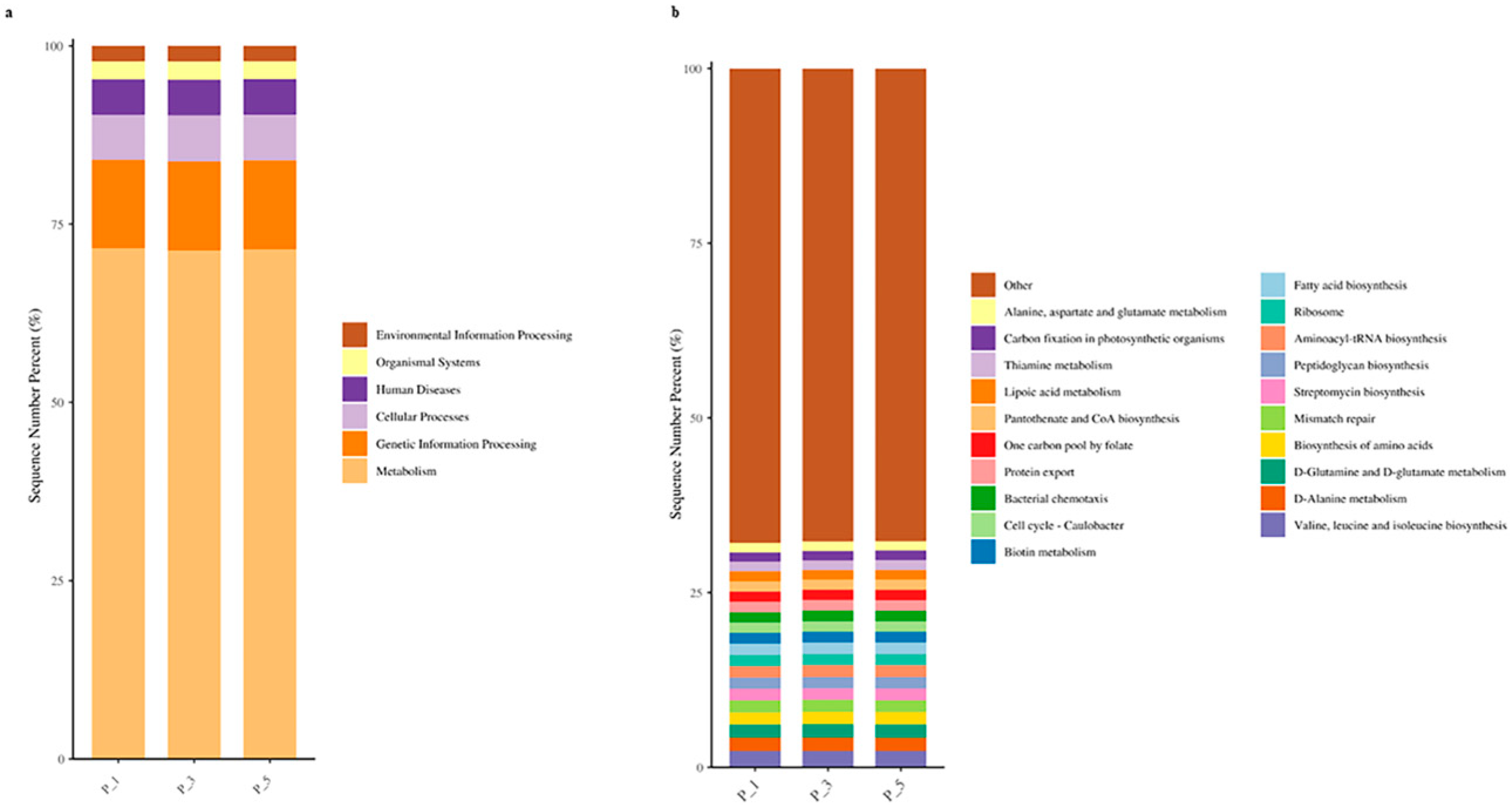
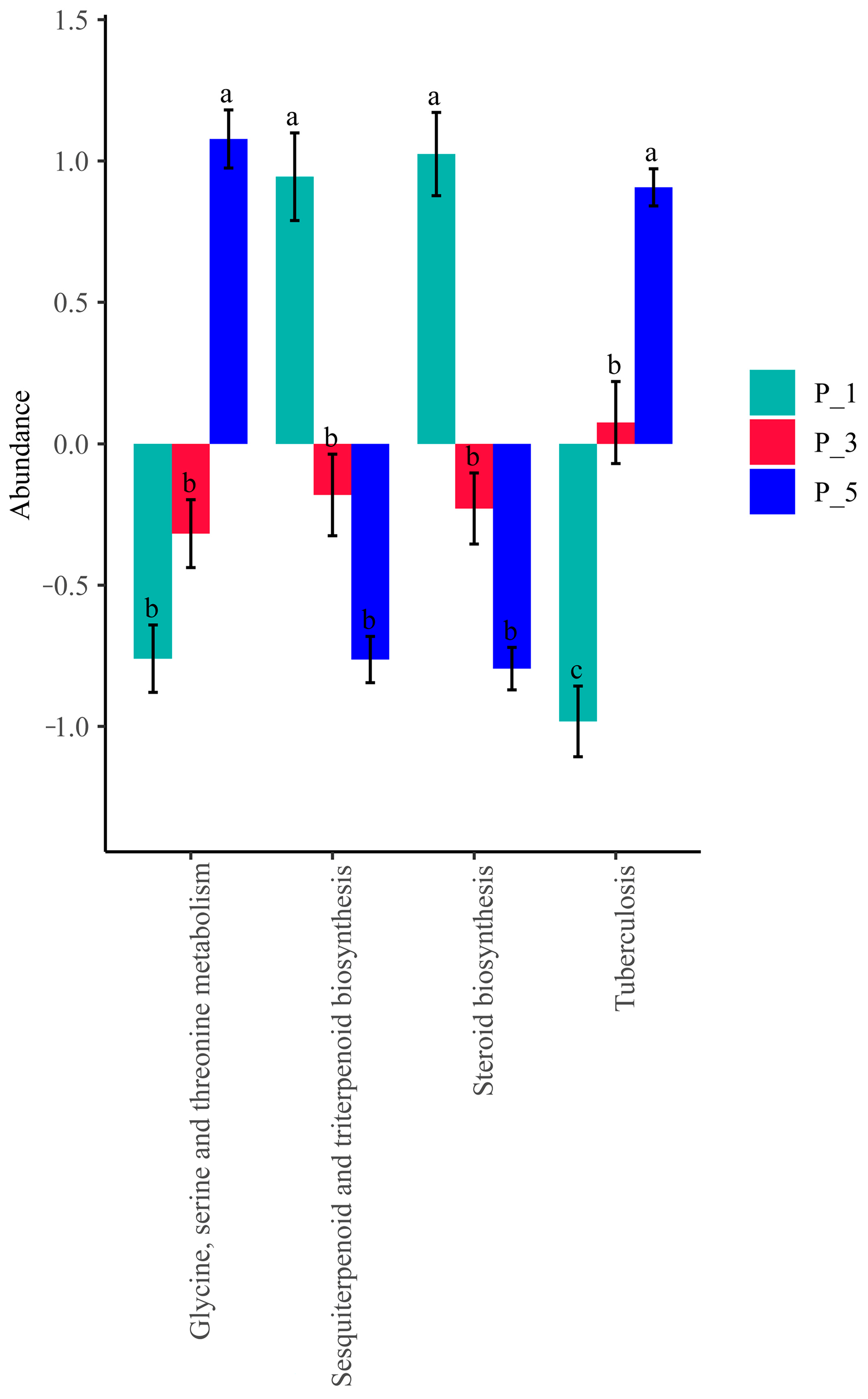
3.4. Dynamics of the KEGG Pathways in the Lactating Camel Feces
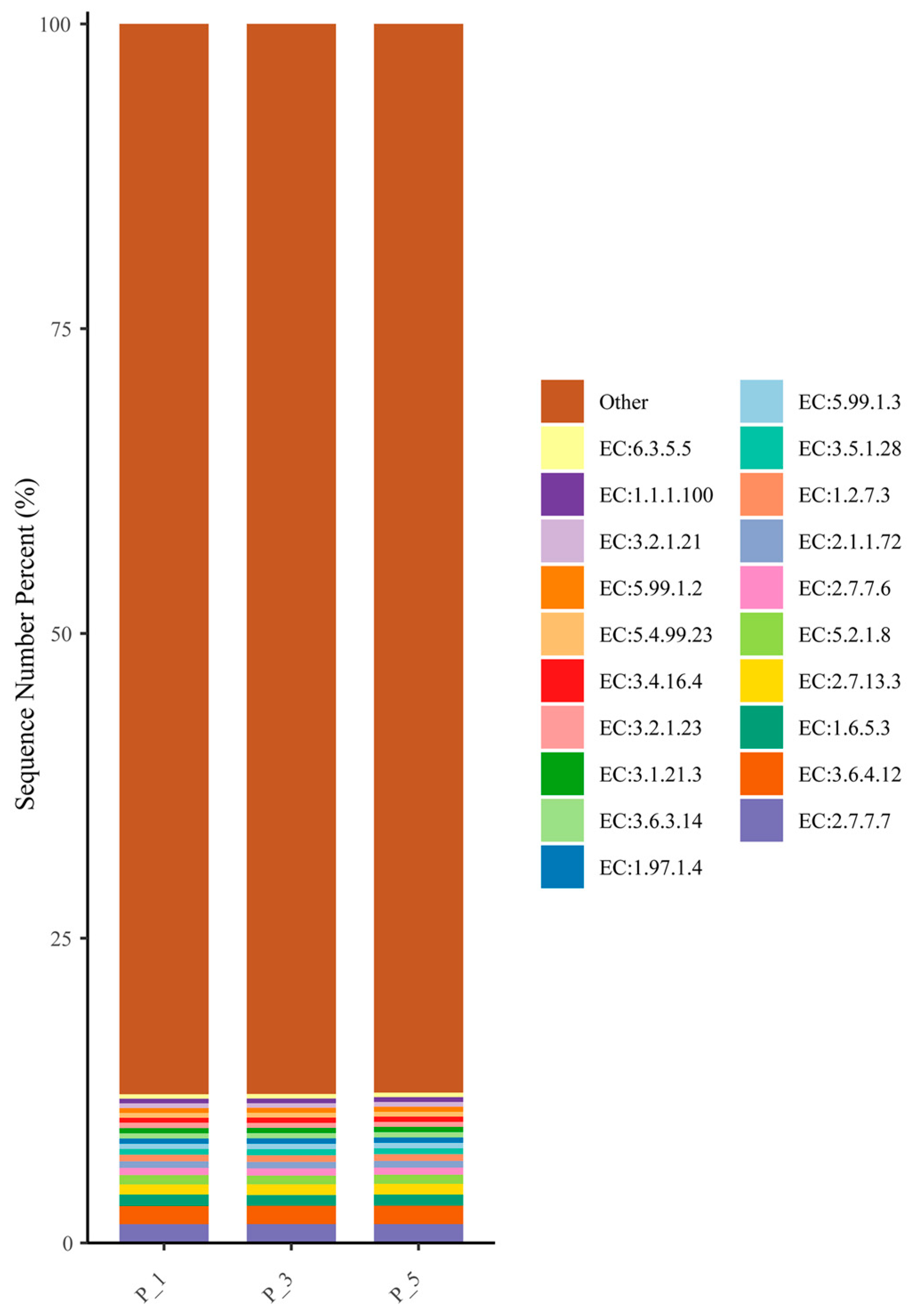
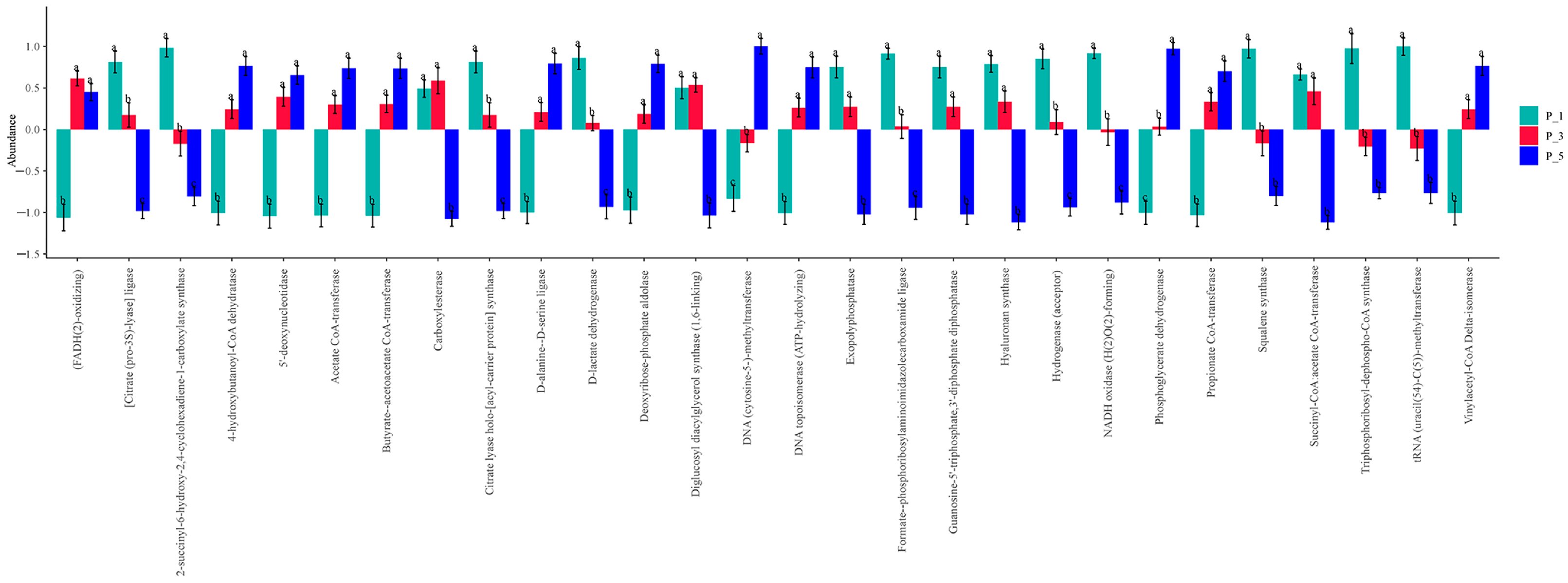
3.5. Dynamics of ECs in Lactating Camel Feces
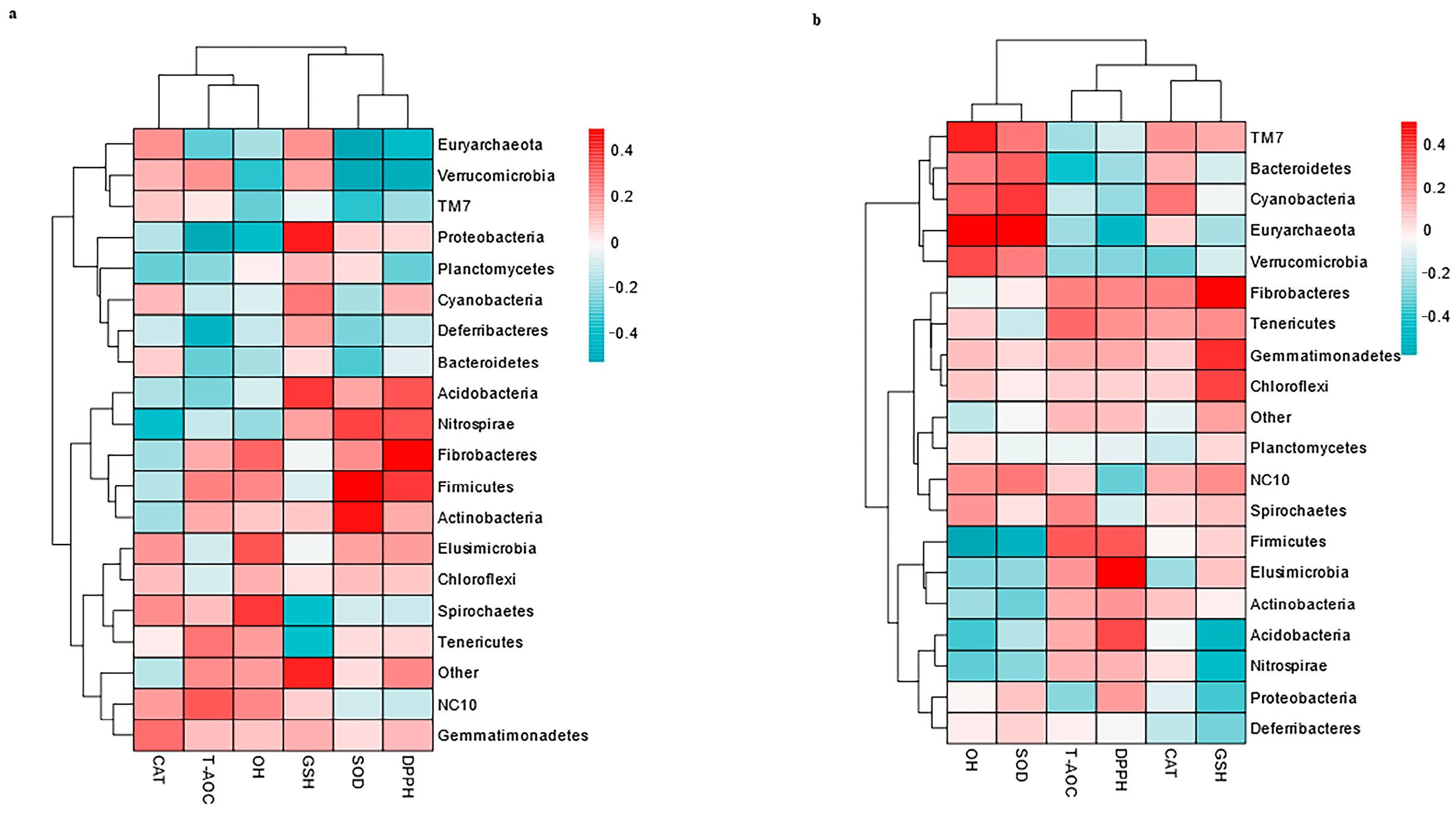
3.6. Correlation Between the Top 20 Genera and Antioxidants in Milk and Serum
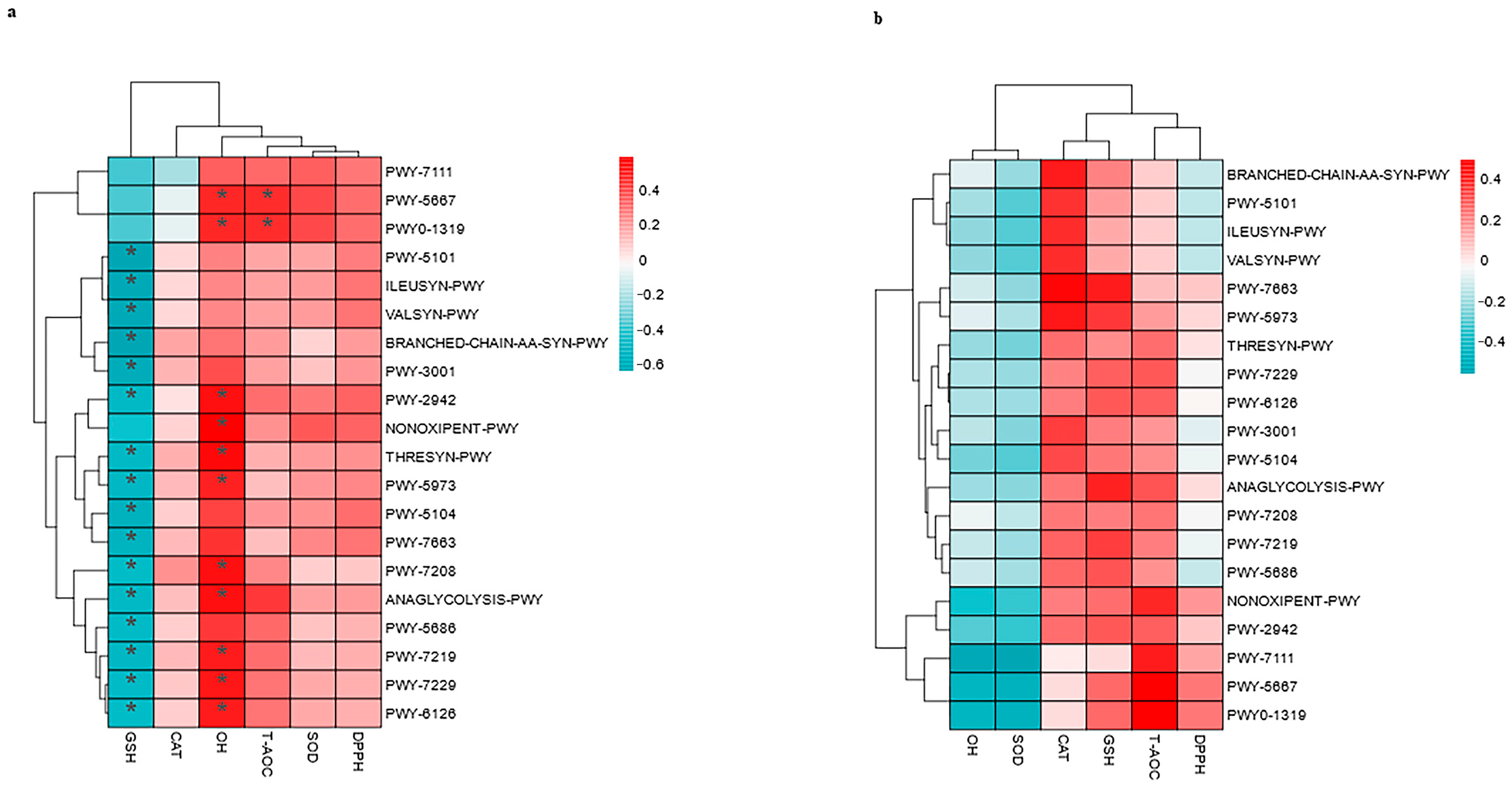
3.7. Correlations Between the Top 20 MetaCyc and Antioxidants in Milk and in Serum
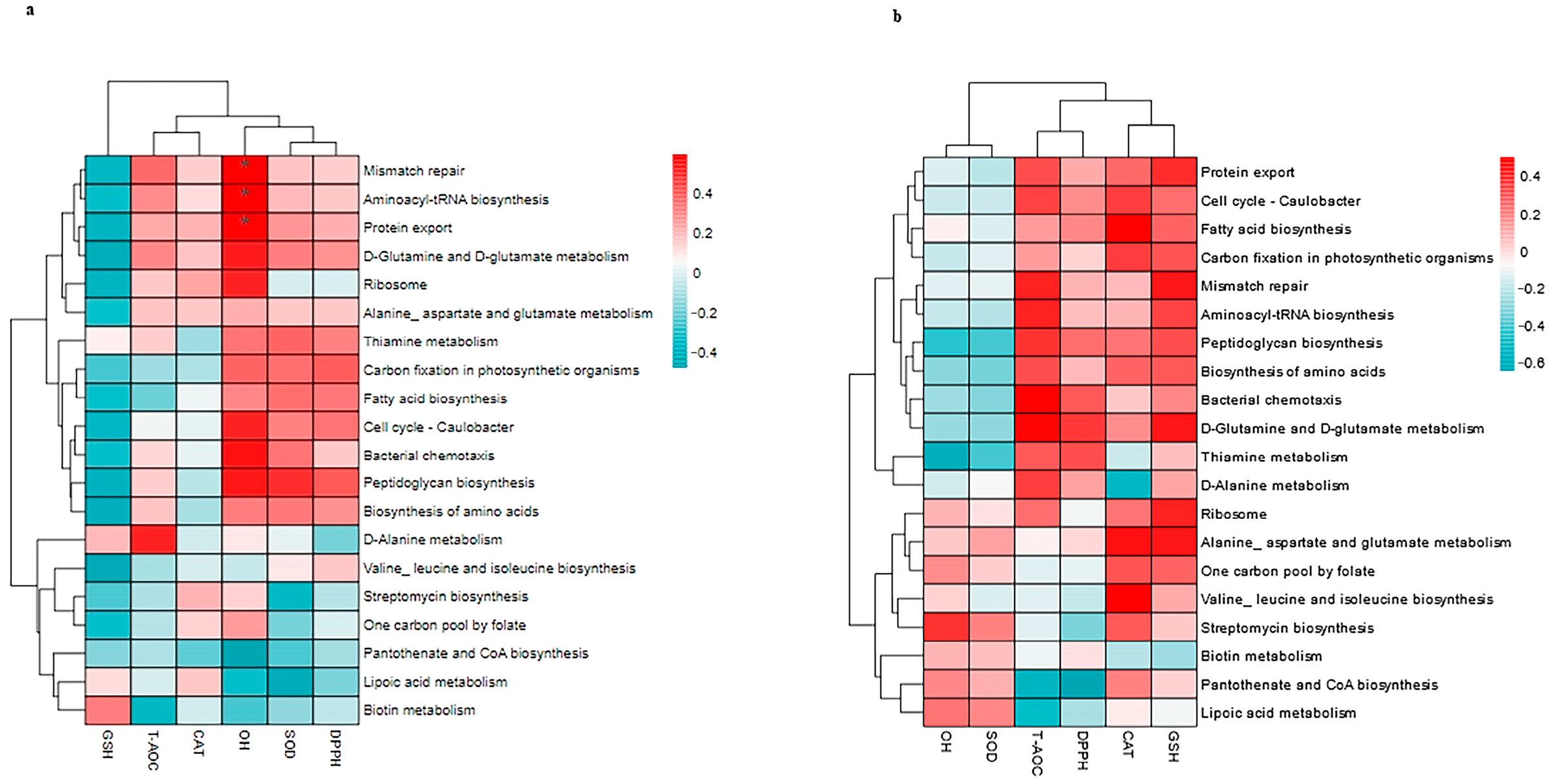
3.8. Correlation Between Top 20 KEGG Pathways and the Antioxidants in Milk and in Serum
3.9. Correlation Between Top 20 ECs and the Antioxidants in Milk and in Serum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T-AOC | Total antioxidant capacity |
| CAT | Catalase |
| OH | Antioxidant activity for hydroxyl radical |
| SOD | Superoxide dismutase |
| GSH | Glutathione peroxidase |
| DPPH | Antioxidant activity for polypeptides |
References
- Volpato, G.; Di Nardo, A. The role of Nucularia perrinii Batt. (Chenopodiaceae) in the camel-based Sahrawi social-ecological system. J. Ethnobiol. Ethnomed. 2017, 13, 12. [Google Scholar] [CrossRef]
- Ali, A.; Baby, B.; Vijayan, R. From desert to medicine: A review of camel genomics and therapeutic products. Front. Genet. 2019, 10, 17. [Google Scholar] [CrossRef]
- Lyu, H.; Na, Q.; Wang, L.; Li, Y.; Zheng, Z.; Wu, Y.; Li, Y.; Hang, G.; Zhu, X.; Ji, R. Effects of muscle type and aging on glycolysis and physicochemical quality properties of bactrian camel (Camelus bactrianus) meat. Animals 2024, 14, 611. [Google Scholar] [CrossRef]
- Khalphallah, A.; Almuhanna, A.H.; Al-Daek, T.; Alhaider, A.; Elmeligy, E.; Mohamed, R.H.; Abdulkarim, A.; Khalifa, M.I.; Bazeed, S.M.; Khesruf, K.A. The clinical importance and correlations of post-partum changes in the clinical findings, reproductive cyclicity, serum-milk oxidant/antioxidant parameters as a stress indicator in female dromedary camel (Camelus dromedarius) and their effect on milk palatability. Vet. Res. Commun. 2024, 48, 1595–1610. [Google Scholar]
- Khalphallah, A.; Elmeligy, E.; Zakaria, A.M.; Ghallab, R.S.; Abdulkarim, A.; Mohamed, R.H. Comparative study of efficacy of prepartum injection of multivitamins and selenium-vitamin E (ά-tocopherol)-combination on post-partum clinical findings, serum steroids, calf and placental weights, and milk antioxidant biomarkers changes in female dromed. Open Vet. J. 2022, 12, 657–667. [Google Scholar] [CrossRef]
- Sies, H. Oxidative eustress: On constant alert for redox homeostasis. Redox Biol. 2021, 41, 101867. [Google Scholar] [CrossRef]
- Nagel, C.; Aurich, C.; Aurich, J. Stress effects on the regulation of parturition in different domestic animal species. Anim. Reprod. Sci. 2019, 207, 153–161. [Google Scholar] [CrossRef]
- Tufarelli, V.; Colonna, M.A.; Losacco, C.; Puvača, N. Biological health markers associated with oxidative stress in dairy cows during lactation period. Metabolites 2023, 13, 405. [Google Scholar] [CrossRef]
- Zheng, S.; Qin, G.; Zhen, Y.; Zhang, X.; Chen, X.; Dong, J.; Li, C.; Aschalew, N.D.; Wang, T.; Sun, Z. Correlation of oxidative stress-related indicators with milk composition and metabolites in early lactating dairy cows. Vet. Med. Sci. 2021, 7, 2250–2259. [Google Scholar] [CrossRef]
- Gessner, D.K.; Sandrock, L.M.; Most, E.; Koch, C.; Ringseis, R.; Eder, K. Performance and metabolic, inflammatory, and oxidative stress-related parameters in early lactating dairy cows with high and low hepatic FGF21 expression. Animals 2022, 13, 131. [Google Scholar] [CrossRef]
- Urh, C.; Denißen, J.; Gerster, E.; Kraus, N.; Stamer, E.; Heitkönig, B.; Spiekers, H.; Sauerwein, H. Pro-and antioxidative indicators in serum of dairy cows during late pregnancy and early lactation: Testing the effects of parity, different dietary energy levels, and farm. J. Dairy Sci. 2019, 102, 6672–6678. [Google Scholar] [CrossRef] [PubMed]
- El-Malky, O.M.; Mostafa, T.H.; Abd El-Salaam, A.M.; Ayyat, M.S. Effect of reproductive disorders on productivity and reproductive efficiency of dromedary she-camels in relation to cytokine concentration. Trop. Anim. Health Prod. 2018, 50, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.A.; Rateb, M.H.; Mostafa, H.I.; Abou-Khalil, N.S.; Hassan, M.S. Circulating oxidative stress status in Bedouin she-camels (Camelus dromedarius) during the peripartum period. Trop. Anim. Health Prod. 2021, 53, 446. [Google Scholar] [CrossRef]
- Mohamed, R.H.; Khalphallah, A.; Nakada, K.; Elmeligy, E.; Hassan, D.; Ebissy, E.A.; Ghandour, R.A.; Mousa, S.A.; Hassaneen, A.S.A. Clinical and correlated responses among steroid hormones and oxidant/antioxidant biomarkers in pregnant, non-pregnant and lactating CIDR-pre-synchronized dromedaries (Camelus dromedarius). Vet. Sci. 2021, 8, 247. [Google Scholar] [CrossRef]
- Iliodromiti, Z.; Triantafyllou, A.-R.; Tsaousi, M.; Pouliakis, A.; Petropoulou, C.; Sokou, R.; Volaki, P.; Boutsikou, T.; Iacovidou, N. Gut microbiome and neurodevelopmental disorders: A link yet to be disclosed. Microorganisms 2023, 11, 487. [Google Scholar] [CrossRef]
- Siddiqui, R.; Khan, N.A. Microbiome and one health: Potential of novel metabolites from the gut microbiome of unique species for human health. Microorganisms 2023, 11, 481. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Smith, R.P.; Easson, C.; Lyle, S.M.; Kapoor, R.; Donnelly, C.P.; Davidson, E.J.; Parikh, E.; Lopez, J.V.; Tartar, J.L. Gut microbiome diversity is associated with sleep physiology in humans. PLoS ONE 2019, 14, e0222394. [Google Scholar] [CrossRef]
- Gharechahi, J.; Zahiri, H.S.; Noghabi, K.A.; Salekdeh, G.H. In-depth diversity analysis of the bacterial community resident in the camel rumen. Syst. Appl. Microbiol. 2015, 38, 67–76. [Google Scholar] [CrossRef]
- Gharechahi, J.; Sarikhan, S.; Han, J.L.; Ding, X.Z.; Salekdeh, G.H. Functional and phylogenetic analyses of camel rumen microbiota associated with different lignocellulosic substrates. NPJ Biofilms Microbiomes 2022, 8, 46. [Google Scholar] [CrossRef]
- Davis, D.L.; Wu, C.; Brown, W.J.; Nohr, E.A. Parity and mode of birth and their relationships with quality of life: A longitudinal study. PLoS ONE 2022, 17, e0273366. [Google Scholar] [CrossRef] [PubMed]
- Kervinen, K.; Holster, T.; Saqib, S.; Virtanen, S.; Stefanovic, V.; Rahkonen, L.; Nieminen, P.; Salonen, A.; Kalliala, I. Parity and gestational age are associated with vaginal microbiota composition in term and late term pregnancies. EBioMedicine 2022, 81, 104107. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yu, D.; Chen, T.; Liu, J.; Tong, X.; Yang, L.; Da, M.; Shen, S.; Fan, C.; Wang, S. Maternal parity and the risk of congenital heart defects in offspring: A dose-response meta-analysis of epidemiological observational studies. PLoS ONE 2014, 9, e108944. [Google Scholar] [CrossRef] [PubMed]
- Bogado Pascottini, O.; Spricigo, J.F.W.; Van Schyndel, S.J.; Mion, B.; Rousseau, J.; Weese, J.S.; LeBlanc, S.J. Effects of parity, blood progesterone, and non-steroidal anti-inflammatory treatment on the dynamics of the uterine microbiota of healthy postpartum dairy cows. PLoS ONE 2021, 16, e0233943. [Google Scholar] [CrossRef]
- Lean, I.J.; Golder, H.M.; LeBlanc, S.J.; Duffield, T.; Santos, J.E.P. Increased parity is negatively associated with survival and reproduction in different production systems. J. Dairy Sci. 2023, 106, 476–499. [Google Scholar] [CrossRef]
- Elbashir, M.H.M.; Elhassan, S.; Abueissa, H.I. Parity effect on camel milk composition under traditional and intensive management systems in Butana area, Sudan. Int. J. Sci. Res. 2018, 6, 1778–1782. [Google Scholar]
- Chamekh, L.; Calvo, M.; Khorchani, T.; Hammadi, M.; Fontecha, J.; Habib Yahyaoui, M. Impact of parity on the profile of fatty acids with potential importance for human health in camel (Camelus dromedarius) milk. Mljekarstvo 2023, 73, 263–270. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Zarrin, M.; Riveros, J.L.; Ahmadpour, A.; de Almeida, A.M.; Konuspayeva, G.; Vargas-Bello-Pérez, E.; Faye, B.; Hernández-Castellano, L.E. Camelids: New players in the international animal production context. Trop. Anim. Health Prod. 2020, 52, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Hamlili, F.Z.; Laroche, M.; Diarra, A.Z.; Lafri, I.; Gassen, B.; Boutefna, B.; Davoust, B.; Bitam, I.; Parola, P. MALDI-TOF MS identification of dromedary camel ticks and detection of associated microorganisms, Southern Algeria. Microorganisms 2022, 10, 2178. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.; Joosten, H.; Faye, B.; Burger, P.A. The flourishing camel milk market and concerns about animal welfare and legislation. Animals 2022, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Damiran, D. Palatability of Mongolian rangeland plants. In Circular of Information No. 3; Eastern Oregon Agricultural Research Center, Oregon State University: Union, OR, USA, 2005. [Google Scholar]
- Du, H.; Tong, J.; Olonuud, M. Comparison between the sustainably- and unsustainably-managed pastures: The case of urad-middle banner in Inner Mongolia, China. Past. Pastor. 2024, 2, 82–100. [Google Scholar] [CrossRef]
- Benmeziane–Derradji, F. Evaluation of camel milk: Gross composition—A scientific overview. Trop. Anim. Health Prod. 2021, 53, 308. [Google Scholar] [CrossRef]
- Arain, M.A.; Khaskheli, G.B.; Shah, A.H.; Marghazani, I.B.; Barham, G.S.; Shah, Q.A.; Khand, F.M.; Buzdar, J.A.; Soomro, F.; Fazlani, S.A. Nutritional significance and promising therapeutic/medicinal application of camel milk as a functional food in human and animals: A comprehensive review. Anim. Biotechnol. 2023, 34, 1988–2005. [Google Scholar] [CrossRef]
- Khan, M.Z.; Xiao, J.; Ma, Y.; Ma, J.; Liu, S.; Khan, A.; Khan, J.M.; Cao, Z. Research development on anti-microbial and antioxidant properties of camel milk and its role as an anti-cancer and anti-hepatitis agent. Antioxidants 2021, 10, 788. [Google Scholar] [CrossRef]
- Behrouz, S.; Saadat, S.; Memarzia, A.; Sarir, H.; Folkerts, G.; Boskabady, M.H. The antioxidant, anti-inflammatory and immunomodulatory effects of camel milk. Front. Immunol. 2022, 13, 855342. [Google Scholar] [CrossRef]
- Zhang, H.; Lai, T.; Du, H.; Li, J.; Wu, S.; Xu, J. Basic components, oxidative stress and inflammatory factors of camel milk obtained from different parity of Gobi red camel. Feed Ind. 2025, 46, 82–86. [Google Scholar]
- Atigui, M.; Brahmi, M.; Marnet, P.G.; Ben Salem, W.; Campagna, M.C.; Borghese, A.; Todde, G.; Caria, M.; Hammadi, M.; Boselli, C. Study of the milkability of the mediterranean italian buffalo and the tunisian maghrebi camel according to parity and lactation stage. Animals 2024, 14, 1055. [Google Scholar] [CrossRef]
- Babiker, W.I.A.; El-Zubeir, I.E.M. Impact of husbandry, stages of lactation and parity number on milk yield and chemical composition of dromedary camel milk. Emir. J. Food Agric. 2014, 26, 333–341. [Google Scholar] [CrossRef]
- Mumford, S.L.; Browne, R.W.; Schliep, K.C.; Schmelzer, J.; Plowden, T.C.; Michels, K.A.; Sjaarda, L.A.; Zarek, S.M.; Perkins, N.J.; Messer, L.C. Serum antioxidants are associated with serum reproductive hormones and ovulation among healthy women. J. Nutr. 2016, 146, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Stocco, G.; Cipolat-Gotet, C.; Biffani, S.; Ablondi, M.; Negro, A.; Summer, A.; Kyriakaki, P.; Mavrommatis, A.; Tsiplakou, E. Stage of lactation, parity, breed, milk composition, and minerals affect the nonenzymatic antioxidant activity of sheep milk. J. Dairy Sci. 2024, 107, 10361–10372. [Google Scholar] [CrossRef] [PubMed]
- Amballi, A.A.; Oyedeji, S.O.; Ogunyemi, E.O.; Adeyemi, A.B.; Anetor, J.I.; Adenaike, F.A. Effects of parity on antioxidant status in pregnant women in a Nigerian population. Ann. Trop. Pathol. 2014, 5, 83–91. [Google Scholar]
- Yehia, S.G.; Ramadan, E.S.; Megahed, E.A.; Salem, N.Y. Effect of parity on metabolic and oxidative stress profiles in Holstein dairy cows. Vet. World 2020, 13, 2780–2786. [Google Scholar] [CrossRef]
- Corset, A.; Remot, A.; Graulet, B.; Poton, P.; Philau, S.; Ricouleau, J.F.; Dhumez, O.; Germon, P.; Boudon, A.; Boutinaud, M. Effects of parity and week after calving on the metabolic, redox, and immune status of dairy cows. J. Dairy Sci. 2024, 107, 8592–8608. [Google Scholar] [CrossRef]
- Radin, L.; Šimpraga, M.; Vince, S.; Kostelić, A.; Milinković-Tur, S. Metabolic and oxidative status of Saanen goats of different parity during the peripartum period. J. Dairy Res. 2015, 82, 426–433. [Google Scholar] [CrossRef]
- Wu, X.; Sun, H.; Xue, M.; Wang, D.; Guan, L.; Liu, J. Days-in-milk and parity affected serum biochemical parameters and hormone profiles in mid-lactation holstein cows. Animals 2019, 9, 230. [Google Scholar] [CrossRef]
- He, J.; Yi, L.; Hai, L.; Ming, L.; Gao, W.; Ji, R. Characterizing the bacterial microbiota in different gastrointestinal tract segments of the Bactrian camel. Sci. Rep. 2018, 8, 654. [Google Scholar] [CrossRef]
- Karnachuk, O.V.; Panova, I.A.; Panov, V.L.; Ikkert, O.P.; Kadnikov, V.V.; Rusanov, I.I.; Avakyan, M.R.; Glukhova, L.B.; Lukina, A.P.; Rakitin, A.V. Active sulfate-reducing bacterial community in the camel gut. Microorganisms 2023, 11, 401. [Google Scholar] [CrossRef]
- He, J.; Hai, L.; Orgoldol, K.; Yi, L.; Ming, L.; Guo, F.; Li, G.; Ji, R. High-throughput sequencing reveals the gut microbiome of the Bactrian camel in different ages. Curr. Microbiol. 2019, 76, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.; Yi, L.; Siriguleng; Hasi, S.; He, J.; Hai, L.; Wang, Z.; Guo, F.; Qiao, X.; Jirimutu. Comparative analysis of fecal microbial communities in cattle and Bactrian camels. PLoS ONE 2017, 12, e0173062. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Liu, H.; Hu, L.; Zhao, N.; Xu, S.; Lin, Z.; Chen, Y. Bacterial community characteristics in the gastrointestinal tract of yak (Bos grunniens) fully grazed on pasture of the Qinghai-Tibetan Plateau of China. Animals 2021, 11, 2243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, L.; He, Y.; Luo, X.; Zhao, S.; Jia, X. Composition of fecal microbiota in grazing and feedlot angus beef cattle. Animals 2021, 11, 3167. [Google Scholar] [CrossRef]
- Weese, J.S.; Jelinski, M. Assessment of the fecal microbiota in beef calves. J. Vet. Intern. Med. 2017, 31, 176–185. [Google Scholar] [CrossRef]
- Ameri, R.; Laville, E.; Potocki-Véronèse, G.; Trabelsi, S.; Mezghani, M.; Elgharbi, F.; Bejar, S. Two new gene clusters involved in the degradation of plant cell wall from the fecal microbiota of Tunisian dromedary. PLoS ONE 2018, 13, e0194621. [Google Scholar] [CrossRef]
- Hinsu, A.T.; Tulsani, N.J.; Panchal, K.J.; Pandit, R.J.; Jyotsana, B.; Dafale, N.A.; Patil, N.V.; Purohit, H.J.; Joshi, C.G.; Jakhesara, S.J. Characterizing rumen microbiota and CAZyme profile of Indian dromedary camel (Camelus dromedarius) in response to different roughages. Sci. Rep. 2021, 11, 9400. [Google Scholar] [CrossRef]
- Minozzi, G.; Biscarini, F.; Dalla Costa, E.; Chincarini, M.; Ferri, N.; Palestrini, C.; Minero, M.; Mazzola, S.; Piccinini, R.; Vignola, G. Analysis of hindgut microbiome of sheep and effect of different husbandry conditions. Animals 2020, 11, 4. [Google Scholar] [CrossRef]
- Tanca, A.; Fraumene, C.; Manghina, V.; Palomba, A.; Abbondio, M.; Deligios, M.; Pagnozzi, D.; Addis, M.F.; Uzzau, S. Diversity and functions of the sheep faecal microbiota: A multi-omic characterization. Microb. Biotechnol. 2017, 10, 541–554. [Google Scholar] [CrossRef]
- Williamson, J.R.; Callaway, T.R.; Lourenco, J.M.; Ryman, V.E. Characterization of rumen, fecal, and milk microbiota in lactating dairy cows. Front. Microbiol. 2022, 13, 984119. [Google Scholar] [CrossRef]
- Jewell, K.A.; McCormick, C.A.; Odt, C.L.; Weimer, P.J.; Suen, G. Ruminal bacterial community composition in dairy cows is dynamic over the course of two lactations and correlates with feed efficiency. Appl. Environ. Microbiol. 2015, 81, 4697–4710. [Google Scholar] [CrossRef] [PubMed]
- Pitta, D.W.; Indugu, N.; Kumar, S.; Vecchiarelli, B.; Sinha, R.; Baker, L.D.; Bhukya, B.; Ferguson, J.D. Metagenomic assessment of the functional potential of the rumen microbiome in Holstein dairy cows. Anaerobe 2016, 38, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, Y.; Gaucher, E.A. Evolution of bacterial steroid biosynthesis and its impact on eukaryogenesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2101276118. [Google Scholar] [CrossRef]
- Zhai, Q.; Feng, S.; Arjan, N.; Chen, W. A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 2019, 59, 3227–3236. [Google Scholar] [CrossRef]
- Macchione, I.G.; Lopetuso, L.R.; Ianiro, G.; Napoli, M.; Gibiino, G.; Rizzatti, G.; Petito, V.; Gasbarrini, A.; Scaldaferri, F. Akkermansia muciniphila: Key player in metabolic and gastrointestinal disorders. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8075–8083. [Google Scholar]
- Rabee, A.E.; Sayed Alahl, A.A.; Lamara, M.; Ishaq, S.L. Fibrolytic rumen bacteria of camel and sheep and their applications in the bioconversion of barley straw to soluble sugars for biofuel production. PLoS ONE 2022, 17, e0262304. [Google Scholar] [CrossRef]
- Rabee, A.E.; Abd El Rahman, T.; Lamara, M. Changes in the bacterial community colonizing extracted and non-extracted tannin-rich plants in the rumen of dromedary camels. PLoS ONE 2023, 18, e0282889. [Google Scholar] [CrossRef]
- Koch, A.; Mizrahi, V. Mycobacterium tuberculosis. Trends Microbiol. 2018, 26, 555–556. [Google Scholar] [CrossRef]
- Infantes-Lorenzo, J.A.; Romero, B.; Rodríguez-Bertos, A.; Roy, A.; Ortega, J.; de Juan, L.; Moreno, I.; Domínguez, M.; Domínguez, L.; Bezos, J. Tuberculosis caused by Mycobacterium caprae in a camel (Camelus dromedarius). BMC Vet. Res. 2020, 16, 435. [Google Scholar] [CrossRef]
- Sawaswong, V.; Chanchaem, P.; Kemthong, T.; Warit, S.; Chaiprasert, A.; Malaivijitnond, S.; Payungporn, S. Alteration of gut microbiota in wild-borne long-tailed macaques after 1-year being housed in hygienic captivity. Sci. Rep. 2023, 13, 5842. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, F.; Liu, X.; Lu, Y.; Yao, K.; Tian, N.; Tong, L.; Figge, D.A.; Wang, X.; Han, Y. Non-oxidative pentose phosphate pathway controls regulatory T cell function by integrating metabolism and epigenetics. Nat. Metab. 2022, 4, 559–574. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Pathway Summary for Pathway ANAGLYCOLYSIS-PWY, Glycolysis III (from Glucose). BioCyc. Available online: https://pubchem.ncbi.nlm.nih.gov/pathway/BioCyc:EVA_ANAGLYCOLYSIS-PWY (accessed on 26 January 2025).
- Parmar, N.R.; Kumar, J.I.N.; Joshi, C.G. Deep insights into carbohydrate metabolism in the rumen of Mehsani buffalo at different diet treatments. Genomics Data 2015, 6, 59–62. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, H.; Xue, M.; Liu, J. Metagenomics reveals differences in microbial composition and metabolic functions in the rumen of dairy cows with different residual feed intake. Anim. Microbiome 2022, 4, 19. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Pathway Summary for Pathway PWY-5667, CDP-Diacylglycerol Biosynthesis I. BioCyc. Available online: https://pubchem.ncbi.nlm.nih.gov/pathway/BioCyc:META_PWY-5667 (accessed on 26 January 2025).
- Horvath, S.E.; Daum, G. Lipids of mitochondria. Prog. Lipid Res. 2013, 52, 590–614. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Pathway Summary for Pathway PWY-7208, Superpathway of Pyrimidine Nucleobases Salvage. BioCyc. Available online: https://pubchem.ncbi.nlm.nih.gov/pathway/BioCyc:META_PWY-7208 (accessed on 13 February 2025).
- National Center for Biotechnology Information. PubChem Pathway Summary for Pathway PWY-7219, Adenosine Ribonucleotides de Novo Biosynthesis. BioCyc. Available online: https://pubchem.ncbi.nlm.nih.gov/pathway/BioCyc:HUMAN_PWY-7219 (accessed on 13 February 2025).
- National Center for Biotechnology Information. PubChem Pathway Summary for Pathway PWY-7229, Superpathway of Adenosine Nucleotides de Novo Biosynthesis I. BioCyc. Available online: https://pubchem.ncbi.nlm.nih.gov/pathway/BioCyc:META_PWY-7229 (accessed on 13 February 2025).
- National Center for Biotechnology Information. PubChem Pathway Summary for Pathway PWY-6126, Adenosine Nucleotides de Novo Biosynthesis. BioCyc. Available online: https://pubchem.ncbi.nlm.nih.gov/pathway/BioCyc:YEAST_PWY-6126 (accessed on 13 February 2025).
- Iglesias Pastrana, C.; Navas González, F.J.; Ciani, E.; Barba Capote, C.J.; Delgado Bermejo, J.V. Effect of research impact on emerging camel husbandry, welfare and social-related awareness. Animals 2020, 10, 780. [Google Scholar] [CrossRef]
- Padalino, B.; Menchetti, L. The first protocol for assessing welfare of camels. Front. Vet. Sci. 2021, 7, 631876. [Google Scholar] [CrossRef]
- Menchetti, L.; Zappaterra, M.; Nanni Costa, L.; Padalino, B. Application of a protocol to assess camel welfare: Scoring system of collected measures, aggregated assessment indices, and criteria to classify a pen. Animals 2021, 11, 494. [Google Scholar] [CrossRef]
- Masebo, N.T.; Zappaterra, M.; Felici, M.; Benedetti, B.; Padalino, B. Dromedary camel’s welfare: Literature from 1980 to 2023 with a text mining and topic analysis approach. Front. Vet. Sci. 2023, 10, 1277512. [Google Scholar] [CrossRef]


| Ingredients | Percentages |
|---|---|
| Corn straw | 34.56 |
| Oat grass | 12.76 |
| Alfalfa grass | 12.32 |
| Whole-plant corn silage | 24.9 |
| Concentrate | 14.38 |
| NaCl | 0.7 |
| Sodium bicarbonate | 0.38 |
| CP | 9.63 |
| ADF | 38 |
| NDF | 54.41 |
| Non-fiber carbohydrate | 24.09 |
| Starch | 10.73 |
| EE | 2.27 |
| TDN | 56 |
| Ca | 0.45 |
| P | 0.31 |
| Parameters | P_1 | P_3 | P_5 | SEM | p Value |
|---|---|---|---|---|---|
| T-AOC (mg/mL) | 59.74 b | 86.09 a | 56.60 b | 4.1194 | 0.0051 |
| CAT (U/mL) | 2.87 a | 2.17 b | 2.09 b | 0.1363 | 0.0246 |
| OH (U/mL) | 10.27 b | 16.75 a | 16.08 a | 0.7475 | <0.0001 |
| SOD (U/mL) | 43.62 b | 68.17 a | 64.49 a | 3.6173 | 0.0084 |
| GSH (U/mL) | 90.05 | 76.88 | 85.24 | 5.9390 | 0.6726 |
| DPPH (μg Trolox/mL) | 192.28 b | 205.43 a | 203.25 a | 1.8610 | 0.0071 |
| Parameters | P_1 | P_3 | P_5 | SEM | p Value |
|---|---|---|---|---|---|
| T-AOC (mg/mL) | 4.82 b | 5.30 a | 4.93 b | 0.0535 | <0.0001 |
| CAT (U/mL) | 6.63 | 6.15 | 7.64 | 0.2600 | 0.0609 |
| OH (U/mL) | 9.62 a | 6.02 b | 6.50 b | 0.4950 | 0.0017 |
| SOD (U/mL) | 13.64 a | 7.97 c | 11.50 b | 0.5144 | <0.0001 |
| GSH (U/mL) | 26.05 | 24.49 | 24.58 | 0.6804 | 0.6000 |
| DPPH (μg Trolox/mL) | 116.59 b | 126.04 a | 121.05 b | 16.109 | 0.0019 |
| Variable | Group 1 | Group 2 | p Value |
|---|---|---|---|
| faith_pd * | P_5 | P_3 | 1.000 |
| P_5 | P_1 | 0.796 | |
| P_3 | P_1 | 0.853 | |
| Observed OTUs | P_5 | P_3 | 0.143 |
| P_5 | P_1 | 0.089 | |
| P_3 | P_1 | 0.247 | |
| shannon_entropy | P_5 | P_3 | 0.393 |
| P_5 a | P_1 b | 0.015 | |
| P_3 a | P_1 b | 0.015 | |
| Simpson | P_5 | P_3 | 0.481 |
| P_5 a | P_1 b | 0.023 | |
| P_3 | P_1 | 0.089 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Xu, J.; Zhang, H.; Li, J.; Wang, F.; Li, H.; Han, S.; Gala, J.; Wang, J. Parity-Associated Differences in the Antioxidants and Fecal Microbiota of Bactrian Camels. Vet. Sci. 2025, 12, 440. https://doi.org/10.3390/vetsci12050440
Du H, Xu J, Zhang H, Li J, Wang F, Li H, Han S, Gala J, Wang J. Parity-Associated Differences in the Antioxidants and Fecal Microbiota of Bactrian Camels. Veterinary Sciences. 2025; 12(5):440. https://doi.org/10.3390/vetsci12050440
Chicago/Turabian StyleDu, Hongxi, Jianxiong Xu, Hongcai Zhang, Jianjun Li, Fei Wang, Huan Li, Sarula Han, Jiri Gala, and Jilite Wang. 2025. "Parity-Associated Differences in the Antioxidants and Fecal Microbiota of Bactrian Camels" Veterinary Sciences 12, no. 5: 440. https://doi.org/10.3390/vetsci12050440
APA StyleDu, H., Xu, J., Zhang, H., Li, J., Wang, F., Li, H., Han, S., Gala, J., & Wang, J. (2025). Parity-Associated Differences in the Antioxidants and Fecal Microbiota of Bactrian Camels. Veterinary Sciences, 12(5), 440. https://doi.org/10.3390/vetsci12050440






