Safety and Efficacy of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Different Vaccines at Phase 3
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Data Extraction and Publication Quality Assessment
2.4. Data Analysis
3. Results
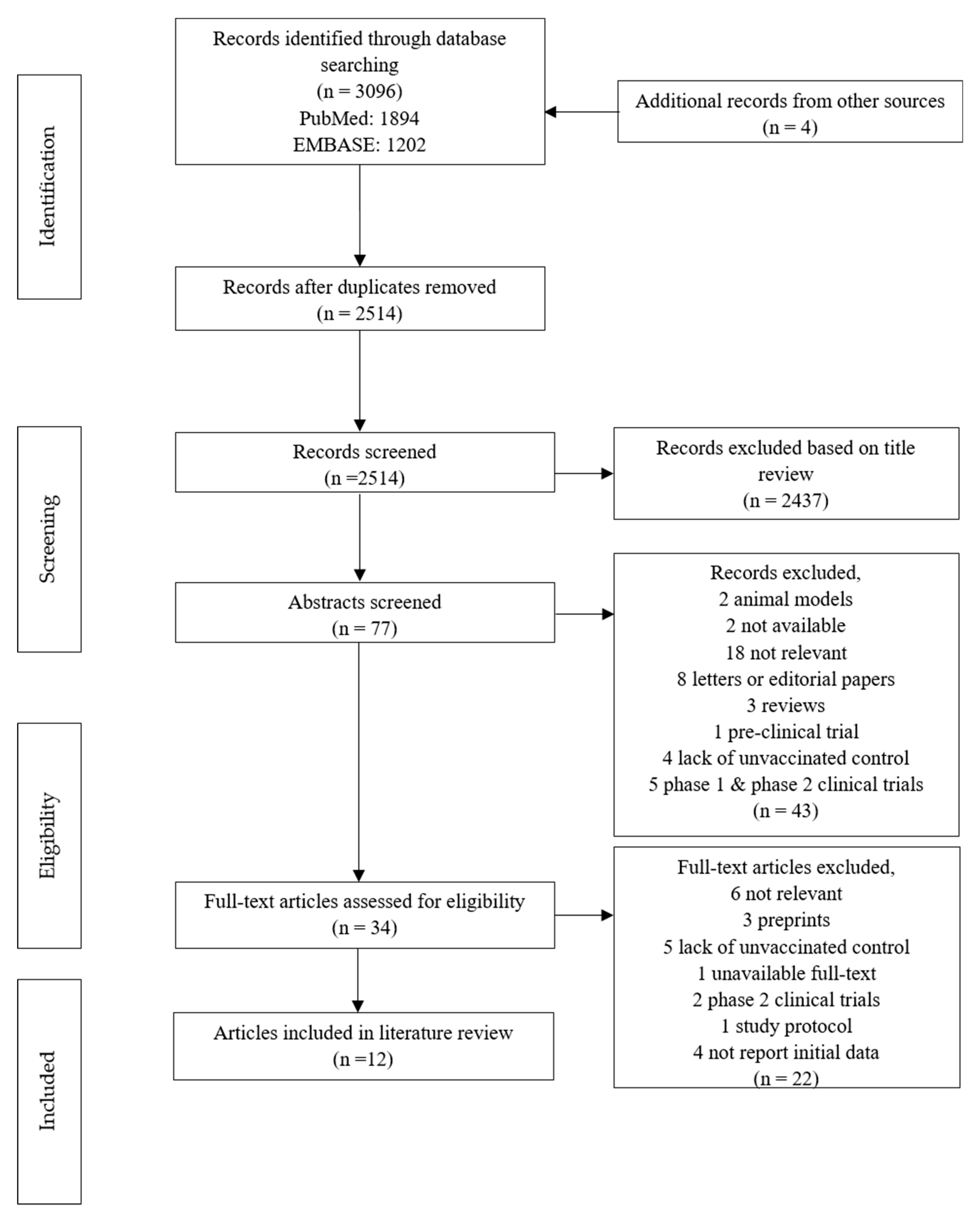
3.1. Study Selection
3.2. Description of Studies
3.3. Meta-Analysis Results
3.3.1. Vaccine Safety
3.3.2. Vaccine Efficacy
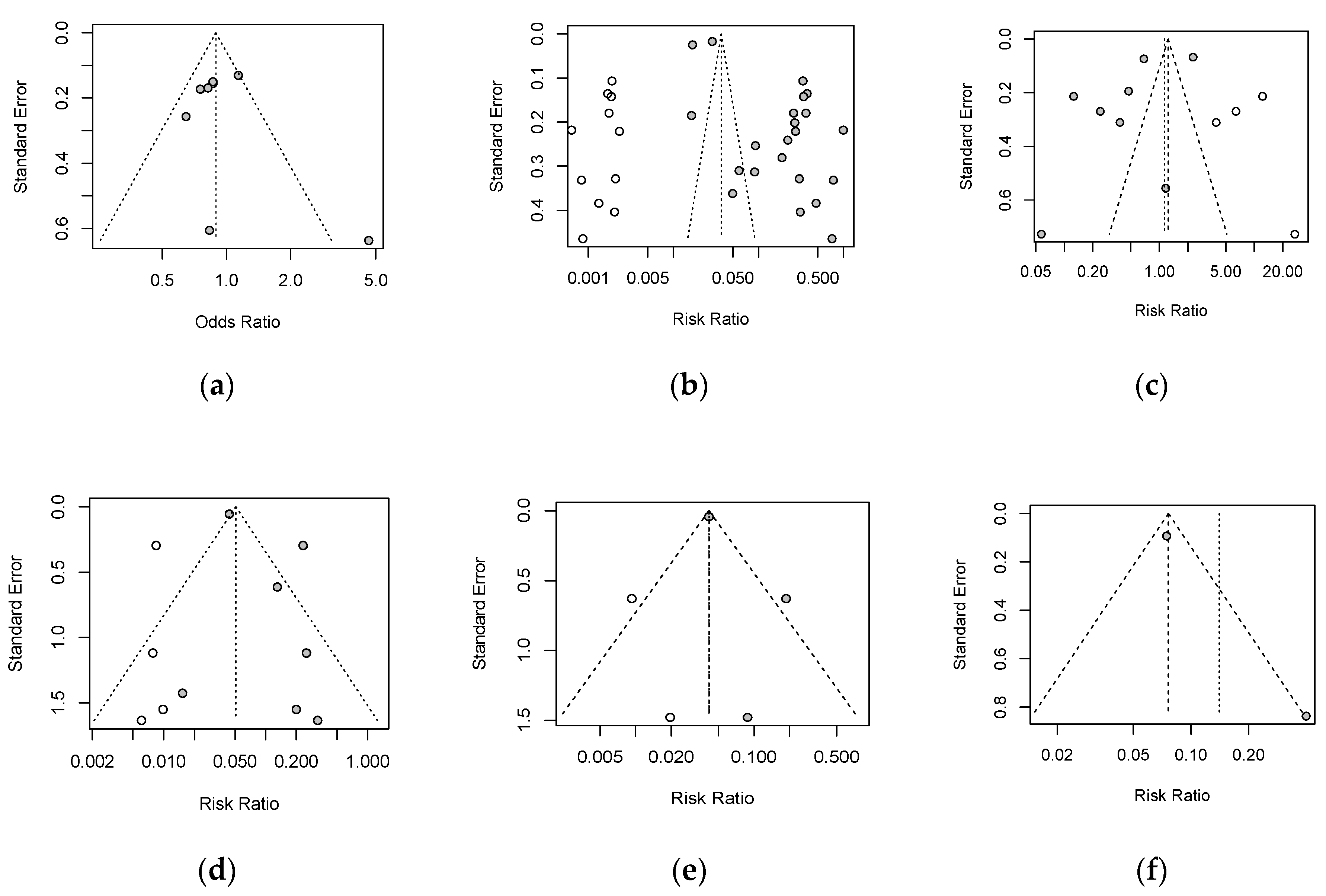
3.4. Publication Bias and Risk of Bias Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Petrosillo, N.; Viceconte, G.; Ergonul, O.; Ippolito, G.; Petersen, E. COVID-19, SARS and MERS: Are they closely related? Clin. Microbiol. Infect. 2020, 26, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Liu, F.; Yen, T.-C.; Lan, X. 18F-FDG PET/CT findings of COVID-19: A series of four highly suspected cases. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1281–1286. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2021. Available online: https://covid19.who.int/ (accessed on 22 May 2021).
- Wilder-Smith, A.; Freedman, D.O. Isolation, quarantine, social distancing and community containment: Pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J. Travel Med. 2020, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Xia, S.; Duan, K.; Zhang, Y.; Zhao, D.; Zhang, H.; Xie, Z.; Li, X.; Peng, C.; Zhang, Y.; Zhang, W.; et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. JAMA 2020, 324, 951–960. [Google Scholar] [CrossRef]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef]
- Palacios, R.; Batista, A.P.; Albuquerque, C.S.N.; Patiño, E.G.; Santos, J.D.P.; Tilli Reis Pessoa Conde, M.; Piorelli, R.D.O.; Pereira Júnior, L.C.; Raboni, S.M.; Ramos, F.; et al. Efficacy and Safety of a COVID-19 Inactivated Vaccine in Healthcare Professionals in Brazil: The PROFISCOV Study. SSRN 2021. [Google Scholar] [CrossRef]
- Feng, L.; Wang, Q.; Shan, C.; Yang, C.; Feng, Y.; Wu, J.; Liu, X.; Zhou, Y.; Jiang, R.; Hu, P.; et al. An adenovirus-vectored COVID-19 vaccine confers protection from SARS-COV-2 challenge in rhesus macaques. Nat. Commun. 2020, 11, 4207. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.-C.; Guan, X.-H.; Li, Y.-H.; Huang, J.-Y.; Jiang, T.; Hou, L.-H.; Li, J.-X.; Yang, B.-F.; Wang, L.; Wang, W.-J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Alberer, M.; Gnad-Vogt, U.; Hong, H.S.; Mehr, K.T.; Backert, L.; Finak, G.; Gottardo, R.; Bica, M.A.; Garofano, A.; Koch, S.D.; et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: An open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet 2017, 390, 1511–1520. [Google Scholar] [CrossRef]
- World Health Organization. What Is COVID-19 Vaccine Efficacy? 2021. Available online: https://www.afro.who.int/news/what-covid-19-vaccine-efficacy (accessed on 22 May 2021).
- Li, J.; Ulitzky, L.; Silberstein, E.; Taylor, D.R.; Viscidi, R. Immunogenicity and Protection Efficacy of Monomeric and Trimeric Recombinant SARS Coronavirus Spike Protein Subunit Vaccine Candidates. Viral Immunol. 2013, 26, 126–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H.; et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, Y.; Dai, L.; Wang, J.; He, P.; Li, C.; Fang, X.; Wang, C.; Zhao, X.; Huang, E.; et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: Two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect. Dis. 2021, 21, 1107–1119. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Hayden, J.A.; Van Der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing Bias in Studies of Prognostic Factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef] [PubMed]
- MedDRA. Introductory Guide for Standardised MedDRA Queries (SMQs) Version 21.0; International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH): Geneva, Switzerland, 2019. [Google Scholar]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Emary, K.R.W.; Golubchik, T.; Aley, P.K.; Ariani, C.V.; Angus, B.; Bibi, S.; Blane, B.; Bonsall, D.; Cicconi, P.; Charlton, S.; et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): An exploratory analysis of a randomised controlled trial. Lancet 2021, 397, 1351–1362. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.K.; Rivett, L.; Seaman, S.; Samworth, R.J.; Warne, B.; Workman, C.; Ferris, M.; Wright, J.; Quinnell, N.; Shaw, A.; et al. Single-dose BNT162b2 vaccine protects against asymptomatic SARS-CoV-2 infection. eLife 2021, 10, e68808. [Google Scholar] [CrossRef]
- Swift, M.D.; Breeher, L.E.; Tande, A.J.; Tommaso, C.P.; Hainy, C.M.; Chu, H.; Murad, M.H.; Berbari, E.F.; Virk, A. Effectiveness of Messenger RNA Coronavirus Disease 2019 (COVID-19) Vaccines Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in a Cohort of Healthcare Personnel. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Al Kaabi, N.; Zhang, Y.; Xia, S.; Yang, Y.; Al Qahtani, M.M.; Abdulrazzaq, N.; Al Nusair, M.; Hassany, M.; Jawad, J.S.; Abdalla, J.; et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. JAMA 2021, 326, 35–45. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials; Food and Drug Administration: Silver Spring, MD, USA; US Department of Health and Human Services: Washington, DC, USA, 2007. [Google Scholar]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]

| PubMed | EMBASE | |
|---|---|---|
| Search terms | ||
| COVID-19 vaccine | “COVID-19 vaccine *” | exp SARS-COV-2 vaccine/or COVID-19 vaccine.mp. |
| AND | AND | |
| Endpoints | ((Efficacy OR Effectiveness) OR Safety) | ((Efficacy.mp. OR Effectiveness.mp.) OR (Safety.mp. or exp Safety/)) |
| Inclusion criteria | ||
| Population | No restrictions on population | |
| Intervention | COVID-19 vaccines | |
| Comparison | The population in control group | |
| Outcomes | Incidence of serious adverse events, and cases infected with COVID-19 after vaccination | |
| Authors, Year [Ref] | Vaccine | Country/ Region | Platform | Population | Study Design | Period | Sample Size | Number of Doses | Route of Administration |
|---|---|---|---|---|---|---|---|---|---|
| L.R. Baden et al., 2021 [19] | mRNA-1273 | US | mRNA | ≥18 years old | Single-blind, randomized, controlled trial | 27 July–23 October 2020 | 30,420 | 2 | Intramuscular |
| Merryn Voysey et al., 2020 [20] | AZD1222 | UK, Brazil, South Africa | Non-Replicating Viral Vector | ≥18 years old | Single-blind, randomized, controlled trial | 24 April–4 November 2020 | 23,848 | 2 | Intramuscular |
| Denis Y Logunov et al., 2021 [21] | Gam-COVID-Vac | Russia | Non-Replicating Viral Vector | ≥18 years old | Double-blind, randomized, controlled trial | 7 September–24 November 2020 | 21,977 | 2 | Intramuscular |
| Fernando P. Polack et al., 2020 [22] | BNT162b2 | US, Argentina, Brazil, South Africa | mRNA | ≥16 years old | Single-blind, randomized, controlled trial | 27 July–14 November 2020 | 44,820 | 2 | Intramuscular |
| Katherine R W Emary et al., 2021 [23] | AZD1222 | UK | Non-Replicating Viral Vector | ≥18 years old | Single-blind, randomized, controlled trial | 31 May–13 November 2020 | 8534 | 2 | Intramuscular |
| J. Sadoff et al., 2021 [24] | Ad26.COV2.S | Argentina, Brazil, Chile, Colombia, Mexico, Peru South Africa, US | Non-Replicating Viral Vector | ≥18 years old | Double-blind, randomized, controlled trial | 21 September 2020–22 January 2021 | 44,325 | 1 | Intramuscular |
| Eric J Haas et al., 2021 [25] | BNT162b2 | Israel | mRNA | ≥16 years old | Observational study | 24 January–3 April, 2021 | 6,538,911 | 2 | Intramuscular |
| Noa Dagan et al., 2021 [26] | BNT162b2 | Israel | mRNA | ≥16 years old healthcare workers | Observational study | 20 December 2020–1 February 2021 | 769,958 | 2 | Intramuscular |
| Nick K Jones et al., 2021 [27] | BNT162b2 | UK | mRNA | Healthcare workers | Observational study | 18 January–31 January 2021 | 8819 | 1 | Intramuscular |
| Melanie D. Swift et al., 2021 [28] | BNT162b2 | US | mRNA | Healthcare workers | Observational study | 1 January–31 March 2021 | 76,000 | 2 | Intramuscular |
| Merryn Voysey et al., 2021 [29] | AZD1222 | UK, Brazil, South Africa | Non-Replicating Viral Vector | ≥18 years old | Randomized, controlled trial | 23 April–6 December 2020 | 24,422 | 2 | Intramuscular |
| Nawal Al Kaabi Et al., 2021 [30] | BBIBP-CorV | UAE, Bahrain | Inactivated | ≥18 years old | Double-blind, randomized, controlled trial | 16 July–31 December 2020 | 40,382 | 2 | Intramuscular |
| mRNA | Non-Replicating Viral Vector | Inactivated | Overall (95% CI) | |
|---|---|---|---|---|
| Safety 1 (Number of participants with serious adverse events/total number of participants in the group) | 0.86 (0.7, 1.06) | |||
| Vaccine Group # | 145/51,466 | 194/50,343 | 123/26,935 | |
| Control Group # | 120/51,352 | 210/38,957 | 156/26,906 | |
| Odds ratio (95% CI) | 1.47 (0.65, 3.29) | 0.76 (0.62, 0.93) | 0.79 (0.62, 1.00) | |
| Efficacy after 2 doses 2 (Number of cases/total number of participants in the group) | 0.17 (0.07, 0.40) | |||
| Vaccine Group * | 6371/5,019,062 | 285/60,477 | 73/25,440 | |
| Control Group * | 111,554/2,107,611 | 788/51,235 | 232/25,444 | |
| Risk ratio (95% CI) | 0.05 (0.02, 0.13) | 0.33 (0.22, 0.50) | 0.32 (0.23, 0.42) | |
| Efficacy after 1 dose 2 (Number of cases/total number of participants in the group) | 0.42 (0.20, 0.89) | |||
| Vaccine Group * | 1077/603,427 | 54/27,492 | / | |
| Control Group * | 904/601,411 | 156/17,426 | / | |
| Risk ratio (95% CI) | 0.53 (0.23, 1.20) | 0.29 (0.11, 0.76) | / | |
| Overall | Inactivated | Non-Replicating Viral Vector | mRNA | SAEs 2 | ||||
|---|---|---|---|---|---|---|---|---|
| HB02 | WIV04 | AZD1222 | Gam-COVID-Vac | Ad26. CoV2.S | mRNA-1273 | BNT162b2 | ||
| 0.01 | 0.19 | 0.14 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | Nutrition and metabolism disorders |
| (0.00, 0.08) | (0.10, 0.31) | (0.07, 0.25) | (0.00, 0.04) | (0.00, 0.08) | (0.00, 0.04) | (0.00, 0.02) | (0.00, 0.60) | |
| 0.05 | 0.12 | 0.13 | 0.10 | 0.09 | 0.00 | 0.02 | 0.00 | Gastrointestinal disorders |
| (0.01, 0.10) | (0.05, 0.23) | (0.06, 0.23) | (0.04, 0.18) | (0.02, 0.20) | (0.00, 0.04) | (0.01, 0.06) | (0.00, 0.60) | |
| 0.04 | 0.09 | 0.13 | 0.06 | 0.02 | 0.02 | 0.01 | 0.00 | Musculoskeletal and connective tissue disorders |
| (0.01, 0.08) | (0.03, 0.19) | (0.06, 0.23) | (0.02, 0.13) | (0.00, 0.11) | (0.00, 0.08) | (0.00, 0.03) | (0.00, 0.60) | |
| 0.03 | 0.17 | 0.08 | 0.05 | 0.04 | 0.00 | 0.00 | 0.00 | Renal and urinary disorders |
| (0.00, 0.08) | (0.08, 0.29) | (0.03, 0.17) | (0.01, 0.12) | (0.01, 0.15) | (0.00, 0.04) | (0.00, 0.03) | (0.00, 0.60) | |
| 0.05 | 0.08 | 0.05 | 0.08 | 0.04 | 0.02 | 0.01 | 0.25 | Nervous system disorders |
| (0.03, 0.10) | (0.03, 0.19) | (0.01, 0.13) | (0.03 0.16) | (0.01, 0.15) | (0.00, 0.08) | (0.00, 0.03) | (0.01, 0.81) | |
| 0.05 | 0.15 | 0.00 | 0.08 | 0.13 | 0.01 | 0.07 | 0.25 | Cardiac disorders |
| (0.01, 0.11) | (0.07, 0.27) | (0.00, 0.06) | (0.03, 0.16) | (0.05, 0.16) | (0.00, 0.06) | (0.04, 0.12) | (0.01, 0.81) | |
| 0.01 | 0.02 | 0.06 | 0.01 | 0.00 | 0.00 | 0.02 | 0.00 | Respiratory system disorders |
| (0.00, 0.02) | (0.00, 0.09) | (0.02, 0.15) | (0.00, 0.06) | (0.00, 0.08) | (0.00, 0.04) | (0.01, 0.05) | (0.00, 0.60) | |
| 0.01 | 0.08 | 0.06 | 0.00 | 0.02 | 0.01 | 0.02 | 0.00 | Blood and immune system disorders |
| (0.00, 0.04) | (0.03, 0.19) | (0.02, 0.15) | (0.00, 0.04) | (0.00, 0.11) | (0.00, 0.06) | (0.01, 0.05) | (0.00, 0.60) | |
| 0.02 | 0.03 | 0.05 | 0.01 | 0.02 | 0.01 | 0.04 | 0.00 | General or administration site conditions |
| (0.01, 0.04) | (0.00, 0.12) | (0.01, 0.13) | (0.00, 0.06) | (0.00, 0.11) | (0.00, 0.06) | (0.02, 0.08) | (0.00, 0.60) | |
| 0.05 | 0.05 | 0.02 | 0.00 | 0.21 | 0.00 | 0.08 | 0.5 | Vessels and lymphatic vessels disorders |
| (0.01, 0.12) | (0.01, 0.14) | (0.00, 0.08) | (0.00, 0.04) | (0.11, 0.36) | (0.00, 0.04) | (0.04, 0.12) | (0.07, 0.93) | |
| 0.00 | 0.03 | 0.00 | 0.00 | 0.06 | 0.00 | 0.01 | 0.00 | Hepatobiliary system disorders |
| (0.00, 0.01) | (0.00, 0.12) | (0.00, 0.06) | (0.00, 0.04) | (0.01, 0.18) | (0.00, 0.04) | (0.00, 0.04) | (0.00, 0.60) | |
| 0.00 | 0.02 | 0.02 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | Skin and subcutaneous tissue disorders |
| (0.00, 0.001) | (0.00, 0.09) | (0.00, 0.08) | (0.00, 0.06) | (0.00, 0.08) | (0.00, 0.04) | (0.00, 0.02) | (0.00, 0.60) | |
| 0.00 | 0.00 | 0.00 | 0.08 | 0.06 | 0.00 | 0.00 | 0.00 | Reproductive system and breast disorders |
| (0.00, 0.03) | (0.00, 0.06) | (0.00, 0.60) | (0.03, 0.16) | (0.01, 0.18) | (0.00, 0.04) | (0.00, 0.02) | (0.00, 0.60) | |
| 0.13 | 0.34 | 0.33 | 0.21 | 0.17 | 0.00 | 0.04 | 0.00 | Infections and infestations |
| (0.02, 0.28) | (0.22, 0.47) | (0.22, 0.47) | (0.13, 0.32) | (0.08, 0.31) | (0.00, 0.04) | (0.02, 0.07) | (0.00, 0.60) | |
| 0.07 | 0.19 | 0.13 | 0.12 | 0.13 | 0.00 | 0.03 | 0.25 | Injury, poisoning and procedural complications |
| (0.02, 0.16) | (0.10, 0.31) | (0.06, 0.23) | (0.06, 0.21) | (0.05, 0.26) | (0.00, 0.04) | (0.01, 0.07) | (0.01, 0.81) | |
| Vaccine Group | Control Group | Risk Ratio (95% CI) | |||
|---|---|---|---|---|---|
| Events (N) | Total (N) | Events (N) | Total (N) | ||
| Severe cases | 382 | 4,917,815 | 3325 | 2,026,409 | 0.12 (0.05, 0.30) |
| Hospitalized cases | 599 | 4,837,007 | 5547 | 1,945,570 | 0.08 (0.02, 0.24) |
| Death | 140 | 4,825,033 | 720 | 1,933,987 | 0.14 (0.03, 0.69) |
| Biases | Study Population | Study Attrition | Prognostic Factor Measurement | Outcome Measurement | Study Confounding | Statistical Analysis and Reporting | Overall |
|---|---|---|---|---|---|---|---|
| L.R. Baden et al., 2021 [19] | low | high | low | low | low | low | low |
| Merryn Voysey et al., 2020 [20] | low | high | low | low | low | low | low |
| Denis Y Logunov et al., 2021 [21] | low | high | low | low | low | low | low |
| Fernando P. Polack et al., 2020 [22] | low | high | low | low | low | low | low |
| Katherine R W Emary et al., 2021 [23] | low | high | low | low | high | low | low |
| J. Sadoff et al., 2021 [24] | low | high | low | low | low | low | low |
| Eric J Haas et al., 2021 [25] | middle | high | low | low | high | low | middle |
| Noa Dagan et al., 2021 [26] | low | low | low | low | low | low | low |
| Nick K Jones et al., 2021 [27] | low | high | low | low | high | middle | middle |
| Melanie D. Swift et al., 2021 [28] | middle | high | low | low | high | low | middle |
| Merryn Voysey et al., 2021 [29] | low | high | low | low | high | middle | middle |
| Nawal Al Kaabi Et al., 2021 [30] | low | middle | low | low | middle | low | low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.-J.; Chan, K.-H.; Hung, I.F.-N. Safety and Efficacy of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Different Vaccines at Phase 3. Vaccines 2021, 9, 989. https://doi.org/10.3390/vaccines9090989
Fan Y-J, Chan K-H, Hung IF-N. Safety and Efficacy of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Different Vaccines at Phase 3. Vaccines. 2021; 9(9):989. https://doi.org/10.3390/vaccines9090989
Chicago/Turabian StyleFan, Yu-Jing, Kwok-Hung Chan, and Ivan Fan-Ngai Hung. 2021. "Safety and Efficacy of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Different Vaccines at Phase 3" Vaccines 9, no. 9: 989. https://doi.org/10.3390/vaccines9090989
APA StyleFan, Y.-J., Chan, K.-H., & Hung, I. F.-N. (2021). Safety and Efficacy of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Different Vaccines at Phase 3. Vaccines, 9(9), 989. https://doi.org/10.3390/vaccines9090989







