What Is the Role of Therapeutic Plasma Exchange as an Adjunctive Treatment in Severe COVID-19: A Systematic Review
Abstract
:1. Introduction
2. Methods
3. Results
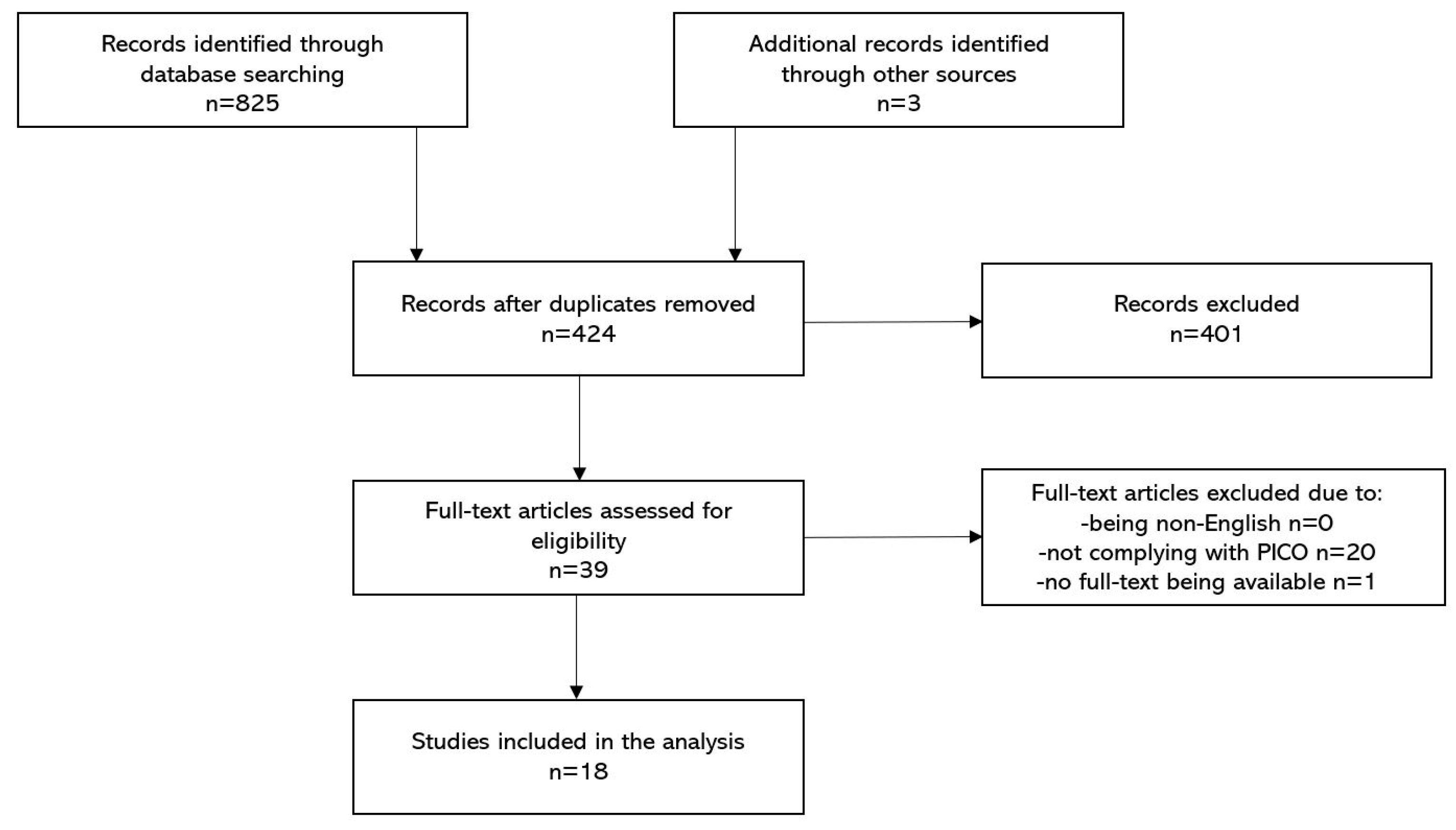
3.1. Included Studies
3.2. Quality Assessment
3.3. Patient Characteristics
3.4. Interventions
3.5. Outcomes
3.6. Side Effects
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cummings, M.J.; Baldwin, M.R.; Abrams, D.; Jacobson, S.D.; Meyer, B.J.; Balough, E.M.; Aaron, J.G.; Claassen, J.; Rabbani, L.E.; Hastie, J.; et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 2020, 395, 1763–1770. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; HLH Across Speciality Collaboration, UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Giannis, D.; Ziogas, I.A.; Gianni, P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J. Clin. Virol. 2020, 127, 104362. [Google Scholar] [CrossRef] [PubMed]
- Rico-Mesa, J.S.; Rosas, D.; Ahmadian-Tehrani, A.; White, A.; Anderson, A.S.; Chilton, R. The Role of Anticoagulation in COVID-19-Induced Hypercoagulability. Curr. Cardiol. Rep. 2020, 22, 53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, M.; Zhang, S.; Xia, P.; Cao, W.; Jiang, W.; Chen, H.; Ding, X.; Zhao, H.; Zhang, H.; et al. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N. Engl. J. Med. 2020, 382, e38. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C. Acute Respiratory Distress Syndrome as an Organ Phenotype of Vascular Microthrombotic Disease: Based on Hemostatic Theory and Endothelial Molecular Pathogenesis. Clin. Appl. Thromb. 2019, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Teachey, D.T.; Lacey, S.F.; Shaw, P.A.; Melenhorst, J.J.; Maude, S.L.; Jeffrey, F.; Pequignot, E.; Gonzalez, V.E.; Chen, F.; Finklestein, J.; et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia. Cancer Discov. 2016, 6, 664–679. [Google Scholar] [CrossRef] [Green Version]
- Henry, B.M.; Aggarwal, G.; Wong, J.; Benoit, S.; Vikse, J.; Plebani, M.; Lippi, G. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis. Am. J. Emerg. Med. 2020, 38, 1722–1726. [Google Scholar] [CrossRef]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020, 19, 102537. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhou, W.; Yan, X.; Guo, T.; Wang, B.; Xia, H.; Ye, L.; Xiong, J.; Jiang, Z.; Liu, Y.; et al. Prognostic Value of C-Reactive Protein in Patients With Coronavirus 2019. Clin. Infect. Dis. 2020, 71, 2174–2179. [Google Scholar] [CrossRef]
- Jose, R.J.P.; Manuel, A. COVID-19 cytokine storm: The interplay between inflammation and coagulation. Lancet Respir. Med. 2020, 8, e46–e47. [Google Scholar] [CrossRef]
- Clark, W.; Huang, S. Introduction to therapeutic plasma exchange. Transfus. Apher. Sci. 2019, 58, 228–229. [Google Scholar] [CrossRef]
- Padmanabhan, A.; Connelly-Smith, L.; Aqui, N.; Balogun, R.A.; Klingel, R.; Meyer, E.; Pham, H.P.; Schneiderman, J.; Witt, V.; Wu, Y.; et al. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice—Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Eighth Special Issue. J. Clin. Apher. 2019, 34, 171–354. [Google Scholar] [CrossRef] [PubMed]
- Gucyetmez, B.; Atalan, H.K.; Sertdemir, I.; Cakir, U.; Telci, L.; COVID-19 Study Group. Therapeutic plasma exchange in patients with COVID-19 pneumonia in intensive care unit: A retrospective study. Crit. Care 2020, 24, 492. [Google Scholar] [CrossRef] [PubMed]
- Keith, P.; Day, M.; Perkins, L.; Moyer, L.; Hewitt, K.; Wells, A. A novel treatment approach to the novel coronavirus: An argument for the use of therapeutic plasma exchange for fulminant COVID-19. Crit. Care 2020, 24, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhai, H.; Ma, S.; Chen, J.; Gao, Y. Efficacy of therapeutic plasma exchange in severe COVID-19 patients. Br. J. Haematol. 2020, 190, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Morath, C.; Weigand, M.A.; Zeier, M.; Speer, C.; Tiwari-Heckler, S.; Merle, U. Plasma exchange in critically ill COVID-19 patients. Crit. Care 2020, 24, 481. [Google Scholar] [CrossRef] [PubMed]
- Faqihi, F.; Alharthy, A.; Alodat, M.; Kutsogiannis, D.J.; Brindley, P.G.; Karakitsos, D. Therapeutic plasma exchange in adult critically ill patients with life-threatening SARS-CoV-2 disease: A pilot study. J. Crit. Care 2020, 60, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Nasa, P.; Raouf, M.; Gupta, M.; Dewedar, H.; Mohammad, H.; Al Rais, Z.; Baqer, M.A.; Alsabbah, A.; Ibrahim, Y.; et al. Therapeutic plasma exchange followed by convalescent plasma transfusion in critical COVID-19—An exploratory study. Int. J. Infect. Dis. 2021, 102, 332–334. [Google Scholar] [CrossRef]
- Gluck, W.L.; Callahan, S.P.; Brevetta, R.A.; Stenbit, A.E.; Smith, W.M.; Martin, J.C.; Blenda, A.V.; Arce, S.; Edenfield, W.J. Efficacy of therapeutic plasma exchange in the treatment of penn class 3 and 4 cytokine release syndrome complicating COVID-19. Respir. Med. 2020, 175, 106188. [Google Scholar] [CrossRef]
- Fernandez, J.; Ginès, J.G.; Olivas, P.; Costa, M.; Nieto, S.; Mateo, D.; Sánchez, M.B.; Aguilar, F.; Bassegoda, O.; Covid Clinic Critical Care (CCCC) Group; et al. Plasma Exchange: An Effective Rescue Therapy in Critically Ill Patients With Coronavirus Disease 2019 Infection. Crit. Care Med. 2020, 48, e1350–e1355. [Google Scholar] [CrossRef]
- Dogan, L.; Kaya, D.; Sarıkaya, Z.T.; Zengin, R.; Dincer, A.; Akinci, I.O.; Afsar, N. Plasmapheresis treatment in COVID-19–related autoimmune meningoencephalitis: Case series. Brain Behav. Immun. 2020, 87, 155–158. [Google Scholar] [CrossRef]
- Adeli, S.H.; Asghari, A.; Tabarraii, R.; Shajari, R.; Afshari, S.; Kalhor, N.; Vafaeimanesh, J. Therapeutic plasma exchange as a rescue therapy in patients with coronavirus disease 2019: A case series. Pol. Arch. Intern. Med. 2020, 130, 455–458. [Google Scholar] [CrossRef] [PubMed]
- de Prost, N.; Bastard, P.; Arrestier, R.; Fourati, S.; Mahévas, M.; Burrel, S.; Dorgham, K.; Gorochov, G.; Tandjaoui-Lambiotte, Y.; Azzaoui, I.; et al. Plasma Exchange to Rescue Patients with Autoantibodies Against Type I Interferons and Life-Threatening COVID-19 Pneumonia. J. Clin. Immunol. 2021, 41, 536–544. [Google Scholar] [CrossRef]
- Hashemian, S.M.; Shafigh, N.; Afzal, G.; Jamaati, H.; Tabarsi, P.; Marjani, M.; Malekmohammad, M.; Mortazavi, S.M.; Khoundabi, B.; Mansouri, D.; et al. Plasmapheresis reduces cytokine and immune cell levels in COVID-19 patients with acute respiratory distress syndrome (ARDS). Pulmonology 2020. [Google Scholar] [CrossRef] [PubMed]
- Keith, P.D.; Scott, L.K.; Weaver, K.E.; Day, M.; Choe, C.; Perkins, L.; Moyer, L.; Hays, E.; French, M.; Hewitt, K.; et al. Treatment of Critically Ill Coronavirus Disease 2019 Patients With Adjunct Therapeutic Plasma Exchange: A Single-Center Retrospective Case Series. Crit. Care Explor. 2020, 2, e0223. [Google Scholar] [CrossRef]
- Matsushita, Y.; Kusaoi, M.; Hiki, M.; Murayama, G.; Abe, Y.; Nozawa, K.; Takahashi, K.; Yamaji, K.; Tamura, N.; Naito, T. Combination therapy with plasma exchange and glucocorticoid may be effective for severe COVID-19 infection: A retrospective observational study. Ther. Apher. Dial. 2021, 25, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Roshandel, E.; Sankanian, G.; Salimi, M.; Jalili, A.; Salari, S.; Sadeghi, A.; Hashemian, S.M.; Moshari, M.R.; Pirsalehi, A.; Hajifathali, A. Plasma exchange followed by convalescent plasma transfusion in COVID-19 patients. Transfus. Apher. Sci. 2021, 103141. [Google Scholar] [CrossRef]
- Truong, A.D.; Auld, S.C.; Barker, N.A.; Friend, S.; Wynn, A.T.; Cobb, J.; Sniecinski, R.M.; Tanksley, C.; Polly, D.M.; Gaddh, M.; et al. Therapeutic plasma exchange for COVID-19-associated hyperviscosity. Transfusion 2021, 61, 1029–1034. [Google Scholar] [CrossRef]
- Khamis, F.; Al-Zakwani, I.; Al Hashmi, S.; Al Dowaiki, S.; Al Bahrani, M.; Pandak, N.; Al Khalili, H.; Memish, Z. Therapeutic plasma exchange in adults with severe COVID-19 infection. Int. J. Infect. Dis. 2020, 99, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Kamran, S.M.; Mirza, Z.-E.-H.; Naseem, A.; Liaqat, J.; Fazal, I.; Alamgir, W.; Saeed, F.; Saleem, S.; Nisar, S.; Yousaf, M.A.; et al. Therapeutic plasma exchange for coronavirus disease-2019 triggered cytokine release syndrome; a retrospective propensity matched control study. PLoS ONE 2021, 16, e0244853. [Google Scholar] [CrossRef] [PubMed]
- Faqihi, F.; Alharthy, A.; Abdulaziz, S.; Balhamar, A.; Alomari, A.; AlAseri, Z.; Tamim, H.; Alqahtani, S.A.; Kutsogiannis, D.J.; Brindley, P.G.; et al. Therapeutic plasma exchange in patients with life-threatening COVID-19: A randomised controlled clinical trial. Int. J. Antimicrob. Agents 2021, 57, 106334. [Google Scholar] [CrossRef]
- Papoutsi, E.; Giannakoulis, V.G.; Xourgia, E.; Routsi, C.; Kotanidou, A.; Siempos, I.I. Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: A systematic review and meta-analysis of non-randomized cohort studies. Crit. Care 2021, 25, 121. [Google Scholar] [CrossRef]
- McGonagle, D.; O’Donnell, J.S.; Sharif, K.; Emery, P.; Bridgewood, C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020, 2, e437–e445. [Google Scholar] [CrossRef]
- Guirao, J.J.; Cabrera, C.M.; Jiménez, N.; Rincón, L.; Urra, J.M. High serum IL-6 values increase the risk of mortality and the severity of pneumonia in patients diagnosed with COVID-19. Mol. Immunol. 2020, 128, 64–68. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, H.; Mu, S.; Wei, W.; Jin, C.; Tong, C.; Song, Z.; Zha, Y.; Xue, Y.; Gu, G. Lactate dehydrogenase, an independent risk factor of severe COVID-19 patients: A retrospective and observational study. Aging 2020, 12, 11245–11258. [Google Scholar] [CrossRef] [PubMed]
- Kernan, K.F.; Carcillo, J.A. Hyperferritinemia and inflammation. Int. Immunol. 2017, 29, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Bowles, L.; Platton, S.; Yartey, N.; Dave, M.; Lee, K.; Hart, D.P.; Macdonald, V.; Green, L.; Sivapalaratnam, S.; Pasi, K.J.; et al. Lupus Anticoagulant and Abnormal Coagulation Tests in Patients with Covid-19. N. Engl. J. Med. 2020, 383, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Shemin, D.; Briggs, D.; Greenan, M. Complications of therapeutic plasma exchange: A prospective study of 1727 procedures. J. Clin. Apher. 2007, 22, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Basic-Jukic, N.; Kes, P.; Glavas-Boras, S.; Brunetta, B.; Bubic-Filipi, L.; Puretic, Z. Complications of Therapeutic Plasma Exchange: Experience with 4857 Treatments. Ther. Apher. Dial. 2005, 9, 391–395. [Google Scholar] [CrossRef]
- Gala-Błądzińska, A.; Mazur, K.; Dębiec, A.; Gargasz, K.; Bartosik-Psujek, H. Safety and tolerability of therapeutic plasma exchange in autoimmune neurological diseases—A retrospective single-centre analysis. Neurol. Neurochir. Polska 2020, 54, 344–349. [Google Scholar] [CrossRef]
- Tabibi, S.; Tabibi, T.; Conic, R.R.Z.; Banisaeed, N.; Streiff, M.B. Therapeutic Plasma Exchange: A potential Management Strategy for Critically Ill COVID-19 Patients. J. Intensiv. Care Med. 2020, 35, 827–835. [Google Scholar] [CrossRef]
- Thölking, G.; Mesters, R.; Dittrich, R.; Pavenstädt, H.; Kümpers, P.; Reuter, S. Assessment of Hemostasis after Plasma Exchange Using Rotational Thrombelastometry (ROTEM). PLoS ONE 2015, 10, e0130402. [Google Scholar] [CrossRef] [Green Version]
- Singhania, N.; Bansal, S.; Nimmatoori, D.P.; Ejaz, A.A.; McCullough, P.A.; Singhania, G. Current Overview on Hypercoagulability in COVID-19. Am. J. Cardiovasc. Drugs 2020, 20, 393–403. [Google Scholar] [CrossRef]
- Spiezia, L.; Boscolo, A.; Poletto, F.; Cerruti, L.; Tiberio, I.; Campello, E.; Navalesi, P.; Simioni, P. COVID-19-Related Severe Hypercoagulability in Patients Admitted to Intensive Care Unit for Acute Respiratory Failure. Thromb. Haemost. 2020, 120, 998–1000. [Google Scholar] [CrossRef]
- Simonovich, V.A.; Pratx, L.D.B.; Scibona, P.; Beruto, M.V.; Vallone, M.G.; Vázquez, C.; Savoy, N.; Giunta, D.H.; Pérez, L.G.; PlasmAr Study Group; et al. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N. Engl. J. Med. 2021, 384, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Pluta, M.; Dziech, M.; Jaworski, T.; Krzych, Ł. Is this TRALI, TACO, or just pneumonia?—A case report of acute respiratory failure. Anestezjol. Intensywna Ter. 2019, 51, 414–416. [Google Scholar] [CrossRef] [PubMed]
| Participants | Patients with severe course of COVID-19. |
| Interventions | Therapeutic plasma exchange (any type) as an adjunctive treatment. |
| Control | Due to limited number of studies and their methodological type, a control group was not required to include in the study. |
| Outcomes | Mortality and changes in various biomarkers, along with which additional attention was given to safety issues in retrieved papers. |
| Author | Study Type | Population | Intervention | Median Time from First Symptoms to TPE Initiation | Replacement Fluid | Adverse Effects of TPE | Outcome |
|---|---|---|---|---|---|---|---|
| Zhang et al. [21] | Case-series | 3 severely ill patients | 1 TPE session | 15 days | FFP | N/A | Mortality (day 14): 0% |
| Morath et al. [22] | Case-series | 5 patients with COVID-19-induced multi-organ failure and ARDS | All patients received 1–2 TPE sessions | 12 days | FFP | N/A | Mortality: 20% |
| Faqihi et al. [23] | Case-series | 10 patients with ARDS, APACHE II score >20, septic shock or cytokine release syndrome | All patients received 5–7 TPE | 6.5 days | 5% albumin or FFP | None | Mortality (day 28): 10% |
| Gucyemetz et al. [17] | Case-control | 73 patients with COVID-19-related pneumonia | 18 patients received 3 TPE sessions | N/A | N/A | N/A | Mortality (non-TPE vs. TPE): 58.3% vs. 8.3% * |
| Khamis et al. [35] | Case-control | 31 critically ill patients with COVID-19-related ARDS, severe pneumonia, septic shock or multiple organ dysfunction syndrome | 11 patients underwent 5 TPE sessions | N/A | FFP | One hypotension episode treated with fluid bolus and hydrocortisone | Mortality (non-TPE vs. TPE, day 28): 35% vs. 0% * |
| Jaiswal et al. [24] | Case-series | 14 patients with severe COVID-19 infection according to WHO classification | All patients received 1 TPE session | 9 days | Convalescent Plasma | 3 cases of hypotension treated with fluid bolus | Mortality (day 28): 28.6% |
| Gluck et al. [25] | Case-series | 10 patients with COVID-19 and Penn class 3 and 4 cytokine release syndrome | All patients received 5 TPE sessions | N/A | 5% albumin or FFP | None | Mortality (day 14): 0% |
| Karman et al. [36] | PSM | 90 patients with severe COVID-19 infection and cytokine release syndrome | 45 patients received one TPE until resolution of the disease | N/A | FFP and normal saline in 2:1 ratio | 1 femoral artery puncture and thrombophlebitis treated accordingly | Mortality (non-TPE vs. TPE, day 28): 38.5% vs. 8.9% * |
| Fernandez et al. [26] | Case-series | 4 critically ill patients with COVID-19 | 2–6 plasma exchange sessions | 20 days | 5% albumin + FFP | 1 episode of hypotension and tachycardia | Mortality (day 28): 0% |
| Dogan et al. [27] | Case-series | 6 patients with COVID-19–related autoimmune meningoencephalitis | 1–9 plasma exchange sessions | N/A | 5% albumin | N/A | Mortality (day 14): 16.7% |
| Adeli et al. [28] | Case-series | 8 patients | 3–5 plasma exchange sessions | N/A | FFP + albumin solution + calcium gluconate | None | Mortality (no specified day): 12.5% |
| De Prost et al. [29] | Case-series | 4 critically-ill patients with high blood concentrations of neutralizing autoantibodies against type I interferons | 3–4 plasma exchange sessions | 18 days | 5% albumin solution | None | Mortality (no specified day): 50% |
| Faqihi et al. [37] | RCT | 87 intubated patients with either ARDS, APACHE II score >20 pts, septic shock or cytokine release syndrome | 43 patients received 1–5 (median 3) plasma exchange sessions | 8 days | FFP | None | Mortality (TPE vs non-TPE, day 35): 20.9% vs. 34.1 % (p = 0.09) |
| Hashemian et al. [30] | Case-series | 15 patients | 1–3 TPE sessions | N/A | 5% albumin solution + 0.9% NaCl/convalescent plasma | N/A | Mortality (no specified day): 40% |
| Keith et al. [31] | Case-series | 8 patients | 1–7 plasma exchange sessions | N/A | FFP | N/A | Mortality (no specified day): 25% |
| Matsushita et al. [32] | Case-series | 5 patients with PaO2/FiO2 ratio of less than 200 mmHg and/or labored respiration and/or tracheal intubation | 3–7 plasma exchange sessions | 14 days | FFP | N/A | Mortality (no specified day): 60% |
| Roshandel et al. [33] | Case-series | 5 COVID-19 patients with respiratory failure | 2 standard plasma exchange sessions | 39 days | FFP + 5% albumin, then 0.9% NaCl/convalescent plasma | N/A | Mortality (no specified day): 20% |
| Truong et al. [34] | Case-series | 6 critically ill patients with plasma hyperviscosity | 2–3 plasma exchange sessions | N/A | FFP | None | Mortality (no specified day): 50% |
| Parameter | Values: Median (IQR) |
|---|---|
| pre-TPE PaO2/FiO2 (mmHg) | 132 (112.5–153.5) [21,23,24,25,29,30,35,37] |
| post-TPE PaO2/FiO2 (mmHg) | 224 (216.5–300) [21,23,24,29,30,35,37] |
| pre-TPE CRP (mg/L) | 132 (79–168.5) [17,21,22,23,24,25,26,27,29,30,31,33,34,35,36,37] |
| post-TPE CRP (mg/L) | 28.5 (11.1–47.5) [17,21,22,23,24,25,30,31,33,34,35,37] |
| pre-TPE Lymphocytes (109/L) | 0.7 (0.58–1.0) [17,21,23,24,25,26,29,35,37] |
| post-TPE Lymphocytes (109/L) | 1.04 (1.0–1.5) [17,21,23,24,35,37] |
| pre-TPE IL-6 (pg/mL) | 118.7 (25.2–295.3) [17,21,22,23,25,26,27,32,35,36,37] |
| post-TPE IL-6 (pg/mL) | 18.5 (5.7–35) [17,21,22,23,26,30,33,35] |
| pre-TPE LDH (U/L) | 576.5 (492.5–849.5) [17,21,22,23,26,27,33,36,37] |
| post-TPE LDH (U/L) | 245.5 (236–440) [17,22,23,26,33,37] |
| pre-TPE D-Dimers (mg/L) | 6.05 (4.5–7.6) [17,22,23,24,25,26,27,28,29,31,34,35,36,37] |
| post-TPE D-Dimers (mg/L) | 2.6 (1.3–4.0) [17,22,23,24,26,31,33,34,35,37] |
| pre-TPE Ferritin (ug/L) | 1332 (1125–1444) [17,22,23,24,26,27,30,31,35,36,37] |
| post-TPE Ferritin (ug/L) | 494 (352–842) [17,22,23,24,26,30,31,35,37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzych, Ł.J.; Putowski, Z.; Czok, M.; Hofman, M. What Is the Role of Therapeutic Plasma Exchange as an Adjunctive Treatment in Severe COVID-19: A Systematic Review. Viruses 2021, 13, 1484. https://doi.org/10.3390/v13081484
Krzych ŁJ, Putowski Z, Czok M, Hofman M. What Is the Role of Therapeutic Plasma Exchange as an Adjunctive Treatment in Severe COVID-19: A Systematic Review. Viruses. 2021; 13(8):1484. https://doi.org/10.3390/v13081484
Chicago/Turabian StyleKrzych, Łukasz J., Zbigniew Putowski, Marcelina Czok, and Mariusz Hofman. 2021. "What Is the Role of Therapeutic Plasma Exchange as an Adjunctive Treatment in Severe COVID-19: A Systematic Review" Viruses 13, no. 8: 1484. https://doi.org/10.3390/v13081484
APA StyleKrzych, Ł. J., Putowski, Z., Czok, M., & Hofman, M. (2021). What Is the Role of Therapeutic Plasma Exchange as an Adjunctive Treatment in Severe COVID-19: A Systematic Review. Viruses, 13(8), 1484. https://doi.org/10.3390/v13081484







