Genotoxicity and Cytotoxicity Assessment of Volatile Organic Compounds in Pathology Professionals Through the Buccal Micronuclei Assay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Samples
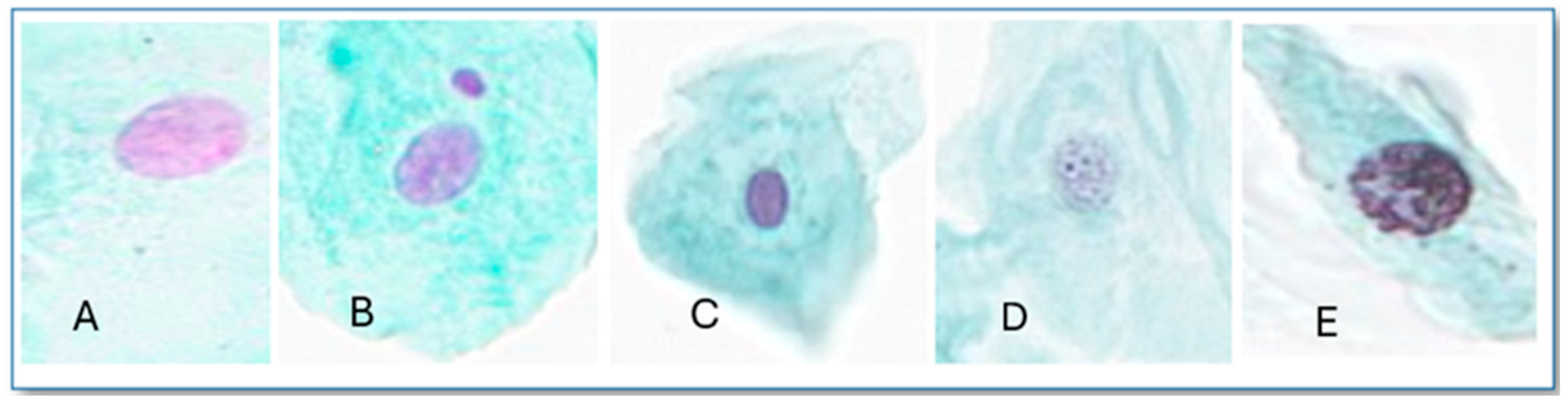
2.2. Exfoliated Buccal MN Cytome Assay
2.3. Visualization and Scoring
2.4. Statistical Analysis
3. Results
3.1. Genotoxicity and Citotoxicity Assessment
3.1.1. Micronucleus (MN)
3.1.2. Other Nuclear Abnormalities (ONAs)
3.2. Genotoxicity Risk Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IARC | International Agency for Research on Cancer |
| MN | Micronucleus |
| ONAs | Other nuclear abnormalities |
| VOCs | Volatile organic compounds |
| RR | Relative risk |
References
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Xylene; U.S. Departmen of Health and Human Services: Washington, DC, USA, 2007. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Formaldehyde; U.S. Departmen of Health and Human Services: Washington, DC, USA, 1999. [Google Scholar]
- International Agency for Research on Cancer (IARC). Monographs on the Evaluation of Carcinogenic Risks to Humans. In Formaldehyde, 2-Butoxyethanol and 1-tert-Butoxypropan-2-ol; IARC: Lyon, France, 2006. [Google Scholar]
- Kandyala, R.; Raghavendra, S.P.; Rajasekharan, S. Xylene: An Overview of Its Health Hazards and Preventive Measures. J. Oral Maxillofac. Pathol. 2010, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Szucs, S.; Tóth, L.; Legoza, J.; Sárváry, A.; Ádány, R. Simultaneous Determination of Styrene, Toluene, and Xylene Metabolites in Urine by Gas Chromatography/Mass Spectrometry. Arch. Toxicol. 2002, 76, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Diário da República. Decree Law No. 38 DL No. 39 de 2018; Diário da República: Lisbon, Portugal, 2018. [Google Scholar]
- Thomas, P.; Fenech, M. Buccal Micronucleus Cytome Assay. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2011; Volume 682, pp. 235–248. ISBN 9781603274081. [Google Scholar]
- Aguiar Torres, L.; dos Santos Rodrigues, A.; Linhares, D.; Camarinho, R.; Nunes Páscoa Soares Rego, Z.M.; Ventura Garcia, P. Buccal Epithelial Cell Micronuclei: Sensitive, Non-Invasive Biomarkers of Occupational Exposure to Low Doses of Ionizing Radiation. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2019, 838, 54–58. [Google Scholar] [CrossRef]
- Garcia, P.V.; Linhares, D.; Amaral, A.F.S.; Rodrigues, A.S. Exposure of Thermoelectric Power-Plant Workers to Volatile Organic Compounds from Fuel Oil: Genotoxic and Cytotoxic Effects in Buccal Epithelial Cells. Mutat. Res. Toxicol. Environ. Mutagen. 2012, 747, 197–201. [Google Scholar] [CrossRef]
- Holland, N.; Bolognesi, C.; Kirsch-Volders, M.; Bonassi, S.; Zeiger, E.; Knasmueller, S.; Fenech, M. The Micronucleus Assay in Human Buccal Cells as a Tool for Biomonitoring DNA Damage: The HUMN Project Perspective on Current Status and Knowledge Gaps. Mutat. Res. Rev. Mutat. Res. 2008, 659, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Cobanoglu, H.; Coskun, M.; Coskun, M.; Çayir, A. Results of Buccal Micronucleus Cytome Assay in Pesticide-Exposed and Non-Exposed Group. Environ. Sci. Pollut. Res. 2019, 26, 19676–19683. [Google Scholar] [CrossRef]
- Kashyap, B.; Reddy, P.S. Micronuclei Assay of Exfoliated Oral Buccal Cells: Means to Assess the Nuclear Abnormalities in Different Diseases. J. Cancer Res. Ther. 2012, 8, 184–191. [Google Scholar] [CrossRef]
- Speit, G.; Schmid, O. Local Genotoxic Effects of Formaldehyde in Humans Measured by the Micronucleus Test with Exfoliated Epithelial Cells. Mutat. Res. Rev. Mutat. Res. 2006, 613, 1–9. [Google Scholar] [CrossRef]
- Viegas, S.; Ladeira, C.; Nunes, C.; Malta-Vacas, J.; Gomes, M.; Brito, M.; Mendonça, P.; Prista, J. Genotoxic Effects in Occupational Exposure to Formaldehyde: A Study in Anatomy and Pathology Laboratories and Formaldehyde-Resins Production. J. Occup. Med. Toxicol. 2010, 5, 25. [Google Scholar] [CrossRef]
- Ladeira, C.; Viegas, S.; Carolino, E.; Prista, J.; Gomes, M.C.; Brito, M. Genotoxicity Biomarkers in Occupational Exposure to Formaldehyde-The Case of Histopathology Laboratories. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011, 721, 15–20. [Google Scholar] [CrossRef]
- Goyer, N.; Beaudry, C.; Buissonnet, S.; Goyer, N.; Beaudry, C.; Bégin, D.; Roberge, S. Impacts d’un Abaissement de La Valeur d’Exposition Admissible au Impacts d’un Abaissement de La Valeur d’Exposition Admissible au Formaldéhyde: Industries de Fabrication de Formaldéhyde et de Formaldéhyde: Industries de Fabrication de Formaldéhyde et de Résines à Base de Formaldéhyde Résines à Base de Formaldéhyde Citation Recommandée Citation Recommandée; National Library of Quebec: Montreal, QC, Canada, 2004. [Google Scholar]
- Orsière, T.; Sari-Minodier, I.; Iarmarcovai, G.; Botta, A. Genotoxic Risk Assessment of Pathology and Anatomy Laboratory Workers Exposed to Formaldehyde by Use of Personal Air Sampling and Analysis of DNA Damage in Peripheral Lymphocytes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2006, 605, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M.; Nersesyan, A.; Knasmueller, S. A Systematic Review of the Association between Occupational Exposure to Formaldehyde and Effects on Chromosomal DNA Damage Measured Using the Cytokinesis-Block Micronucleus Assay in Lymphocytes. Mutat. Res. Rev. Mutat. Res. 2016, 770, 46–57. [Google Scholar] [CrossRef] [PubMed]
- de Aquino, T.; Zenkner, F.F.; Ellwanger, J.H.; Prá, D.; Rieger, A. DNA Damage and Cytotoxicity in Pathology Laboratory Technicians Exposed to Organic Solvents. An. Acad. Bras. Cienc. 2016, 88, 227–236. [Google Scholar] [CrossRef]
- Ladeira, C.; Gajski, G.; Meneses, M.; Gerić, M.; Viegas, S. The Genotoxicity of an Organic Solvent Mixture: A Human Biomonitoring Study and Translation of a Real-Scenario Exposure to in Vitro. Regul. Toxicol. Pharmacol. 2020, 116, 104726. [Google Scholar] [CrossRef]
- Dos Reis Filho, A.P.; Silveira, M.A.D.; Demarco, N.R.; D’Arce, L.P.G. Increased DNA Damage, Instability and Cytokinesis Defects in Occupationally Exposed Car Painters. In Vivo 2019, 33, 1807–1811. [Google Scholar] [CrossRef]
- Cavallo, D.; Ursini, C.L.; Fresegna, A.M.; Ciervo, A.; Maiello, R.; Buresti, G.; Paci, E.; Pigini, D.; Gherardi, M.; Carbonari, D.; et al. Occupational Exposure in Industrial Painters: Sensitive and Noninvasive Biomarkers to Evaluate Early Cytotoxicity, Genotoxicity and Oxidative Stress. Int. J. Environ. Res. Public Health 2021, 18, 4645. [Google Scholar] [CrossRef]
- de Oliveira, F.M.; Carmona, A.M.; Ladeira, C. Genotoxicity Assessment Data for Exfoliated Buccal Cells Exposed to Mobile Phone Radiation. Data Brief. 2017, 15, 344–347. [Google Scholar] [CrossRef]
- Ferri, A.P.N.; Gomide, B.; de Figueiredo, F.A.T.; Carta, C.F.L.; Balducci, I.; Almeida, J.D. Effect of Phenol Derivatives in the Oral Mucosa of University Laboratory Technicians. Braz. Arch. Biol. Technol. 2023, 66, e23210014. [Google Scholar] [CrossRef]
- Sommer, S.; Buraczewska, I.; Kruszewski, M. Micronucleus Assay: The State of Art, and Future Directions. Int. J. Mol. Sci. 2020, 21, 1534. [Google Scholar] [CrossRef]
- Mayocchi, K.; Arcuri, M.; Giménez, J.; Arcuri, A.; Mayocchi, M.; Blasetti, N.; Levalle, M.J.; Darrigran, L.; Sirimarco, K.; Echeverría, N.; et al. Diagnostic Value of Exfoliative Cytology for Oral Cancer. Rev. Fac. Odontol. 2022, 49–52. Available online: https://sedici.unlp.edu.ar/bitstream/handle/10915/155360/Revista_completa.pdf-PDFA.pdf?sequence=1&isAllowed=y (accessed on 8 April 2025).
- Kim, D.; Thrall, M.J.; Michelow, P.; Schmitt, F.C.; Vielh, P.R.; Siddiqui, M.T.; Sundling, K.E.; Virk, R.; Alperstein, S.; Bui, M.M.; et al. The Current State of Digital Cytology and Artificial Intelligence (AI): Global Survey Results from the American Society of Cytopathology Digital Cytology Task Force. J. Am. Soc. Cytopathol. 2024, 13, 319–328. [Google Scholar] [CrossRef]
- Tolbert, P.E.; Shy, C.M.; Allen, J.W. Micronuclei and Other Nuclear Anomalies in Buccal Smears: Methods Development. Mutat. Res. Mutagen. Relat. Subj. 1992, 271, 69–77. [Google Scholar] [CrossRef]
- Thomas, P.; Holland, N.; Bolognesi, C.; Kirsch-Volders, M.; Bonassi, S.; Zeiger, E.; Knasmueller, S.; Fenech, M. Buccal Micronucleus Cytome Assay. Nat. Protoc. 2009, 4, 825–837. [Google Scholar] [CrossRef]
- Ladeira, C. The Use of Effect Biomarkers in Chemical Mixtures Risk Assessment—Are They Still Important? Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2024, 896, 503768. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.O.; Arcaro, K.; Spink, D.C. Understanding the Human Health Effects of Chemical Mixtures. Environ. Health Perspect. 2002, 110, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Moreira, S.; Silva Santos, C.; Ramos, C.; Borges, T.; Teio, E.; Almeida, T.; Manzano, M.J.; Nogueira, A.; Carreira de Oliveira, A.; Sacadura Leite, E.; et al. Technical Guide No. 2 Health Surveillance of Workers Exposed to Carcinogenic, Mutagenic or Reproductive Toxic Chemical Agents. Programa Nac. Saúde Ocup. 2018, 1–121. Available online: https://www.dgs.pt/saude-ocupacional/referenciais-tecnicos-e-normativos/guias-tecnicos/guia-tecnico-n-2-1.aspx (accessed on 8 April 2025).
- Sarto, F.; Finotto, S.; Giacomelli, L.; Mazzotti, D.; Tomanin, R.; Levis, A.G. The Micronucleus Assay in Exfoliated Cells of the Human Buccal Mucosa. Mutagenesis 1987, 2, 11–17. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Arruda, M.S.C.; Garcia, P.V. Evidence of DNA Damage in Humans Inhabiting a Volcanically Active Environment: A Useful Tool for Biomonitoring. Environ. Int. 2012, 49, 51–56. [Google Scholar] [CrossRef]
- Bolognesi, C.; Lando, C.; Forni, A.; Landini, E.; Scarpato, R.; Migliore, L.; Bonassi, S. Chromosomal Damage and Ageing: Effect on Micronuclei Frequency in Peripheral Blood Lymphocytes. Age Ageing 1999, 28, 393–397. [Google Scholar] [CrossRef]
- Ziȩtkiewicz, E.; Wojda, A.; Witt, M. Cytogenetic Perspective of Ageing and Longevity in Men and Women. J. Appl. Genet. 2009, 50, 261–273. [Google Scholar] [CrossRef]
- Barnett, Y.A.; King, C.M. An Investigation of Antioxidant Status, DNA Repair Capacity and Mutation as a Function of Age in Humans. Mutat. Res. DNAging 1995, 338, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Linhares, D.; Rocha, J.; Rodrigues, A.; Camarinho, R.; Garcia, P. Evaluation of Respiratory, Genotoxic and Cytotoxic Effects from Occupational Exposure to Typography Activities. Atmosphere 2023, 14, 562. [Google Scholar] [CrossRef]
- Caponio, V.C.A.; Silva, F.F.-V.e.; Popolo, F.; Giugliano, S.; Spizzirri, F.; Lorenzo-Pouso, A.I.; Padín-Iruegas, M.E.; Zhurakivska, K.; Muzio, L.L.; López-Pintor, R.M. State of Art of Micronuclei Assay in Exfoliative Cytology as a Clinical Biomarker of Genetic Damage in Oral Carcinogenesis: A Systematic Review and Meta-Analysis. Mutat. Res. Rev. Mutat. Res. 2024, 794, 108508. [Google Scholar] [CrossRef] [PubMed]
- Bonassi, S.; Coskun, E.; Ceppi, M.; Lando, C.; Bolognesi, C.; Burgaz, S.; Holland, N.; Kirsh-Volders, M.; Knasmueller, S.; Zeiger, E.; et al. The HUman MicroNucleus Project on EXfoLiated Buccal Cells (HUMN XL): The Role of Life-Style, Host Factors, Occupational Exposures, Health Status, and Assay Protocol. Mutat. Res. Rev. Mutat. Res. 2011, 728, 88–97. [Google Scholar] [CrossRef]
- Bonassi, S.; Milić, M.; Neri, M. Frequency of Micronuclei and Other Biomarkers of DNA Damage in Populations Exposed to Dusts, Asbestos and Other Fibers. A Systematic Review. Mutat. Res. Rev. Mutat. Res. 2016, 770, 106–118. [Google Scholar] [CrossRef]
- Bolognesi, C.; Bonassi, S.; Knasmueller, S.; Fenech, M.; Bruzzone, M.; Lando, C.; Ceppi, M. Clinical Application of Micronucleus Test in Exfoliated Buccal Cells: A Systematic Review and Metanalysis. Mutat. Res. Rev. Mutat. Res. 2015, 766, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.A.; Cury, F.D.P.; Scapulatempo Neto, C.; Marques, M.M.C.; Silveira, H.C.S. Micronucleus Evaluation of Exfoliated Buccal Epithelial Cells Using Liquid-Based Cytology Preparation. Acta Cytol. 2014, 58, 582–588. [Google Scholar] [CrossRef]
- Nersesyan, A.; Kundi, M.; Atefie, K.; Schulte-Hermann, R.; Knasmüller, S. Effect of Staining Procedures on the Results of Micronucleus Assays with Exfoliated Oral Mucosa Cells. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1835–1840. [Google Scholar] [CrossRef]
- Çelik, A.; Çavaş, T.; Ergene-Gözükara, S. Cytogenetic Biomonitoring in Petrol Station Attendants: Micronucleus Test in Exfoliated Buccal Cells. Mutagenesis 2003, 18, 417–421. [Google Scholar] [CrossRef]
- de los A. Gutiérrez, M.; Palmieri, M.A.; Giuliani, D.S.; Colman Lerner, J.E.; Maglione, G.; Andrinolo, D.; Tasat, D.R. Monitoring Human Genotoxicity Risk Associated to Urban and Industrial Buenos Aires Air Pollution Exposure. Environ. Sci. Pollut. Res. 2020, 27, 13995–14006. [Google Scholar] [CrossRef]
- Akbar-Khanzadeh, F.; Ulises Vaquerano, M.; Akbar-Khanzadeh, M.; Bisesi, M.S. Formaldehyde Exposure, Acute Pulmonary Response, and Exposure Control Options in a Gross Anatomy Laboratory. Am. J. Ind. Med. 1994, 26, 61–75. [Google Scholar] [CrossRef]
- Sivasankari, N.P.; Sundarapandian, S.; Sakthivel, E. Micronucleus Assay in Formalin Exposed Individuals. Indian J. Clin. Anat. Physiol. 2020, 5, 81–84. [Google Scholar] [CrossRef]
- Bolognesi, C.; Knasmueller, S.; Nersesyan, A.; Thomas, P.; Fenech, M. The HUMNxl Scoring Criteria for Different Cell Types and Nuclear Anomalies in the Buccal Micronucleus Cytome Assay—An Update and Expanded Photogallery. Mutat. Res. Rev. Mutat. Res. 2013, 753, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Antonio, E.L.; Nascimento, A.J.D.; Lima, A.A.S.; Leonart, M.S.S.; Fernandes, Â. Genotoxicity and Cytotoxicity of X-Rays in Children Exposed to Panoramic Radiography. Rev. Paul. Pediatr. 2017, 35, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Songur, A.; Ozen, O.A.; Sarsilmaz, M. The Toxic Effects of Formaldehyde on the Nervous System. Rev. Environ. Contam. Toxicol. 2010, 203, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Rovira, J.; Roig, N.; Nadal, M.; Schuhmacher, M.; Domingo, J.L. Human Health Risks of Formaldehyde Indoor Levels: An Issue of Concern. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2016, 51, 357–363. [Google Scholar] [CrossRef]
- Dick, F.D. Solvent Neurotoxicity. Occup. Environ. Med. 2006, 63, 221–226. [Google Scholar] [CrossRef]

| Exposed Group (n = 21) | Control Group (n = 50) | p-Value 1 | |
|---|---|---|---|
| Years (mean ± SD) | 39.38 ± 2.60 | 47.72 ± 1.53 | 0.009 * |
| Sex | |||
| Males | 5 (23.8%) | 8 (16%) | 0.437 |
| Females | 16 (76.2%) | 42 (84%) | |
| Tobacco consumption | |||
| Yes | 5 (23.8%) | 10 (20%) | 0.720 |
| No | 16 (76.2%) | 40 (80%) | |
| Alcohol consumption | |||
| Yes | 15 (71.4%) | 27 (54%) | 0.173 |
| No | 6 (28.6%) | 23 (46%) | |
| Mouthwash use | |||
| Yes | 8 (38.1%) | 27 (59%) | 0.221 |
| No | 13 (61.9%) | 23 (46%) |
| All (n = 71) (C; p-Value) | Exposed (n = 21) (C; p-Value) | Controls (n = 50) (C; p-Value) | |
|---|---|---|---|
| Age | −0.021; 0.861 | 0.303; 0.181 | 0.141; 0.328 |
| Sex | −0.079; 0.514 | −0.067; 0.774 | −0.142; 0.324 |
| Tobacco consumption | −0.28; 0.818 | 0.10; 0.967 | −0.053; 0.717 |
| Alcohol consumption | 0.026; 0.830 | 0.206; 0.369 | −0.118; 0.416 |
| Mouthwash use | 0.041; 0.734 | −0.117; 0.614 | 0.174; 0.226 |
| Poisson Regression (GLZ) | N: 71 Prob ˃ χ2 ˂ 0.001 | ||
|---|---|---|---|
| N (%) | RR (95% CI) a | p-Value | |
| Age | 1.03 (1.01–1.04) | 0.002 * | |
| Gender | |||
| Male | 13 (18.3) | 0.76 (0.48–1.20) | 0.236 |
| Female | 58 (81.7) | 1 | |
| Mouthwash use | |||
| Yes | 35 (49.3) | 1.03 (0.71–1.47) | 0.896 |
| No | 36 (50.7) | 1 | |
| Tobacco consumption | |||
| Yes | 15 (21.1) | 1.24 (0.83–1.83) | 0.293 |
| No | 56 (78.9) | 1 | |
| Alcohol consumption | |||
| Yes | 42 (59.2) | 1.30 (0.88–1.93) | 0.188 |
| No | 29 (40.8) | 1 | |
| Occupational exposure to VOCs | |||
| Exposed group | 21 (29.6) | 3.77 (2.52–5.65) | <0.001 * |
| Control group | 50 (70.4) | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baptista, F.; Garcia, P.V.; Rodrigues, A.S.; Ladeira, C. Genotoxicity and Cytotoxicity Assessment of Volatile Organic Compounds in Pathology Professionals Through the Buccal Micronuclei Assay. Toxics 2025, 13, 411. https://doi.org/10.3390/toxics13050411
Baptista F, Garcia PV, Rodrigues AS, Ladeira C. Genotoxicity and Cytotoxicity Assessment of Volatile Organic Compounds in Pathology Professionals Through the Buccal Micronuclei Assay. Toxics. 2025; 13(5):411. https://doi.org/10.3390/toxics13050411
Chicago/Turabian StyleBaptista, Fátima, Patrícia V. Garcia, Armindo S. Rodrigues, and Carina Ladeira. 2025. "Genotoxicity and Cytotoxicity Assessment of Volatile Organic Compounds in Pathology Professionals Through the Buccal Micronuclei Assay" Toxics 13, no. 5: 411. https://doi.org/10.3390/toxics13050411
APA StyleBaptista, F., Garcia, P. V., Rodrigues, A. S., & Ladeira, C. (2025). Genotoxicity and Cytotoxicity Assessment of Volatile Organic Compounds in Pathology Professionals Through the Buccal Micronuclei Assay. Toxics, 13(5), 411. https://doi.org/10.3390/toxics13050411









