Subcellular Partitioning of Trace Elements Is Related to Metal Ecotoxicological Classes in Livers of Fish (Esox lucius; Coregonus clupeaformis) from the Yellowknife Area (Northwest Territories, Canada)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Fish Collection
2.3. Subcellular Partitioning of Trace Elements in Liver Cells
2.4. Trace Element Measurements and Quality Controls
2.5. Calculations and Statistical Analysis
3. Results
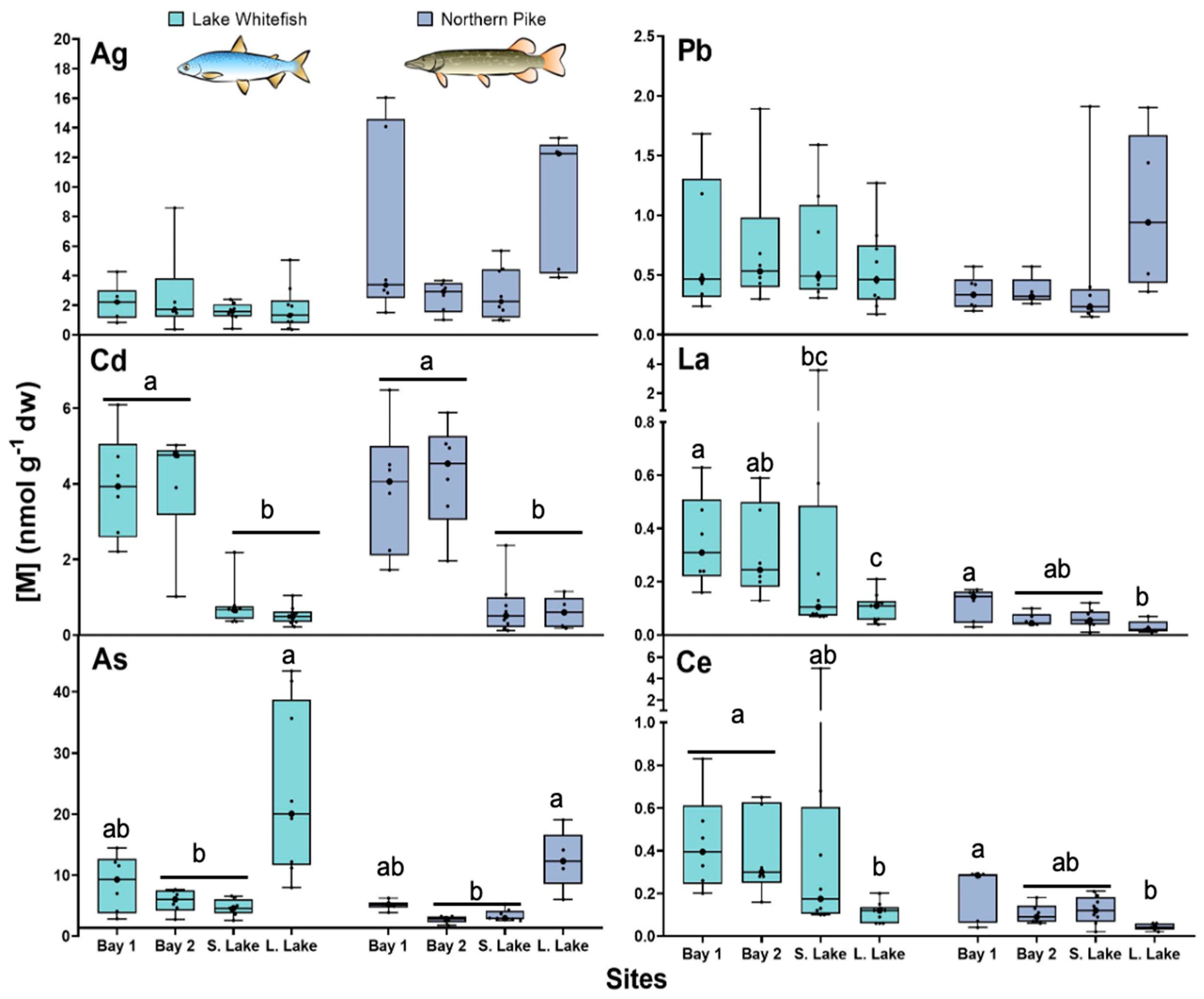
3.1. Total Trace Element Concentrations
3.2. Subcellular Metal Partitioning
3.2.1. Silver and Cadmium

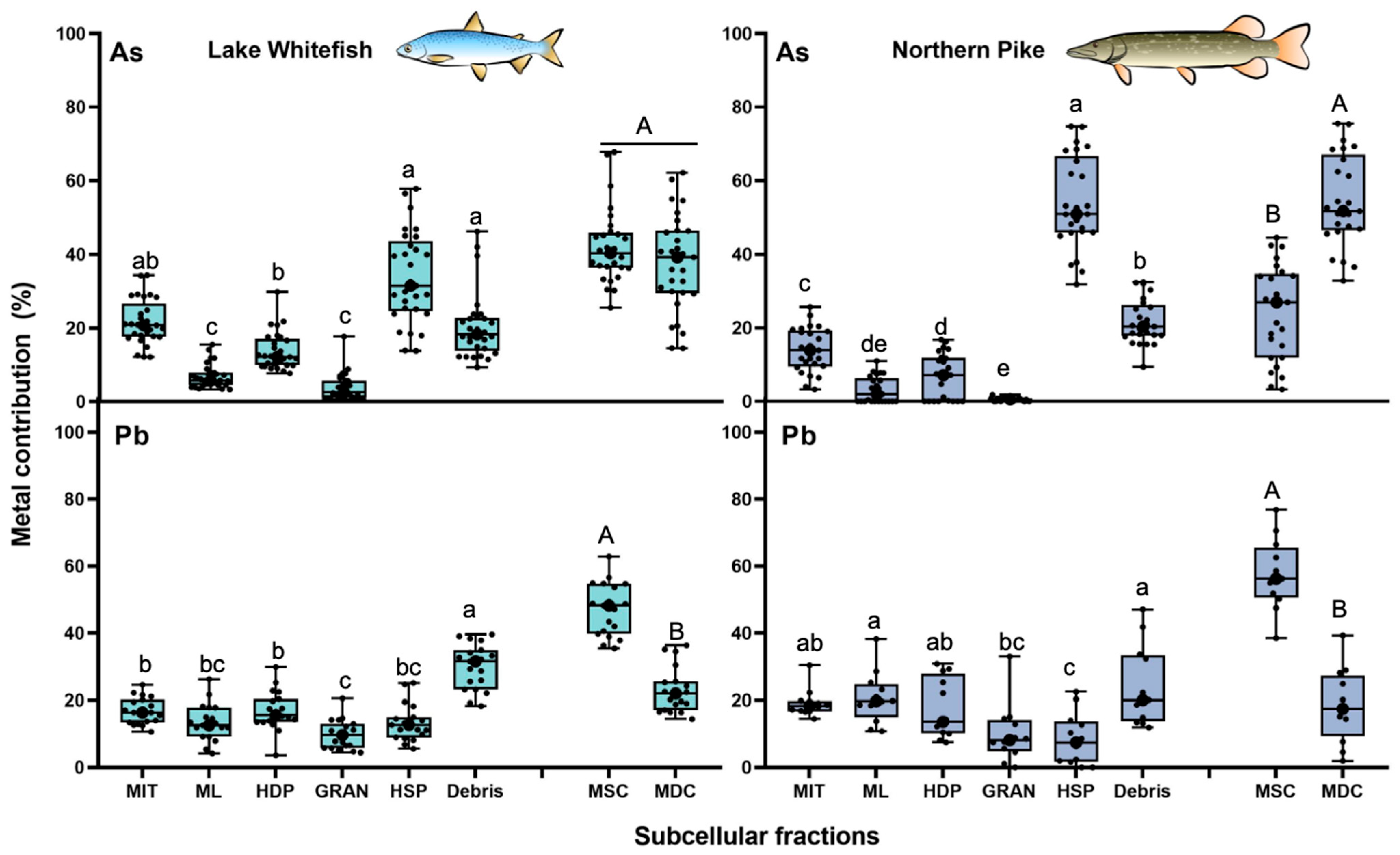
3.2.2. Arsenic and Lead
3.2.3. Cerium and Lanthanum

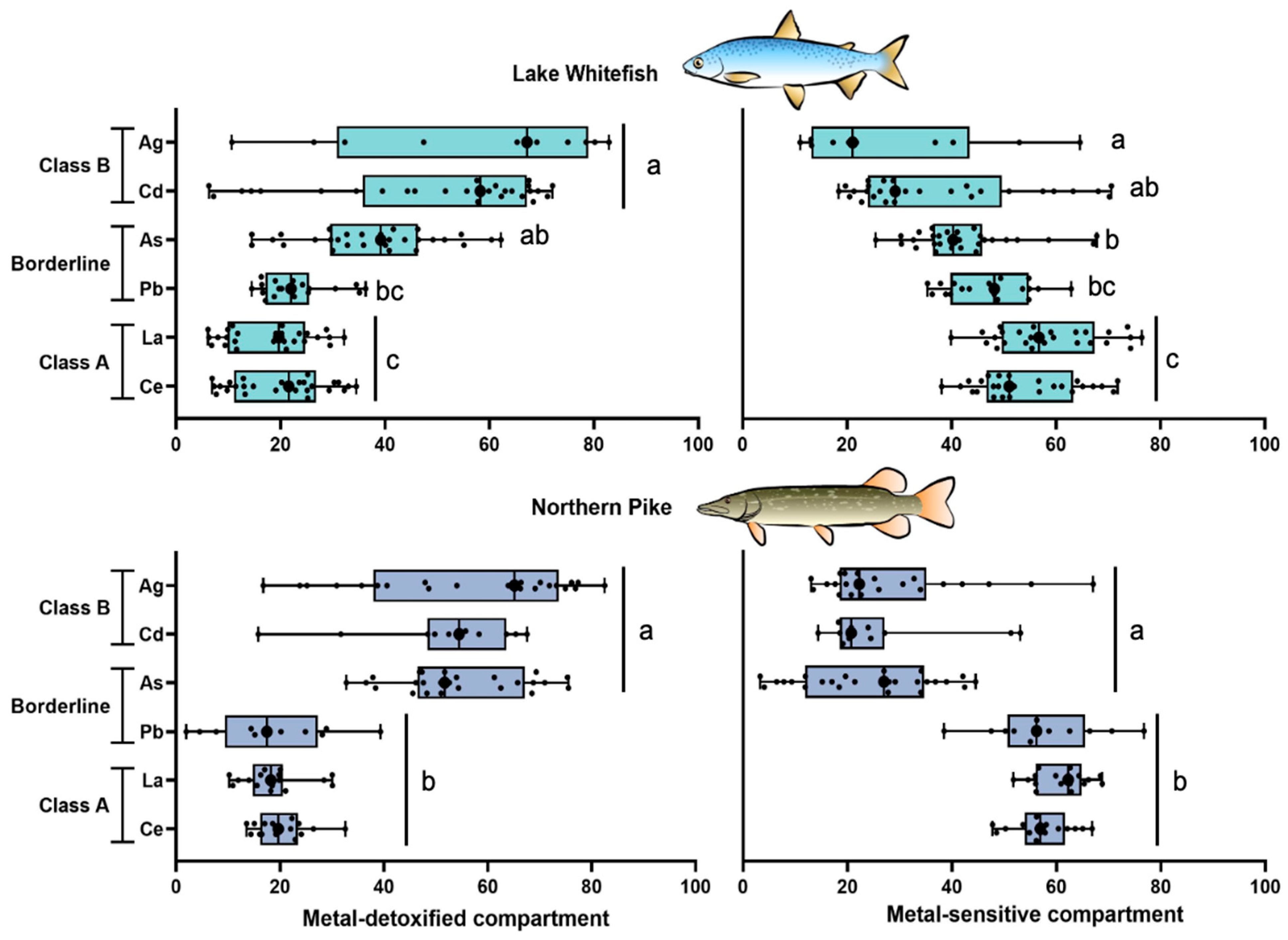
3.2.4. Differences in the Subcellular Partitioning Among Trace Elements
4. Discussion
4.1. Trace Metal Concentrations in Fish Liver
4.2. Subcellular Partitioning of Studied Metals
4.2.1. Class B Elements
4.2.2. Borderline Elements
4.2.3. Class A Elements
4.3. Emerging Classification of Metal Partitioning in Fish Liver
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le Faucheur, S.; Mertens, J.; Van Genderen, E.; Boullemant, A.; Fortin, C.; Campbell, P.G.C. Development of Quantitative Ion Character–Activity Relationship Models to Address the Lack of Toxicological Data for Technology-Critical Elements. Environ. Toxicol. Chem. 2021, 40, 1139–1148. [Google Scholar] [CrossRef]
- Wolterbeek, H.T.; Verburg, T.G. Predicting metal toxicity revisited: General properties vs. specific effects. Sci. Total Environ. 2001, 279, 87–115. [Google Scholar] [CrossRef]
- Kinraide, T.B. Improved scales for metal ion softness and toxicity. Environ. Toxicol. Chem. 2009, 28, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Nieboer, E.; Richardson, D.H.S. The replacement of the nondescript term ‘heavy metals’ by a biologically and chemically significant classification of metal ions. Environ. Pollut. Ser. B Chem. Phys. 1980, 1, 3–26. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. In Survey of Progress in Chemistry; Arthur, F.S., Ed.; Elsevier: Amsterdam, The Netherlands, 1969; Volume 5, pp. 1–52. [Google Scholar]
- Duffus, J.H. “Heavy metals”—A meaningless term? (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 793–807. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, F.; Mu, Y.; Zeng, E.Y.; Meng, W.; Zhao, X.; Giesy, J.P.; Feng, C.; Wang, P.; Liao, H.; et al. Directly Predicting Water Quality Criteria from Physicochemical Properties of Transition Metals. Sci. Rep. 2016, 6, 22515. [Google Scholar] [CrossRef]
- Meng, X.; Wang, X.; Zhao, X.; Wang, Y.; Wu, F. Predicting metal toxicity to aquatic life:a first step towards integrating a QICAR approach with the BLM framework. J. Clean. Prod. 2020, 246, 118973. [Google Scholar] [CrossRef]
- Walker, Y.D.; Newman, M.C.; Enache, M. Fundamental QSARs for Metal Ions; CRC Press: Boca Raton, FL, USA, 2012; pp. 1–302. [Google Scholar]
- Mason, A.Z.; Jenkins, K.D. Metal detoxification in aquatic organisms. In Metal Speciation and Bioavailability in Aquatic Systems; Tessier, A., Turner, D., Buffle, J., van Leeuwen, H.P., Eds.; IUPAC Series on Analytical and Physical Chemistry of Environmental Systems; John Wiley & Sons: Chichester, UK, 1995; pp. 479–608. [Google Scholar]
- Wallace, W.G.; Lee, B.G.; Luoma, S.N. Subcellular compartmentalization of Cd and Zn in two bivalves. I. Significance of metal-sensitive fractions (MSF) and biologically detoxified metal (BDM). Mar. Ecol. Prog. Ser. 2003, 249, 183–197. [Google Scholar] [CrossRef]
- Campbell, P.G.C.; Hare, L. Metal detoxification in freshwater animals. Roles of metallothioneins. In Metallothioneins and Related Chelators; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Metal Ions in Life Sciences; Royal Society of Chemistry: Cambridge, UK, 2009; Volume 5, pp. 239–277. [Google Scholar]
- Vijver, M.G.; Van Gestel, C.A.M.; Lanno, R.P.; Van Straalen, N.M.; Peijnenburg, W.J.G.M. Internal metal sequestration and its ecotoxicological relevance: A review. Environ. Sci. Technol. 2004, 38, 4705–4712. [Google Scholar] [CrossRef]
- Rosabal, M.; Hare, L.; Campbell, P.G.C. Subcellular metal partitioning in larvae of the insect Chaoborus collected along an environmental metal exposure gradient (Cd, Cu, Ni and Zn). Aquat. Toxicol. 2012, 120–121, 67–78. [Google Scholar] [CrossRef]
- Giguère, A.; Campbell, P.G.C.; Hare, L.; Couture, P. Sub-cellular partitioning of cadmium, copper, nickel and zinc in indigenous yellow perch (Perca flavescens) sampled along a polymetallic gradient. Aquat. Toxicol. 2006, 77, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Rainbow, P.S. Subcellular partitioning and the prediction of cadmium toxicity to aquatic organisms. Environ. Chem. 2006, 3, 395–399. [Google Scholar] [CrossRef]
- Rosabal, M. Subcellular Metal-Handling Strategies in Two Groups of Aquatic Animals (Insect, Fish): Contributions to the Understanding of Metal Toxicity. Ph.D. Thesis, Université du Québec, INRS, Québec, QC, Canada, 2015. Available online: https://espace.inrs.ca/id/eprint/4032/1/T742.pdf (accessed on 12 May 2025).
- Hauser-Davis, R.A. Subcellular Cadmium, Lead and Mercury Partitioning Assessments in Aquatic Organisms as a Tool for Assessing Actual Toxicity and Trophic Transfer. In Lead, Mercury and Cadmium in the Aquatic Environment: Worldwide Occurrence, Fate and Toxicity; CRC Press: Boca Raton, FL, USA, 2023; pp. 1–22. [Google Scholar]
- Barst, B.D.; Rosabal, M.; Campbell, P.G.C.; Muir, D.G.C.; Wang, X.; Köck, G.; Drevnick, P.E. Subcellular distribution of trace elements and liver histology of landlocked Arctic char (Salvelinus alpinus) sampled along a mercury contamination gradient. Environ. Pollut. 2016, 212, 574–583. [Google Scholar] [CrossRef]
- Barst, B.D.; Rosabal, M.; Drevnick, P.E.; Campbell, P.G.C.; Basu, N. Subcellular distributions of trace elements (Cd, Pb, As, Hg, Se) in the livers of Alaskan yelloweye rockfish (Sebastes ruberrimus). Environ. Pollut. 2018, 242, 63–72. [Google Scholar] [CrossRef]
- Urien, N.; Cooper, S.; Caron, A.; Sonnenberg, H.; Rozon-Ramilo, L.; Campbell, P.G.C.; Couture, P. Subcellular partitioning of metals and metalloids (As, Cd, Cu, Se and Zn) in liver and gonads of wild white suckers (Catostomus commersonii) collected downstream from a mining operation. Aquat. Toxicol. 2018, 202, 105–116. [Google Scholar] [CrossRef]
- Desjardins, K.; Ponton, D.E.; Bilodeau, F.; Rosabal, M.; Amyot, M. Methylmercury in northern pike (Esox lucius) liver and hepatic mitochondria is linked to lipid peroxidation. Sci. Total Environ. 2024, 931, 172703. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, K.; Khadra, M.; Caron, A.; Ponton, D.E.; Rosabal, M.; Amyot, M. Significance of chemical affinity on metal subcellular distribution in yellow perch (Perca flavescens) livers from Lake Saint-Pierre (QUEBEC, Canada). Environ. Pollut. 2022, 312, 120077. [Google Scholar] [CrossRef]
- Wagemann, R.; Snow, N.B.; Rosenberg, D.M.; Lutz, A. Arsenic in sediments, water and aquatic biota from lakes in the vicinity of Yellowknife, Northwest Territories, Canada. Arch. Environ. Contam. Toxicol. 1978, 7, 169–191. [Google Scholar] [CrossRef]
- Chételat, J.; Amyot, M.; Muir, D.; Black, M.; Richardson, M.; Evans, M.; Palmer, M. Arsenic, Antimony, and Metal Concentrations in Water and Sediment of Yellowknife Bay; NWT Open File 2017-05; Northwest Territories Geological Survey: Yellowknife, NT, Canada, 2018; p. 40.
- Fawcett, S.E.; Jamieson, H.E.; Nordstrom, D.K.; McCleskey, R.B. Arsenic and antimony geochemistry of mine wastes, associated waters and sediments at the Giant Mine, Yellowknife, Northwest Territories, Canada. Appl. Geochem. 2015, 62, 3–17. [Google Scholar] [CrossRef]
- Stantec. Analysis of Contaminants in Tissues of Fish Captured in the Yellowknife Bay Area, NT; Stantec Consulting Ltd.: Yellowknife, NT, Canada, 2014. [Google Scholar]
- Andrade, C.F.; Jamieson, H.E.; Kyser, T.K.; Praharaj, T.; Fortin, D. Biogeochemical redox cycling of arsenic in mine-impacted lake sediments and co-existing pore waters near Giant Mine, Yellowknife Bay, Canada. Appl. Geochem. 2010, 25, 199–211. [Google Scholar] [CrossRef]
- Cott, P.A.; Zajdlik, B.A.; Palmer, M.J.; McPherson, M.D. Arsenic and mercury in lake whitefish and burbot near the abandoned Giant Mine on Great Slave Lake. J. Great Lakes Res. 2016, 42, 223–232. [Google Scholar] [CrossRef]
- Rohonczy, J.; Cott, P.A.; Benwell, A.; Forbes, M.R.; Robinson, S.A.; Rosabal, M.; Amyot, M.; Chételat, J. Trophic structure and mercury transfer in the subarctic fish community of Great Slave Lake, Northwest Territories, Canada. J. Great Lakes Res. 2020, 46, 402–413. [Google Scholar] [CrossRef]
- Chételat, J.; Cott, P.A.; Rosabal, M.; Houben, A.; McClelland, C.; Rose, E.B.; Amyot, M. Arsenic bioaccumulation in subarctic fishes of a mine-impacted bay on Great Slave Lake, Northwest Territories, Canada. PLoS ONE 2019, 14, e0221361. [Google Scholar] [CrossRef] [PubMed]
- Schuh, C.E.; Jamieson, H.E.; Palmer, M.J.; Martin, A.J. Solid-phase speciation and post-depositional mobility of arsenic in lake sediments impacted by ore roasting at legacy gold mines in the Yellowknife area, Northwest Territories, Canada. Appl. Geochem. 2018, 91, 208–220. [Google Scholar] [CrossRef]
- Pinard, J.-P.; Trimble, A. Yellowknife Area Wind Potential; Aurora Research Institute: Inuvik, NT, Canada, 2017. [Google Scholar]
- Wang, J.; Guo, M.; Liu, M.; Wei, X. Long-term outlook for global rare earth production. Resour. Policy 2020, 65, 101569. [Google Scholar] [CrossRef]
- García, M.V.R.; Krzemień, A.; Bárcena, L.C.S.; Álvarez, I.D.; Fernández, C.C. Scoping studies of rare earth mining investments: Deciding on further project developments. Resour. Policy 2019, 64, 101525. [Google Scholar] [CrossRef]
- Cott, P.A.; Johnston, T.A.; Gunn, J.M. Food web position of burbot relative to lake trout, northern pike, and lake whitefish in four sub-Arctic boreal lakes. J. Appl. Ichthyol. 2011, 27, 49–56. [Google Scholar] [CrossRef]
- Morrow, J.E. The Freshwater Fishes of Alaska; Alaska Northwest Publishing Company: Anchorage, AK, USA, 1980. [Google Scholar]
- Pedlar, R.M.; Klaverkamp, J.F. Accumulation and distribution of dietary arsenic in lake whitefish (Coregonus clupeaformis). Aquat. Toxicol. 2002, 57, 153–166. [Google Scholar] [CrossRef]
- Koelz, W. Coregonid Fishes of the Great Lakes; US Government Printing Office: Washington, DC, USA, 1929; Volume 43.
- Hart, J.L. The growth of the whitefish Coregonus clupeaformis (Mitchill). Contrib. Can. Biol. Fish. 1931, 6, 427–444. [Google Scholar] [CrossRef]
- Treble, M.A.; Campana, S.E.; Wastle, R.J.; Jones, C.M.; Boje, J. An assessment of age determination methods, with age validation of Greenland halibut from the Northwest Atlantic. Sci. Counc. Res. Doc. NAFO 2005, 05/43, 1–22. [Google Scholar]
- Cardon, P.Y.; Caron, A.; Rosabal, M.; Fortin, C.; Amyot, M. Enzymatic validation of species-specific protocols for metal subcellular fractionation in freshwater animals. Limnol. Oceanogr. Methods 2018, 16, 537–555. [Google Scholar] [CrossRef]
- Rosabal, M.; Pierron, F.; Couture, P.; Baudrimont, M.; Hare, L.; Campbell, P.G.C. Subcellular partitioning of non-essential trace metals (Ag, As, Cd, Ni, Pb, and Tl) in livers of American (Anguilla rostrata) and European (Anguilla anguilla) yellow eels. Aquat. Toxicol. 2015, 160, 128–141. [Google Scholar] [CrossRef]
- Khadra, M.; Caron, A.; Planas, D.; Ponton, D.E.; Rosabal, M.; Amyot, M. The fish or the egg: Maternal transfer and subcellular partitioning of mercury and selenium in Yellow Perch (Perca flavescens). Sci. Total Environ. 2019, 675, 604–614. [Google Scholar] [CrossRef] [PubMed]
- de Rosemond, S.; Xie, Q.; Liber, K. Arsenic concentration and speciation in five freshwater fish species from Back Bay near Yellowknife, NT, CANADA. Environ. Monit. Assess. 2008, 147, 199–210. [Google Scholar] [CrossRef]
- Golder Associates Ltd. Investigation of the Distribution of Historic Tailings in North Yellowknife Bay; Golder Associates Ltd.: Yellowknife, NT, Canada, 2005. [Google Scholar]
- Mason, R.P.; Laporte, J.M.; Andres, S. Factors controlling the bioaccumulation of mercury, methylmercury, arsenic, selenium, and cadmium by freshwater invertebrates and fish. Arch. Environ. Contam. Toxicol. 2000, 38, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Amundsen, P.A.; Kashulin, N.A.; Terentjev, P.; Gjelland, K.Ø.; Koroleva, I.M.; Dauvalter, V.A.; Sandimirov, S.; Kashulin, A.; Knudsen, R. Heavy metal contents in whitefish (Coregonus lavaretus) along a pollution gradient in a subarctic watercourse. Environ. Monit. Assess. 2011, 182, 301–316. [Google Scholar] [CrossRef]
- Caron, A.; Pannetier, P.; Rosabal, M.; Budzinski, H.; Lauzent, M.; Labadie, P.; Nasri, B.; Pierron, F.; Baudrimont, M.; Couture, P. Organic and inorganic contamination impacts on metabolic capacities in American and European yellow eels. Can. J. Fish. Aquat. Sci. 2016, 73, 1557–1566. [Google Scholar] [CrossRef]
- Mayfield, D.B.; Fairbrother, A. Examination of rare earth element concentration patterns in freshwater fish tissues. Chemosphere 2015, 120, 68–74. [Google Scholar] [CrossRef]
- Lafrenière, M.-C.; Lapierre, J.-F.; Ponton, D.E.; Cabana, G.; Winkler, G.; Lefranc, M.; Amyot, M. Rare earth elements accumulation and patterns in abiotic and biotic compartments of a large river system influenced by natural and anthropogenic sources in Eastern Canada. Environ. Pollut. 2025, 367, 125558. [Google Scholar] [CrossRef]
- Nørregaard, R.D.; Kaarsholm, H.; Bach, L.; Nowak, B.; Geertz-Hansen, O.; Søndergaard, J.; Sonne, C. Bioaccumulation of rare earth elements in juvenile arctic char (Salvelinus alpinus) under field experimental conditions. Sci. Total Environ. 2019, 688, 529–535. [Google Scholar] [CrossRef]
- Gashkina, N.A. Metal Toxicity: Effects on Energy Metabolism in Fish. Int. J. Mol. Sci. 2024, 25, 5015. [Google Scholar] [CrossRef] [PubMed]
- Amiard, J.C.; Amiard-Triquet, C.; Barka, S.; Pellerin, J.; Rainbow, P.S. Metallothioneins in aquatic invertebrates: Their role in metal detoxifixation and their use as biomarkers. Aquat. Toxicol. 2006, 76, 160–202. [Google Scholar] [CrossRef]
- Wang, W.C.; Mao, H.; Ma, D.D.; Yang, W.X. Characteristics, functions, and applications of metallothionein in aquatic vertebrates. Front. Mar. Sci. 2014, 1, 34. [Google Scholar] [CrossRef]
- Caron, A.; Rosabal, M.; Drevet, O.; Couture, P.; Campbell, P.G.C. Binding of trace elements (Ag, Cd, Co, Cu, Ni, and Tl) to cytosolic biomolecules in livers of juvenile yellow perch (Perca flavescens) collected from lakes representing metal contamination gradients. Environ. Toxicol. Chem. 2018, 37, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Pannetier, P.; Caron, A.; Campbell, P.G.C.; Pierron, F.; Baudrimont, M.; Couture, P. A comparison of metal concentrations in the tissues of yellow American eel (Anguilla rostrata) and European eel (Anguilla anguilla). Sci. Total Environ. 2016, 569–570, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Gong, Z.; Li, X.-F.; Cullen, W.R.; Le, X.C. Interaction of Trivalent Arsenicals with Metallothionein. Chem. Res. Toxicol. 2003, 16, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Pourzadi, N.; Gailer, J. The emerging application of LC-based metallomics techniques to unravel the bioinorganic chemistry of toxic metal(loid)s. J. Chromatogr. A 2024, 1736, 465409. [Google Scholar] [CrossRef]
- Cardon, P.Y.; Triffault-Bouchet, G.; Caron, A.; Rosabal, M.; Fortin, C.; Amyot, M. Toxicity and Subcellular Fractionation of Yttrium in Three Freshwater Organisms: Daphnia magna, Chironomus riparius, and Oncorhynchus mykiss. ACS Omega 2019, 4, 13747–13755. [Google Scholar] [CrossRef]
- Marigómez, I.; Soto, M.; Cajaraville, M.P.; Angulo, E.; Giamberini, L. Cellular and subcellular distribution of metals in molluscs. Microsc. Res. Tech. 2002, 56, 358–392. [Google Scholar] [CrossRef]
- Lanno, R.P.; Hicks, B.; Hilton, J.W. Histological observations on intrahepatocytic copper-containing granules in rainbow trout reared on diets containing elevated levels of copper. Aquat. Toxicol. 1987, 10, 251–263. [Google Scholar] [CrossRef]
- Gandahi, N.S.; Gandahi, J.A.; Yang, P.; Tarique, I.; Vistro, W.A.; Haseeb, A.; Huang, Y.; Yu, L.; Bai, X.; Chen, Q. Ultrastructural Evidence of Melanomacrophagic Centers and Lipofuscin in the Liver of Zebrafish (Denio rerio). Zebrafish 2020, 17, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, K.; Li, J.; Zeng, X.; Yu, Z.; Zhang, G. An assessment of polyurethane foam passive samplers for atmospheric metals compared with active samplers. Environ. Pollut. 2018, 236, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Valcheva-Traykova, M.; Saso, L.; Kostova, I. Involvement of lanthanides in the free radicals homeostasis. Curr. Top. Med. Chem. 2014, 14, 2508–2519. [Google Scholar] [CrossRef] [PubMed]
- Cardon, P.Y.; Roques, O.; Caron, A.; Rosabal, M.; Fortin, C.; Amyot, M. Role of prey subcellular distribution on the bioaccumulation of yttrium (Y) in the rainbow trout. Environ. Pollut. 2020, 258, 113804. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rolland, A.; Palmer, M.; Chételat, J.; Amyot, M.; Rosabal, M. Subcellular Partitioning of Trace Elements Is Related to Metal Ecotoxicological Classes in Livers of Fish (Esox lucius; Coregonus clupeaformis) from the Yellowknife Area (Northwest Territories, Canada). Toxics 2025, 13, 410. https://doi.org/10.3390/toxics13050410
Rolland A, Palmer M, Chételat J, Amyot M, Rosabal M. Subcellular Partitioning of Trace Elements Is Related to Metal Ecotoxicological Classes in Livers of Fish (Esox lucius; Coregonus clupeaformis) from the Yellowknife Area (Northwest Territories, Canada). Toxics. 2025; 13(5):410. https://doi.org/10.3390/toxics13050410
Chicago/Turabian StyleRolland, Aymeric, Mike Palmer, John Chételat, Marc Amyot, and Maikel Rosabal. 2025. "Subcellular Partitioning of Trace Elements Is Related to Metal Ecotoxicological Classes in Livers of Fish (Esox lucius; Coregonus clupeaformis) from the Yellowknife Area (Northwest Territories, Canada)" Toxics 13, no. 5: 410. https://doi.org/10.3390/toxics13050410
APA StyleRolland, A., Palmer, M., Chételat, J., Amyot, M., & Rosabal, M. (2025). Subcellular Partitioning of Trace Elements Is Related to Metal Ecotoxicological Classes in Livers of Fish (Esox lucius; Coregonus clupeaformis) from the Yellowknife Area (Northwest Territories, Canada). Toxics, 13(5), 410. https://doi.org/10.3390/toxics13050410








