Cell Culture Systems and Drug Targets for Hepatitis A Virus Infection
Abstract
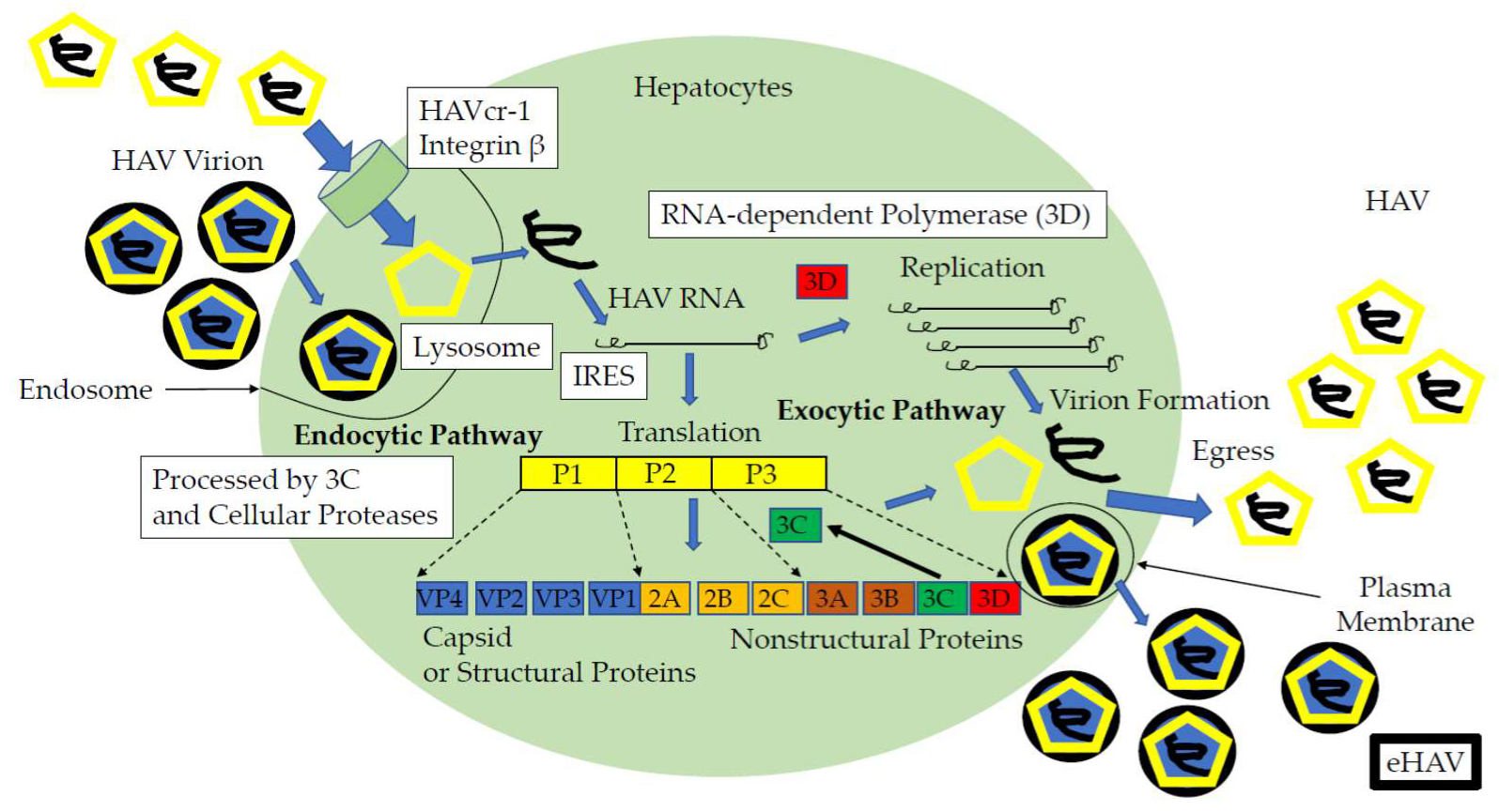
:1. Introduction
2. Cell Culture Systems for HAV Replication
2.1. Cell Lines Permissive for HAV Replication
2.2. Cell Culture for HAV Vaccine Development
2.3. Cell Culture for the Development of Anti-HAV Drugs
3. HAV Subgenomic Replicon for the Study of Antiviral Drugs
4. Blocking the Entry Pathway as an Antiviral Strategy
5. Inhibiting the HAV IRES-Mediated Translation in Human Hepatoma Cell Lines
6. HAV 3C Protease and 3D Polymerase May Be other Candidates for Anti-HAV Drug Targets
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gupta, E.; Ballani, N.; Kumar, M.; Sarin, S.K. Role of non-hepatotropic viruses in acute sporadic viral hepatitis and acute-on-chronic liver failure in adults. Indian J. Gastroenterol. 2015, 34, 448–452. [Google Scholar] [CrossRef]
- Tominaga, A.; Kanda, T.; Akiike, T.; Komoda, H.; Ito, K.; Abe, A.; Aruga, A.; Kaneda, S.; Saito, M.; Kiyohara, T.; et al. Hepatitis A outbreak associated with a revolving sushi bar in Chiba, Japan: Application of molecular epidemiology. Hepatol. Res. 2012, 42, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, P.; Brett, C.; Batool, A.; Sapra, A. Hepatitis A Vaccine; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554604/ (accessed on 11 May 2020).
- Tsukada, R.; Ono, S.; Kobayashi, H.; Wada, Y.; Nishizawa, K.; Fujii, M.; Takeuchi, M.; Kuroiwa, K.; Kobayashi, Y.; Ishii, K.; et al. A Cluster of Hepatitis A Infections Presumed to be Related to Asari Clams and Investigation of the Spread of Viral Contamination from Asari Clams. Jpn. J. Infect. Dis. 2019, 72, 44–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, H.; Shiota, T.; Yoshizaki, S.; Saito-Obata, M.; Malbas, F.F., Jr.; Lupisan, S.P.; Oshitani, H.; Takeda, N.; Muramatsu, M.; Wakita, T.; et al. Detection of Subgenotype IA and IIIA Hepatitis A Viruses in Rivers Flowing through Metro Manila, the Philippines. Jpn. J. Infect. Dis. 2019, 72, 53–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinohara, N.; Owada, T.; Tanaka, A.; Matsubayashi, K.; Nagai, T.; Satake, M. Hepatitis A virus and hepatitis E virus prevalence relates to Human Immunodeficiency Virus infection in Japanese male blood donors. Microbiol. Immunol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.A.; Hofmeister, M.G.; Kupronis, B.A.; Lin, Y.; Xia, G.L.; Yin, S.; Teshale, E. Increase in Hepatitis A Virus Infections-United States, 2013–2018. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 413–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamura, T.; Ishii, K.; Kanda, T.; Tawada, A.; Sekimoto, T.; Wu, S.; Nakamoto, S.; Arai, M.; Fujiwara, K.; Imazeki, F.; et al. Possible widespread presence of hepatitis A virus subgenotype IIIA in Japan: Recent trend of hepatitis A causing acute liver failure. Hepatol. Res. 2012, 42, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Maki, Y.; Kimizuka, Y.; Sasaki, H.; Yamamoto, T.; Hamakawa, Y.; Tagami, Y.; Miyata, J.; Hayashi, N.; Fujikura, Y.; Kawana, A. Hepatitis A virus-associated fulminant hepatitis with human immunodeficiency virus coinfection. J. Infect. Chemother. 2020, 26, 282–285. [Google Scholar] [CrossRef]
- Feng, Z.; Hensley, L.; McKnight, K.L.; Hu, F.; Madden, V.; Ping, L.; Jeong, S.H.; Walker, C.; Lanford, R.E.; Lemon, S.M. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature 2013, 496, 367–371. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.I.; Rosenblum, B.; Ticehurst, J.R.; Daemer, R.J.; Feinstone, S.M.; Purcell, R.H. Complete nucleotide sequence of an attenuated hepatitis A virus: Comparison with wild-type virus. Proc. Natl. Acad. Sci. USA 1987, 84, 2497–2501. [Google Scholar] [CrossRef] [Green Version]
- Debing, Y.; Neyts, J.; Thibaut, H.J. Molecular biology and inhibitors of hepatitis A virus. Med. Res. Rev. 2014, 34, 895–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanda, T.; Nakamoto, S.; Wu, S.; Nakamura, M.; Jiang, X.; Haga, Y.; Sasaki, R.; Yokosuka, O. Direct-acting Antivirals and Host-targeting Agents against the Hepatitis A Virus. J. Clin. Transl. Hepatol. 2015, 3, 205–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanda, T.; Zhang, B.; Kusov, Y.; Yokosuka, O.; Gauss-Müller, V. Suppression of hepatitis A virus genome translation and replication by siRNAs targeting the internal ribosomal entry site. Biochem. Biophys. Res. Commun. 2005, 330, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Yokosuka, O.; Imazeki, F.; Fujiwara, K.; Nagao, K.; Saisho, H. Amantadine inhibits hepatitis A virus internal ribosomal entry site-mediated translation in human hepatoma cells. Biochem. Biophys. Res. Commun. 2005, 331, 621–629. [Google Scholar] [CrossRef]
- Kanda, T.; Imazeki, F.; Nakamoto, S.; Okitsu, K.; Fujiwara, K.; Yokosuka, O. Internal ribosomal entry-site activities of clinical isolate-derived hepatitis A virus and inhibitory effects of amantadine. Hepatol. Res. 2010, 40, 415–423. [Google Scholar] [CrossRef]
- Yang, L.; Kiyohara, T.; Kanda, T.; Imazeki, F.; Fujiwara, K.; Gauss-Müller, V.; Ishii, K.; Wakita, T.; Yokosuka, O. Inhibitory effects on HAV IRES-mediated translation and replication by a combination of amantadine and interferon-alpha. Virol. J. 2010, 7, 212. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Isaacson, J.; Patick, A.K.; Blair, W.S. High-throughput human immunodeficiency virus type 1 (HIV-1) full replication assay that includes HIV-1 Vif as an antiviral target. Antimicrob. Agents Chemother. 2005, 49, 3833–3841. [Google Scholar] [CrossRef] [Green Version]
- Verrier, E.R.; Colpitts, C.C.; Schuster, C.; Zeisel, M.B.; Baumert, T.F. Cell Culture Models for the Investigation of Hepatitis B and D Virus Infection. Viruses 2016, 8, 261. [Google Scholar] [CrossRef]
- Pintó, R.M.; Pérez-Rodríguez, F.J.; D’Andrea, L.; de Castellarnau, M.; Guix, S.; Bosch, A. Hepatitis A Virus Codon Usage: Implications for Translation Kinetics and Capsid Folding. Cold Spring Harb. Perspect. Med. 2018, 8. [Google Scholar] [CrossRef]
- Provost, P.J.; Hilleman, M.R. Propagation of human hepatitis A virus in cell culture in vitro. Proc. Soc. Exp. Biol. Med. 1979, 160, 213–221. [Google Scholar] [CrossRef]
- Frösner, G.G.; Deinhardt, F.; Scheid, R.; Gauss-Müller, V.; Holmes, N.; Messelberger, V.; Siegl, G.; Alexander, J.J. Propagation of human hepatitis A virus in a hepatoma cell line. Infection 1979, 7, 303–305. [Google Scholar] [CrossRef]
- Gauss-Müller, V.; Frösner, G.G.; Deinhardt, F. Propagation of hepatitis A virus in human embryo fibroblasts. J. Med. Virol. 1981, 7, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Shibayama, T.; Sato, A.; Suzuki, S.; Ichida, F.; Hamada, C. Propagation of human hepatitis A virus in conventional cell lines. J. Med. Virol. 1981, 7, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Daemer, R.J.; Feinstone, S.M.; Gust, I.D.; Purcell, R.H. Propagation of human hepatitis A virus in African green monkey kidney cell culture: Primary isolation and serial passage. Infect. Immun. 1981, 32, 388–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemon, S.M.; Binn, L.N.; Marchwicki, R.H. Radioimmunofocus assay for quantitation of hepatitis A virus in cell cultures. J. Clin. Microbiol. 1983, 17, 834–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheeler, C.M.; Fields, H.A.; Schable, C.A.; Meinke, W.J.; Maynard, J.E. Adsorption, purification, and growth characteristics of hepatitis A virus strain HAS-15 propagated in fetal rhesus monkey kidney cells. J. Clin. Microbiol. 1986, 23, 434–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crance, J.M.; Passagot, J.; Biziagos, E.; Deloince, R. Continuous production of hepatitis A virus in PLC/PRF/5 cell cultures: Use of antigen for serology. J. Virol. Methods 1987, 18, 193–203. [Google Scholar] [CrossRef]
- Robertson, B.H.; Khanna, B.; Brown, V.K.; Margolis, H.S. Large scale production of hepatitis A virus in cell culture: Effect of type of infection on virus yield and cell integrity. J. Gen. Virol. 1988, 69, 2129–2134. [Google Scholar] [CrossRef]
- Tsarev, S.A.; Emerson, S.U.; Balayan, M.S.; Ticehurst, J.; Purcell, R.H. Simian hepatitis A virus (HAV) strain AGM-27: Comparison of genome structure and growth in cell culture with other HAV strains. J. Gen. Virol. 1991, 72, 1677–1683. [Google Scholar] [CrossRef]
- Cohen, J.I.; Ticehurst, J.R.; Feinstone, S.M.; Rosenblum, B.; Purcell, R.H. Hepatitis A virus cDNA and its RNA transcripts are infectious in cell culture. J. Virol. 1987, 61, 3035–3039. [Google Scholar] [CrossRef] [Green Version]
- Emerson, S.U.; Huang, Y.K.; McRill, C.; Lewis, M.; Shapiro, M.; London, W.T.; Purcell, R.H. Molecular basis of virulence and growth of hepatitis A virus in cell culture. Vaccine 1992, 10 (Suppl. 1), S36–S39. [Google Scholar] [CrossRef]
- Emerson, S.U.; Huang, Y.K.; McRill, C.; Lewis, M.; Purcell, R.H. Mutations in both the 2B and 2C genes of hepatitis A virus are involved in adaptation to growth in cell culture. J. Virol. 1992, 66, 650–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morace, G.; Pisani, G.; Beneduce, F.; Divizia, M.; Panà, A. Mutations in the 3A genomic region of two cytopathic strains of hepatitis A virus isolated in Italy. Virus Res. 1993, 28187–28194. [Google Scholar] [CrossRef]
- Venuti, A.; Di Russo, C.; del Grosso, N.; Patti, A.M.; Ruggeri, F.; De Stasio, P.R.; Martiniello, M.G.; Pagnotti, P.; Degener, A.M.; Midulla, M.; et al. Isolation and molecular cloning of a fast-growing strain of human hepatitis A virus from its double-stranded replicative form. J. Virol. 1985, 56, 579–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemon, S.M.; Murphy, P.C.; Shields, P.A.; Ping, L.H.; Feinstone, S.M.; Cromeans, T.; Jansen, R.W. Antigenic and genetic variation in cytopathic hepatitis A virus variants arising during persistent infection: Evidence for genetic recombination. J. Virol. 1991, 65, 2056–2065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graff, J.; Normann, A.; Feinstone, S.M.; Flehmig, B. Nucleotide sequence of wild-type hepatitis A virus GBM in comparison with two cell culture-adapted variants. J. Virol. 1994, 68, 548–554. [Google Scholar] [CrossRef] [Green Version]
- Graff, J.; Kasang, C.; Normann, A.; Pfisterer-Hunt, M.; Feinstone, S.M.; Flehmig, B. Mutational events in consecutive passages of hepatitis A virus strain GBM during cell culture adaptation. Virology 1994, 204, 60–68. [Google Scholar] [CrossRef]
- Zhang, H.; Chao, S.F.; Ping, L.H.; Grace, K.; Clarke, B.; Lemon, S.M. An infectious cDNA clone of a cytopathic hepatitis A virus: Genomic regions associated with rapid replication and cytopathic effect. Virology 1995, 212, 686–697. [Google Scholar] [CrossRef] [Green Version]
- Funkhouser, A.W.; Raychaudhuri, G.; Purcell, R.H.; Govindarajan, S.; Elkins, R.; Emerson, S.U. Progress toward the development of a genetically engineered attenuated hepatitis A virus vaccine. J. Virol. 1996, 70, 7948–7957. [Google Scholar] [CrossRef] [Green Version]
- Baba, M.; Takegawa, M.; Kaito, M.; Miyamoto, K.; Suzuki, S. Propagation of hepatitis A virus in a renal cell line JTC-12.P3 of cynomolgus monkey origin. Acta Virol. 1993, 37, 209–222. [Google Scholar]
- Dotzauer, A.; Feinstone, S.M.; Kaplan, G. Susceptibility of nonprimate cell lines to hepatitis A virus infection. J. Virol. 1994, 68, 6064–6068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feigelstock, D.A.; Thompson, P.; Kaplan, G.G. Growth of hepatitis A virus in a mouse liver cell line. J. Virol. 2005, 79, 2950–2955. [Google Scholar] [CrossRef] [Green Version]
- Win, N.N.; Kanda, T.; Nakamoto, S.; Moriyama, M.; Jiang, X.; Suganami, A.; Tamura, Y.; Okamoto, H.; Shirasawa, H. Inhibitory effect of Japanese rice-koji miso extracts on hepatitis A virus replication in association with the elevation of glucose-regulated protein 78 expression. Int. J. Med. Sci. 2018, 15, 1153–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakita, T.; Pietschmann, T.; Kato, T.; Date, T.; Miyamoto, M.; Zhao, Z.; Murthy, K.; Habermann, A.; Kräusslich, H.G.; Mizokami, M.; et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 2005, 11, 791–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, J.; Gastaminza, P.; Cheng, G.; Kapadia, S.; Kato, T.; Burton, D.R.; Wieland, S.F.; Uprichard, S.L.; Wakita, T.; Chisari, F.V. Robust hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. USA 2005, 102, 9294–9299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanda, T.; Basu, A.; Steele, R.; Wakita, T.; Ryerse, J.S.; Ray, R.; Ray, R.B. Generation of infectious hepatitis C virus in immortalized human hepatocytes. J. Virol. 2006, 80, 4633–4639. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Rodríguez, F.J.; D’Andrea, L.; de Castellarnau, M.; Costafreda, M.I.; Guix, S.; Ribes, E.; Quer, J.; Gregori, J.; Bosch, A.; Pintó, R.M. Improving virus production through quasispecies genomic selection and molecular breeding. Sci. Rep. 2016, 6, 35962. [Google Scholar] [CrossRef] [Green Version]
- Konduru, K.; Kaplan, G.G. Stable growth of wild-type hepatitis A virus in cell culture. J. Virol. 2006, 80, 1352–1360. [Google Scholar] [CrossRef] [Green Version]
- Kusov, Y.; Kanda, T.; Palmenberg, A.; Sgro, J.Y.; Gauss-Müller, V. Silencing of hepatitis A virus infection by small interfering RNAs. J. Virol. 2006, 80, 5599–5610. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Kanda, T.; Wu, S.; Nakamoto, S.; Saito, K.; Shirasawa, H.; Kiyohara, T.; Ishii, K.; Wakita, T.; Okamoto, H.; et al. Suppression of La antigen exerts potential antiviral effects against hepatitis A virus. PLoS ONE 2014, 9, e101993. [Google Scholar] [CrossRef] [Green Version]
- Hirai-Yuki, A.; Hensley, L.; Whitmire, J.K.; Lemon, S.M. Biliary Secretion of Quasi-Enveloped Human Hepatitis A Virus. mBio 2016, 7, e01998-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provost, P.J.; Hughes, J.V.; Miller, W.J.; Giesa, P.A.; Banker, F.S.; Emini, E.A. An inactivated hepatitis A viral vaccine of cell culture origin. J. Med. Virol. 1986, 19, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Flehmig, B.; Vallbracht, A.; Wurster, G. Hepatitis A virus in cell culture. III. Propagation of hepatitis A virus in human embryo kidney cells and human embryo fibroblast strains. Med. Microbiol. Immunol. 1981, 170, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Flehmig, B.; Heinricy, U.; Pfisterer, M. Immunogenicity of a killed hepatitis A vaccine in seronegative volunteers. Lancet 1989, 1, 1039–1041. [Google Scholar] [CrossRef]
- Widell, A.; Hansson, B.G.; Oberg, B.; Nordenfelt, E. Influence of twenty potentially antiviral substances on in vitro multiplication of hepatitis A virus. Antivir. Res. 1986, 6, 103–112. [Google Scholar] [CrossRef]
- Biziagos, E.; Crance, J.M.; Passagot, J.; Deloince, R. Effect of antiviral substances on hepatitis A virus replication in vitro. J. Med. Virol. 1987, 22, 57–66. [Google Scholar] [CrossRef]
- Biziagos, E.; Crance, J.M.; Passagot, J.; Deloince, R. Inhibitory effects of atropine, protamine, and their combination on hepatitis A virus replication in PLC/PRF/5 cells. Antimicrob. Agents Chemother. 1990, 34, 1112–1117. [Google Scholar] [CrossRef] [Green Version]
- Crance, J.M.; Biziagos, E.; Passagot, J.; van Cuyck-Gandré, H.; Deloince, R. Inhibition of hepatitis A virus replication in vitro by antiviral compounds. J. Med. Virol. 1990, 31, 155–160. [Google Scholar] [CrossRef]
- Girond, S.; Crance, J.M.; Van Cuyck-Gandre, H.; Renaudet, J.; Deloince, R. Antiviral activity of carrageenan on hepatitis A virus replication in cell culture. Res. Virol. 1991, 142, 261–270. [Google Scholar] [CrossRef]
- Crance, J.M.; Lévêque, F.; Chousterman, S.; Jouan, A.; Trépo, C.; Deloince, R. Antiviral activity of recombinant interferon-alpha on hepatitis A virus replication in human liver cells. Antivir. Res. 1995, 28, 69–80. [Google Scholar] [CrossRef]
- Kanda, T.; Wu, S.; Kiyohara, T.; Nakamoto, S.; Jiang, X.; Miyamura, T.; Imazeki, F.; Ishii, K.; Wakita, T.; Yokosuka, O. Interleukin-29 suppresses hepatitis A and C viral internal ribosomal entry site-mediated translation. Viral Immunol. 2012, 25, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Kanda, T.; Nakamoto, S.; Saito, K.; Nakamura, M.; Wu, S.; Haga, Y.; Sasaki, R.; Sakamoto, N.; Shirasawa, H.; et al. The JAK2 inhibitor AZD1480 inhibits hepatitis A virus replication in Huh7 cells. Biochem. Biophys. Res. Commun. 2015, 458, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Sasaki, R.; Nakamoto, S.; Haga, Y.; Nakamura, M.; Shirasawa, H.; Okamoto, H.; Yokosuka, O. The sirtuin inhibitor sirtinol inhibits hepatitis A virus (HAV) replication by inhibiting HAV internal ribosomal entry site activity. Biochem. Biophys. Res. Commun. 2015, 466, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Kanda, T.; Suganami, A.; Nakamoto, S.; Win, N.N.; Tamura, Y.; Nakamura, M.; Matsuoka, S.; Yokosuka, O.; Kato, N.; et al. Antiviral activity of zinc sulfate against hepatitis A virus replication. Future Virol. 2019, 14, 399–406. [Google Scholar] [CrossRef]
- Yi, M.; Lemon, S.M. Replication of subgenomic hepatitis A virus RNAs expressing firefly luciferase is enhanced by mutations associated with adaptation of virus to growth in cultured cells. J. Virol. 2002, 76, 1171–1180. [Google Scholar] [CrossRef] [Green Version]
- Gauss-Müller, V.; Kusov, Y.Y. Replication of a hepatitis A virus replicon detected by genetic recombination in vivo. J. Gen. Virol. 2002, 83, 2183–2192. [Google Scholar] [CrossRef]
- Kusov, Y.Y.; Shatirishvili, G.; Klinger, M.; Gauss-Müller, V. A vaccinia virus MVA-T7-mediated recovery of infectious hepatitis A virus from full-size cDNA or from two cDNAs, both by themselves unable to complete the virus life cycle. Virus Res. 2002, 89, 75–88. [Google Scholar] [CrossRef]
- Yang, Y.; Yi, M.; Simonds, P.; Lemon, S.M. Identification of a conserved RNA replication element (cre) within the 3Dpol-coding sequence of hepatoviruses. J. Virol. 2008, 82, 10118–10128. [Google Scholar] [CrossRef] [Green Version]
- Kanda, T.; Kusov, Y.; Yokosuka, O.; Gauss-Müller, V. Interference of hepatitis A virus replication by small interfering RNAs. Biochem. Biophys. Res. Commun. 2004, 318, 341–345. [Google Scholar] [CrossRef]
- Kaplan, G.; Totsuka, A.; Thompson, P.; Akatsuka, T.; Moritsugu, Y.; Feinstone, S.M. Identification of a surface glycoprotein on African green monkey kidney cells as a receptor for hepatitis A virus. EMBO J. 1996, 15, 4282–4296. [Google Scholar] [CrossRef]
- BioGPS. HAVCR1 (Hepatitis A Virus Cellular Receptor 1), Gene Expression/Activity Chart. Available online: http://biogps.org/#goto=genereport&id=26762 (accessed on 26 March 2020).
- McIntire, J.J.; Umetsu, S.E.; Akbari, O.; Potter, M.; Kuchroo, V.K.; Barsh, G.S.; Freeman, G.J.; Umetsu, D.T.; DeKruyff, R.H. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat. Immunol. 2001, 2, 1109–1116. [Google Scholar] [CrossRef]
- Umetsu, S.E.; Lee, W.L.; McIntire, J.J.; Downey, L.; Sanjanwala, B.; Akbari, O.; Berry, G.J.; Nagumo, H.; Freeman, G.J.; Umetsu, D.T.; et al. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat. Immunol. 2005, 6, 447–454. [Google Scholar] [CrossRef]
- Rivera-Serrano, E.E.; González-López, O.; Das, A.; Lemon, S.M. Cellular entry and uncoating of naked and quasi-enveloped human hepatoviruses. Elife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Costafreda, M.I.; Kaplan, G. HAVCR1 (CD365) and Its Mouse Ortholog Are Functional Hepatitis A Virus (HAV) Cellular Receptors That Mediate HAV Infection. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.; Maury, W.; Lemon, S.M. TIM1 (HAVCR1): An Essential “Receptor” or an “Accessory Attachment Factor” for Hepatitis A Virus? J. Virol. 2019, 93. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Hirai-Yuki, A.; González-López, O.; Rhein, B.; Moller-Tank, S.; Brouillette, R.; Hensley, L.; Misumi, I.; Lovell, W.; Cullen, J.M.; et al. TIM1 (HAVCR1) Is Not Essential for Cellular Entry of Either Quasi-enveloped or Naked Hepatitis A Virions. mBio 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, E.A.; Zajac, A.J.; Lemon, S.M. In vitro characterization of an internal ribosomal entry site (IRES) present within the 5’ nontranslated region of hepatitis A virus RNA: Comparison with the IRES of encephalomyocarditis virus. J. Virol. 1994, 68, 1066–1074. [Google Scholar] [CrossRef] [Green Version]
- Kassem, A.F.; Batran, R.Z.; Abbas, E.M.H.; Elseginy, S.A.; Shaheen, M.N.F.; Elmahdy, E.M. New 4-phenylcoumarin derivatives as potent 3C protease inhibitors: Design, synthesis, anti-HAV effect and molecular modeling. Eur. J. Med. Chem. 2019, 168, 447–460. [Google Scholar] [CrossRef]
- Banerjee, K.; Bhat, R.; Rao, V.U.B.; Nain, A.; Rallapalli, K.L.; Gangopadhyay, S.; Singh, R.P.; Banerjee, M.; Jayaram, B. Toward development of generic inhibitors against the 3C proteases of picornaviruses. FEBS J. 2019, 286, 765–787. [Google Scholar] [CrossRef]
- Konduru, K.; Kaplan, G.G. Determinants in 3Dpol modulate the rate of growth of hepatitis A virus. J. Virol. 2010, 84, 8342–8347. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.A.; Day, S.P.; Jansen, R.W.; Lemon, S.M. The 5′ nontranslated region of hepatitis A virus RNA: Secondary structure and elements required for translation in vitro. J. Virol. 1991, 65, 5828–5838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whetter, L.E.; Day, S.P.; Elroy-Stein, O.; Brown, E.A.; Lemon, S.M. Low efficiency of the 5′ nontranslated region of hepatitis A virus RNA in directing cap-independent translation in permissive monkey kidney cells. J. Virol. 1994, 68, 5253–5263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanda, T.; Lau, G.K.K.; Wei, L.; Moriyama, M.; Yu, M.L.; Chuang, W.L.; Ibrahim, A.; Lesmana, C.R.A.; Sollano, J.; Kumar, M.; et al. APASL clinical practice recommendation: How to treat HCV-infected patients with renal impairment? Hepatol. Int. 2019, 13, 103–109. [Google Scholar] [CrossRef] [Green Version]
- AASLD-IDSA HCV Guidance Panel. Hepatitis C Guidance 2018 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin. Infect. Dis. 2018, 67, 1477–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Fang, L.; Wei, D.; Zhang, H.; Luo, R.; Chen, H.; Li, K.; Xiao, S. Hepatitis A virus 3C protease cleaves NEMO to impair induction of beta interferon. J. Virol. 2014, 88, 10252–10258. [Google Scholar] [CrossRef] [Green Version]
- Shubin, A.V.; Demidyuk, I.V.; Lunina, N.A.; Komissarov, A.A.; Roschina, M.P.; Leonova, O.G.; Kostrov, S.V. Protease 3C of hepatitis A virus induces vacuolization of lysosomal/endosomal organelles and caspase-independent cell death. BMC Cell Biol. 2015, 16, 4. [Google Scholar] [CrossRef] [Green Version]
- Qu, L.; Feng, Z.; Yamane, D.; Liang, Y.; Lanford, R.E.; Li, K.; Lemon, S.M. Disruption of TLR3 signaling due to cleavage of TRIF by the hepatitis A virus protease-polymerase processing intermediate, 3CD. PLoS Pathog. 2011, 7, e1002169. [Google Scholar] [CrossRef]
| Authors (Year) [References] | Cell Lines | HAV Strain | Duration of HAV Infection | Cytopathy |
|---|---|---|---|---|
| Provost, P.J., et al. (1979) [21] | Primary marmoset liver and fetal rhesus kidney (FRhK6) cells | Marmoset-adapted CR326 | NA | No |
| Frösner, G.G., et al. (1979) [22] | Alexander (PLC/PRF/5) cells | MS-1 | 7 weeks | No |
| Gauss-Müller, V., et al. (1981) [23] | Human embryo fibroblasts | Cell culture-adapted strain | 90 and 210 days | No |
| Kojima, H., et al. (1981) [24] | FL or Vero cells | HAV derived from fecal extracts | 18 days | No |
| Daemer, R.J., et al. (1981) [25] | Primary African green monkey kidney (AGMK) cells | MS-1, SD-1, HM-175 | 11 weeks | No |
| Lemon, S.M., et al. (1983) [26] | BS-C-1 cells | HM-175, PA-21 | 30 days | No |
| Wheeler, C.M., et al. (1986) [27] | FRhK-4 cells | HAS-15 | 20 × 7 passages | No |
| Cohen, J.I., et al. (1987) [31] | AGMK or CV-1 cells | HAV cDNA HM-175n MK-5 | 5 weeks | No |
| Crance, J.M., et al. (1987) [28] | PLC/PRF/5 cells | CF53 | 6–12 months | No |
| Robertson, B.H., et al. (1988) [29] | FRhK-4 cells | HAS-15 | 2–3 months | No |
| Tsarev, S.A., et al. (1991) [30] | Primary AGMK or FRhK-4 cells | AGM-27 | 14 days | No |
| Emerson, S.U., et al. (1992) [32] | FRhK-4 cells | HM175 | 2 months | No |
| Emerson, S.U., et al. (1992) [33] | AGMK cells | HM175 | 120 days | No |
| Morace, G., et al. (1993) [34] | Frp/3 cells | HM175 cytopathic clone | 7–9 days | Yes |
| Baba, M., et al. (1993) [41] | JTC-12.P3 cells | HAV | 8 weeks | No |
| Graff, J., et al. (1994) [37] | FRhK-4 or HFS cells | GBM/WT, GBM/FRhK, GBM/HFS | 14 days | No |
| Dotzauer, A., et al. (1994) [42] | GPE or SP 1K cells | HM175 | 42 days | No |
| Graff, J., et al. (1994) [38] | FRhK-4 cells | GBM/Fp2 | 60 days | No |
| Zhang, H., et al. (1995) [39] | BS-C-1 cells | HM175/18f | 14 days | Yes |
| Funkhouser, A.W., et al. (1996) [40] | MRC-5 cells | MR8 or MRC-5/9 | 160 days | No |
| Feigelstock, D.A., et al. (2005) [43] | GL37 or MMH-D3 cells | HM175 | 14–50 days | No |
| Konduru, K., et al. (2006) [49] | Huh7-A-I cells | WT HM175 | 16 days | No |
| Kusov, Y., et al. (2006) [50] | Huh7 cells | Huh-7/HAV | 14 days | No |
| Jiang, X., et al. (2014) [51] | Huh7 or GL37 cells | HA11-1299 GT IIIA or KRM003 GT IIIB | 4 days | No |
| Hirai-Yuki, A., et al. (2016) [52] | Caco-2 or HepG2-N cells | HM175/p16 | 4–7 days | No |
| Pérez-Rodríguez, F.J., et al. (2016) [48] | FRhK-4 | HM175-HP, F0.05LA | 7 days | No |
| Win, N.N., et al. (2018) [44] | PXB cells | HA11-1299 GT IIIA | 7 days | No |
| Authors (Year) [References] | Cell Lines | HAV Strain | Effective Anti-HAV Drugs |
|---|---|---|---|
| Widell, A., et al. (1986) [56] | Frhk-4 | H 141 | Arabinosylcytosine, amantadine, ribavirin |
| Biziagos, E., et al. (1987) [57] | PLC/PRF/5 | CF53 | Taxifolin, atropine |
| Biziagos, E., et al. (1990) [58] | PLC/PRF/5 | CF53 | Atropine, protamine, atropine/protamine combination |
| Crance, J.M., et al. (1990) [59] | PLC/PRF/5 | CF53 | Ribavirin, amantadine, pyrazofurin, glycyrrhizin |
| Girond, S., et al. (1991) [60] | PLC/PRF/5 | CF53 | Sulphated polysaccharides |
| Crance, J.M., et al. (1995) [61] | PLC/PRF/5 | CF53 | Interferon-alpha |
| Kusov Y., et al. (2006) [50] | Huh7 | Huh-7/HAV | siRNA |
| Yang, L., et al. (2010) [17] | GL37 | KRM003 | Amantadine, Interferon-alpha |
| Kanda, T., et al. (2010) [62] | GL37 | KRM003 | Interferon-lambda |
| Jiang, X., et al. (2015) [63] | Huh7 | HA11-1299 | AZD1480 |
| Kanda, T., et al. (2015) [64] | Huh7 | HA11-1299 | Sirtinol |
| Win, N.N., et al. (2019) [44] | Huh7 PXB | HA11-1299 | Japanese rice koji miso extracts |
| Ogawa, M., et al. (2019) [65] | Huh7 | HA11-1299 | Zinc sulfate |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanda, T.; Sasaki, R.; Masuzaki, R.; Matsumoto, N.; Ogawa, M.; Moriyama, M. Cell Culture Systems and Drug Targets for Hepatitis A Virus Infection. Viruses 2020, 12, 533. https://doi.org/10.3390/v12050533
Kanda T, Sasaki R, Masuzaki R, Matsumoto N, Ogawa M, Moriyama M. Cell Culture Systems and Drug Targets for Hepatitis A Virus Infection. Viruses. 2020; 12(5):533. https://doi.org/10.3390/v12050533
Chicago/Turabian StyleKanda, Tatsuo, Reina Sasaki, Ryota Masuzaki, Naoki Matsumoto, Masahiro Ogawa, and Mitsuhiko Moriyama. 2020. "Cell Culture Systems and Drug Targets for Hepatitis A Virus Infection" Viruses 12, no. 5: 533. https://doi.org/10.3390/v12050533
APA StyleKanda, T., Sasaki, R., Masuzaki, R., Matsumoto, N., Ogawa, M., & Moriyama, M. (2020). Cell Culture Systems and Drug Targets for Hepatitis A Virus Infection. Viruses, 12(5), 533. https://doi.org/10.3390/v12050533






