Incidence and Risk Factors of COVID-19 Vaccine Breakthrough Infections: A Prospective Cohort Study in Belgium
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Data Sources
2.3. Outcomes
2.4. Risk Factors
2.5. Statistical Analysis
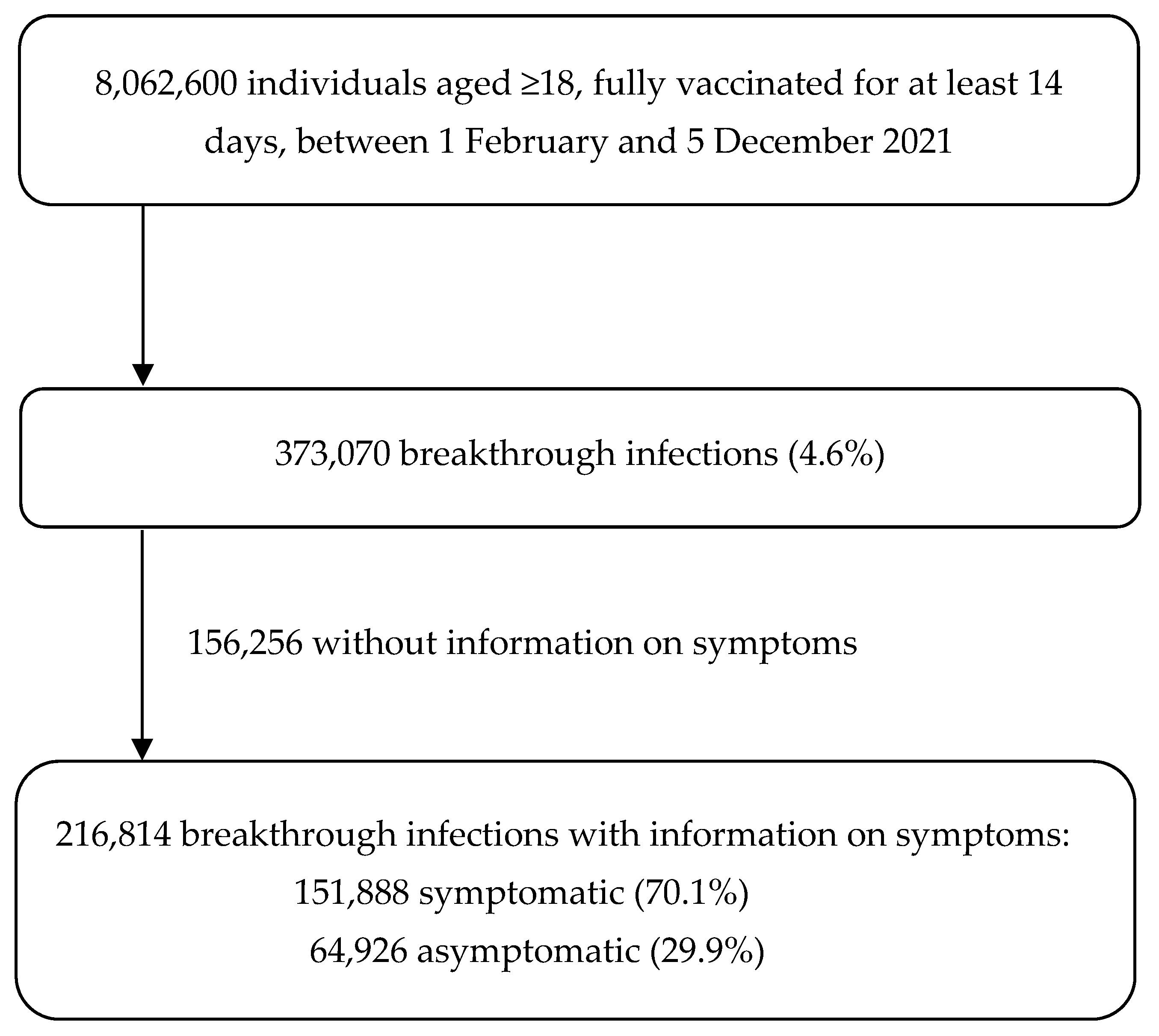
3. Results
3.1. Population Characteristics
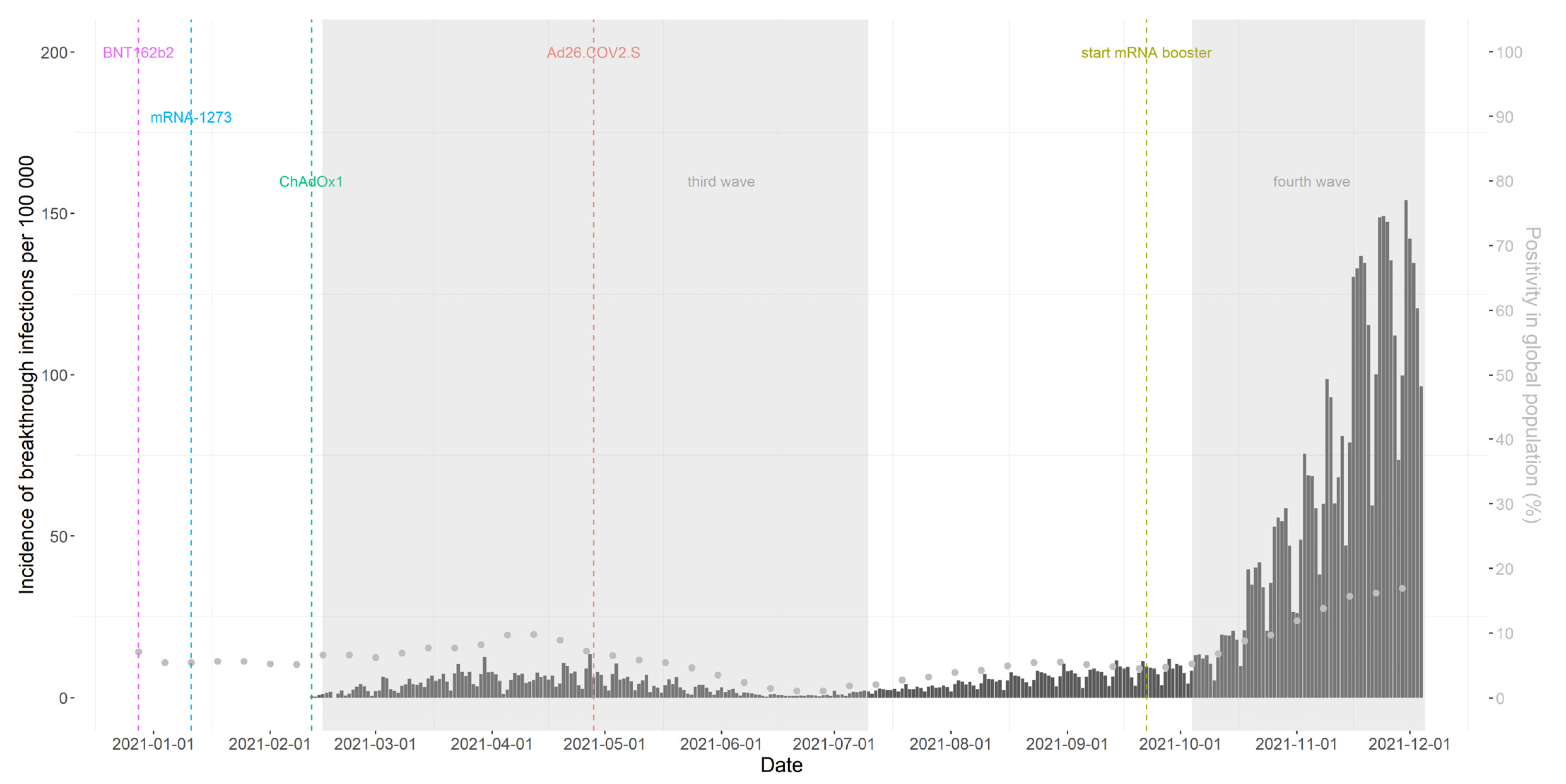
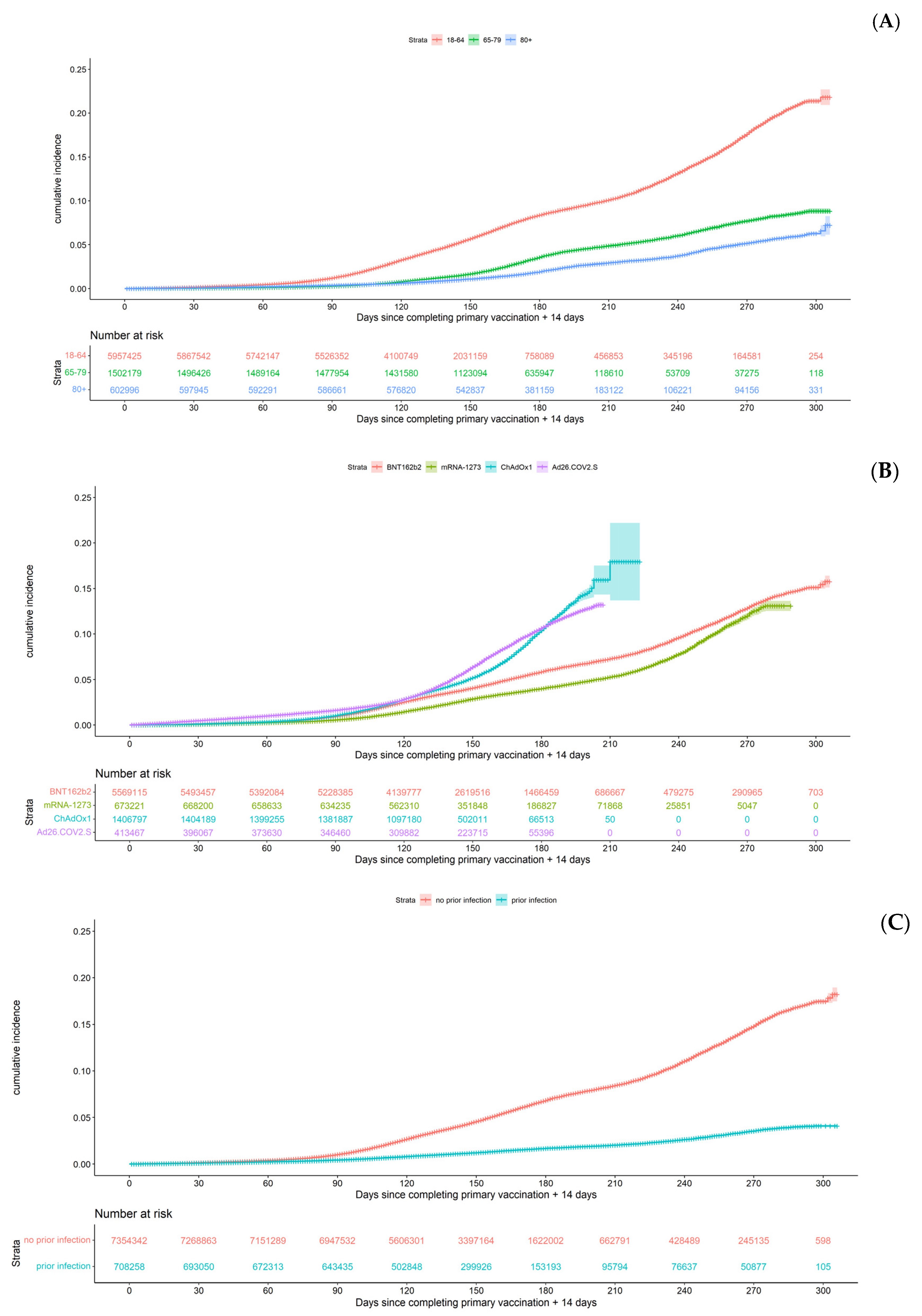
3.2. Occurrence of Breakthrough Infections and Persons’ Characteristics
3.3. Occurrence of Symptomatic Breakthrough Infections
3.4. Risk Factors of a Breakthrough Infection
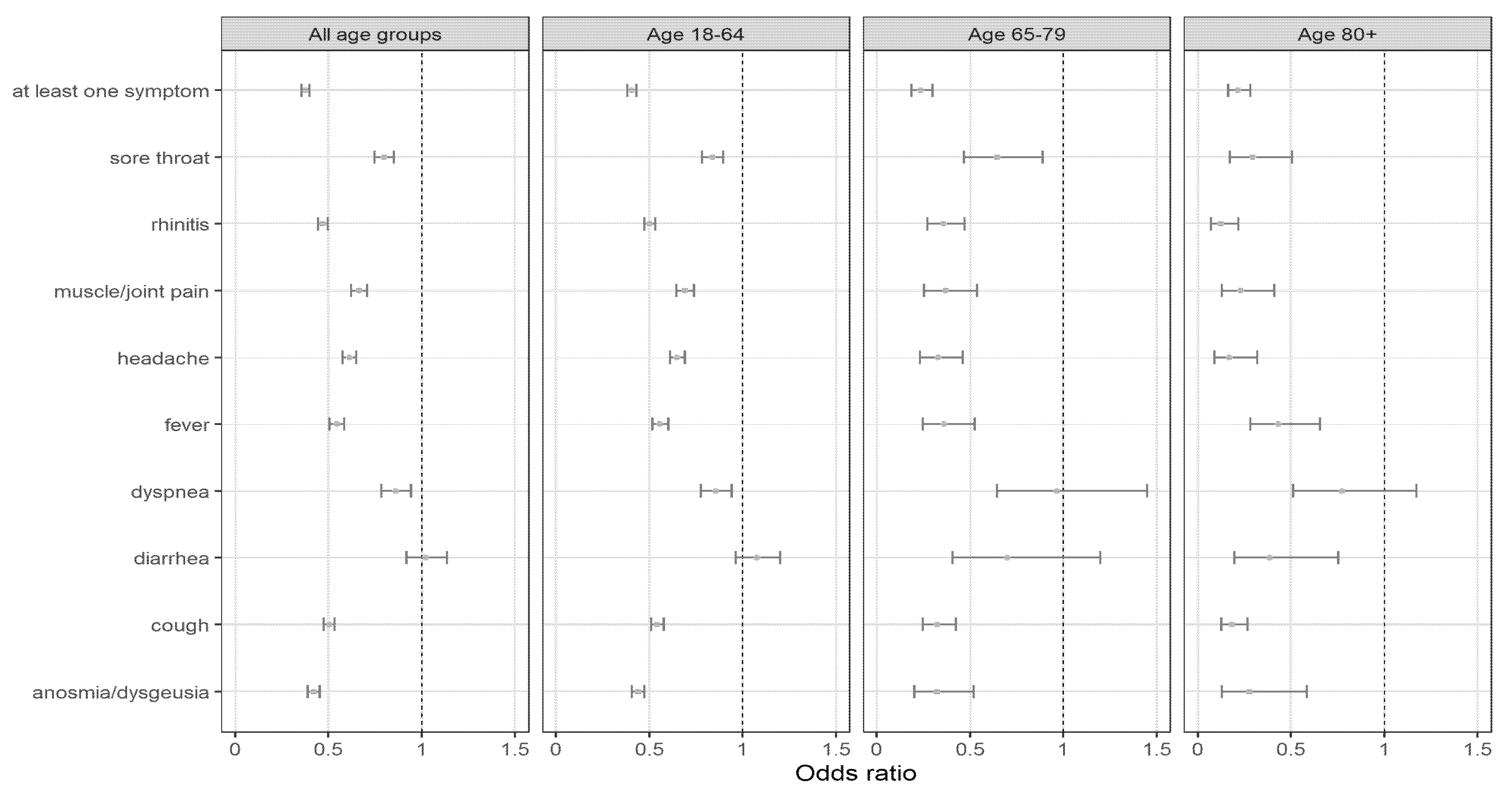
3.5. Symptomatic Profile of Breakthrough Infections
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 83, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2020, 397, 99–111. [Google Scholar]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Iheanacho, C.O.; Eze, U.I.H.; Adida, E.A. A systematic review of effectiveness of BNT162b2 mRNA and ChAdOx1 adenoviral vector COVID-19 vaccines in the general population. Bull. Natl. Res. Cent. 2021, 45, 150. [Google Scholar] [CrossRef] [PubMed]
- Harder, T.; Külper-Schiek, W.; Reda, S.; Treskova-Schwarzbach, M.; Koch, J.; Vygen-Bonnet, S.; Wichmann, O. Effectiveness of COVID-19 vaccines against SARS-CoV-2 infection with the Delta (B.1.617.2) variant: Second interim results of a living systematic review and meta-analysis, 1 January to 25 August 2021. Eurosurveillance 2021, 26, 2100920. [Google Scholar] [CrossRef] [PubMed]
- Catteau, L.; van Loenhout, J.; Stouten, V.; Billuart, M.; Hubin, P.; Haarhuis, F.; Wyndham, T.C. Couverture Vaccinale et Impact Épidémiologique de la Campagne de Vaccination COVID-19 en Belgique; Données Jusqu’au 31 Octobre 2021 Inclus; Sciensano: Brussels, Belgium, 2021; Available online: https://covid-19.sciensano.be/sites/default/files/Covid19/COVID-19_THEMATIC_REPORT_VaccineCoverageAndImpactReport_FR.pdf (accessed on 10 March 2022).
- Sharma, A.; Oda, G.; Holodniy, M. COVID-19 Vaccine Breakthrough Infections in the Veterans Health Administration. medRxiv 2021. [Google Scholar] [CrossRef]
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, S.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: A prospective, community-based, nested, case-control study. Lancet Infect Dis. 2022, 22, 43–55. [Google Scholar] [CrossRef]
- Peter, J.K.; Wegner, F.; Gsponer, S.; Helfenstein, F.; Roloff, T.; Tarnutzer, R.; Grosheintz, K.; Back, M.; Schaubhut, C.; Wagner, S.; et al. SARS-CoV-2 vaccine Alpha and Delta variant breakthrough infections are rare and mild, but happen relative early after vaccination. medRxiv 2021. [Google Scholar] [CrossRef]
- Fisman, D.N.; Lee, N.; Tuite, A.R. Timing of Breakthrough Infection Risk After Vaccination Against SARS-CoV-2. medRxiv 2022. [Google Scholar] [CrossRef]
- Uschner, D.; Bott, M.; Santacatterina, M.; Gunaratne, M.; Fette, L.M.; Burke, B.; Strylewicz, G.; Edelstein, S.L.; Lagarde, W.H.; Miller, K.; et al. Breakthrough SARS-CoV-2 Infections after Vaccination in North Carolina. medRxiv 2021. [Google Scholar] [CrossRef]
- Liu, C.; Lee, J.; Ta, C.; Soroush, A.; Rogers, J.R.; Kim, J.H.; Natarajan, K.; Zucker, J.; Weng, C. A Retrospective Analysis of COVID-19 mRNA Vaccine Breakthrough Infections—Risk Factors and Vaccine Effectiveness. medRxiv 2021. [Google Scholar] [CrossRef]
- Van Goethem, N.; Serrien, B.; Vandromme, M.; Wyndham-Thomas, C.; Catteau, L.; Brondeel, R.; Klamer, S.; Meurisse, M.; Cuypers, L.; André, E.; et al. Conceptual causal framework to assess the effect of SARS-CoV-2 variants on COVID-19 disease severity among hospitalized patients. Arch. Public Health 2021, 79, 185. [Google Scholar] [CrossRef] [PubMed]
- Proesmans, K.; Hancart, S.; Braeye, T.; Klamer, S.; Robesyn, E.; Djiena, A.; De Leeuw, F.; Mahieu, R.; Dreuw, A.; Hammami, N.; et al. COVID-19 Contact Tracing in Belgium: Main Indicators and Performance, January–September 2021. 2022. Available online: https://www.researchsquare.com/article/rs-1326456/v1 (accessed on 7 March 2022).
- COVID-19—Gevalsdefinitie en Testing|Coronavirus COVID-19. Available online: https://covid-19.sciensano.be/nl/covid-19-gevalsdefinitie-en-testing (accessed on 10 February 2022).
- Braeye, T.; Catteau, L.; Brondeel, R.; van Loenhout, J.; Proesmans, K.; Cornelissen, L.; Van Oyen, H.; Stouten, V.; Hubin, P.; Billuart, M.; et al. Vaccine Effectiveness against COVID19-Infection and Onward Transmission by Variant of Concern and Time Since Vaccination, Belgian Contact Tracing, 2021; Report No.: ID 4000559; Social Science Research Network: Rochester, NY, USA, 2022; Available online: https://papers.ssrn.com/abstract=4000559 (accessed on 31 January 2022).
- Population Movement|Statbel. Available online: https://statbel.fgov.be/en/themes/population/population-movement (accessed on 11 January 2022).
- Green, A.C.; Curtis, H.J.; Hulme, W.J.; Williamson, E.J.; McDonald, H.I.; Bhaskaran, K.; Rentsch, C.; Schultze, A.; MacKenna, B.; Mahalingasivam, V.; et al. Describing the population experiencing COVID-19 vaccine breakthrough following second vaccination in England: A cohort study from OpenSAFELY. medRxiv 2021. [Google Scholar] [CrossRef]
- Israel, A.; Merzon, E.; Schäffer, A.A.; Shenhar, Y.; Green., I.; Golan-Cohen, A.; Ruppin, E.; Magen, E.; Vinker, S. Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection in a large cohort. medRxiv 2021. [Google Scholar] [CrossRef]
- Reynolds, M.W.; Xie, Y.; Knuth, K.B.; Mack, C.D.; Brinkley, E.; Toovey, S.; Dreyer, N.A. COVID-19 vaccination breakthrough infections in a real-world setting: Using community reporters to evaluate vaccine effectiveness. medRxiv 2022. [Google Scholar] [CrossRef]
- Stouten, V.; Blot, K.; Haarhuis, F.; Serrien, B.; Hubin, P.; Vandromme, M.; Litzroth, A.; Chung, J.; Billuart, M.; Van Goethem, N.; et al. Occurrence and Patient Characteristics of COVID-19 Breakthrough Infections; Sciensano: Ixelles, Belgium, 2021. [Google Scholar]
- Shenai, M.B.; Rahme, R.; Noorchashm, H. Equivalency of Protection from Natural Immunity in COVID-19 Recovered Versus Fully Vaccinated Persons: A Systematic Review and Pooled Analysis. Cureus 2021, 10, e19102. [Google Scholar] [CrossRef]
- Cavanaugh, A.M.; Spicer, K.B.; Thoroughman, D.; Glick, C.; Winter, K. Reduced Risk of Reinfection with SARS-CoV-2 after COVID-19 Vaccination—Kentucky, May–June 2021. MMWR Morb. Mortal Wkly. Rep. 2021, 70, 1081–1083. [Google Scholar] [CrossRef]
- Gazit, S.; Shlezinger, R.; Perez, G.; Lotan, R.; Peretz, A.; Ben-Tov, A.; Herzel, E.; Alapi, H.; Cohen, D.; Muhsen, K.; et al. The Incidence of SARS-CoV-2 Reinfection in Persons with Naturally Acquired Immunity with and without Subsequent Receipt of a Single Dose of BNT162b2 Vaccine. Ann. Intern. Med. 2022. [Google Scholar] [CrossRef]
- Cohn, B.A.; Cirillo, P.M.; Murphy, C.C.; Krigbaum, N.Y.; Wallace, A.W. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science 2022, 375, 331–336. [Google Scholar] [CrossRef]
- Corchado-Garcia, J.; Zemmour, D.; Hughes, T.; Bandi, H.; Cristea-Platon, T.; Lenehan, P.; Pawlowski, C.; Bade, S.; O’Horo, J.C.; Gores, G.J.; et al. Analysis of the Effectiveness of the Ad26.COV2.S Adenoviral Vector Vaccine for Preventing COVID-19. JAMA Netw. Open 2021, 11, e2132540. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J.L.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabreraet, G.; et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N. Engl. J. Med. 2021, 385, e92. [Google Scholar]
- Bernal, J.L.; Andrews, N.; Gower, C.; Stowe, J.; Tessier, E.; Simmons, R.; Ramsay, M. Effectiveness of BNT162b2 mRNA vaccine and ChAdOx1 adenovirus vector vaccine on mortality following COVID-19. medRxiv 2021. [Google Scholar] [CrossRef]
- Cohn, B.A.; Cirillo, P.M.; Murphy, C.C.; Krigbaum, N.Y.; Wallace, A.W. Breakthrough SARS-CoV-2 infections in 620,000 U.S. Veterans, February 1, 2021 to August 13, 2021. medRxiv 2021. [Google Scholar] [CrossRef]
- Kshirsagar, M.; Mukherjee, S.; Nasir, M.; Becker, N.; Ferres, J.L.; Richardson, B.A. Risk of hospitalization and mortality after breakthrough SARS-CoV-2 infection by vaccine type and previous SARS-CoV-2 infection utilizing medical claims data. medRxiv 2021. [Google Scholar] [CrossRef]
- Würtz, A.M.; Kinnerup, M.B.; Pugdahl, K.; Schlünssen, V.; Vestergaard, J.M.; Nielsen, K.; Cramer, C.; Bonde, J.P.; Biering, K.; Carstensen, O.; et al. Healthcare workers’ SARS-CoV-2 infection rates during the second wave of the pandemic: Prospective follow-up study. medRxiv 2022. [Google Scholar] [CrossRef]
| Fully Vaccinated 1 (>14 Days) | Breakthrough Infection, n (%) | Incidence per 100 Person Years (95%CI) | Symptomatic Breakthrough Infection, n (%) | Incidence per 100 Person Years (95%CI) | |
|---|---|---|---|---|---|
| Overall | 8,062,600 | 373,070 | 11.2 (11.2–11.3) | 151,888 | 4.6 (4.6–4.6) |
| Sex | |||||
| Female | 4,139,926 (51.3%) | 199,900 (53.6%) | 11.3 (11.3–11.4) | 86,178 (56.7%) | 4.9 (4.8–4.9) |
| Male | 3,922,674 (48.7%) | 173,170 (46.4%) | 11.2 (11.1–11.2) | 65,710 (43.3%) | 4.2 (4.2–4.3) |
| Age categories (years) | |||||
| 18–24 | 742,418 (9.2%) | 31,133 (8.3%) | 12.9 (12.8–13.1) | 11,803 (7.8%) | 4.9 (4.8–5.0) |
| 25–34 | 1,178,450 (14.6%) | 63,119 (16.9%) | 15.3 (15.1–15.4) | 26,796 (17.6%) | 6.5 (6.4–6.6) |
| 35–44 | 1,256,702 (15.6%) | 86,505 (23.2%) | 18.4 (18.3–18.5) | 39,680 (26.1%) | 8.4 (8.4–8.5) |
| 45–54 | 1,361,440 (16.9%) | 71,714 (19.2%) | 13.0 (12.9–13.1) | 31,014 (20.4%) | 5.6 (5.6–5.7) |
| 55–64 | 1,418,415 (17.6%) | 57,292 (15.4%) | 9.4 (9.3–9.5) | 22,955 (15.1%) | 3.8 (3.7–3.8) |
| 65–74 | 1,124,756 (14.0%) | 35,622 (9.5%) | 6.9 (6.8–6.9) | 12,826 (8.4%) | 2.5 (2.4–2.5) |
| 75–84 | 671,571 (8.3%) | 19,761 (5.3%) | 5.7 (5.6–5.8) | 5651 (3.7%) | 1.6 (1.6–1.7) |
| ≥85 | 308,848 (3.8%) | 7924 (2.1%) | 4.6 (4.5–4.7) | 1163 (0.8%) | 0.7 (0.6–0.7) |
| Brand of primary vaccine | |||||
| BNT162b2 | 5,569,115 (69.1%) | 257,119 (68.9%) | 11.0 (10.9–11.0) | 102,860 (67.7%) | 4.4 (4.4–4.4) |
| mRNA-1273 | 673,221 (8.3%) | 21,614 (5.8%) | 7.6 (7.5–7.7) | 8866 (5.8%) | 3.1 (3.0–3.2) |
| ChAdOx1 | 1,406,797 (17.4%) | 68,525 (18.4%) | 12.7 (12.7–12.8) | 29,390 (19.3%) | 5.5 (5.4–5.5) |
| Ad26.COV2.S | 413,467 (5.1%) | 25,812 (6.9%) | 16.5 (16.3–16.7) | 10,772 (7.1%) | 6.9 (6.8–7.0) |
| Healthcare worker | |||||
| No | 7,577,369 (94.0%) | 339,041 (90.9%) | 11.1 (11.1–11.2) | 136,183 (89.7%) | 4.5 (4.4–4.5) |
| Yes | 485,231 (6.0%) | 34,029 (9.1%) | 12.4 (12.2–12.5) | 15,705 (10.3%) | 5.7 (5.6–5.8) |
| Prior COVID-19 infection 2 | |||||
| No | 7,354,342 (91.2%) | 363,710 (97.5%) | 12.0 (12.0–12.0) | 149,259 (98.3%) | 4.9 (4.9–5.0) |
| Yes | 708,258 (8.8%) | 9360 (2.5%) | 3.2 (3.2–3.3) | 2629 (1.7%) | 0.9 (0.9–0.9) |
| Number of COVID-19 tests 3 | |||||
| 0–3 | 7,928,881 (98.3%) | 345,544 (92.6%) | 10.6 (10.6–10.6) | 142,756 (94.0%) | 4.4 (4.4–4.4) |
| ≥4 | 133,719 (1.7%) | 27,526 (7.4%) | 45.0 (44.5–45.5) | 9132 (6.0%) | 14.9 (14.6–15.2) |
| Booster vaccine received | |||||
| No | 7,104,588 (88.1%) | 353,608 (94.8%) | 12.7 (12.6–12.7) | 146,431 (96.4%) | 5.2 (5.2–5.3) |
| Yes | 958,012 (11.9%) | 19,462 (5.2%) | 3.7 (3.6–3.7) | 5457 (3.6%) | 1.0 (1.0–1.1) |
| Factor | Hazard Ratio (95%CI) | p-Value |
|---|---|---|
| Age (per 10-year increase) | 0.88 (0.88–0.88) | <0.001 |
| Male sex | 0.99 (0.98–0.99) | <0.001 |
| Brand primary vaccine (ref: BNT162b2) | ||
| mRNA-1273 | 0.68 (0.67–0.69) | <0.001 |
| ChAdOx1 | 1.68 (1.66–1.69) | <0.001 |
| Ad26.COV2.S | 1.54 (1.52–1.56) | <0.001 |
| Healthcare worker | 0.60 (0.60–0.61) | <0.001 |
| Prior COVID-19 infection | 0.23 (0.23–0.24) | <0.001 |
| Received mRNA booster | 0.44 (0.43–0.45) | <0.001 |
| Background positivity rate | 1.33 (1.32–1.33) | <0.001 |
| High frequency testing profile | 3.87 (3.82–3.92) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stouten, V.; Hubin, P.; Haarhuis, F.; van Loenhout, J.A.F.; Billuart, M.; Brondeel, R.; Braeye, T.; Van Oyen, H.; Wyndham-Thomas, C.; Catteau, L. Incidence and Risk Factors of COVID-19 Vaccine Breakthrough Infections: A Prospective Cohort Study in Belgium. Viruses 2022, 14, 802. https://doi.org/10.3390/v14040802
Stouten V, Hubin P, Haarhuis F, van Loenhout JAF, Billuart M, Brondeel R, Braeye T, Van Oyen H, Wyndham-Thomas C, Catteau L. Incidence and Risk Factors of COVID-19 Vaccine Breakthrough Infections: A Prospective Cohort Study in Belgium. Viruses. 2022; 14(4):802. https://doi.org/10.3390/v14040802
Chicago/Turabian StyleStouten, Veerle, Pierre Hubin, Freek Haarhuis, Joris A. F. van Loenhout, Matthieu Billuart, Ruben Brondeel, Toon Braeye, Herman Van Oyen, Chloé Wyndham-Thomas, and Lucy Catteau. 2022. "Incidence and Risk Factors of COVID-19 Vaccine Breakthrough Infections: A Prospective Cohort Study in Belgium" Viruses 14, no. 4: 802. https://doi.org/10.3390/v14040802
APA StyleStouten, V., Hubin, P., Haarhuis, F., van Loenhout, J. A. F., Billuart, M., Brondeel, R., Braeye, T., Van Oyen, H., Wyndham-Thomas, C., & Catteau, L. (2022). Incidence and Risk Factors of COVID-19 Vaccine Breakthrough Infections: A Prospective Cohort Study in Belgium. Viruses, 14(4), 802. https://doi.org/10.3390/v14040802






