Nanovaccines against Animal Pathogens: The Latest Findings
Abstract
:1. Introduction
2. An Effective Broad-Coverage Immune Response
2.1. Considerations Regarding Host Immune Response to Various Pathogens
2.2. Genetic Variability and Immune Response
2.3. Nanovaccine Considerations Regarding the Immune Response
3. Nanoplatforms: Modern Approaches for Producing Real Anti-Pathogen Vaccines
3.1. Protein Nanoparticles
3.1.1. Self-Assembling Proteins
3.1.2. Virus-like Nanoparticles (VLPs)
3.2. Lipid-Derived Nanoparticles (LNPs) and Nanomaterials: Liposomes and Virosomes
3.3. Polymeric Nanoparticles (PNPs)
3.4. Inorganic and Other Nanoparticles
4. Latest Nanovaccine Applications Regarding One Health Relevant Pathogens
4.1. Nanovaccines and Viral Infections
4.2. Nanovaccines and Bacterial Infections
4.3. Nanovaccines and Parasitic Infections and Infestations
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, C.M.C.; Plotkin, S.A. Impact of Vaccines; Health, Economic and Social Perspectives. Front. Microbiol. 2020, 11, 1526. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.A.; Franzel, L.; Atwell, J.; Datta, S.D.; Friberg, I.K.; Goldie, S.J.; Reef, S.E.; Schwalbe, N.; Simons, E.; Strebel, P.M.; et al. The estimated mortality impact of vaccinations forecast to be administered during 2011–2020 in 73 countries supported by the GAVI Alliance. Vaccine 2013, 31 (Suppl. S2), B61–B72. [Google Scholar] [CrossRef] [PubMed]
- WHO. Child Mortality and Causes of Death. 2020. Available online: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/child-mortality-and-causes-of-death (accessed on 26 July 2021).
- Heaton, P.M. Challenges of Developing Novel Vaccines with Particular Global Health Importance. Front. Immunol. 2020, 11, 517290. [Google Scholar] [CrossRef]
- Hotez, P.J. The global fight to develop antipoverty vaccines in the anti-vaccine era. Hum. Vaccines Immunother. 2018, 14, 2128–2131. [Google Scholar] [CrossRef] [Green Version]
- Trovato, M.; Sartorius, R.; D’Apice, L.; Manco, R.; De Berardinis, P. Viral Emerging Diseases: Challenges in Developing Vaccination Strategies. Front. Immunol. 2020, 11, 2130. [Google Scholar] [CrossRef]
- Gebre, M.S.; Brito, L.A.; Tostanoski, L.H.; Edwards, D.K.; Carfi, A.; Barouch, D.H. Novel approaches for vaccine development. Cell 2021, 184, 1589–1603. [Google Scholar] [CrossRef]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19); StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Singh, J.; Pandit, P.; McArthur, A.G.; Banerjee, A.; Mossman, K. Evolutionary trajectory of SARS-CoV-2 and emerging variants. Virol. J. 2021, 18, 166. [Google Scholar] [CrossRef]
- Schnierle, B.S. Cellular Attachment and Entry Factors for Chikungunya Virus. Viruses 2019, 11, 1078. [Google Scholar] [CrossRef] [Green Version]
- Peng, R.; Wu, L.-A.; Wang, Q.; Qi, J.; Gao, G.F. Cell entry of SARS-CoV-2. Trends Biochem. Sci. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Sharun, K.; Dhama, K.; Pawde, A.M.; Gortázar, C.; Tiwari, R.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J.; de la Fuente, J.; Michalak, I.; Attia, Y.A. SARS-CoV-2 in animals: Potential for unknown reservoir hosts and public health implications. Veter. Q. 2021, 41, 181–201. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.J.; Diamond, M.S. Zika Virus Pathogenesis and Tissue Tropism. Cell Host Microbe 2017, 21, 134–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, G.; He, S.; Leung, A.; Cao, W.; Bi, Y.; Zhang, Z.; Zhu, W.; Wang, L.-F.; Zhao, Y.; Cheng, K.; et al. Naturally Occurring Single Mutations in Ebola Virus Observably Impact Infectivity. J. Virol. 2019, 93, e01098-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halabi, K.; Mayrose, I. Mechanisms Underlying Host Range Variation in Flavivirus: From Empirical Knowledge to Predictive Models. J. Mol. Evol. 2021, 89, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Bagnoli, F.; Rappuoli, R.; Serruto, D. The role of vaccines in combatting antimicrobial resistance. Nat. Rev. Genet. 2021, 19, 287–302. [Google Scholar] [CrossRef]
- Buchy, P.; Ascioglu, S.; Buisson, Y.; Datta, S.; Nissen, M.; Tambyah, P.A.; Vong, S. Impact of vaccines on antimicrobial resistance. Int. J. Infect. Dis. 2019, 90, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Aida, V.; Pliasas, V.C.; Neasham, P.J.; North, J.F.; McWhorter, K.L.; Glover, S.R.; Kyriakis, C.S. Novel Vaccine Technologies in Veterinary Medicine: A Herald to Human Medicine Vaccines. Front. Veter. Sci. 2021, 8. [Google Scholar] [CrossRef]
- Clemmons, E.A.; Alfson, K.J.; Dutton, J.W., 3rd. Transboundary Animal Diseases, an Overview of 17 Diseases with Potential for Global Spread and Serious Consequences. Animals 2021, 11, 2039. [Google Scholar] [CrossRef]
- Amanna, I.J.; Slifka, M.K. Successful Vaccines. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2020; Volume 428, pp. 1–30. [Google Scholar] [CrossRef]
- D’Amico, C.; Fontana, F.; Cheng, R.; Santos, H.A. Development of vaccine formulations: Past, present, and future. Drug Deliv. Transl. Res. 2021, 11, 353–372. [Google Scholar] [CrossRef]
- Zhang, C.; Maruggi, G.; Shan, H.; Li, J. Advances in mRNA Vaccines for Infectious Diseases. Front. Immunol. 2019, 10, 594. [Google Scholar] [CrossRef] [Green Version]
- Maina, T.W.; Grego, E.A.; Boggiatto, P.M.; Sacco, R.E.; Narasimhan, B.; McGill, J.L. Applications of Nanovaccines for Disease Prevention in Cattle. Front. Bioeng. Biotechnol. 2020, 8, 608050. [Google Scholar] [CrossRef]
- Hoelzer, K.; Bielke, L.; Blake, D.P.; Cox, E.; Cutting, S.M.; Devriendt, B.; Erlacher-Vindel, E.; Goossens, E.; Karaca, K.; Lemiere, S.; et al. Vaccines as alternatives to antibiotics for food producing animals. Part 2: New approaches and potential solutions. Veter. Res. 2018, 49, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewangan, H.K. Rational application of nanoadjuvant for mucosal vaccine delivery system. J. Immunol. Methods 2020, 481–482, 112791. [Google Scholar] [CrossRef]
- Kheirollahpour, M.; Mehrabi, M.; Dounighi, N.M.; Mohammadi, M.; Masoudi, A. Nanoparticles and Vaccine Development. Pharm. Nanotechnol. 2020, 8, 6–21. [Google Scholar] [CrossRef]
- Warimwe, G.M.; Francis, M.J.; Bowden, T.A.; Thumbi, S.M.; Charleston, B. Using cross-species vaccination approaches to counter emerging infectious diseases. Nat. Rev. Immunol. 2021, 1–8, in press. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Arranz-Solís, D.; Saeij, J.P.J. Influence of the Host and Parasite Strain on the Immune Response during Toxoplasma Infection. Front. Cell. Infect. Microbiol. 2020, 10, 580425. [Google Scholar] [CrossRef]
- Tomlin, H.; Piccinini, A.M. A complex interplay between the extracellular matrix and the innate immune response to microbial pathogens. Immunology 2018, 155, 186–201. [Google Scholar] [CrossRef]
- Shepherd, F.R.; McLaren, J.E. T Cell Immunity to Bacterial Pathogens: Mechanisms of Immune Control and Bacterial Evasion. Int. J. Mol. Sci. 2020, 21, 6144. [Google Scholar] [CrossRef]
- Gan, Y.; Li, C.; Peng, X.; Wu, S.; Li, Y.; Tan, J.P.K.; Yang, Y.Y.; Yuan, P.; Ding, X. Fight bacteria with bacteria: Bacterial membrane vesicles as vaccines and delivery nanocarriers against bacterial infections. Nanomed. Nanotechnol. Biol. Med. 2021, 35, 102398. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Mattei, A.A.; Karuppannan, A.K.; Halbur, P.G. Future perspectives on swine viral vaccines: Where are we headed? Porc. Health Manag. 2021, 7, 1. [Google Scholar] [CrossRef]
- Thakur, A.; Mikkelsen, H.; Jungersen, G. Intracellular Pathogens: Host Immunity and Microbial Persistence Strategies. J. Immunol. Res. 2019, 2019, 1356540. [Google Scholar] [CrossRef]
- Netea, M.G.; Domínguez-andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; Van Der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef] [Green Version]
- Byrne, K.A.; Loving, C.L.; McGill, J.L. Innate Immunomodulation in Food Animals: Evidence for Trained Immunity? Front. Immunol. 2020, 11, 1099. [Google Scholar] [CrossRef]
- Viana, I.M.D.O.; Roussel, S.; Defrêne, J.; Lima, E.M.; Barabé, F.; Bertrand, N. Innate and adaptive immune responses toward nanomedicines. Acta Pharm. Sin. B 2021, 11, 852–870. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Bhatia, E.; Sharma, S.; Ahamad, N.; Banerjee, R. Advancements in prophylactic and therapeutic nanovaccines. Acta Biomater. 2020, 108, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Raphael, I.; Nalawade, S.; Eagar, T.N.; Forsthuber, T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015, 74, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Chang, Y.-M.; Stell, A.J.; Priestnall, S.L.; Sharma, E.; Goulart, M.R.; Gribben, J.; Xia, D.; Garden, O.A. Phenotypic characterisation of regulatory T cells in dogs reveals signature transcripts conserved in humans and mice. Sci. Rep. 2019, 9, 13478. [Google Scholar] [CrossRef] [PubMed]
- Bohorquez, J.A.; Muñoz-González, S.; Pérez-Simó, M.; Revilla, C.; Domínguez, J.; Ganges, L. Identification of an Immunosuppressive Cell Population during Classical Swine Fever Virus Infection and Its Role in Viral Persistence in the Host. Viruses 2019, 11, 822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, L.; Canelli, E.; De Angelis, E.; Catella, A.; Ferrarini, G.; Ogno, G.; Bonati, L.; Nardini, R.; Borghetti, P.; Martelli, P. A highly pathogenic porcine reproductive and respiratory syndrome virus type 1 (PRRSV-1) strongly modulates cellular innate and adaptive immune subsets upon experimental infection. Veter. Microbiol. 2018, 216, 85–92. [Google Scholar] [CrossRef]
- Sánchez-Cordón, P.J.; Jabbar, T.; Chapman, D.; Dixon, L.K.; Montoya, M. Absence of Long-Term Protection in Domestic Pigs Immunized with Attenuated African Swine Fever Virus Isolate OURT88/3 or BeninΔMGF Correlates with Increased Levels of Regulatory T Cells and Interleukin-10. J. Virol. 2020, 94, e00350-20. [Google Scholar] [CrossRef] [PubMed]
- Veiga-Parga, T. Regulatory T Cells and Their Role in Animal Disease. Veter. Pathol. 2016, 53, 737–745. [Google Scholar] [CrossRef] [Green Version]
- Guzman, E.; Hope, J.; Taylor, G.; Smith, A.L.; Cubillos-Zapata, C.; Charleston, B. Bovine γδ T Cells Are a Major Regulatory T Cell Subset. J. Immunol. 2014, 193, 208–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peddireddy, V.; Doddam, S.N.; Ahmed, N. Mycobacterial Dormancy Systems and Host Responses in Tuberculosis. Front. Immunol. 2017, 8, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Habibe, I.; Celis-Giraldo, C.; Patarroyo, M.E.; Avendaño, C.; Patarroyo, M.A. A Comprehensive Review of the Immunological Response against Foot-and-Mouth Disease Virus Infection and Its Evasion Mechanisms. Vaccines 2020, 8, 764. [Google Scholar] [CrossRef]
- Anderson, C.; Kendall, M.M. Salmonella enterica Serovar Typhimurium Strategies for Host Adaptation. Front. Microbiol. 2017, 8, 1983. [Google Scholar] [CrossRef] [Green Version]
- Caugant, D.A.; Brynildsrud, O.B. Neisseria meningitidis: Using genomics to understand diversity, evolution and pathogenesis. Nat. Rev. Genet. 2019, 18, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Vahedi, S.M.; Nikbakht, G.; Jamshidi, S.; Lankarani, L.; Alimi, N.; Esmailnejad, A. Association between DLA-DRB1.2 allelic diversity and development of mammary gland tumors in dogs. Acta Veter. Scand. 2019, 61, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Hammer, S.E.; Ho, C.-S.; Ando, A.; Rogel-Gaillard, C.; Charles, M.; Tector, M.; Tector, A.J.; Lunney, J.K. Importance of the Major Histocompatibility Complex (Swine Leukocyte Antigen) in Swine Health and Biomedical Research. Annu. Rev. Anim. Biosci. 2020, 8, 171–198. [Google Scholar] [CrossRef]
- Bohórquez, M.; Ordoñez, D.; Suárez, C.F.; Vicente, B.; Vieira, C.; López-Abán, J.; Muro, A.; Ordóñez, I.; Patarroyo, M.A. Major Histocompatibility Complex Class II (DRB3) Genetic Diversity in Spanish Morucha and Colombian Normande Cattle Compared to Taurine and Zebu Populations. Front. Genet. 2020, 10, 1293. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Dijkstra, J.M. Major Histocompatibility Complex (MHC) Genes and Disease Resistance in Fish. Cells 2019, 8, 378. [Google Scholar] [CrossRef] [Green Version]
- Celis-Giraldo, C.T.; Bohórquez, M.D.; Camargo, M.; Suárez, C.F.; Camargo, A.; Rodríguez-Obediente, K.; Martínez, A.; Lucero, C.E.; Hernández, B.; Manzano-Román, R.; et al. A comparative analysis of SLA-DRB1 genetic diversity in Colombian (creoles and commercial line) and worldwide swine populations. Sci. Rep. 2021, 11, 4340. [Google Scholar] [CrossRef]
- Manczinger, M.; Boross, G.; Kemény, L.; Müller, V.; Lenz, T.L.; Papp, B.; Pál, C. Pathogen diversity drives the evolution of generalist MHC-II alleles in human populations. PLoS Biol. 2019, 17, e3000131. [Google Scholar] [CrossRef] [Green Version]
- Hammer, S.E.; Duckova, T.; Groiss, S.; Stadler, M.; Jensen-Waern, M.; Golde, W.T.; Gimsa, U.; Saalmueller, A. Comparative analysis of swine leukocyte antigen gene diversity in European farmed pigs. Anim. Genet. 2021, 52, 523–531. [Google Scholar] [CrossRef]
- Techakriengkrai, N.; Nedumpun, T.; Golde, W.T.; Suradhat, S. Diversity of the Swine Leukocyte Antigen Class I and II in Commercial Pig Populations. Front. Veter. Sci. 2021, 8, 637682. [Google Scholar] [CrossRef]
- Giovambattista, G.; Moe, K.K.; Polat, M.; Borjigin, L.; Hein, S.T.; Moe, H.H.; Takeshima, S.-N.; Aida, Y. Characterization of bovine MHC DRB3 diversity in global cattle breeds, with a focus on cattle in Myanmar. BMC Genet. 2020, 21, 95. [Google Scholar] [CrossRef]
- Mandefro, A.; Sisay, T.; Edea, Z.; Uzzaman, M.R.; Kim, K.-S.; Dadi, H. Genetic assessment of BoLA-DRB3 polymorphisms by comparing Bangladesh, Ethiopian, and Korean cattle. J. Anim. Sci. Technol. 2021, 63, 248–261. [Google Scholar] [CrossRef]
- Takeshima, S.-N.; Corbi-Botto, C.; Giovambattista, G.; Aida, Y. Genetic diversity of BoLA-DRB3 in South American Zebu cattle populations. BMC Genet. 2018, 19, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daous, H.; Mitoma, S.; Elhanafy, E.; Nguyen, H.T.; Mai, N.T.; Notsu, K.; Kaneko, C.; Norimine, J.; Sekiguchi, S. Relationship between Allelic Heterozygosity in BoLA-DRB3 and Proviral Loads in Bovine Leukemia Virus-Infected Cattle. Animals 2021, 11, 647. [Google Scholar] [CrossRef]
- Patarroyo, M.E.; Arevalo-Pinzon, G.; Reyes, C.; Moreno-Vranich, A.; Patarroyo, M.A. Malaria Parasite Survival Depends on Conserved Binding Peptides’ Critical Biological Functions. Curr. Issues Mol. Biol. 2016, 18, 57–78. [Google Scholar]
- Aza-Conde, J.; Reyes, C.; Suárez, C.F.; Patarroyo, M.A.; Patarroyo, M.E. The molecular basis for peptide-based antimalarial vaccine development targeting erythrocyte invasion by P. falciparum. Biochem. Biophys. Res. Commun. 2021, 534, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Reyes, C.; Molina-Franky, J.; Aza-Conde, J.; Suárez, C.F.; Pabón, L.; Moreno-Vranich, A.; Patarroyo, M.A.; Patarroyo, M.E. Malaria: Paving the way to developing peptide-based vaccines against invasion in infectious diseases. Biochem. Biophys. Res. Commun. 2020, 527, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Arenas, M.; Araujo, N.M.; Branco, C.; Castelhano, N.; Castro-Nallar, E.; Pérez-Losada, M. Mutation and recombination in pathogen evolution: Relevance, methods and controversies. Infect. Genet. Evol. 2018, 63, 295–306. [Google Scholar] [CrossRef]
- Dos Santos, W.G. Impact of virus genetic variability and host immunity for the success of COVID-19 vaccines. Biomed. Pharmacother. 2021, 136, 111272. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, Y.; Chen, Y.; Huang, K. Nano-based approaches in the development of antiviral agents and vaccines. Life Sci. 2021, 265, 118761. [Google Scholar] [CrossRef]
- Cotugno, N.; Ruggiero, A.; Santilli, V.; Manno, E.C.; Rocca, S.; Zicari, S.; Amodio, D.; Colucci, M.; Rossi, P.; Levy, O.; et al. OMIC Technologies and Vaccine Development: From the Identification of Vulnerable Individuals to the Formulation of Invulnerable Vaccines. J. Immunol. Res. 2019, 2019, 8732191. [Google Scholar] [CrossRef] [Green Version]
- Mottram, L.; Chakraborty, S.; Cox, E.; Fleckenstein, J. How genomics can be used to understand host susceptibility to enteric infection, aiding in the development of vaccines and immunotherapeutic interventions. Vaccine 2019, 37, 4805–4810. [Google Scholar] [CrossRef] [PubMed]
- Moise, L.; Gutiérrez, A.H.; Khan, S.; Tan, S.; Ardito, M.; Martin, W.D.; De Groot, A.S. New Immunoinformatics Tools for Swine: Designing Epitope-Driven Vaccines, Predicting Vaccine Efficacy, and Making Vaccines on Demand. Front. Immunol. 2020, 11, 563362. [Google Scholar] [CrossRef]
- Nielsen, M.; Connelley, T.; Ternette, N. Improved Prediction of Bovine Leucocyte Antigens (BoLA) Presented Ligands by Use of Mass-Spectrometry-Determined Ligand and in Vitro Binding Data. J. Proteome Res. 2017, 17, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Fisch, A.; Reynisson, B.; Benedictus, L.; Nicastri, A.; Vasoya, D.; Morrison, I.; Buus, S.; Ferreira, B.R.; Ferreira de Miranda Santos, I.K.; Ternette, N.; et al. Integral Use of Immunopeptidomics and Immunoinformatics for the Characterization of Antigen Presentation and Rational Identification of BoLA-DR–Presented Peptides and Epitopes. J. Immunol. 2021, 206, 2489–2497. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Good, M.F.; Toth, I. Nanovaccines and their mode of action. Methods 2013, 60, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, T.; Pal, K.; Zaheer, I. Topical review on nano-vaccinology: Biochemical promises and key challenges. Process. Biochem. 2020, 100, 237–244. [Google Scholar] [CrossRef]
- Mohammed, G.M.; ElZorkany, H.E.; Farroh, K.Y.; Abd El-Aziz, W.R.; Elshoky, H.A. Potential improvement of the immune response of chickens against E. coli vaccine by using two forms of chitosan nanoparticles. Int. J. Biol. Macromol. 2020, 167, 395–404. [Google Scholar] [CrossRef]
- Monrad, J.T.; Sandbrink, J.B.; Cherian, N.G. Promoting versatile vaccine development for emerging pandemics. NPJ Vaccines 2021, 6, 26. [Google Scholar] [CrossRef]
- Butkovich, N.; Li, E.; Ramirez, A.; Burkhardt, A.M.; Wang, S.W. Advancements in protein nanoparticle vaccine platforms to combat infectious disease. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 13, e1681. [Google Scholar] [CrossRef]
- Zottig, X.; Côté-Cyr, M.; Arpin, D.; Archambault, D.; Bourgault, S. Protein Supramolecular Structures: From Self-Assembly to Nanovaccine Design. Nanomaterials 2020, 10, 1008. [Google Scholar] [CrossRef]
- Vijayan, V.; Mohapatra, A.; Uthaman, S.; Park, I.-K. Recent Advances in Nanovaccines Using Biomimetic Immunomodulatory Materials. Pharmaceutics 2019, 11, 534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufmann, S.H.E. Immunology’s Coming of Age. Front. Immunol. 2019, 10, 684. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2020, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.B.; Schneerson, R.; Szu, S.C.; Fattom, A.; Yang, Y.; Lagergard, T.; Chu, C.; Sørensen, U.S. Prevention of Invasive Bacterial Diseases by Immunization with Polysaccharide-Protein Conjugates. Curr. Top Microbiol. Immunol. 1989, 146, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kingstad-Bakke, B.; Paulson, B.; Larsen, A.; Overmyer, K.; Marinaik, C.B.; Dulli, K.; Toy, R.; Vogel, G.; Mueller, K.P.; et al. Carbomer-based adjuvant elicits CD8 T-cell immunity by inducing a distinct metabolic state in cross-presenting dendritic cells. PLOS Pathog. 2021, 17, e1009168. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.R.; Hassett, K.J.; Brito, L.A. Overview of Vaccine Adjuvants: Introduction, History, and Current Status. Methods Mol. Biol. 2017, 1494, 1–13. [Google Scholar] [CrossRef]
- Rambe, D.S.; Del Giudice, G.; Rossi, S.; Sanicas, M. Safety and Mechanism of Action of Licensed Vaccine Adjuvants. Int. Curr. Pharm. J. 2015, 4, 420–431. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, C.; Guan, Y.; Wei, X.; Sha, M.; Yi, M.; Jing, M.; Lv, M.; Guo, W.; Xu, J.; et al. Manganese salts function as potent adjuvants. Cell. Mol. Immunol. 2021, 18, 1222–1234. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Luo, J.; Guo, M.; Hu, X.; Chen, X.; Chen, Z.; Lu, X.; Mao, L.; Zhang, K.; et al. Engineering a self-navigated MnARK nanovaccine for inducing potent protective immunity against novel coronavirus. Nano Today 2021, 38, 101139. [Google Scholar] [CrossRef]
- Garçon, N.; Chomez, P.; Van Mechelen, M. GlaxoSmithKline Adjuvant Systems in vaccines: Concepts, achievements and perspectives. Expert Rev. Vaccines 2007, 6, 723–739. [Google Scholar] [CrossRef]
- Casella, C.R.; Mitchell, T.C. Putting endotoxin to work for us: Monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell. Mol. Life Sci. 2008, 65, 3231–3240. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wang, G.-X.; Zhu, B. Application of antigen presenting cell-targeted nanovaccine delivery system in rhabdovirus disease prophylactics using fish as a model organism. J. Nanobiotechnol. 2020, 18, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sayed, A.; Kamel, M. Advanced applications of nanotechnology in veterinary medicine. Environ. Sci. Pollut. Res. 2020, 27, 19073–19086. [Google Scholar] [CrossRef]
- Van Riel, D.; De Wit, E. Next-generation vaccine platforms for COVID-19. Nat. Mater. 2020, 19, 810–812. [Google Scholar] [CrossRef] [PubMed]
- Boyoglu-Barnum, S.; Ellis, D.; Gillespie, R.A.; Hutchinson, G.B.; Park, Y.-J.; Moin, S.M.; Acton, O.J.; Ravichandran, R.; Murphy, M.; Pettie, D.; et al. Quadrivalent influenza nanoparticle vaccines induce broad protection. Nature 2021, 592, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Munira, S.L.; Hendriks, J.T.; Atmosukarto, I.I.; Friede, M.H.; Carter, L.M.; Butler, J.R.; Clements, A.C. A cost analysis of producing vaccines in developing countries. Vaccine 2019, 37, 1245–1251. [Google Scholar] [CrossRef]
- Genito, C.J.; Batty, C.J.; Bachelder, E.M.; Ainslie, K.M. Considerations for Size, Surface Charge, Polymer Degradation, Co-Delivery, and Manufacturability in the Development of Polymeric Particle Vaccines for Infectious Diseases. Adv. NanoBiomed Res. 2020, 1, 2000041. [Google Scholar] [CrossRef]
- Petkar, K.C.; Patil, S.M.; Chavhan, S.S.; Kaneko, K.; Sawant, K.K.; Kunda, N.; Saleem, I. An Overview of Nanocarrier-Based Adjuvants for Vaccine Delivery. Pharmaceutics 2021, 13, 455. [Google Scholar] [CrossRef] [PubMed]
- Skwarczynski, M.; Zhao, G.; Boer, J.C.; Ozberk, V.; Azuar, A.; Cruz, J.G.; Giddam, A.K.; Khalil, Z.G.; Pandey, M.; Shibu, M.A.; et al. Poly(amino acids) as a potent self-adjuvanting delivery system for peptide-based nanovaccines. Sci. Adv. 2020, 6, eaax2285. [Google Scholar] [CrossRef] [Green Version]
- Azuar, A.; Li, Z.; Shibu, M.A.; Zhao, L.; Luo, Y.; Shalash, A.O.; Khalil, Z.G.; Capon, R.J.; Hussein, W.M.; Toth, I.; et al. Poly(hydrophobic amino acid)-Based Self-Adjuvanting Nanoparticles for Group A Streptococcus Vaccine Delivery. J. Med. Chem. 2021, 64, 2648–2658. [Google Scholar] [CrossRef] [PubMed]
- Nevagi, R.J.; Dai, W.; Khalil, Z.G.; Hussein, W.M.; Capon, R.J.; Skwarczynski, M.; Toth, I. Self-assembly of trimethyl chitosan and poly(anionic amino acid)-peptide antigen conjugate to produce a potent self-adjuvanting nanovaccine delivery system. Bioorganic Med. Chem. 2019, 27, 3082–3088. [Google Scholar] [CrossRef] [PubMed]
- Nevagi, R.J.; Dai, W.; Khalil, Z.G.; Hussein, W.; Capon, R.J.; Skwarczynski, M.; Toth, I. Structure-activity relationship of group A streptococcus lipopeptide vaccine candidates in trimethyl chitosan-based self-adjuvanting delivery system. Eur. J. Med. Chem. 2019, 179, 100–108. [Google Scholar] [CrossRef]
- Liu, D.; Liu, J.; Ma, B.; Deng, B.; Leng, X.; Kong, D.; Liu, L. A simple self-adjuvanting biomimetic nanovaccine self-assembled with the conjugate of phospholipids and nucleotides can induce a strong cancer immunotherapeutic effect. Biomater. Sci. 2021, 9, 84–92. [Google Scholar] [CrossRef]
- Sahu, R.; Verma, R.; Dixit, S.; Igietseme, J.U.; Black, C.M.; Duncan, S.; Singh, S.R.; Al Dennis, V. Future of human Chlamydia vaccine: Potential of self-adjuvanting biodegradable nanoparticles as safe vaccine delivery vehicles. Expert Rev. Vaccines 2018, 17, 217–227. [Google Scholar] [CrossRef]
- Liang, X.; Duan, J.; Li, X.; Zhu, X.; Chen, Y.; Wang, X.; Sun, H.; Kong, D.; Li, C.; Yang, J. Improved vaccine-induced immune responses via a ROS-triggered nanoparticle-based antigen delivery system. Nanoscale 2018, 10, 9489–9503. [Google Scholar] [CrossRef]
- Sinani, G.; Sessevmez, M.; Gök, M.K.; Özgümüş, S.; Alpar, H.O.; Cevher, E. Modified chitosan-based nanoadjuvants enhance immunogenicity of protein antigens after mucosal vaccination. Int. J. Pharm. 2019, 569, 118592. [Google Scholar] [CrossRef]
- Bartlett, S.; Skwarczynski, M.; Toth, I. Lipids as Activators of Innate Immunity in Peptide Vaccine Delivery. Curr. Med. Chem. 2020, 27, 2887–2901. [Google Scholar] [CrossRef]
- Bartlett, S.; Eichenberger, R.M.; Nevagi, R.J.; Ghaffar, K.A.; Marasini, N.; Dai, Y.; Loukas, A.; Toth, I.; Skwarczynski, M. Lipopeptide-Based Oral Vaccine Against Hookworm Infection. J. Infect. Dis. 2019, 221, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Liu, J.; Guan, J.; Wang, H.; Ding, T.; Qian, J.; Zhan, C.; Jiang, Z.; Liu, J.; Guan, J.; et al. Self-Adjuvant Effect by Manipulating the Bionano Interface of Liposome-Based Nanovaccines. Nano Lett. 2021, 21, 4744–4752. [Google Scholar] [CrossRef]
- Maruggi, G.; Zhang, C.; Li, J.; Ulmer, J.B.; Yu, D. mRNA as a Transformative Technology for Vaccine Development to Control Infectious Diseases. Mol. Ther. 2019, 27, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Cao, F.; Liu, X.; Wang, H.; Zhang, C.; Sun, H.; Wang, C.; Leng, X.; Song, C.; Kong, D.; et al. Hyaluronic Acid-Modified Cationic Lipid–PLGA Hybrid Nanoparticles as a Nanovaccine Induce Robust Humoral and Cellular Immune Responses. ACS Appl. Mater. Interfaces 2016, 8, 11969–11979. [Google Scholar] [CrossRef]
- De Medeiros, A.S.A.; Torres-Rêgo, M.; Lacerda, A.F.; Rocha, H.A.O.; Egito, E.S.T.D.; Cornélio, A.M.; Tambourgi, D.V.; Fernandes-Pedrosa, M.D.F.; Da Silva, A.A., Jr. Self-Assembled Cationic-Covered Nanoemulsion as A Novel Biocompatible Immunoadjuvant for Antiserum Production Against Tityus serrulatus Scorpion Venom. Pharmaceutics 2020, 12, 927. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Zhang, H.; Zhou, Y.; Umeshappa, C.S.; Gao, H. Nanovaccine-Based Strategies to Overcome Challenges in the Whole Vaccination Cascade for Tumor Immunotherapy. Small 2021, 17, 2006000. [Google Scholar] [CrossRef] [PubMed]
- Bakkari, M.A.; Valiveti, C.K.; Kaushik, R.S.; Tummala, H. Toll-like Receptor-4 (TLR4) Agonist-Based Intranasal Nanovaccine Delivery System for Inducing Systemic and Mucosal Immunity. Mol. Pharm. 2021, 18, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.; Tolia, N.H. Protein-based antigen presentation platforms for nanoparticle vaccines. NPJ Vaccines 2021, 6, 70. [Google Scholar] [CrossRef]
- Kim, J.; Narayana, A.; Patel, S.; Sahay, G. Advances in intracellular delivery through supramolecular self-assembly of oligonucleotides and peptides. Theranostics 2019, 9, 3191–3212. [Google Scholar] [CrossRef] [PubMed]
- Abudula, T.; Bhatt, K.; Eggermont, L.J.; O’Hare, N.; Memic, A.; Bencherif, S.A. Supramolecular Self-Assembled Peptide-Based Vaccines: Current State and Future Perspectives. Front. Chem. 2020, 8, 598160. [Google Scholar] [CrossRef]
- Cimica, V.; Galarza, J.M. Adjuvant formulations for virus-like particle (VLP) based vaccines. Clin. Immunol. 2017, 183, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.; Fehling, S.K.; Dorna, J.; Urbanowicz, R.A.; Oestereich, L.; Krebs, Y.; Kolesnikova, L.; Schauflinger, M.; Krähling, V.; Magassouba, N.; et al. Adjuvant formulated virus-like particles expressing native-like forms of the Lassa virus envelope surface glycoprotein are immunogenic and induce antibodies with broadly neutralizing activity. NPJ Vaccines 2020, 5, 1–17. [Google Scholar] [CrossRef]
- Tan, M.; Jiang, X. Subviral particle as vaccine and vaccine platform. Curr. Opin. Virol. 2014, 6, 24–33. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef]
- Matsuda, T.; Tanijima, T.; Hirose, A.; Masumi-Koizumi, K.; Katsuda, T.; Yamaji, H. Production of influenza virus-like particles using recombinant insect cells. Biochem. Eng. J. 2020, 163, 107757. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, Y.; Gaoa, J.; Saeeda, M.; Lia, T.; Wanga, W.; Yuac, H. Nanobiomaterial-based vaccination immunotherapy of cancer. Biomaterials 2021, 270, 120709. [Google Scholar] [CrossRef]
- Zhang, H.; Leal, J.; Soto, M.R.; Smyth, H.D.C.; Ghosh, D. Aerosolizable Lipid Nanoparticles for Pulmonary Delivery of mRNA through Design of Experiments. Pharmaceutics 2020, 12, 1042. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, M.; Wang, T. Liposomes used as a vaccine adjuvant-delivery system: From basics to clinical immunization. J. Control. Release 2019, 303, 130–150. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.; Gao, M.; Li, F.; Li, Z.; Zhang, X.Q.; Xu, X. Next-Generation Vaccines: Nanoparticle-Mediated DNA and mRNA Delivery. Adv. Health Mater. 2021, 10, e2001812. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, C.; Walker, P.G.; Dong, Y. Formulation and Delivery Technologies for mRNA Vaccines. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- CUREVAC. CEPI Awards US$ 34M Contract to CureVac to Advance the RNA Printer™—A Disruptive, Transportable mRNA Vaccine Manufacturing Platform That Can Rapidly Combat Multiple Diseases. 2019. Available online: https://www.curevac.com/en/2019/02/27/cepi-awards-us-34m-contract-to-curevac-to-advance-the-rna-printer-a-disruptive-transportable-mrna-vaccine-manufacturing-platform-that-can-rapidly-combat-multiple-diseases/ (accessed on 30 July 2021).
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles—From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Jackson, N.A.C.; Kester, K.E.; Casimiro, D.; Gurunathan, S.; DeRosa, F. The promise of mRNA vaccines: A biotech and industrial perspective. NPJ Vaccines 2020, 5, 11. [Google Scholar] [CrossRef]
- Pilkington, E.H.; Suys, E.J.; Trevaskis, N.L.; Wheatley, A.K.; Zukancic, D.; Algarni, A.; Al-Wassiti, H.; Davis, T.P.; Pouton, C.W.; Kent, S.J.; et al. From influenza to COVID-19: Lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases. Acta Biomater. 2021, 131, 16–40. [Google Scholar] [CrossRef]
- Nguyen, Q.; Kikuchi, K.; Maity, B.; Ueno, T. The Versatile Manipulations of Self-Assembled Proteins in Vaccine Design. Int. J. Mol. Sci. 2021, 22, 1934. [Google Scholar] [CrossRef]
- Asadi, K.; Gholami, A. Virosome-based nanovaccines; a promising bioinspiration and biomimetic approach for preventing viral diseases: A review. Int. J. Biol. Macromol. 2021, 182, 648–658. [Google Scholar] [CrossRef]
- Asadikaram, G.; Poustforoosh, A.; Pardakhty, A.; Torkzadeh-Mahani, M.; Nematollahi, M.H. Niosomal virosome derived by vesicular stomatitis virus glycoprotein as a new gene carrier. Biochem. Biophys. Res. Commun. 2020, 534, 980–987. [Google Scholar] [CrossRef]
- Peleteiro, M.; Presas, E.; González-Aramundiz, J.V.; Sánchez-Correa, B.; Simón-Vázquez, R.; Csaba, N.; Alonso, M.J.; González-Fernández. Polymeric Nanocapsules for Vaccine Delivery: Influence of the Polymeric Shell on the Interaction with the Immune System. Front. Immunol. 2018, 9, 791. [Google Scholar] [CrossRef]
- Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.N.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the Encapsulation of Natural Products: The Case of Chitosan Biopolymer as a Matrix. Pharmaceutics 2020, 12, 669. [Google Scholar] [CrossRef]
- Diego-González, L.; Crecente-Campo, J.; Paul, M.J.; Singh, M.; Reljic, R.; Alonso, M.J.; González-Fernández, Á.; Simón-Vázquez, R. Design of Polymeric Nanocapsules for Intranasal Vaccination against Mycobacterium Tuberculosis: Influence of the Polymeric Shell and Antigen Positioning. Pharmaceutics 2020, 12, 489. [Google Scholar] [CrossRef] [PubMed]
- Sulczewski, F.; Liszbinski, R.B.; Romão, P.R.T.; Rodrigues, L.C., Jr. Nanoparticle vaccines against viral infections. Arch. Virol. 2018, 163, 2313–2325. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.; Ng, S.W.; Singh, S.K.; Gulati, M.; Gupta, G.; Chaudhary, S.K.; Hing, G.B.; Collet, T.; MacLoughlin, R.; Löbenberg, R.; et al. Revolutionizing polymer-based nanoparticle-linked vaccines for targeting respiratory viruses: A perspective. Life Sci. 2021, 280, 119744. [Google Scholar] [CrossRef]
- Pippa, N.; Gazouli, M.; Pispas, S. Recent Advances and Future Perspectives in Polymer-Based Nanovaccines. Vaccines 2021, 9, 558. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Gao, S.; Cui, X.; Sun, D.; Zhao, K. Adjuvants and delivery systems based on polymeric nanoparticles for mucosal vaccines. Int. J. Pharm. 2019, 572, 118731. [Google Scholar] [CrossRef]
- Yan, X.; Zhou, M.; Yu, S.; Jin, Z.; Zhao, K. An overview of biodegradable nanomaterials and applications in vaccines. Vaccine 2020, 38, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhu, Z.; Ma, L.; Li, Y. O/W Nanoemulsion as an Adjuvant for an Inactivated H3N2 Influenza Vaccine: Based on Particle Properties and Mode of Carrying. Int. J. Nanomed. 2020, ume15, 2071–2083. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Sun, Y.; Yu, M.; Lu, X.; Komarneni, S.; Yang, C. Emulsions stabilized by highly hydrophilic TiO2 nanoparticles via van der Waals attraction. J. Colloid Interface Sci. 2021, 589, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Franklyne, J.S.; Gopinath, P.M.; Mukherjee, A.; Chandrasekaran, N. Nanoemulsions: The rising star of antiviral therapeutics and nanodelivery system—Current status and prospects. Curr. Opin. Colloid Interface Sci. 2021, 54, 101458. [Google Scholar] [CrossRef]
- Minakshi, P.; Ghosh, M.; Brar, B.; Kumar, R.; Lambe, U.P.; Ranjan, K.; Manoj, J.; Prasad, G. Nano-antimicrobials: A New Paradigm for Combating Mycobacterial Resistance. Curr. Pharm. Des. 2019, 25, 1554–1579. [Google Scholar] [CrossRef]
- Şenel, S. Nanotechnology and Animal Health. Pharm. Nanotechnol. 2021, 9, 26–35. [Google Scholar] [CrossRef]
- WHO. Prioritizing Diseases for Research and Development in Emergency Contexts. Available online: https://www.who.int/activities/prioritizing-diseases-for-research-and-development-in-emergency-contexts (accessed on 15 July 2021).
- Kim, D.; Wu, Y.; Kim, Y.B.; Oh, Y.-K. Advances in vaccine delivery systems against viral infectious diseases. Drug Deliv. Transl. Res. 2021, 11, 1401–1419. [Google Scholar] [CrossRef]
- Zhang, Q.; Honko, A.; Zhou, J.; Gong, H.; Downs, S.N.; Vasquez, J.H.; Fang, R.H.; Gao, W.; Griffiths, A.; Zhang, L. Cellular Nanosponges Inhibit SARS-CoV-2 Infectivity. Nano Lett. 2020, 20, 5570–5574. [Google Scholar] [CrossRef]
- Zheng, B.; Peng, W.; Guo, M.; Huang, M.; Gu, Y.; Wang, T.; Ni, G.; Ming, D. Inhalable nanovaccine with biomimetic coronavirus structure to trigger mucosal immunity of respiratory tract against COVID-19. Chem. Eng. J. 2021, 418, 129392. [Google Scholar] [CrossRef] [PubMed]
- Rasool, M.H.; Mehmood, A.; Saqalein, M.; Nisar, M.A.; Almatroudi, A.; Khurshid, M. Development, Biological Characterization, and Immunological Evaluation of Virosome Vaccine against Newcastle Disease in Pakistan. BioMed Res. Int. 2021, 2021, 8879277. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Chen, Y.; Li, H.; Fang, K.; Chen, H.; Li, X.; Qian, P. A Self-Assembling Ferritin Nanoplatform for Designing Classical Swine Fever Vaccine: Elicitation of Potent Neutralizing Antibody. Vaccines 2021, 9, 45. [Google Scholar] [CrossRef]
- Ding, P.; Jin, Q.; Chen, X.; Yang, S.; Guo, J.; Xing, G.; Deng, R.; Wang, A.; Zhang, G. Nanovaccine Confers Dual Protection Against Influenza A Virus and Porcine Circovirus Type 2. Int. J. Nanomed. 2019, ume14, 7533–7548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, P.; Jin, Q.; Zhou, W.; Chai, Y.; Liu, X.; Wang, Y.; Chen, X.; Guo, J.; Deng, R.; Gao, G.F.; et al. A Universal Influenza Nanovaccine for “Mixing Vessel” Hosts Confers Potential Ability to Block Cross-Species Transmission. Adv. Health Mater. 2019, 8, e1900456. [Google Scholar] [CrossRef] [PubMed]
- Fontana, D.; Garay, E.; Cervera, L.; Kratje, R.; Prieto, C.; Gòdia, F. Chimeric VLPs Based on HIV-1 Gag and a Fusion Rabies Glycoprotein Induce Specific Antibodies against Rabies and Foot-and-Mouth Disease Virus. Vaccines 2021, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.-H.; Wu, S.-Y.; Lin, C.-H. Oral immunization with cell-free self-assembly virus-like particles against orange-spotted grouper nervous necrosis virus in grouper larvae, Epinephelus coioides. Veter. Immunol. Immunopathol. 2018, 197, 69–75. [Google Scholar] [CrossRef]
- Jeong, K.-H.; Kim, H.J. Current status and future directions of fish vaccines employing virus-like particles. Fish Shellfish Immunol. 2020, 100, 49–57. [Google Scholar] [CrossRef]
- Barsøe, S.; Toffan, A.; Pascoli, F.; Stratmann, A.; Pretto, T.; Marsella, A.; Er-Rafik, M.; Vendramin, N.; Olesen, N.; Sepúlveda, D.; et al. Long-Term Protection and Serologic Response of European Sea Bass Vaccinated with a Betanodavirus Virus-Like Particle Produced in Pichia pastoris. Vaccines 2021, 9, 447. [Google Scholar] [CrossRef]
- Chan, Y.; Jazayeri, S.D.; Ramanathan, B.; Poh, C.L. Enhancement of Tetravalent Immune Responses to Highly Conserved Epitopes of a Dengue Peptide Vaccine Conjugated to Polystyrene Nanoparticles. Vaccines 2020, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Babych, M.; Bertheau-Mailhot, G.; Zottig, X.; Dion, J.; Gauthier, L.; Archambault, D.; Bourgault, S.; Gauhier, L. Engineering and evaluation of amyloid assemblies as a nanovaccine against the Chikungunya virus. Nanoscale 2018, 10, 19547–19556. [Google Scholar] [CrossRef] [PubMed]
- Kose, N.; Fox, J.M.; Sapparapu, G.; Bombardi, R.; Tennekoon, R.N.; de Silva, A.D.; Elbashir, S.M.; Theisen, M.A.; Humphris-Narayanan, E.; Ciaramella, G.; et al. A lipid-encapsulated mRNA encoding a potently neutralizing human monoclonal antibody protects against chikungunya infection. Sci. Immunol. 2019, 4, eaaw6647. [Google Scholar] [CrossRef] [PubMed]
- Favaro, M.T.P.; Rodrigues-Jesus, M.J.; Venceslau-Carvalho, A.A.; Alves, R.P.D.S.; Pereira, L.R.; Pereira, S.S.; Andreata-Santos, R.; Souza Ferreira, L.C. Nanovaccine based on self-assembling nonstructural protein 1 boosts antibody responses to Zika virus. Nanomed. Nanotechnol. Biol. Med. 2020, 32, 102334. [Google Scholar] [CrossRef] [PubMed]
- Pires de Souza, G.A.; Prado Rocha, R.; Lemes Gonçalves, R.; Silva Ferreira, C.; de Mello Silva, B.; Fróes Goulart de Castro, R.; Vitório Rodrigues, J.F.; Vilela Vieira, J.C., Jr.; Cosme Cotta Malaquias, L.; Santos Abrahão, J.; et al. Nanoparticles as Vaccines to Prevent Arbovirus Infection: A Long Road Ahead. Pathogens 2021, 10, 36. [Google Scholar] [CrossRef]
- Chen, G.; Bai, Y.; Li, Z.; Wang, F.; Fan, X.; Zhou, X. Bacterial extracellular vesicle-coated multi-antigenic nanovaccines protect against drug-resistant Staphylococcus aureus infection by modulating antigen processing and presentation pathways. Theranostics 2020, 10, 7131–7149. [Google Scholar] [CrossRef]
- Hu, R.; Liu, H.; Wang, M.; Li, J.; Lin, H.; Liang, M.; Gao, Y.; Yang, M. An OMV-Based Nanovaccine Confers Safety and Protection against Pathogenic Escherichia coli via Both Humoral and Predominantly Th1 Immune Responses in Poultry. Nanomaterials 2020, 10, 2293. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, L.S.; Turini, C.A.; Santos, P.S.; de Morais, M.A.; Souza, A.G.; Barbosa, M.B.; Martins, E.M.D.N.; Coutinho, L.B.; Furtado, C.A.; Ladeira, L.O.; et al. Balanced Th1/Th2 immune response induced by MSP1a functional motif coupled to multiwalled carbon nanotubes as anti-anaplasmosis vaccine in murine model. Nanomed. Nanotechnol. Biol. Med. 2019, 24, 102137. [Google Scholar] [CrossRef]
- Thukral, A.; Ross, K.; Hansen, C.; Phanse, Y.; Narasimhan, B.; Steinberg, H.; Talaat, A.M. A single dose polyanhydride-based nanovaccine against paratuberculosis infection. NPJ Vaccines 2020, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Boggiatto, P.M.; Schaut, R.G.; Kanipe, C.; Kelly, S.M.; Narasimhan, B.; Jones, D.E.; Olsen, S.C. Sustained antigen release polyanhydride-based vaccine platform for immunization against bovine brucellosis. Heliyon 2019, 5, e02370. [Google Scholar] [CrossRef] [Green Version]
- Maleki, M.; Salouti, M.; Ardestani, M.S.; Talebzadeh, A. Preparation of a nanovaccine against Brucella melitensis M16 based on PLGA nanoparticles and oligopolysaccharide antigen. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4248–4256. [Google Scholar] [CrossRef] [Green Version]
- Acevedo-Villanueva, K.; Renu, S.; Gourapura, R.; Selvaraj, R. Efficacy of a nanoparticle vaccine administered in-ovo against Salmonella in broilers. PLoS ONE 2021, 16, e0247938. [Google Scholar] [CrossRef]
- Kelly, S.M.; Larsen, K.R.; Darling, R.; Petersen, A.C.; Bellaire, B.H.; Wannemuehler, M.J.; Narasimhan, B. Single-dose combination nanovaccine induces both rapid and durable humoral immunity and toxin neutralizing antibody responses against Bacillus anthracis. Vaccine 2021, 39, 3862–3870. [Google Scholar] [CrossRef]
- Ross, K.; Senapati, S.; Alley, J.; Darling, R.; Goodman, J.; Jefferson, M.; Uz, M.; Guo, B.; Yoon, K.-J.; Verhoeven, D.; et al. Single dose combination nanovaccine provides protection against influenza A virus in young and aged mice. Biomater. Sci. 2019, 7, 809–821. [Google Scholar] [CrossRef] [Green Version]
- Kitiyodom, S.; Trullàs, C.; Rodkhum, C.; Thompson, K.D.; Katagiri, T.; Temisak, S.; Namdee, K.; Yata, T.; Pirarat, N. Modulation of the mucosal immune response of red tilapia (Oreochromis sp.) against columnaris disease using a biomimetic-mucoadhesive nanovaccine. Fish Shellfish Immunol. 2021, 112, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, G.; Zhao, Y. Biomimetic nanoparticles as universal influenza vaccine. Smart Mater. Med. 2020, 1, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Renu, S.; Han, Y.; Dhakal, S.; Lakshmanappa, Y.S.; Ghimire, S.; Feliciano-Ruiz, N.; Senapati, S.; Narasimhan, B.; Selvaraj, R.; Renukaradhya, G.J. Chitosan-adjuvanted Salmonella subunit nanoparticle vaccine for poultry delivered through drinking water and feed. Carbohydr. Polym. 2020, 243, 116434. [Google Scholar] [CrossRef]
- Han, Y.; Renu, S.; Patil, V.; Schrock, J.; Feliciano-Ruiz, N.; Selvaraj, R.; Renukaradhya, G.J. Immune Response to Salmonella Enteritidis Infection in Broilers Immunized Orally with Chitosan-Based Salmonella Subunit Nanoparticle Vaccine. Front. Immunol. 2020, 11, 935. [Google Scholar] [CrossRef] [PubMed]
- Renu, S.; Renukaradhya, G.J. Chitosan Nanoparticle Based Mucosal Vaccines Delivered Against Infectious Diseases of Poultry and Pigs. Front. Bioeng. Biotechnol. 2020, 8, 1316. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.A. Gold nanoparticles for preparation of antibodies and vaccines against infectious diseases. Expert Rev. Vaccines 2020, 19, 465–477. [Google Scholar] [CrossRef] [Green Version]
- Bettencourt, P. Current Challenges in the Identification of Pre-Erythrocytic Malaria Vaccine Candidate Antigens. Front. Immunol. 2020, 11, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Ray, P.C.; Datta, D.; Bansal, G.P.; Angov, E.; Kumar, N. Nanovaccines for malaria using Plasmodium falciparum antigen Pfs25 attached gold nanoparticles. Vaccine 2015, 33, 5064–5071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wetzel, D.; Chan, J.-A.; Suckow, M.; Barbian, A.; Weniger, M.; Jenzelewski, V.; Reiling, L.; Richards, J.S.; Anderson, D.A.; Kouskousis, B.; et al. Display of malaria transmission-blocking antigens on chimeric duck hepatitis B virus-derived virus-like particles produced in Hansenula polymorpha. PLoS ONE 2019, 14, e0221394. [Google Scholar] [CrossRef] [Green Version]
- Kurtovic, L.; Wetzel, D.; Reiling, L.; Drew, D.R.; Palmer, C.; Kouskousis, B.; Hanssen, E.; Wines, B.D.; Hogarth, P.M.; Suckow, M.; et al. Novel Virus-Like Particle Vaccine Encoding the Circumsporozoite Protein of Plasmodium falciparum Is Immunogenic and Induces Functional Antibody Responses in Mice. Front. Immunol. 2021, 12, 788. [Google Scholar] [CrossRef] [PubMed]
- Tamborrini, M.; Hauser, J.; Schäfer, A.; Amacker, M.; Favuzza, P.; Kyungtak, K.; Fleury, S.; Pluschke, G. Vaccination with virosomally formulated recombinant CyRPA elicits protective antibodies against Plasmodium falciparum parasites in preclinical in vitro and in vivo models. NPJ Vaccines 2020, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, I.H.; Lokugamage, N.; Garg, N.J. Experimental Nanovaccine Offers Protection against Repeat Exposures to Trypanosoma cruzi through Activation of Polyfunctional T Cell Response. Front. Immunol. 2020, 11, 595039. [Google Scholar] [CrossRef]
- Tosyali, O.A.; Allahverdiyev, A.; Bagirova, M.; Abamor, E.S.; Aydogdu, M.; Dinparvar, S.; Acar, T.; Mustafaeva, Z.; Derman, S. Nano-co-delivery of lipophosphoglycan with soluble and autoclaved leishmania antigens into PLGA nanoparticles: Evaluation of in vitro and in vivo immunostimulatory effects against visceral leishmaniasis. Mater. Sci. Eng. C 2021, 120, 111684. [Google Scholar] [CrossRef]
- Lacasta, A.; Mody, K.T.; De Goeyse, I.; Yu, C.; Zhang, J.; Nyagwange, J.; Mwalimu, S.; Awino, E.; Saya, R.; Njoroge, T.; et al. Synergistic Effect of Two Nanotechnologies Enhances the Protective Capacity of the Theileria parva Sporozoite p67C Antigen in Cattle. J. Immunol. 2021, 206, 686–699. [Google Scholar] [CrossRef]
- El Bissati, K.; Zhou, Y.; Paulillo, S.M.; Raman, S.K.; Karch, C.P.; Reed, S.; Estes, A.; Estes, A.; Lykins, J.; Burkhard, P.; et al. Engineering and characterization of a novel Self Assembling Protein for Toxoplasma peptide vaccine in HLA-A*11:01, HLA-A*02:01 and HLA-B*07:02 transgenic mice. Sci. Rep. 2020, 10, 16984. [Google Scholar] [CrossRef]
- Mody, K.T.; Zhang, B.; Li, X.; Fletcher, N.L.; Akhter, D.T.; Jarrett, S.; Zhang, J.; Yu, C.; Thurecht, K.J.; Mahony, T.J.; et al. Characterization of the Biodistribution of a Silica Vesicle Nanovaccine Carrying a Rhipicephalus (Boophilus) microplus Protective Antigen with in vivo Live Animal Imaging. Front. Bioeng. Biotechnol. 2021, 8, 1455. [Google Scholar] [CrossRef]
- Bernstein, J.; Dutkiewicz, J. A Public Health Ethics Case for Mitigating Zoonotic Disease Risk in Food Production. Food Ethic. 2021, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, P. The significance of bioengineered nanoplatforms against SARS-CoV-2: From detection to genome editing. Life Sci. 2021, 274, 119289. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.; Yao, Y.; Nair, V.; Luo, J. Latest Advances of Virology Research Using CRISPR/Cas9-Based Gene-Editing Technology and Its Application to Vaccine Development. Viruses 2021, 13, 779. [Google Scholar] [CrossRef] [PubMed]
 ), viruses (
), viruses (  ), and parasites (
), and parasites (  ) is via their pathogen-associated molecular patterns (PAMPs). Endogenous particle recognition from dead or apoptotic cells is by damage-associated molecular patterns (DAMPs), thus activating the arms of the immune response: (a) innate immunity. After PAMPs interaction with host pattern recognition receptors (PRRs) (i.e., Toll-like receptors (1)), the action of complement proteins (i.e., C5a and C3b) and the final effect of recruited immune cells such as dendritic cells (DC), macrophages (MØ) and LTϒ/δ (specially in swine and ruminants (LTϒ/δ-WC1+)) starts; natural killer cells (NK), monocytes (M), and neutrophils (N) eliminate infectious agents, mainly by phagocytosis, cell locomotion, the formation of the membrane attack complex (MAC) and lysis. (b) Adaptive immunity. Antigen presenting cells (APCs) migrate to secondary lymphoid organs such as the spleen (i) and lymph nodes (ii) with subsequent antigen presentation (via MHC-I) to the TCR (2) of CD8+ cytotoxic T-lymphocytes (triggering cell-mediated apoptosis) and via MHC-II presentation to CD4+ T cells which leads to their maturation. IFN-ϒ and IL-2 secretion then promotes commitment to a Th1 profile increasing phagocytic and cytotoxic activity and promoting opsonising Ab production by B-cells. Th2 cell responses promote B-cell proliferation, plasma cell (Pc) maturation, and consequent production of neutralising Abs (NAbs). After infection control, most immune cells die by apoptosis and a small percentage differentiate to memory T (Tmem) and memory B (Bmem) cells. (c) Immunological response to nanovaccines. These vaccines have several advantages aimed at eliciting long-term balanced protective immune responses, including (i) different administration routes (intramuscular [A], subcutaneous [B], intravenous [C], oral [D]) (ii) different nanomaterial types and properties (i.e., polymer nanoparticles [E], protein-based nanoparticles [F] and liposomes [G]) which prolong interaction time with immune system cells, protect the antigen and potentially serve as adjuvants and immunomodulators and (iii) stimulate APC migration to the secondary lymphoid organs and increase lymphocyte maturation. Designed by: Sofía Chaves-Vargas and Sofía Riaño-Riaño, Animal Science Faculty, U.D.C.A. Created with BioRender.com, accessed on 26 July 2021.
) is via their pathogen-associated molecular patterns (PAMPs). Endogenous particle recognition from dead or apoptotic cells is by damage-associated molecular patterns (DAMPs), thus activating the arms of the immune response: (a) innate immunity. After PAMPs interaction with host pattern recognition receptors (PRRs) (i.e., Toll-like receptors (1)), the action of complement proteins (i.e., C5a and C3b) and the final effect of recruited immune cells such as dendritic cells (DC), macrophages (MØ) and LTϒ/δ (specially in swine and ruminants (LTϒ/δ-WC1+)) starts; natural killer cells (NK), monocytes (M), and neutrophils (N) eliminate infectious agents, mainly by phagocytosis, cell locomotion, the formation of the membrane attack complex (MAC) and lysis. (b) Adaptive immunity. Antigen presenting cells (APCs) migrate to secondary lymphoid organs such as the spleen (i) and lymph nodes (ii) with subsequent antigen presentation (via MHC-I) to the TCR (2) of CD8+ cytotoxic T-lymphocytes (triggering cell-mediated apoptosis) and via MHC-II presentation to CD4+ T cells which leads to their maturation. IFN-ϒ and IL-2 secretion then promotes commitment to a Th1 profile increasing phagocytic and cytotoxic activity and promoting opsonising Ab production by B-cells. Th2 cell responses promote B-cell proliferation, plasma cell (Pc) maturation, and consequent production of neutralising Abs (NAbs). After infection control, most immune cells die by apoptosis and a small percentage differentiate to memory T (Tmem) and memory B (Bmem) cells. (c) Immunological response to nanovaccines. These vaccines have several advantages aimed at eliciting long-term balanced protective immune responses, including (i) different administration routes (intramuscular [A], subcutaneous [B], intravenous [C], oral [D]) (ii) different nanomaterial types and properties (i.e., polymer nanoparticles [E], protein-based nanoparticles [F] and liposomes [G]) which prolong interaction time with immune system cells, protect the antigen and potentially serve as adjuvants and immunomodulators and (iii) stimulate APC migration to the secondary lymphoid organs and increase lymphocyte maturation. Designed by: Sofía Chaves-Vargas and Sofía Riaño-Riaño, Animal Science Faculty, U.D.C.A. Created with BioRender.com, accessed on 26 July 2021.
 ), viruses (
), viruses (  ), and parasites (
), and parasites (  ) is via their pathogen-associated molecular patterns (PAMPs). Endogenous particle recognition from dead or apoptotic cells is by damage-associated molecular patterns (DAMPs), thus activating the arms of the immune response: (a) innate immunity. After PAMPs interaction with host pattern recognition receptors (PRRs) (i.e., Toll-like receptors (1)), the action of complement proteins (i.e., C5a and C3b) and the final effect of recruited immune cells such as dendritic cells (DC), macrophages (MØ) and LTϒ/δ (specially in swine and ruminants (LTϒ/δ-WC1+)) starts; natural killer cells (NK), monocytes (M), and neutrophils (N) eliminate infectious agents, mainly by phagocytosis, cell locomotion, the formation of the membrane attack complex (MAC) and lysis. (b) Adaptive immunity. Antigen presenting cells (APCs) migrate to secondary lymphoid organs such as the spleen (i) and lymph nodes (ii) with subsequent antigen presentation (via MHC-I) to the TCR (2) of CD8+ cytotoxic T-lymphocytes (triggering cell-mediated apoptosis) and via MHC-II presentation to CD4+ T cells which leads to their maturation. IFN-ϒ and IL-2 secretion then promotes commitment to a Th1 profile increasing phagocytic and cytotoxic activity and promoting opsonising Ab production by B-cells. Th2 cell responses promote B-cell proliferation, plasma cell (Pc) maturation, and consequent production of neutralising Abs (NAbs). After infection control, most immune cells die by apoptosis and a small percentage differentiate to memory T (Tmem) and memory B (Bmem) cells. (c) Immunological response to nanovaccines. These vaccines have several advantages aimed at eliciting long-term balanced protective immune responses, including (i) different administration routes (intramuscular [A], subcutaneous [B], intravenous [C], oral [D]) (ii) different nanomaterial types and properties (i.e., polymer nanoparticles [E], protein-based nanoparticles [F] and liposomes [G]) which prolong interaction time with immune system cells, protect the antigen and potentially serve as adjuvants and immunomodulators and (iii) stimulate APC migration to the secondary lymphoid organs and increase lymphocyte maturation. Designed by: Sofía Chaves-Vargas and Sofía Riaño-Riaño, Animal Science Faculty, U.D.C.A. Created with BioRender.com, accessed on 26 July 2021.
) is via their pathogen-associated molecular patterns (PAMPs). Endogenous particle recognition from dead or apoptotic cells is by damage-associated molecular patterns (DAMPs), thus activating the arms of the immune response: (a) innate immunity. After PAMPs interaction with host pattern recognition receptors (PRRs) (i.e., Toll-like receptors (1)), the action of complement proteins (i.e., C5a and C3b) and the final effect of recruited immune cells such as dendritic cells (DC), macrophages (MØ) and LTϒ/δ (specially in swine and ruminants (LTϒ/δ-WC1+)) starts; natural killer cells (NK), monocytes (M), and neutrophils (N) eliminate infectious agents, mainly by phagocytosis, cell locomotion, the formation of the membrane attack complex (MAC) and lysis. (b) Adaptive immunity. Antigen presenting cells (APCs) migrate to secondary lymphoid organs such as the spleen (i) and lymph nodes (ii) with subsequent antigen presentation (via MHC-I) to the TCR (2) of CD8+ cytotoxic T-lymphocytes (triggering cell-mediated apoptosis) and via MHC-II presentation to CD4+ T cells which leads to their maturation. IFN-ϒ and IL-2 secretion then promotes commitment to a Th1 profile increasing phagocytic and cytotoxic activity and promoting opsonising Ab production by B-cells. Th2 cell responses promote B-cell proliferation, plasma cell (Pc) maturation, and consequent production of neutralising Abs (NAbs). After infection control, most immune cells die by apoptosis and a small percentage differentiate to memory T (Tmem) and memory B (Bmem) cells. (c) Immunological response to nanovaccines. These vaccines have several advantages aimed at eliciting long-term balanced protective immune responses, including (i) different administration routes (intramuscular [A], subcutaneous [B], intravenous [C], oral [D]) (ii) different nanomaterial types and properties (i.e., polymer nanoparticles [E], protein-based nanoparticles [F] and liposomes [G]) which prolong interaction time with immune system cells, protect the antigen and potentially serve as adjuvants and immunomodulators and (iii) stimulate APC migration to the secondary lymphoid organs and increase lymphocyte maturation. Designed by: Sofía Chaves-Vargas and Sofía Riaño-Riaño, Animal Science Faculty, U.D.C.A. Created with BioRender.com, accessed on 26 July 2021.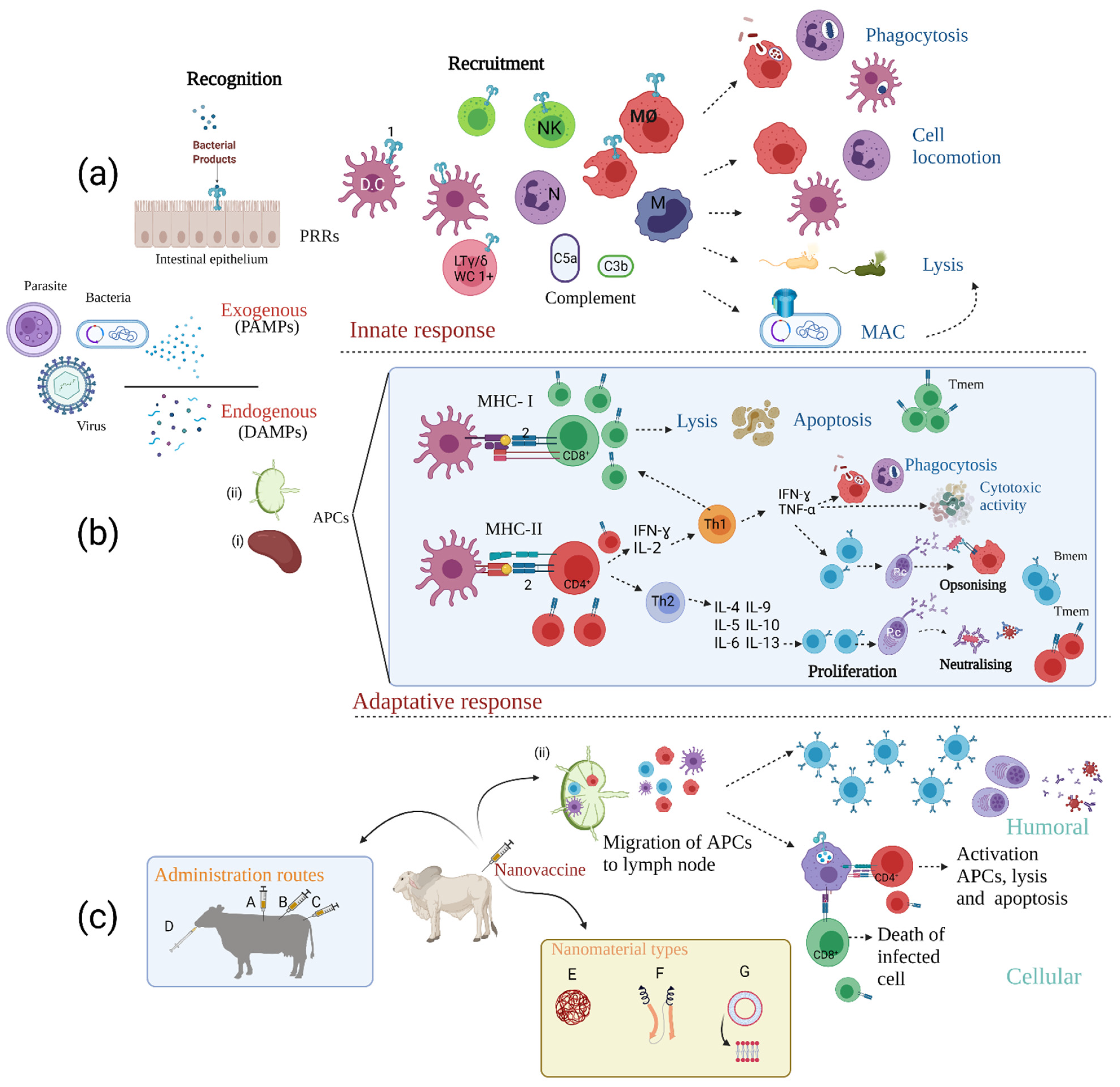
| Vaccine Type | Benefits/Advantages | Constrains/Inadequacies/Shortcomings |
|---|---|---|
| Inactivated/live-attenuated vaccines |
|
|
| Subunit or recombinant vaccines |
|
|
| All | - Beneficial effects for society in terms of reducing disease transmission and outbreaks |
|
| Nanovaccine Complex | Compound/Adjuvant Properties | References |
|---|---|---|
| Self-adjuvanting moieties | Poly and polyhydrophobic amino acids acting as self-adjuvants inducing specific antibodies able to clear bacterial load | [96,97] |
| Trimethyl chitosan alone or self-assembled with poly (anionic amino acid) can stimulate the highest levels of serum protective antibodies and nanovaccines’ opsonin-mediated killing potential. | [98,99] | |
| Biomimetic nanoparticles self-assembled with Toll-like receptor phospholipids and nucleotides agonist activating strong immune responses and serving as a safe, simple, and efficient approach for anti-tumour immunotherapy | [100] | |
| Biodegradable polymeric nanoparticles | PLGA, PLA-PEG a copolymer of polylactic acid (PLA), polyethylene glycol (PEG) and their adjuvanted derivatives facilitate their release upon degradation of the matrix, having prolonged biodegradation properties | [101] |
| A ROS-triggered nanoparticle-based antigen delivery system consisting of three-armed PLGA, conjugated to PEG via the peroxalate ester bond (3s-PLGA-PO-PEG) and PEI as a cationic adjuvant (PPO NPs) | [102] | |
| Novel chitosan derivatives (aminated chitosan and aminated plus thiolated chitosan) promote strong mucoadhesiveness and thereby systemic and local immune responses following nasal vaccination | [103] | |
| Liposomes-mRNAs | Incorporating lipid moieties into peptide epitopes increases antigen immunogenicity | [104,105] |
| IgM (after spontaneous absorption on the nanosurface) serve as self-adjuvant by regulating antigen-presenting cell recognition and complement activation | [106] | |
| Carriers consisting of non-encoding RNA complexed with protamine (a cationic protein activating TLR7) naked 1-methylpseudouridine modified mRNA to small-molecule TLR2 and TLR7 agonists | [107] | |
| Lipid-PLGA nanoparticles | Hyaluronic acid (HA)-decorated cationic lipid-poly(lactide-co-glycolide) acid (PLGA) hybrid nanoparticles (HA-DOTAP-PLGA NPs) | [108] |
| Nanoemulsions | A self-assembled biocompatible cationic-covered with hyper-branched poly(ethyleneimine) nanoemulsion has superior adjuvant activity than the non-cationic and traditional adjuvants in vivo. | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celis-Giraldo, C.T.; López-Abán, J.; Muro, A.; Patarroyo, M.A.; Manzano-Román, R. Nanovaccines against Animal Pathogens: The Latest Findings. Vaccines 2021, 9, 988. https://doi.org/10.3390/vaccines9090988
Celis-Giraldo CT, López-Abán J, Muro A, Patarroyo MA, Manzano-Román R. Nanovaccines against Animal Pathogens: The Latest Findings. Vaccines. 2021; 9(9):988. https://doi.org/10.3390/vaccines9090988
Chicago/Turabian StyleCelis-Giraldo, Carmen Teresa, Julio López-Abán, Antonio Muro, Manuel Alfonso Patarroyo, and Raúl Manzano-Román. 2021. "Nanovaccines against Animal Pathogens: The Latest Findings" Vaccines 9, no. 9: 988. https://doi.org/10.3390/vaccines9090988
APA StyleCelis-Giraldo, C. T., López-Abán, J., Muro, A., Patarroyo, M. A., & Manzano-Román, R. (2021). Nanovaccines against Animal Pathogens: The Latest Findings. Vaccines, 9(9), 988. https://doi.org/10.3390/vaccines9090988






