Safeguarding Patients, Relatives, and Nurses: A Screening Approach for Detecting 5-FU Residues on Elastomeric Infusion Pumps Using HPLC-DAD
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Method Development and Optimization
3.2. Method Validation
3.2.1. Linearity
3.2.2. Detection and Quantification Limits
3.2.3. Precision
3.2.4. Accuracy
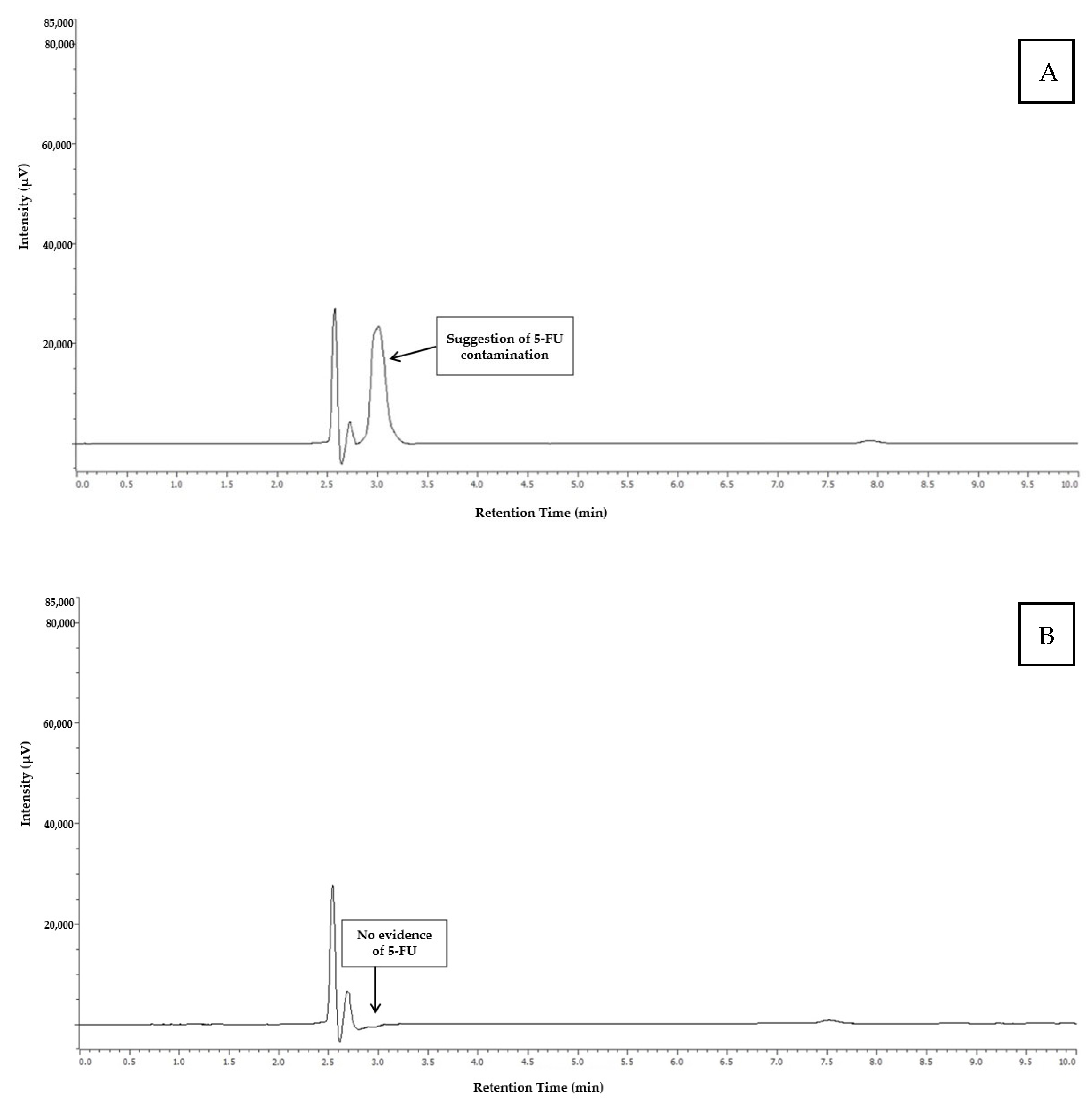
3.2.5. Specificity/Selectivity
3.2.6. Robustness
3.2.7. Stability
3.3. Analysis of Real Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The Ever-Increasing Importance of Cancer as a Leading Cause of Premature Death Worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Kloor, M.; Pox, C.P. Colorectal Cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef]
- Goldstein, D.A.; Chen, Q.; Ayer, T.; Howard, D.H.; Lipscomb, J.; Harvey, R.D.; El-Rayes, B.F.; Flowers, C.R. Cost Effectiveness Analysis of Pharmacokinetically-Guided 5-Fluorouracil in FOLFOX Chemotherapy for Metastatic Colorectal Cancer. Clin. Color. Cancer 2014, 13, 219–225. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO Consensus Guidelines for the Management of Patients with Metastatic Colorectal Cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Modest, D.P.; Pant, S.; Sartore-Bianchi, A. Treatment Sequencing in Metastatic Colorectal Cancer. Eur. J. Cancer 2019, 109, 70–83. [Google Scholar] [CrossRef]
- Casale, J.; Patel, P. Fluorouracil. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lee, J.J.; Beumer, J.H.; Chu, E. Therapeutic Drug Monitoring of 5-Fluorouracil. Cancer Chemother. Pharmacol. 2016, 78, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Chefchaouni, A.C.; Ouedraogo, J.-M.; Bechar, H.; Belahcen, M.J.; Rahali, Y. Retrospective Analysis of Failures of Ambulatory Elastomeric Pumps Containing 5-FU in a Hospital Pharmacy Unit. J. Oncol. Pharm. Pract. 2023, 29, 125–129. [Google Scholar] [CrossRef]
- Leventon Guia Clínico Dosi-Fuser®. Available online: https://www.dosi-fuser.com/pt/pharmacy/?accept=1 (accessed on 25 September 2023).
- Sabbagh Dit Hawasli, R.; Barton, S.; Nabhani-Gebara, S. Ambulatory Chemotherapy: Past, Present, and Future. J. Oncol. Pharm. Pract. 2021, 27, 962–973. [Google Scholar] [CrossRef]
- Hobbs, J.G.; Ryan, M.K.; Ritchie, B.; Sluggett, J.K.; Sluggett, A.J.; Ralton, L.; Reynolds, K.J. Protocol for a Randomised Crossover Trial to Evaluate Patient and Nurse Satisfaction with Electronic and Elastomeric Portable Infusion Pumps for the Continuous Administration of Antibiotic Therapy in the Home: The Comparing Home Infusion Devices (CHID) Study. BMJ Open 2017, 7, e016763. [Google Scholar] [CrossRef]
- Skryabina, E.A.; Dunn, T.S. Disposable Infusion Pumps. Am. J. Health Syst. Pharm. 2006, 63, 1260–1268. [Google Scholar] [CrossRef]
- Aristizabal-Pachon, A.F.; Castillo, W.O. Genotoxic Evaluation of Occupational Exposure to Antineoplastic Drugs. Toxicol. Res. 2020, 36, 29–36. [Google Scholar] [CrossRef]
- Burgaz, S.; Karahalil, B.; Bayrak, P.; Taşkin, L.; Yavuzaslan, F.; Bökesoy, I.; Anzion, R.B.; Bos, R.P.; Platin, N. Urinary Cyclophosphamide Excretion and Micronuclei Frequencies in Peripheral Lymphocytes and in Exfoliated Buccal Epithelial Cells of Nurses Handling Antineoplastics. Mutat. Res. 1999, 439, 97–104. [Google Scholar] [CrossRef]
- Undeğer, U.; Başaran, N.; Kars, A.; Güç, D. Assessment of DNA Damage in Nurses Handling Antineoplastic Drugs by the Alkaline COMET Assay. Mutat. Res. 1999, 439, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Villarini, M.; Gianfredi, V.; Levorato, S.; Vannini, S.; Salvatori, T.; Moretti, M. Occupational Exposure to Cytostatic/Antineoplastic Drugs and Cytogenetic Damage Measured Using the Lymphocyte Cytokinesis-Block Micronucleus Assay: A Systematic Review of the Literature and Meta-Analysis. Mutat. Res. Rev. Mutat. Res. 2016, 770, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Young, B.; Jansen, R.; Kirk, J.; Dellavalle, R. 5-Fluorouracil Toxicosis in Our Pets: A Review and Recommendations. J. Am. Acad. Dermatol. 2024, 90, 1051–1052. [Google Scholar] [CrossRef]
- Chen, S.; Cao, Z.; Prettner, K.; Kuhn, M.; Yang, J.; Jiao, L.; Wang, Z.; Li, W.; Geldsetzer, P.; Bärnighausen, T.; et al. Estimates and Projections of the Global Economic Cost of 29 Cancers in 204 Countries and Territories From 2020 to 2050. JAMA Oncol. 2023, 9, 465–472. [Google Scholar] [CrossRef]
- Viegas, S.; Pádua, M.; Veiga, A.C.; Carolino, E.; Gomes, M. Antineoplastic Drugs Contamination of Workplace Surfaces in Two Portuguese Hospitals. Environ. Monit. Assess. 2014, 186, 7807–7818. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.; Akhtar, M.F.; Naeem, S.; Wahid, M.; Shehzad, M.A.; Saadullah, M.; Nasir, B.; Afzal, S. Development and Validation of a New HPLC Method For the Detection of 5- Fluorouracil in Mobile Phase and in Plasma. Curr. Pharm. Anal. 2018, 14, 3–7. [Google Scholar] [CrossRef]
- Sinha, V.R.; Kumar, R.V.; Bhinge, J.R. A Stability-Indicating RP-HPLC Assay Method for 5-Fluorouracil. Indian J. Pharm. Sci. 2009, 71, 630. [Google Scholar] [CrossRef]
- Mavromatis, P.; Stampouli, K.; Vliora, A.; Mayilyan, A.; Samanidou, V.; Touraki, M. Development of an HPLC-DAD Method for the Extraction and Quantification of 5-Fluorouracil, Uracil, and 5-Fluorodeoxyuridin Monophosphate in Cells and Culture Media of Lactococcus Lactis. Separations 2022, 9, 376. [Google Scholar] [CrossRef]
- Alanazi, F.K.; Eldeen Yassin, A.; El-Badry, M.; Mowafy, H.A.; Alsarra, I.A. Validated High-Performance Liquid Chromatographic Technique for Determination of 5-Fluorouracil: Applications to Stability Studies and Simulated Colonic Media. J. Chromatogr. Sci. 2009, 47, 558–563. [Google Scholar] [CrossRef]
- Pi, C.; Wei, Y.; Yang, H.; Zhou, Y.; Fu, J.; Yang, S.; Ye, Y.; Zhao, L. Development of a HPLC Method to Determine 5-Fluorouracil in Plasma: Application in Pharmacokinetics and Steady-State Concentration Monitoring. Int. J. Clin. Pharmacol. Ther. 2014, 52, 1093–1101. [Google Scholar] [CrossRef]
- Kim, S.; Youssef, S.H.; Song, Y.; Garg, S. Development and Application of a Chromatographic Method for Simultaneous Quantification of 5-Fluorouracil and Imiquimod in Drug-in-Adhesive Topical Patches. Sustain. Chem. Pharm. 2022, 27, 100711. [Google Scholar] [CrossRef]
- Viegas, S.; Oliveira, d.A.C.; Carolino, E.; Pádua, M. Occupational Exposure to Cytotoxic Drugs: The Importance of Surface Cleaning to Prevent or Minimise Exposure. Arh. Hig. Rada Toksikol. 2018, 69, 238–249. [Google Scholar] [CrossRef] [PubMed]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Validation of Analytical Procedures: Q2(R2).; European Medicines Agency: Amsterdam, The Netherlands, 2024.
- Gustavo González, A.; Ángeles Herrador, M. A Practical Guide to Analytical Method Validation, Including Measurement Uncertainty and Accuracy Profiles. TrAC Trends Anal. Chem. 2007, 26, 227–238. [Google Scholar] [CrossRef]
- Chavan, S.D.; Desai, D.M. Analytical Method Validation: A Brief Review. World J. Adv. Res. Rev. 2022, 16, 389–402. [Google Scholar] [CrossRef]
- Araujo, P. Key Aspects of Analytical Method Validation and Linearity Evaluation. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2009, 877, 2224–2234. [Google Scholar] [CrossRef] [PubMed]
- Maurer, H.H. Position of Chromatographic Techniques in Screening for Detection of Drugs or Poisons in Clinical and Forensic Toxicology and/or Doping Control. Clin. Chem. Lab. Med. 2004, 42, 1310–1324. [Google Scholar] [CrossRef]
- Hollá, M.; Bílková, A.; Jakubec, P.; Košková, S.; Kočová Vlčková, H.; Šatínský, D.; Švec, F.; Sklenářová, H. Benefits and Pitfalls of HPLC Coupled to Diode-Array, Charged Aerosol, and Coulometric Detections: Effect of Detection on Screening of Bioactive Compounds in Apples. Molecules 2021, 26, 3246. [Google Scholar] [CrossRef]
- Kosjek, T.; Perko, S.; Žigon, D.; Heath, E. Fluorouracil in the Environment: Analysis, Occurrence, Degradation and Transformation. J. Chromatogr. A 2013, 1290, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Wielińska, J.; Nowacki, A.; Liberek, B. 5-Fluorouracil—Complete Insight into Its Neutral and Ionised Forms. Molecules 2019, 24, 3683. [Google Scholar] [CrossRef]
- Kaliszan, R.; Wiczling, P.; Markuszewski, M.J. pH Gradient High-Performance Liquid Chromatography: Theory and Applications. J. Chromatogr. A 2004, 1060, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Aragão, N.M.d.; Veloso, M.C.d.C.; Bispo, M.S.; Andrade, J.B. de The Role of Acidity and Organic Modifiers in the Methylxanthines Determination: A HPLC Experiment. Quím. Nova 2009, 32, 2482–2486. [Google Scholar] [CrossRef]
- Ornaf, R.M.; Dong, M.W. 2—Key Concepts of HPLC in Pharmaceutical Analysis. In Separation Science and Technology; Ahuja, S., Dong, M.W., Eds.; Handbook of Pharmaceutical Analysis by HPLC; Academic Press: Cambridge, MA, USA, 2005; Volume 6, pp. 19–45. [Google Scholar]
- Budak, N.H.; Aykin, E.; Seydim, A.C.; Greene, A.K.; Guzel-Seydim, Z.B. Functional Properties of Vinegar. J. Food Sci. 2014, 79, R757–R764. [Google Scholar] [CrossRef]
- Trček, J.; Mira, N.P.; Jarboe, L.R. Adaptation and Tolerance of Bacteria against Acetic Acid. Appl. Microbiol. Biotechnol. 2015, 99, 6215–6229. [Google Scholar] [CrossRef]
- Minhas, M.; Ahmad, M.; Sohail, M.; Siddique, F. Simple HPLC-UV Method of 5-Fluorouracil in Human and Rabbit Plasma; Validation and Comparison. Pak. Vet. J. 2015, 35, 71–75. [Google Scholar]
- Escoriaza, J.; Aldaz, A.; Calvo, E.; Giráldez, J. Simple and Sensitive Determination of 5-Fluorouracil in Plasma by High-Performance Liquid Chromatography: Application to Clinical Pharmacokinetic Studies. J. Chromatogr. B Biomed. Sci. Appl. 1999, 736, 97–102. [Google Scholar] [CrossRef]
- Chaudhari, U.; Sahu, J.K.; Dande, P.R. Analytical Method Development, Validation and Forced Degradation Study of Dapagliflozin by RP-HPLC. Drug Metab. Bioanal. Lett. 2023, 16, 140–152. [Google Scholar] [CrossRef]
- Rao, W.; Li, L.; Zhang, C.; Zheng, J.; Fan, X.; Luan, B.; Sun, J.; Qiu, M.; Wu, S.; Li, Y.; et al. Development and Validation of a Stability-Indicating RP-HPLC Method for the Determination of Fifteen Impurities in Rivaroxaban. J. Pharm. Biomed. Anal. 2023, 228, 115325. [Google Scholar] [CrossRef]
- You, H.; Ireland, B.; Moeszinger, M.; Zhang, H.; Snow, L.; Krepich, S.; Takagawa, V. Determination of Bioactive Nonvolatile Ginger Constituents in Dietary Supplements by a Rapid and Economic HPLC Method: Analytical Method Development and Single-Laboratory Validation. Talanta 2019, 194, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Analakkattillam, S.; Langsi, V.K.; Hanrahan, J.P.; Moore, E. Analytical Method Validation for Assay Determination of Cannabidiol and Tetrahydrocannabinol in Hemp Oil Infused Products by RP-HPLC. Sci. Rep. 2022, 12, 12453. [Google Scholar] [CrossRef] [PubMed]
- Reddy Saddala, M.P.; Konduru, N.; Gundla, R.; Kowtharapu, L.P. Development and Validation of Novel RP-HPLC Method for Midostaurin Determination Using Analytical Quality by Design Approach from Regulatory Perspective and Determination of Major Degradation Compounds of Midostaurin Using LC-MS. Biomed. Chromatogr. 2022, 36, e5486. [Google Scholar] [CrossRef]
- Peters, F.T.; Drummer, O.H.; Musshoff, F. Validation of New Methods. Forensic Sci. Int. 2007, 165, 216–224. [Google Scholar] [CrossRef]
- Moosavi, S.M.; Ghassabian, S. Linearity of Calibration Curves for Analytical Methods: A Review of Criteria for Assessment of Method Reliability. In Calibration and Validation of Analytical Methods; Stauffer, M.T., Ed.; IntechOpen: Rijeka, Croatia, 2018; Chapter 6; ISBN 978-1-78923-085-7. [Google Scholar]
- Epshtein, N.A. Validation of Analytical Procedures: Graphic and Calculated Criteria for Assessment of Methods Linearity in Practice. Drug Dev. Regist. 2019, 8, 122–130. [Google Scholar] [CrossRef]
- Ermer, J. Analytical Validation within the Pharmaceutical Environment. In Method Validation in Pharmaceutical Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; pp. 1–19. ISBN 978-3-527-60468-5. [Google Scholar]
- Labrèche, F.; Ouellet, C.; Roberge, B.; Caron, N.J.; Yennek, A.; Bussières, J.-F. Occupational Exposure to Antineoplastic Drugs: What about Hospital Sanitation Personnel? Int. Arch. Occup. Environ. Health 2021, 94, 1877–1888. [Google Scholar] [CrossRef]
- Pinet, E.; Cirtiu, C.M.; Caron, N.; Bussières, J.-F.; Tanguay, C. Canadian Monitoring Program of the Surface Contamination with 11 Antineoplastic Drugs in 124 Centers. J. Oncol. Pharm. Pract. 2024, 30, 19–29. [Google Scholar] [CrossRef]
- Rossignol, E.; Amiand, M.B.; Sorrieul, J.; Bard, J.M.; Bobin-Dubigeon, C. A Fully Validated Simple New Method for Environmental Monitoring by Surface Sampling for Cytotoxics. J. Pharmacol. Toxicol. Methods 2020, 101, 106652. [Google Scholar] [CrossRef] [PubMed]
- Chauchat, L.; Tanguay, C.; Caron, N.J.; Gagné, S.; Labrèche, F.; Bussières, J.F. Surface Contamination with Ten Antineoplastic Drugs in 83 Canadian Centers. J. Oncol. Pharm. Pract. 2019, 25, 1089–1098. [Google Scholar] [CrossRef]
- Kåredal, M.; Jönsson, R.; Wetterling, M.; Björk, B.; Hedmer, M. A Quantitative LC-MS Method to Determine Surface Contamination of Antineoplastic Drugs by Wipe Sampling. J. Occup. Environ. Hyg. 2022, 19, 50–66. [Google Scholar] [CrossRef]
- Ribani, M.; Bottoli, C.B.G.; Collins, C.H.; Jardim, I.C.S.F.; Melo, L.F.C. Validação em métodos cromatográficos e eletroforéticos. Quím. Nova 2004, 27, 771–780. [Google Scholar] [CrossRef]
- Attimarad, M.; Venugopala, K.N.; Sreeharsha, N.; Chohan, M.S.; Shafi, S.; Nair, A.B.; Pottathil, S. A Rapid HPLC Method for the Concurrent Determination of Several Antihypertensive Drugs from Binary and Ternary Formulations. Separations 2021, 8, 86. [Google Scholar] [CrossRef]
- Jaicharoensub, J.; Sakpakdeejaroen, I.; Panthong, S. Validation of HPLC Method for Quantitative Determination of Active Compounds in Thai Traditional Herbal Medicine to Treat Gastrointestinal Disease. Talanta Open 2023, 7, 100227. [Google Scholar] [CrossRef]
- Carvalho, D.; Jesus, Â.; Pinho, C.; Oliveira, R.F.; Moreira, F.; Oliveira, A.I. Validation of an HPLC-DAD Method for Quercetin Quantification in Nanoparticles. Pharmaceuticals 2023, 16, 1736. [Google Scholar] [CrossRef]
- ISOPP Standards for the Safe Handling of Cytotoxics. J. Oncol. Pharm. Pract. 2022, 28, S1–S126. [CrossRef] [PubMed]
- Campos, D.; Silva, I.; Rego, M.; Correia, P.; Moreira, F. Characterization of Education, Technical Practices and Attitudes of Portuguese Pharmacy Technicians towards Manipulation of Cytotoxic Drugs. J. Oncol. Pharm. Pract. 2023, 30, 893–901. [Google Scholar] [CrossRef]
- Li, S.; Tian, M.; Row, K.H. Effect of Mobile Phase Additives on the Resolution of Four Bioactive Compounds by RP-HPLC. Int. J. Mol. Sci. 2010, 11, 2229–2240. [Google Scholar] [CrossRef]
- Rider, B.J. 5 Fluorouracil. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–5. ISBN 978-0-08-055232-3. [Google Scholar]
- Gurbeta, L.; Dzemic, Z.; Bego, T.; Sejdic, E.; Badnjevic, A. Testing of Anesthesia Machines and Defibrillators in Healthcare Institutions. J. Med. Syst. 2017, 41, 133. [Google Scholar] [CrossRef]
- Gurbeta, L.; Alic, B.; Dzemic, Z.; Badnjevic, A. Testing of Dialysis Machines in Healthcare Institutions in Bosnia and Herzegovina. In Proceedings of the EMBEC & NBC 2017, Tampere, Finland, 11–15 June 2017; Eskola, H., Väisänen, O., Viik, J., Hyttinen, J., Eds.; Springer: Singapore, 2018; pp. 470–473. [Google Scholar]
- Dorman, D.C.; Coddington, K.A.; Richardson, R.C. 5-Fluorouracil Toxicosis in the Dog. J. Vet. Intern. Med. 1990, 4, 254–257. [Google Scholar] [CrossRef]
- Fry, M.M.; Forman, M.A. 5-Fluorouracil Toxicity with Severe Bone Marrow Suppression in a Dog. Vet. Hum. Toxicol. 2004, 46, 178–180. [Google Scholar]
- Sayre, R.S.; Barr, J.W.; Bailey, E.M. Accidental and Experimentally Induced 5-Fluorouracil Toxicity in Dogs. J. Vet. Emerg. Crit. Care 2012, 22, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.S.; Connor, T.H. Monitoring Occupational Exposure to Cancer Chemotherapy Drugs. Am. J. Health Syst. Pharm. 1996, 53, 2713–2723. [Google Scholar] [CrossRef]
- Sessink, P.J.; Bos, R.P. Drugs Hazardous to Healthcare Workers. Evaluation of Methods for Monitoring Occupational Exposure to Cytostatic Drugs. Drug Saf. 1999, 20, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, M.; Anderson, D. Monitoring of Occupational Exposure to Cytostatic Anticancer Agents. Mutat. Res. 1996, 355, 253–261. [Google Scholar] [CrossRef]
- Kopp, B.; Schierl, R.; Nowak, D. Evaluation of Working Practices and Surface Contamination with Antineoplastic Drugs in Outpatient Oncology Health Care Settings. Int. Arch. Occup. Environ. Health 2013, 86, 47–55. [Google Scholar] [CrossRef]
- Suspiro, A.; Prista, J. Exposição Ocupacional a Citostáticos e Efeitos Sobre a Saúde. Rev. Port. Saúde Pública 2012, 30, 76–88. [Google Scholar] [CrossRef]
- Polovich, M.; Olsen, M. Safe Handling of Hazardous Drugs, 3rd ed.; Oncology Nursing Society: Pittsburgh, PA, USA, 2011; ISBN 978-1-63593-010-8. [Google Scholar]
- Kieffer, C.; Verhaeghe, P.; Lagrassa, S.; Grégoire, R.; Moussaoui, Z.; Casteras-Ducros, C.; Clark, J.E.; Vanelle, P.; Rathelot, P. Preventing the Contamination of Hospital Personnel by Cytotoxic Agents: Evaluation and Training of the Para-Professional Healthcare Workers in Oncology Units. Eur. J. Cancer Care 2015, 24, 404–410. [Google Scholar] [CrossRef]
- Creta, M.; Verscheure, E.; Tans, B.; Devriese, H.; Devriendt, A.; Devolder, D.; Lebegge, R.; Poels, K.; Godderis, L.; Duca, R.-C.; et al. An Assessment of Surface Contamination and Dermal Exposure to 5-Fluorouracil in Healthcare Settings by UPLC-MS/MS Using a New Atmospheric Pressure Ionization Source. Toxics 2024, 12, 766. [Google Scholar] [CrossRef]

| 5-FU Concentration (µg/cm2) | Obtained 5-FU (µg/cm2) Concentration After Back-Calculation | Accordance (%) |
|---|---|---|
| 0.150 | 0.154 | 102 |
| 0.375 | 0.364 | 97 |
| 0.750 | 0.783 | 104 |
| 1.125 | 1.138 | 101 |
| 1.500 | 1.427 | 95 |
| 2.250 | 2.289 | 102 |
| 3.000 | 2.995 | 100 |
| Calibration Standards Concentration (µg/cm2) | Repeatability | Intermediate Precision | ||
|---|---|---|---|---|
| Mean ± Standard Deviation | RSD (%) | Mean ± Standard Deviation | RSD (%) | |
| 0.375 | 154,474.8 ± 4507.64 | 2.9 | 141,524.3 ± 17,912.83 | 12.7 |
| 1.125 | 331,210.9 ± 6055.42 | 1.8 | 308,320.7 ± 14,758.78 | 4.8 |
| 2.250 | 578,824.6 ± 18,221.2 | 3.2 | 520,997.1 ± 12,636.53 | 2.4 |
| 5-FU Real Concentration (µg/cm2) | 5-FU Experimentally Obtained Concentration (µg/cm2) | Relative Error (%) | Accuracy (%) |
|---|---|---|---|
| 0.375 | 0.319 | −14.9 | 85.1 |
| 1.125 | 1.094 | −2.8 | 97.2 |
| 2.250 | 2.243 | −0.3 | 99.7 |
| Parameter | Parameter Value | Peak Area | % of Peak Area in Relation to Optimized Method | Retention Time (min) | Difference of Retention Time in Relation to Optimized Method (min) |
|---|---|---|---|---|---|
| pH | 2.4 | 321,416.33 ± 1532.37 | NA | 3.0 | NA |
| 2.2 | 323,696.67 ± 512.60 | 100.7 | 3.0 | 0.0 min | |
| Flow rate | 0.8 mL/min | 326,116.67 ± 677.66 | NA | 3.0 | NA |
| 1.0 mL/min | 226,990.33 ± 601.42 | 69.6 | 2.8 | −0.2 min |
| 5-FU Concentration (µg/cm2) | Room Temperature | 4 °C | −20 °C | |||
|---|---|---|---|---|---|---|
| Day 3 (%) | Day 5 (%) | Day 3 (%) | Day 5 (%) | Day 3 (%) | Day 5 (%) | |
| 0.150 | 109 | 77 | 94 | 71 | 96 | 61 |
| 0.750 | 105 | 96 | 97 | 89 | 95 | 85 |
| 3.000 | 90 | 86 | 84 | 83 | 83 | 84 |
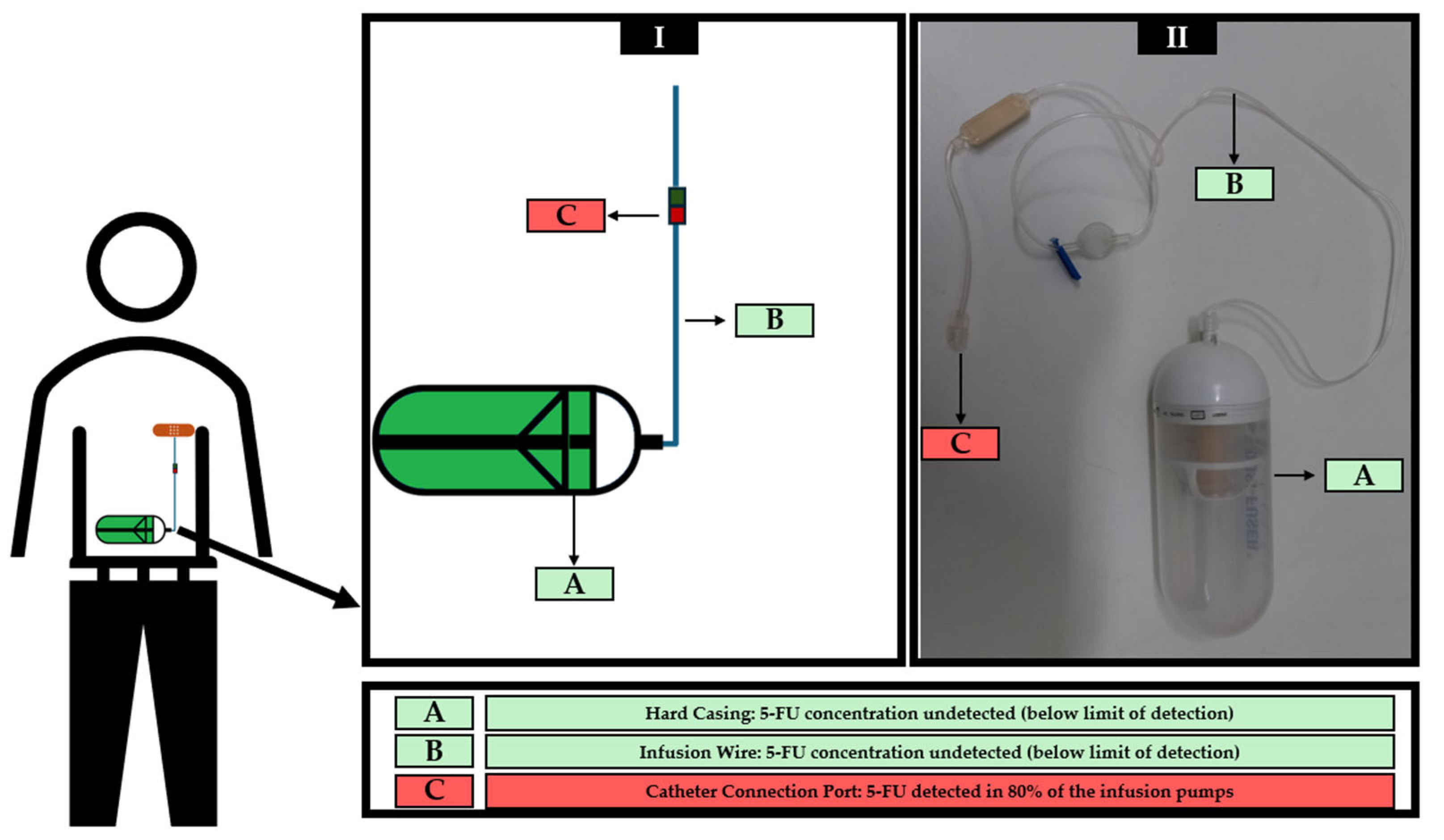
| Infusion Pump ID | Concentration of 5-FU (µg/cm2) in the Analyzed Areas of the Infusion Pumps | ||
|---|---|---|---|
| Outside of the Pump (Hard Casing) | Infusion Wire | Catheter Connection Port | |
| Infusion Pump 1 | <LOD | <LOD | <LOD |
| Infusion Pump 2 | <LOD | <LOD | 0.39 * |
| Infusion Pump 3 | <LOD | <LOD | 0.22 * |
| Infusion Pump 4 | <LOD | <LOD | 0.62 * |
| Infusion Pump 5 | <LOD | <LOD | <LOD |
| Infusion Pump 6 | <LOD | <LOD | 7.06 * |
| Infusion Pump 7 | <LOD | <LOD | 0.22 * |
| Infusion Pump 8 | <LOD | <LOD | 0.73 * |
| Infusion Pump 9 | <LOD | <LOD | 0.22 * |
| Infusion Pump 10 | <LOD | <LOD | 0.18 * |
| Reference | LOD | LOQ | Analytical Method | Injection Volume | Study Main Goal |
|---|---|---|---|---|---|
| Present Study | 0.050 µg/cm2 | 0.14 µg/cm2 | HPLC-DAD | 10 µL | Detection of 5-FU residues in external parts of infusion pumps |
| Viegas et al. [20,27] | 0.010 µg/cm2 | 0.033 µg/cm2 | HPLC-DAD | 100 µL | Detection of 5-FU residues in room surfaces |
| Labrèche et al. [52] | 0.040 ng/cm2 | 0.14 ng/cm2 | LC-MS/MS | Not reported | Detection of 5-FU residues in room surfaces |
| Pinet et al. [53] | 0.04 ng/cm2 | 0.099 ng/cm2 | LC-MS/MS | Not reported | Detection of 5-FU residues in room surfaces |
| Rossignol et al. [54] | 0.0013 ng/cm2 | 0.025 ng/cm2 | LC-MS/MS | 7 µL | Detection of 5-FU residues in room surfaces |
| Chauchat et al. [55] | 0.040 ng/cm2 | 0.14 ng/cm2 | LC-MS/MS | Not reported | Detection of 5-FU residues in room surfaces |
| Kåredal et al. [56] | 0.0018 ng/cm2 | 0.0035 ng/cm2 | LC-MS/MS | 20 µL | Detection of 5-FU residues in room surfaces |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, A.; Jesus, Â.; Barreiros, L.; Carvalho, D.; Sá, M.d.A.; Carvalho, S.; Correia, P.; Moreira, F. Safeguarding Patients, Relatives, and Nurses: A Screening Approach for Detecting 5-FU Residues on Elastomeric Infusion Pumps Using HPLC-DAD. Toxics 2025, 13, 416. https://doi.org/10.3390/toxics13050416
Cardoso A, Jesus Â, Barreiros L, Carvalho D, Sá MdA, Carvalho S, Correia P, Moreira F. Safeguarding Patients, Relatives, and Nurses: A Screening Approach for Detecting 5-FU Residues on Elastomeric Infusion Pumps Using HPLC-DAD. Toxics. 2025; 13(5):416. https://doi.org/10.3390/toxics13050416
Chicago/Turabian StyleCardoso, Andreia, Ângelo Jesus, Luísa Barreiros, Daniel Carvalho, Maria dos Anjos Sá, Susana Carvalho, Patrícia Correia, and Fernando Moreira. 2025. "Safeguarding Patients, Relatives, and Nurses: A Screening Approach for Detecting 5-FU Residues on Elastomeric Infusion Pumps Using HPLC-DAD" Toxics 13, no. 5: 416. https://doi.org/10.3390/toxics13050416
APA StyleCardoso, A., Jesus, Â., Barreiros, L., Carvalho, D., Sá, M. d. A., Carvalho, S., Correia, P., & Moreira, F. (2025). Safeguarding Patients, Relatives, and Nurses: A Screening Approach for Detecting 5-FU Residues on Elastomeric Infusion Pumps Using HPLC-DAD. Toxics, 13(5), 416. https://doi.org/10.3390/toxics13050416






