PABPC4 Inhibits SADS-CoV Replication by Degrading the Nucleocapsid Protein Through Selective Autophagy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies, Reagents, Plasmids, and siRNAs
2.2. Cell Culture and Viruses
2.3. RNA Isolation and Quantitative PCR Analysis
2.4. Coimmunoprecipitation Assay
2.5. Western Blotting Analysis
2.6. Confocal Immunofluorescence Assay
2.7. Cell Viability Assay
2.8. Statistical Analysis
3. Results
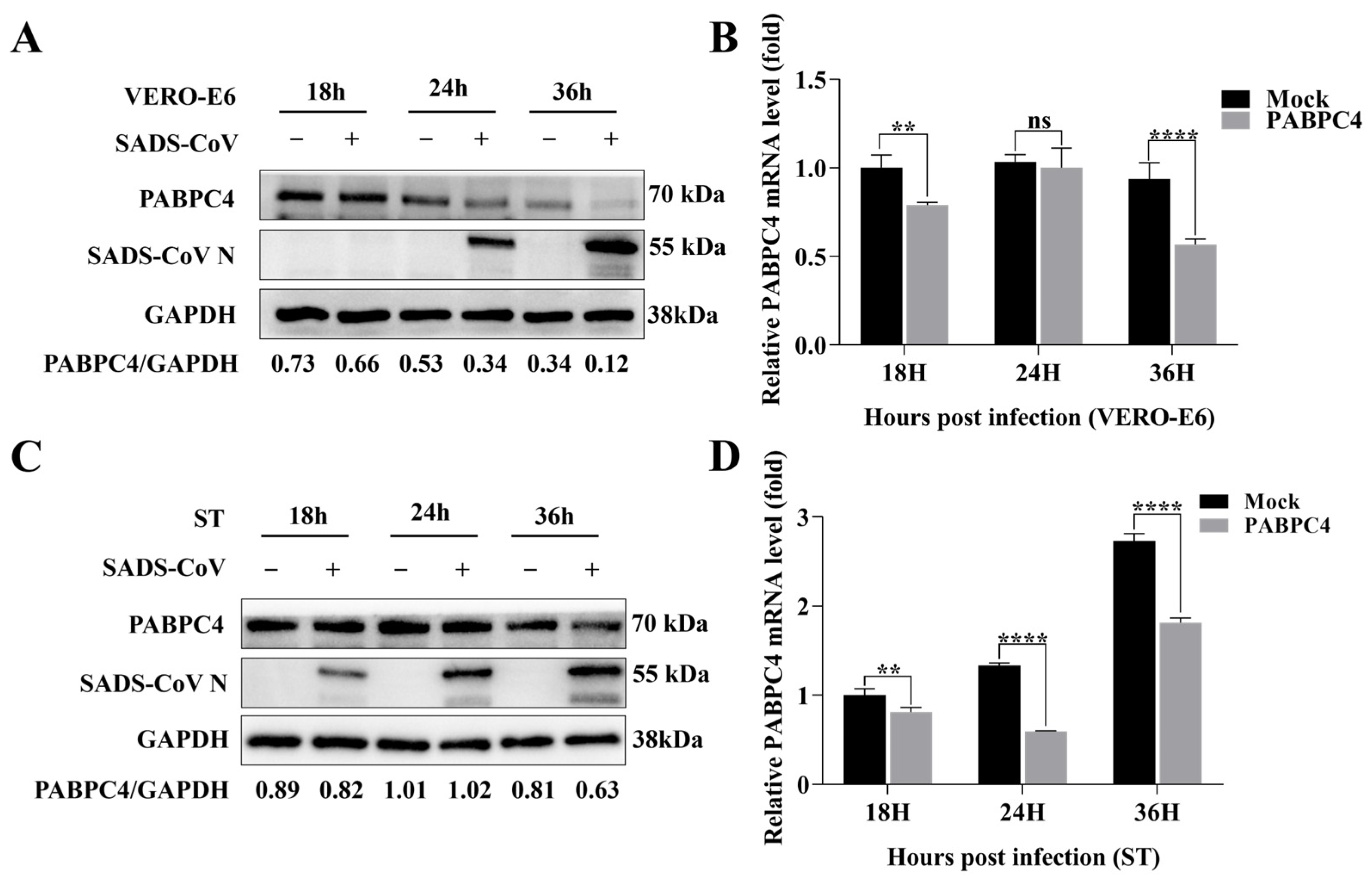
3.1. SADS-CoV Inhibits the Endogenous Expression of PABPC4
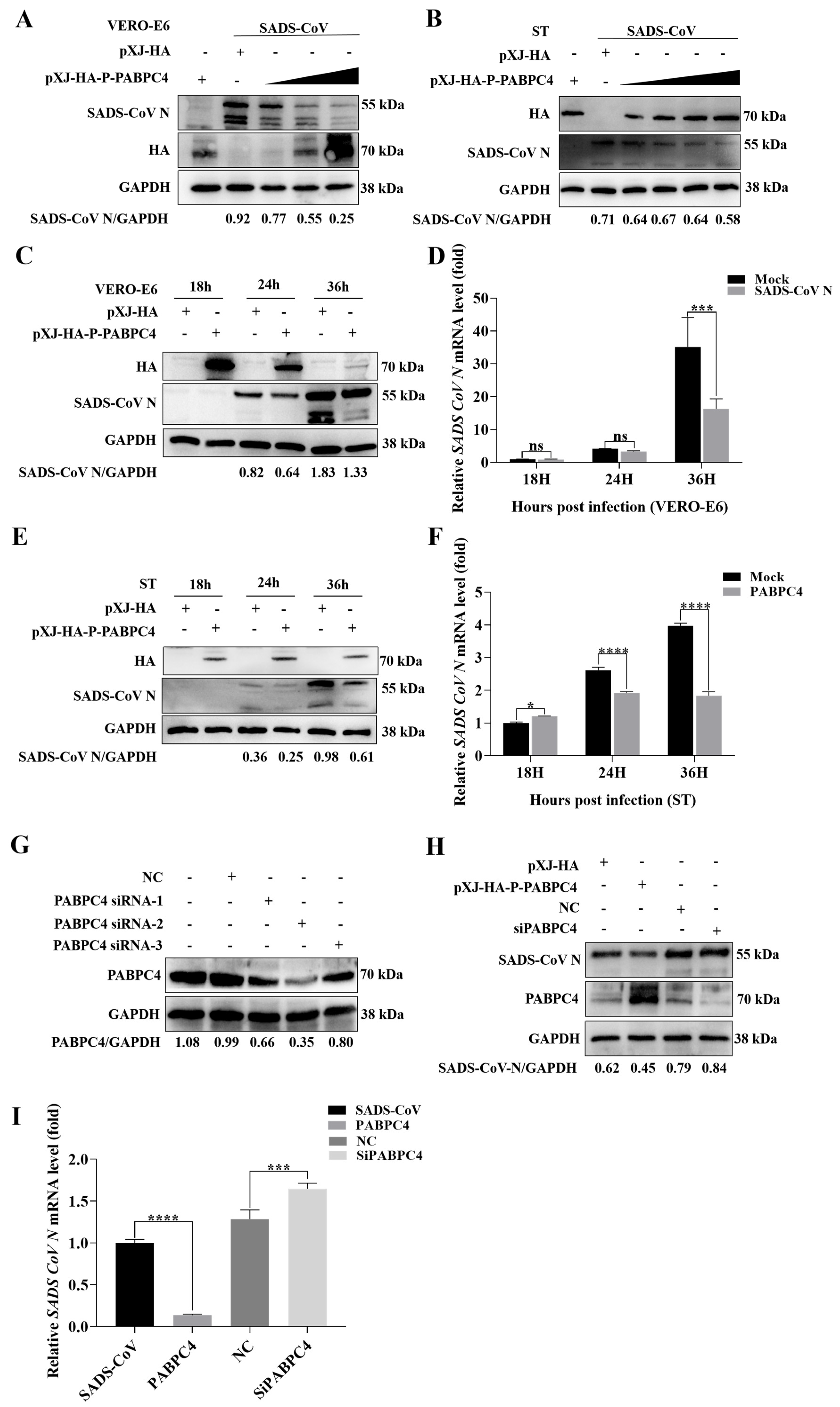
3.2. PABPC4 Inhibits SADS-CoV Replication
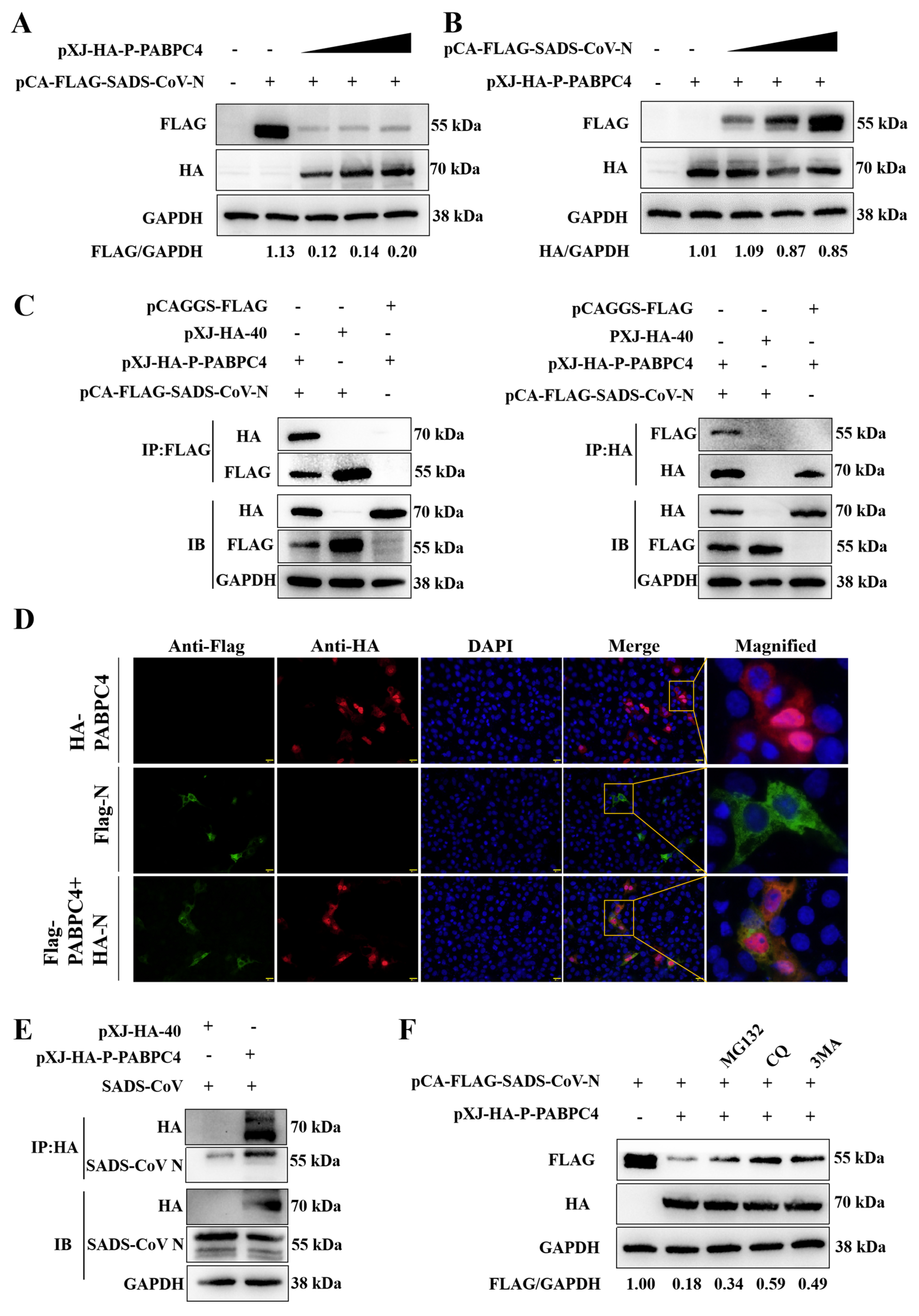
3.3. PABPC4 Targets and Degrades the SADS-CoV N Protein
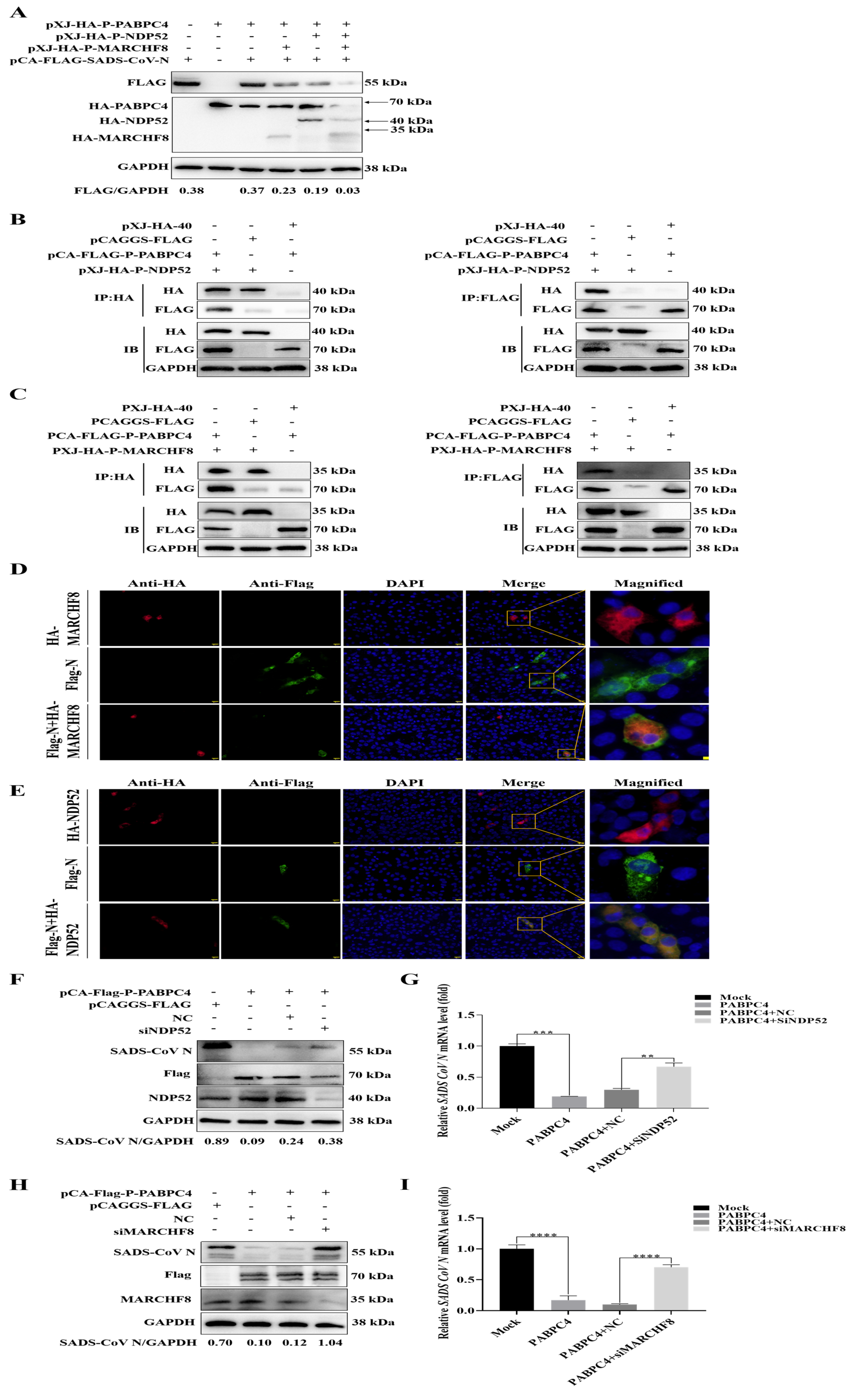
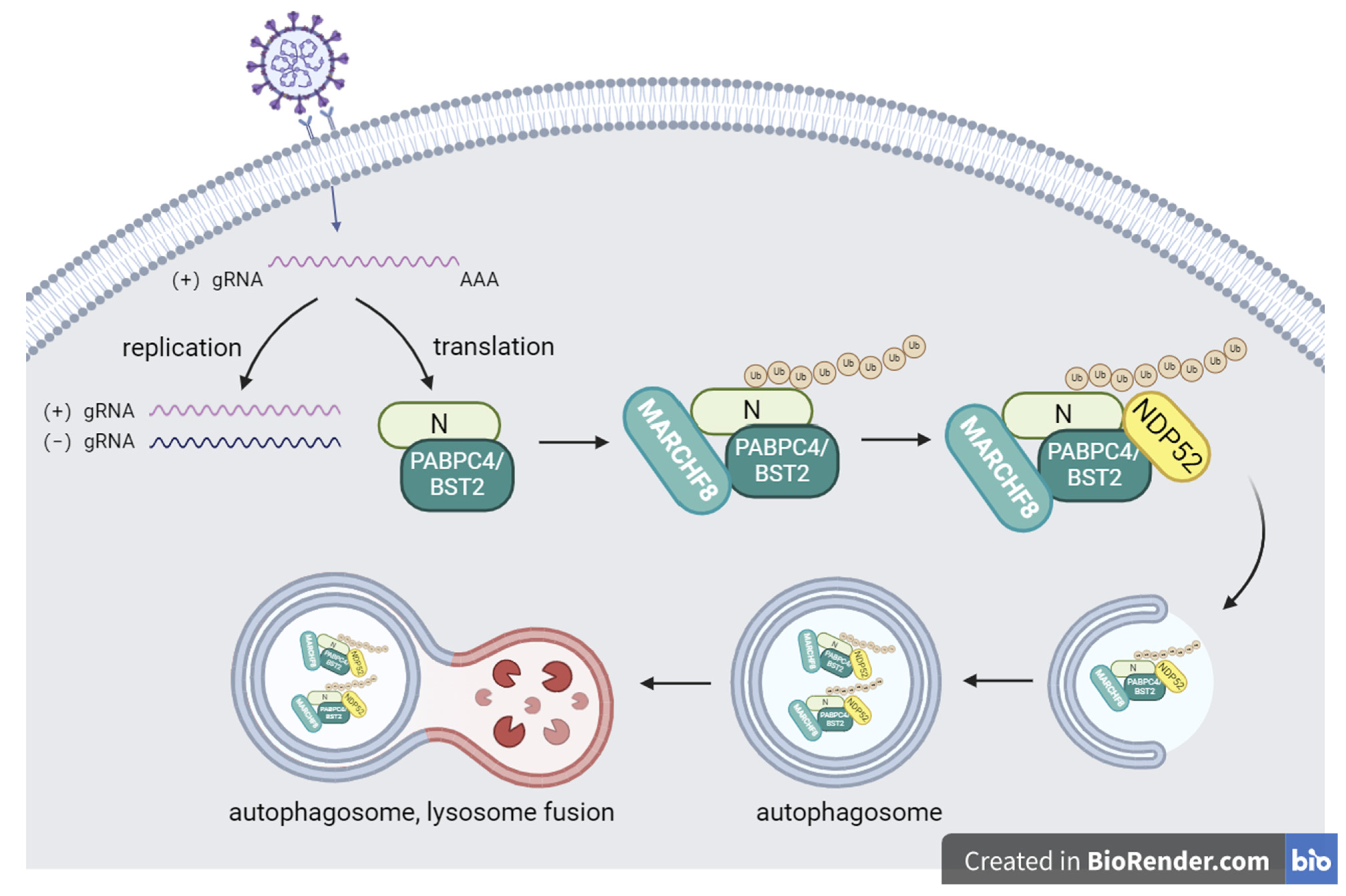
3.4. PABPC4 Degrades the SADS-CoV N Protein by Activating the PABPC4/MARCH8/NDP52 Autophagosome Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, Y.; Tian, X.; Qin, P.; Wang, B.; Zhao, P.; Yang, Y.-L.; Wang, L.; Wang, D.; Song, Y.; Zhang, X.; et al. Discovery of a novel swine enteric alphacoronavirus (SeACoV) in southern China. Vet. Microbiol. 2017, 211, 15–21. [Google Scholar] [CrossRef]
- Sun, Y.; Xing, J.; Xu, Z.-Y.; Gao, H.; Xu, S.-J.; Liu, J.; Zhu, D.-H.; Guo, Y.-F.; Yang, B.-S.; Chen, X.-N.; et al. Re-emergence of Severe Acute Diarrhea Syndrome Coronavirus (SADS-CoV) in Guangxi, China, 2021. J. Infect. 2022, 85, e130–e133. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, H.; Bi, Z.; Gu, J.; Gong, W.; Luo, S.; Zhang, F.; Song, D.; Ye, Y.; Tang, Y. Complete Genome Sequence of a Novel Swine Acute Diarrhea Syndrome Coronavirus, CH/FJWT/2018, Isolated in Fujian, China, in 2018. Genome Announc. 2018, 7, e01259-18. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, F.; Sanna, D.; Azzena, I.; Cossu, P.; Giovanetti, M.; Benvenuto, D.; Coradduzza, E.; Alexiev, I.; Casu, M.; Fiori, P.L.; et al. Update on the Phylodynamics of SADS-CoV. Life 2021, 11, 820. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, Q.N.; Su, J.N.; Chen, G.H.; Wu, Z.X.; Luo, Y.; Wu, R.T.; Sun, Y.; Lan, T.; Ma, J.Y. The re-emerging of SADS-CoV infection in pig herds in Southern China. Transbound. Emerg. Dis. 2019, 66, 2180–2183. [Google Scholar] [CrossRef]
- Edwards, C.E.; Yount, B.L.; Graham, R.L.; Leist, S.R.; Hou, Y.J.; Dinnon, K.H.; Sims, A.C.; Swanstrom, J.; Gully, K.; Scobey, T.D.; et al. Swine acute diarrhea syndrome coronavirus replication in primary human cells reveals potential susceptibility to infection. Proc. Natl. Acad. Sci. USA 2020, 117, 26915–26925. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Yu, J.-Q.; Huang, Y.-W. Swine enteric alphacoronavirus (swine acute diarrhea syndrome coronavirus): An update three years after its discovery. Virus Res. 2020, 285, 198024. [Google Scholar] [CrossRef]
- McBride, R.; Van Zyl, M.; Fielding, B.C. The coronavirus nucleocapsid is a multifunctional protein. Viruses 2014, 6, 2991–3018. [Google Scholar] [CrossRef]
- Grunewald, M.E.; Fehr, A.R.; Athmer, J.; Perlman, S. The coronavirus nucleocapsid protein is ADP-ribosylated. Virology 2018, 517, 62–68. [Google Scholar] [CrossRef]
- Choi, A.M.; Ryter, S.W.; Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 2013, 368, 651–662. [Google Scholar] [CrossRef]
- Matoba, K.; Noda, N.N. Structural catalog of core Atg proteins opens new era of autophagy research. J. Biochem. 2021, 169, 517–525. [Google Scholar] [CrossRef]
- Lamark, T.; Johansen, T. Mechanisms of Selective Autophagy. Annu. Rev. Cell Dev. Biol. 2021, 37, 143–169. [Google Scholar] [CrossRef] [PubMed]
- Wurzer, B.; Zaffagnini, G.; Fracchiolla, D.; Turco, E.; Abert, C.; Romanov, J.; Martens, S.; Laboratories, M.F.P.; Biocenter, V. Austria Oligomerization of p62 allows for selection of ubiquitinated cargo and isolation membrane during selective autophagy. eLife 2015, 4, e08941. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, D.A.; Manna, P.T.; Allen, M.; Bycroft, M.; Arden, S.D.; Kendrick-Jones, J.; Buss, F. The Autophagy Receptor TAX1BP1 and the Molecular Motor Myosin VI Are Required for Clearance of Salmonella Typhimurium by Autophagy. PLoS Pathog. 2016, 11, e1005174. [Google Scholar] [CrossRef]
- Yamano, K.; Kikuchi, R.; Kojima, W.; Hayashida, R.; Koyano, F.; Kawawaki, J.; Shoda, T.; Demizu, Y.; Naito, M.; Tanaka, K.; et al. Critical role of mitochondrial ubiquitination and the OPTN–ATG9A axis in mitophagy. J. Cell Biol. 2020, 219. [Google Scholar] [CrossRef] [PubMed]
- Boyle, K.B.; Randow, F. The role of ‘eat-me’ signals and autophagy cargo receptors in innate immunity. Curr. Opin. Microbiol. 2013, 16, 339–348. [Google Scholar] [CrossRef]
- Kaushik, S.; Cuervo, A.M. The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 365–381. [Google Scholar] [CrossRef]
- Kong, N.; Shan, T.; Wang, H.; Jiao, Y.; Zuo, Y.; Li, L.; Tong, W.; Yu, L.; Jiang, Y.; Zhou, Y.; et al. BST2 suppresses porcine epidemic diarrhea virus replication by targeting and degrading virus nucleocapsid protein with selective autophagy. Autophagy 2020, 16, 1737–1752. [Google Scholar] [CrossRef]
- Ames, J.; Yadavalli, T.; Suryawanshi, R.; Hopkins, J.; Agelidis, A.; Patil, C.; Fredericks, B.; Tseng, H.; Valyi-Nagy, T.; Shukla, D. OPTN is a host intrinsic restriction factor against neuroinvasive HSV-1 infection. Nat. Commun. 2021, 12, 5401. [Google Scholar] [CrossRef]
- Zeng, Y.; Xu, S.; Wei, Y.; Zhang, X.; Wang, Q.; Jia, Y.; Wang, W.; Han, L.; Chen, Z.; Wang, Z.; et al. The PB1 protein of influenza A virus inhibits the innate immune response by targeting MAVS for NBR1-mediated selective autophagic degradation. PLoS Pathog. 2021, 17, e1009300. [Google Scholar] [CrossRef]
- Ylä-Anttila, P.; Gupta, S.; Masucci, M.G. The Epstein-Barr virus deubiquitinase BPLF1 targets SQSTM1/p62 to inhibit selective autophagy. Autophagy 2021, 17, 3461–3474. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Oliveira, L.; Cruz, M.; Guimarães, D.; Lima, T.; Santos-Fávero, B.; Luchessi, A.; Pauletti, B.; Leme, A.; Bajgelman, M.; et al. Interaction between poly(A)–binding protein PABPC4 and nuclear receptor corepressor NCoR1 modulates a metabolic stress response. J. Biol. Chem. 2023, 299, 104702. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Han, T.; Li, Q.; Zhang, M.; Guo, R.; Yang, Y.; Lu, W.; Li, Z.; Peng, C.; Wu, P.; et al. MKRN3-mediated ubiquitination of Poly(A)-binding proteins modulates the stability and translation of GNRH1 mRNA in mammalian puberty. Nucleic Acids Res. 2021, 49, 3796–3813. [Google Scholar] [CrossRef] [PubMed]
- Kajjo, S.; Sharma, S.; Chen, S.; Brothers, W.R.; Cott, M.; Hasaj, B.; Jovanovic, P.; Larsson, O.; Fabian, M.R. PABP prevents the untimely decay of select mRNA populations in human cells. EMBO J. 2022, 41, e108650. [Google Scholar] [CrossRef]
- Jiao, Y.; Kong, N.; Wang, H.; Sun, D.; Dong, S.; Chen, X.; Zheng, H.; Tong, W.; Yu, H.; Yu, L.; et al. PABPC4 Broadly Inhibits Coronavirus Replication by Degrading Nucleocapsid Protein through Selective Autophagy. Microbiol. Spectr. 2021, 9, e0090821. [Google Scholar] [CrossRef]
- Tiwari, R.; de la Torre, J.C.; McGavern, D.B.; Nayak, D. Beyond Tethering the Viral Particles: Immunomodulatory Functions of Tetherin (BST-2). DNA Cell Biol. 2019, 38, 1170–1177. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, L.; Zhou, Z.; Yang, B.; He, Q.; Zhu, C.; Cao, J. The Role of Membrane-Associated E3 Ubiquitin Ligases in Cancer. Front. Pharmacol. 2022, 13, 928794. [Google Scholar] [CrossRef]
- Guo, L.; Yu, H.; Gu, W.; Luo, X.; Li, R.; Zhang, J.; Xu, Y.; Yang, L.; Shen, N.; Feng, L.; et al. Autophagy Negatively Regulates Transmissible Gastroenteritis Virus Replication. Sci. Rep. 2016, 6, 23864. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Wang, X.; Zhou, J.; Ma, L.; Li, J.; Yang, L.; Ouyang, H.; Yuan, H.; Pang, D. Transmissible Gastroenteritis Virus: An Update Review and Perspective. Viruses 2023, 15, 359. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Ge, X.; Wang, J.; Wei, Z.; Feng, W.-H.; Wang, J. Ergosterol peroxide exhibits antiviral and immunomodulatory abilities against porcine deltacoronavirus (PDCoV) via suppression of NF-κB and p38/MAPK signaling pathways in vitro. Int. Immunopharmacol. 2021, 93, 107317. [Google Scholar] [CrossRef]
- Shi, D.; Zhou, L.; Shi, H.; Zhang, J.; Zhang, J.; Zhang, L.; Liu, D.; Feng, T.; Zeng, M.; Chen, J.; et al. Autophagy is induced by swine acute diarrhea syndrome coronavirus through the cellular IRE1-JNK-Beclin 1 signaling pathway after an interaction of viral membrane-associated papain-like protease and GRP78. PLoS Pathog. 2023, 19, e1011201. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Zhao, Y.; Peng, O.; Xia, Y.; Xu, Q.; Li, H.; Xue, C.; Cao, Y.; Zhang, H. Swine acute diarrhea syndrome coronavirus induces autophagy to promote its replication via the Akt/mTOR pathway. iScience 2022, 25, 105394. [Google Scholar] [CrossRef] [PubMed]
| Name | Sequence (5′–3′) |
|---|---|
| si-PABPC4 sense | GAAACAUUCUAUCCUGCAA (dT)(dT) |
| si-PABPC4 antisense | UUGCAGGAUAGAAUGUUUC (dT)(dT) |
| si-MARCHF8 sense | GGAAAGAGACUCAAGGCCUA (dT)(dT) |
| si-MARCHF8 antisense | UAGGCCUUGAGUCUCUUCC (dT)(dT) |
| si-NDP52 sense | GCAGUUAGAGCACCUGAAA (dT)(dT) |
| si-NDP52 antisense | UUUCAGGUGCUCUAACUGC (dT)(dT) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Qin, Y.; Huang, H.; Chen, W.; Hu, Y.; Zhang, X.; Li, Y.; Lan, T.; Sun, W. PABPC4 Inhibits SADS-CoV Replication by Degrading the Nucleocapsid Protein Through Selective Autophagy. Vet. Sci. 2025, 12, 257. https://doi.org/10.3390/vetsci12030257
Zhao C, Qin Y, Huang H, Chen W, Hu Y, Zhang X, Li Y, Lan T, Sun W. PABPC4 Inhibits SADS-CoV Replication by Degrading the Nucleocapsid Protein Through Selective Autophagy. Veterinary Sciences. 2025; 12(3):257. https://doi.org/10.3390/vetsci12030257
Chicago/Turabian StyleZhao, Chenchen, Yan Qin, Haixin Huang, Wei Chen, Yanqing Hu, Xinyu Zhang, Yuying Li, Tian Lan, and Wenchao Sun. 2025. "PABPC4 Inhibits SADS-CoV Replication by Degrading the Nucleocapsid Protein Through Selective Autophagy" Veterinary Sciences 12, no. 3: 257. https://doi.org/10.3390/vetsci12030257
APA StyleZhao, C., Qin, Y., Huang, H., Chen, W., Hu, Y., Zhang, X., Li, Y., Lan, T., & Sun, W. (2025). PABPC4 Inhibits SADS-CoV Replication by Degrading the Nucleocapsid Protein Through Selective Autophagy. Veterinary Sciences, 12(3), 257. https://doi.org/10.3390/vetsci12030257






