Characteristics of Neonicotinoids in Colostrum from Shanghai, China (2007–2019): Concentration Levels, Temporal Trends, and Potential Health Risk
Highlights
- The main neonicotinoids found in this study include a parent compound of imidacloprid and a metabolite of N-desmethyl-acetamiprid.
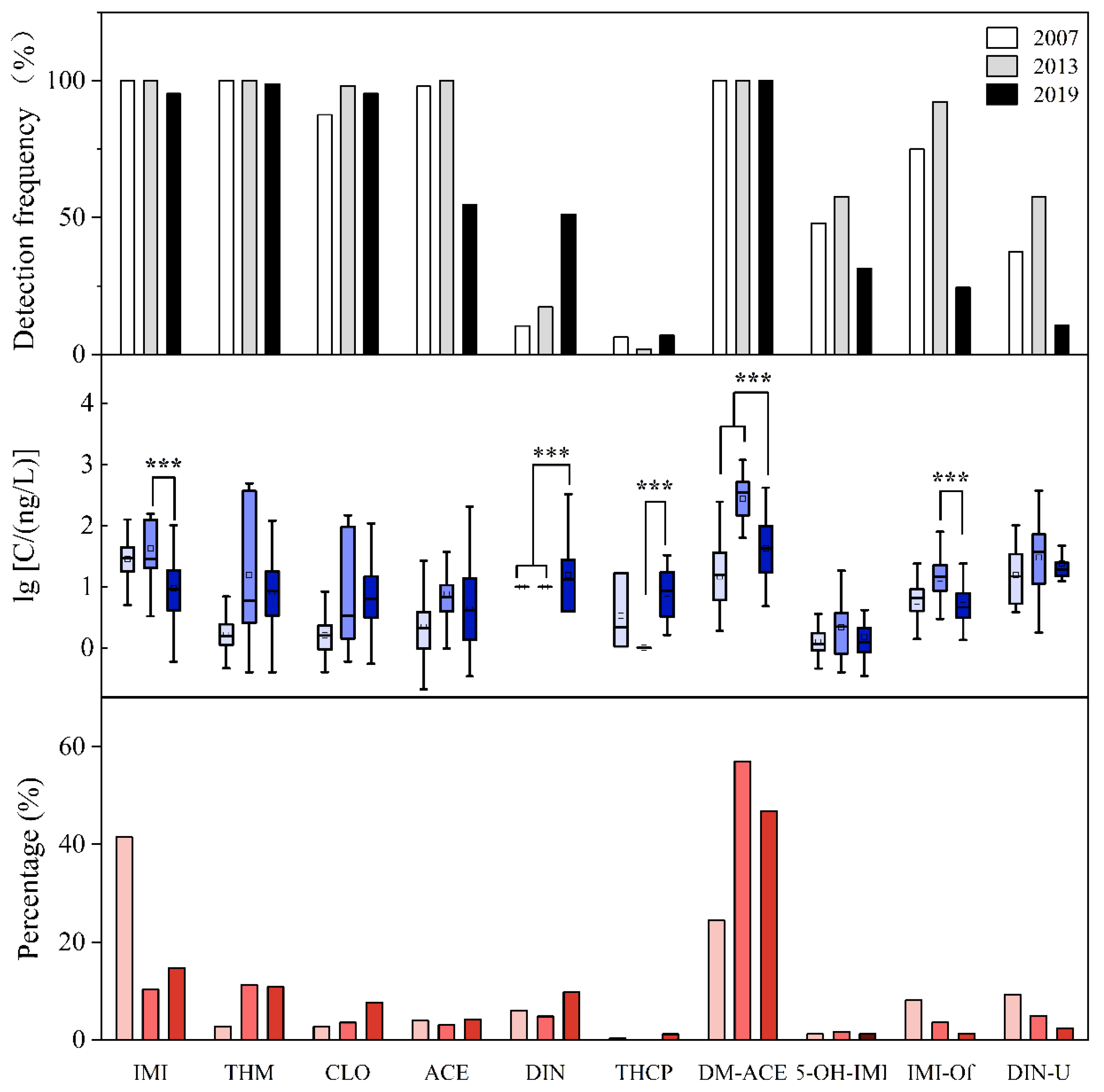
- Dinotefuran levels and detection frequency increased significantly from 2013 to 2019; in comparison, total concentrations of neonicotinoids declined.
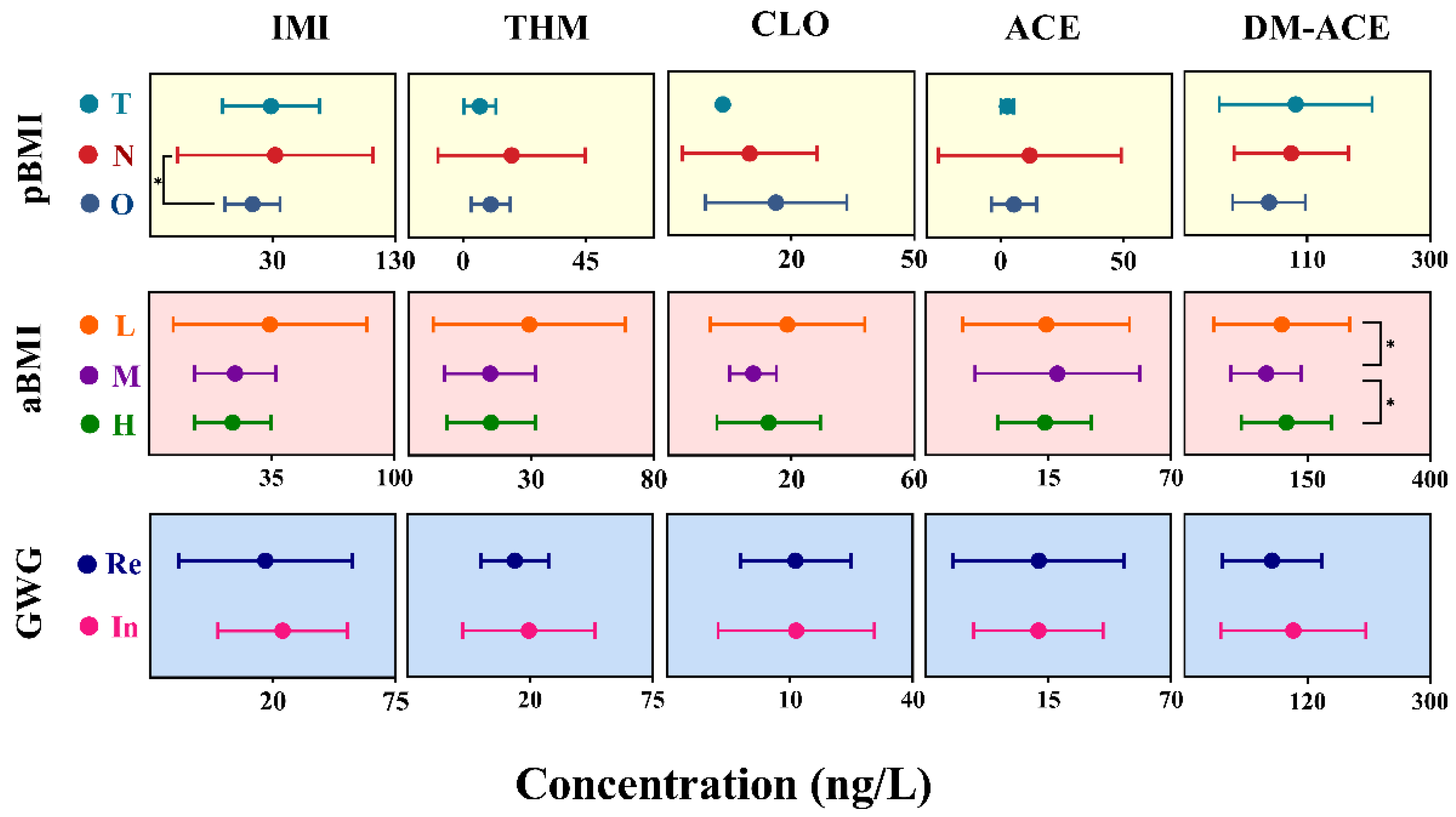
- Maternal dietary habits during pregnancy may influence colostrum concentrations.
- Declining total neonicotinoid levels align with regulatory shifts; however, rising dinotefuran levels highlight evolving exposure risks.
- Maternal dietary habits and weight management during pregnancy may modulate neonatal exposure to specific neonicotinoids, highlighting potential avenues for exposure reduction strategies.
- Our data support biomonitoring and policies to protect infants from emerging pesticide threats.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards
2.2. Sampling and Information Collection
2.3. Sample Treatment Protocols
2.4. Instrumental Analysis
2.5. Estimated Daily Intake and Health Risk Assessment of Exposure to NEOs
2.6. Quality Assurance and Quality Control
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Study Populations
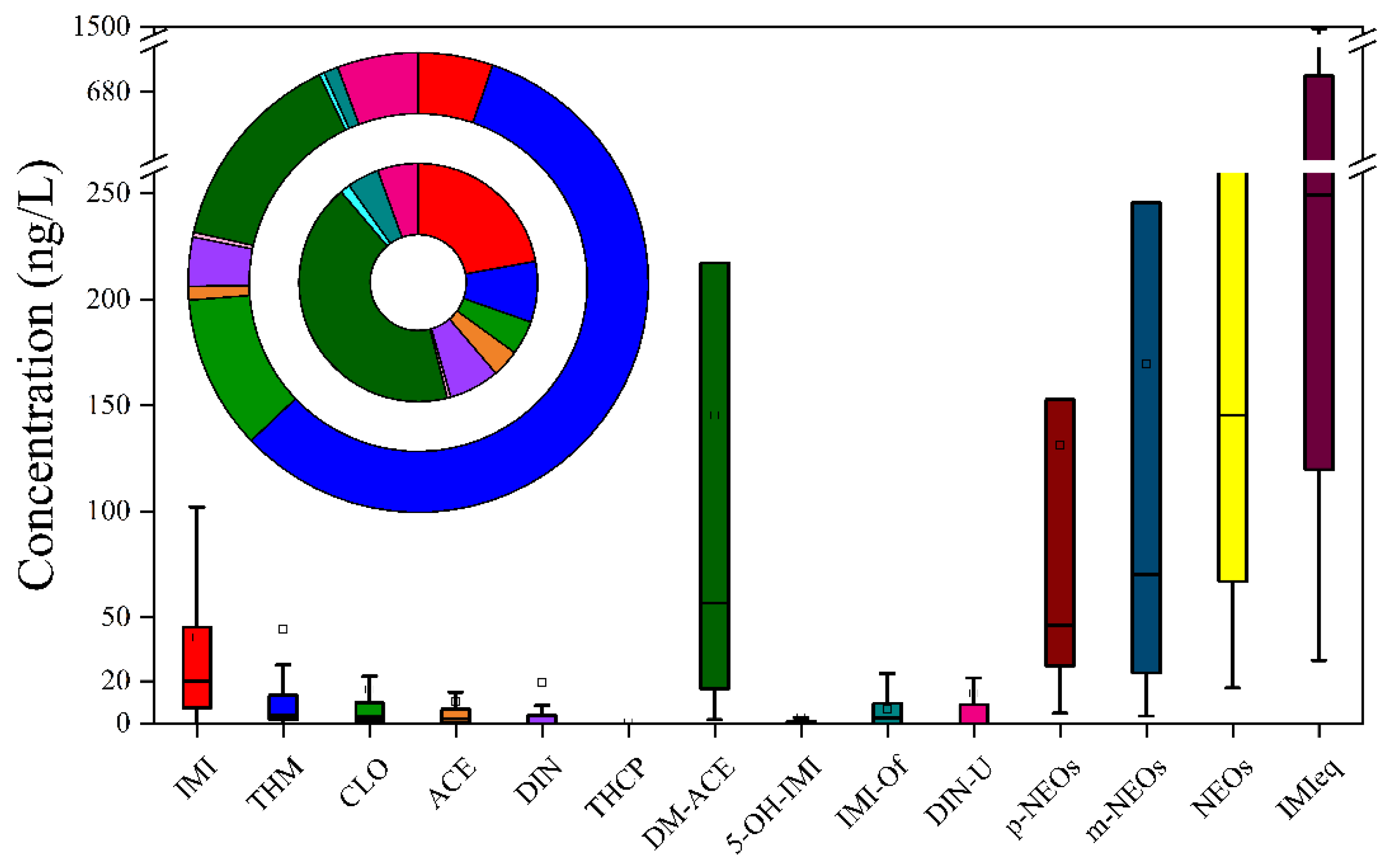
3.2. Occurrence and Distribution Patterns
3.3. Time Variations
3.4. Associations of Demographic Characteristics
3.5. Influence of Colostrum Exposure on Birth Parameter
3.6. Health Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Huang, Y.H.; Chen, W.J.; Wu, S.Y.; Lei, Q.Q.; Zhou, Z.; Zhang, W.P.; Mishra, S.; Bhatt, P.; Chen, S.H. Environmental occurrence, toxicity concerns, and biodegradation of neonicotinoid insecticides. Environ. Res. 2023, 218, 114953. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, L.; Zhang, Z.; An, L.; Hough, R.; Hu, P.; Li, Y.-F.; Zhang, F.; Wang, S.; Zhao, Y.; et al. A review of spatiotemporal patterns of neonicotinoid insecticides in water, sediment, and soil across China. Environ. Sci. Pollut. Res. 2022, 29, 55336–55347. [Google Scholar] [CrossRef]
- Xu, L.; Granger, C.; Dong, H.; Mao, Y.; Duan, S.; Li, J.; Qiang, Z. Occurrences of 29 pesticides in the Huangpu River, China: Highest ecological risk identified in Shanghai metropolitan area. Chemosphere 2020, 251, 126411. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Z.; Chang, C.H.; Yu, C.; Wang, X.; Lu, C. Dietary risk of neonicotinoid insecticides through fruit and vegetable consumption in school-age children. Environ. Int. 2019, 126, 672–681. [Google Scholar] [CrossRef]
- Wang, A.Z.; Wan, Y.J.; Qi, W.; Mahai, G.; Qian, X.; Zheng, T.Z.; Li, Y.Y.; Xu, S.Q.; Xiao, H.; Xia, W. Urinary biomarkers of exposure to organophosphate, pyrethroid, neonicotinoid insecticides and oxidative stress: A repeated measurement analysis among pregnant women. Sci. Total Environ. 2024, 912, 169565. [Google Scholar] [CrossRef]
- Huang, K.Q.; Lin, M.Q.; Yi, J.; Liu, G.C.; Hua, R.; Liu, Y.Y.; Qu, Y.J.; Chen, C.R.; Ma, S.T. Comparison of prenatal and postnatal exposure to neonicotinoids and their temporal trends in breast milk. Sci. Total Environ. 2024, 950, 175386. [Google Scholar] [CrossRef]
- Xu, M.M.; Zhang, Z.P.; Li, Z.Y.; Kan, S.Y.; Liu, Z.X.; Wang, D.S.; Liu, Q.H.; Zhang, H. Profiles of neonicotinoid insecticides and characteristic metabolites in paired urine and blood samples: Partitioning between urine and blood and implications for human exposure. Sci. Total Environ. 2021, 773, 145582. [Google Scholar] [CrossRef]
- Crall, J.D.; Switzer, C.M.; Oppenheimer, R.L.; Ford, V.A.N.; Dey, B.; Brown, A.; Eyster, M.; Guérin, C.; Pierce, N.E.; Combes, S.A.; et al. Neonicotinoid exposure disrupts bumblebee nest behavior, social networks, and thermoregulation. Science 2018, 362, 683–686. [Google Scholar] [CrossRef]
- Yan, S.; Meng, Z.Y.; Tian, S.N.; Teng, M.M.; Yan, J.; Jia, M.; Li, R.S.; Zhou, Z.Q.; Zhu, W.T. Neonicotinoid insecticides exposure cause amino acid metabolism disorders, lipid accumulation and oxidative stress in ICR mice. Chemosphere 2020, 246, 125661. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU) 2018/783 of 29 May 2018 Amending Implementing Regulation (EU) No 540/2011 as Regards the Conditions of Approval of the Active Substance Imidacloprid. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32018R0783 (accessed on 27 February 2025).
- EPA Actions to Protect Pollinators. Available online: https://www.epa.gov/pollinator-protection/epa-actions-protect-pollinators (accessed on 27 February 2025).
- Burke, A.P.; Niibori, Y.; Terayama, H.; Ito, M.; Pidgeon, C.; Arsenault, J.; Camarero, P.R.; Cummins, C.L.; Mateo, R.; Sakabe, K.; et al. Mammalian susceptibility to a neonicotinoid insecticide after fetal and early postnatal exposure. Sci. Rep. 2018, 8, 16639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Wang, Y.; Zhu, H.M.; Lu, S.Y.; Wang, Y.; Xue, J.C.; Zhang, T.; Kannan, K.; Sun, H.W. Infantile internal and external exposure to neonicotinoid insecticides: A comparison of levels across various sources. Environ. Sci. Technol. 2023, 57, 5358–5367. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.Y.; Yu, J.X.; Yao, Q.; Lin, N.; Lu, Z.P.; Zhang, Y.; Zhao, S.S.; Wang, Z.X.; Lei, X.N.; Tian, Y.; et al. Prenatal neonicotinoid insecticides exposure, oxidative stress, and birth outcomes. Environ. Int. 2022, 163, 107180. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, S.L.; Yang, W.; Roberts, E.; Kegley, S.E.; Padula, A.M.; English, P.B.; Lammer, E.J.; Shaw, G.M. Residential agricultural pesticide exposures and risk of selected congenital heart defects among offspring in the San Joaquin Valley of California. Environ. Res. 2014, 135, 133–138. [Google Scholar] [CrossRef]
- Chen, D.W.; Liu, Z.B.; Barrett, H.; Han, J.J.; Lv, B.; Li, Y.; Li, J.G.; Zhao, Y.F.; Wu, Y.N. Nationwide biomonitoring of neonicotinoid insecticides in breast milk and health risk assessment to nursing infants in the Chinese population. J. Agric. Food Chem. 2020, 68, 13906–13915. [Google Scholar] [CrossRef]
- Wang, P.W.; Huang, Y.F.; Fang, L.J.; Chen, M.L. Prenatal and childhood neonicotinoid exposure and neurodevelopment: A study in a young Taiwanese cohort. Sci. Total Environ. 2024, 946, 174232. [Google Scholar] [CrossRef]
- Zhang, Q.; Mo, X.J.; Lou, J.L.; Ying, Z.T.; Wang, Y.; Dai, W. Occurrence, distribution and potential risk to infants of neonicotinoids in breast milk: A case study in Hangzhou, China. Sci. Total Environ. 2023, 878, 163044. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, S.Y. Human exposure to neonicotinoids and the associated health risks: A review. Environ. Int. 2022, 163, 107201. [Google Scholar] [CrossRef]
- Anand, N.; Chakraborty, P.; Ray, S. Human exposure to organochlorine, pyrethroid and neonicotinoid pesticides: Comparison between urban and semi-urban regions of India. Environ. Pollut. 2021, 270, 116156. [Google Scholar] [CrossRef]
- Ospina, M.; Wong, L.Y.; Baker, S.E.; Serafim, A.B.; Morales, A.P.; Calafat, A.M. Exposure to neonicotinoid insecticides in the U.S. general population: Data from the 2015–2016 national health and nutrition examination survey. Environ. Res. 2019, 176, 108555. [Google Scholar] [CrossRef]
- Li, F.X.; Lin, X.; Liu, J. Variability of urinary biomarkers of neonicotinoid insecticides in Chinese population: Implications for human exposure assessment. Chemosphere 2022, 307, 135705. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, J.; Harada, K.H.; Koizumi, A.; Sugiura, Y.; Kondo, T.; Saito, I.; Kamijima, M. Temporal levels of urinary neonicotinoid and dialkylphosphate concentrations in Japanese women between 1994 and 2011. Environ. Sci. Technol. 2015, 49, 14522–14528. [Google Scholar] [CrossRef] [PubMed]
- U.S.EPA. 2010 Development of a Relative Potency Factor (RPF) Approach for Polycyclic Aromatic Hydrocarbon (PAH) Mixtures. U.S, EPA, Washington, DC, USA. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P100HO25.txt (accessed on 12 April 2025).
- US Environmental Protection Agency. Thiamethoxam; Pesticide Tolerance. 2017. Available online: https://www.federalregister.gov/documents/2017/02/15/2017-03075/thiamethoxam-pesticide-tolerance (accessed on 5 April 2025).
- US Environmental Protection Agency. Acetamiprid; Pesticide Tolerances. 2020. Available online: https://www.federalregister.gov/documents/2020/02/14/2020-02038/acetamiprid-pesticide-tolerances (accessed on 5 April 2025).
- Müller, M.H.B.; Polder, A.; Brynildsrud, O.B.; Lie, E.; Løken, K.B.; Manyilizu, W.B.; Mdegela, R.H.; Mokiti, F.; Murtadha, M.; Nonga, H.E.; et al. Brominated flame retardants (BFRs) in breast milk and associated health risks to nursing infants in Northern Tanzania. Environ. Int. 2016, 89, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Lachat, L.; Glauser, G. Development and validation of an Ultra-sensitive UHPLC-MS/MS method for neonicotinoid analysis in milk. J. Agric. Food Chem. 2018, 66, 8639–8646. [Google Scholar] [CrossRef]
- Pedersen, T.L.; Smilowitz, J.T.; Winter, C.K.; Emami, S.; Schmidt, R.J.; Bennett, D.H.; Hertz-Picciotto, I.; Taha, A.Y. Quantification of nonpersistent pesticides in small volumes of human breast milk with ultrahigh performance liquid chromatography coupled to tandem mass spectrometry. J. Agric. Food Chem. 2021, 69, 6676–6689. [Google Scholar] [CrossRef]
- Wrobel, S.A.; Bury, D.; Koslitz, S.; Hayen, H.; Koch, H.M.; Bruning, T.; Kafferlein, H.U. Quantitative metabolism and urinary elimination kinetics of seven neonicotinoids and neonicotinoid-like compounds in humans. Environ. Sci. Technol. 2023, 57, 19285–19294. [Google Scholar] [CrossRef]
- Pan, D.X.; Lin, M.R.; Mu, C.H.; Yu, C.X.; Ye, B.W.; Liang, J.; Sheng, Y.H.; Huang, D.P.; Liu, S.; Zeng, X.Y.; et al. Maternal exposure to neonicotinoid insecticides and fetal growth restriction: A nested case-control study in the Guangxi Zhuang birth cohort. Chemosphere 2023, 336, 139217. [Google Scholar] [CrossRef]
- Zhang, H.L.; Bai, X.Y.; Zhang, T.; Song, S.M.; Zhu, H.K.; Lu, S.Y.; Kannan, K.; Sun, H.W. Neonicotinoid insecticides and their metabolites can pass through the human placenta unimpeded. Environ. Sci. Technol. 2022, 56, 17143–17152. [Google Scholar] [CrossRef]
- Mendy, A.; Pinney, S.M. Exposure to neonicotinoids and serum testosterone in men, women, and children. Environ. Toxicol. 2022, 37, 1521–1528. [Google Scholar] [CrossRef]
- Wang, A.Z.; Wan, Y.J.; Zhou, L.X.; Xia, W.; Guo, Y.S.; Mahai, G.; Yang, Z.; Xu, S.Q.; Zhang, R. Neonicotinoid insecticide metabolites in seminal plasma: Associations with semen quality. Sci. Total Environ. 2022, 811, 151407. [Google Scholar] [CrossRef]
- Chen, D.W.; Zhang, Y.P.; Lv, B.; Liu, Z.B.; Han, J.J.; Li, J.G.; Zhao, Y.F.; Wu, Y.N. Dietary exposure to neonicotinoid insecticides and health risks in the Chinese general population through two consecutive total diet studies. Environ. Int. 2020, 135, 105399. [Google Scholar] [CrossRef] [PubMed]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ Sci. Pollut. Res. 2015, 22, 5. [Google Scholar] [CrossRef] [PubMed]
- Standard of Recommendation for Weight Gain During Pregnancy Period. Available online: http://www.nhc.gov.cn/wjw/fyjk (accessed on 27 February 2025).
- Wang, H.X.; Yang, D.J.; Fang, H.J.; Han, M.H.; Tang, C.X.; Wu, J.G.; Chen, Y.; Jiang, Q.W. Predictors, sources, and health risk of exposure to neonicotinoids in Chinese school children: A biomonitoring-based study. Environ. Int. 2020, 143, 105918. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wang, Y.; Wang, Y.; Lin, G.; Wen, X.; Xu, X.; Hong, S.; Chen, Y.; Lin, H.; Yang, Z.; et al. Exposure of pregnant women to neonicotinoids in Wenzhou city, East China: A biomonitoring study. Environ. Int. 2024, 189, 108811. [Google Scholar] [CrossRef]
- Mahai, G.; Wan, Y.J.; Xia, W.; Wang, A.Z.; Qian, X.; Li, Y.; He, Z.Y.; Li, Y.Y.; Xu, S.Q. Exposure assessment of neonicotinoid insecticides and their metabolites in Chinese women during pregnancy: A longitudinal study. Sci. Total Environ. 2022, 818, 151806. [Google Scholar] [CrossRef]
- Wang, Y.; Gesang, Y.; Wang, Y.; Yang, Z.; Zhao, K.; Liu, J.; Li, C.; Ouzhu, L.; Wang, H.; Chen, Y.; et al. Source and health risk of urinary neonicotinoids in Tibetan pregnant women. Chemosphere 2024, 349, 140774. [Google Scholar] [CrossRef]
- Snijder, C.A.; Heederik, D.; Pierik, F.H.; Hofman, A.; Jaddoe, V.W.; Koch, H.M.; Longnecker, M.P.; Burdorf, A. Fetal growth and prenatal exposure to bisphenol A: The generation R study. Environ. Health Perspect. 2013, 121, 393–398. [Google Scholar] [CrossRef]
- Lee, Y.M.; Hong, Y.; Ha, M.; Kim, Y.; Park, H.; Kim, H.S.; Ha, E. Prenatal bisphenol-A exposure affects fetal length growth by maternal glutathione transferase polymorphisms, and neonatal exposure affects child volume growth by sex: From multiregional prospective birth cohort MPCEH study. Sci. Total Environ. 2018, 612, 1433–1441. [Google Scholar] [CrossRef]
- Lu, Z.P.; Hu, Y.; Tse, L.A.; Yu, J.X.; Xia, Z.N.; Lei, X.N.; Zhang, Y.; Shi, R.; Tian, Y.; Gao, Y. Urinary neonicotinoid insecticides and adiposity measures among 7-year-old children in northern China: A cross-sectional study. Int. J. Hyg. Environ. Health 2023, 251, 114188. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Clothianidin; Pesticide Tolerance. 2012. Available online: https://www.federalregister.gov/documents/2012/08/29/2012-21215/clothianidin-pesticide-tolerances (accessed on 5 April 2025).
- US Environmental Protection Agency. Dinotefuran; Pesticide Tolerance. 2013. Available online: https://www.federalregister.gov/documents/2013/04/10/2013-08400/dinotefuran-pesticide-tolerances (accessed on 5 April 2025).
- US Environmental Protection Agency. Imidacloprid; Pesticide Tolerances for Emergency Exemptions. 2013. Available online: https://www.federalregister.gov/documents/2013/06/05/201313203/imidacloprid-pesticide-tolerances (accessed on 1 May 2020).
- US Environmental Protection Agency. Thiacloprid; Pesticide Tolerances. 2013. Available online: https://www.federalregister.gov/documents/2013/02/06/2013-02692/thiacloprid-pesticidetolerances (accessed on 5 April 2025).
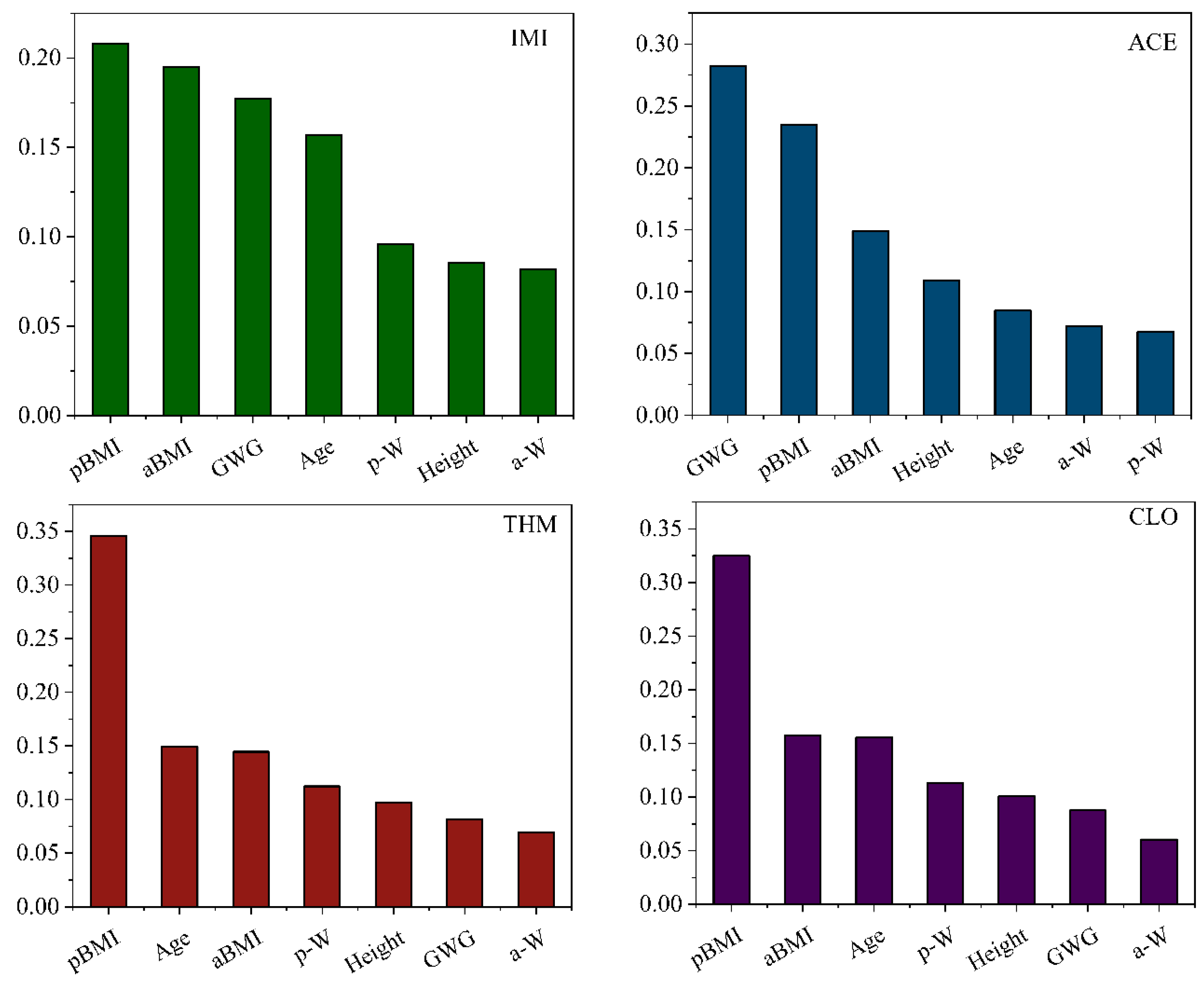
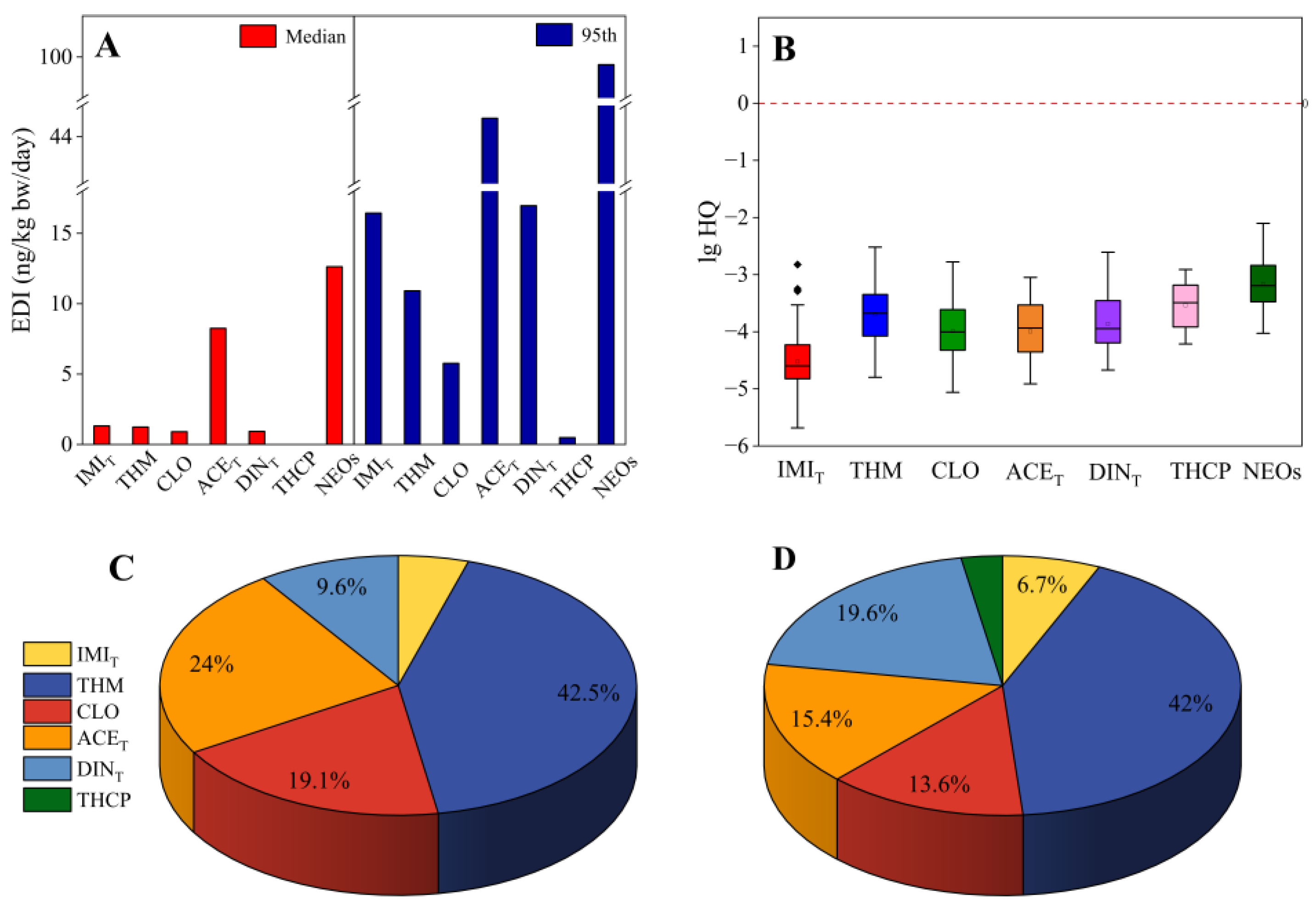
| Characteristic | 2019 (n = 86) | 2013 (n = 52) | 2007 (n = 48) | All (n = 186) |
|---|---|---|---|---|
| Mothers | ||||
| Age (years) | 30.0 ± 3.3 # | 29.2 ± 3.5 # | 27.7 ± 3.6 | 29.2 ± 3.5 |
| ≤30 | 59 (68.6) | 34(65.4) | 32 (66.7) | 125 |
| >30 | 27 (31.4) | 18 (34.6) | 16 (33.3) | 61 |
| pBMI (kg/m2) | 22.1 ± 2.7 | 22.0 ± 2.8 | / | 22.1 ± 2.6 |
| <25 | 62 (72.1) | 32 (61.5) | / | 94 |
| ≥25 | 11 (12.8) | 8 (15.4) | / | 19 |
| Information lost | 13 (15.1) | 12 (23.1) | / | 73 |
| aBMI (kg/m2) | 27.3 ± 2.8 | 28.0 ± 3.7 | / | 27.6 ± 3.2 |
| <25 | 19 (22.1) | 10 (19.2) | / | 29 |
| ≥25 | 61 (71.0) | 37 (71.2) | / | 98 |
| Information lost | 6 (6.9) | 5 (9.6) | / | 59 |
| Education | ||||
| Under college | 11 (12.8) & | 19 (36.5) | / | 30 |
| College and above | 66 (76.7) & | 20 (38.5) | / | 86 |
| Information lost | 9 (10.5) | 13(25.0) | / | 70 |
| Infants | ||||
| Boys | 53 (59.6) c | 27 (51.9) | / | 80 c |
| Girls | 34 (38.2) c | 25 (48.1) | / | 59 c |
| Information lost | 2 (2.3) | 0 | / | 50 |
| Fetal age | ||||
| less than 37 weeks | 21 (23.6) | / | / | 21 |
| over 37 weeks | 67 (75.3) | / | / | 67 |
| Information lost | 1 (1.1) | / | / | 101 |
| Birth weight (g) | 3080 ± 645 & | 3390 ± 409 | / | 3160 ± 596 |
| Infants | 87 (97.8) | 52 (100) | / | 139 |
| Information lost | 2 (2.2) | 0 | / | 50 |
| Birth length (cm) | 48.5 ± 2.2 & | 50.1 ± 0.5 | / | 49.2 ± 1.8 |
| Infants | 61 (68.5) | 52 (100) | / | 113 |
| Information lost | 28 (31.5) | 0 | / | 76 |
| Ponderal index | 0.027 ± 0.003 | 0.027 ± 0.003 | / | 0.027 ± 0.003 |
| Infant | 57 (64.0) | 52 | / | 109 |
| Information lost | 32 (36.0) | / | / | 80 |
| Boys | Girls | All | ||||
|---|---|---|---|---|---|---|
| β (95%CI) | p-Value | β (95%CI) | p-Value | β (95%CI) | p-Value | |
| Birth weight (n = 65, including 35 boys and 30 girls) | ||||||
| IMI | −2.28 (−11.97, 7.42) | 0.612 | 2.44 (−4.43, 9.31) | 0.454 | −0.59 (−3.92, 2.74) | 0.721 |
| THM | −5.67 (−22.01, 10.69) | 0.458 | 2.67 (−24.05, 29.38) | 0.831 | 2.67 (−7.59, 12.93) | 0.600 |
| CLO | 9.04 (−20.03, 38.11) | 0.504 | 3.95 (−24.01, 31.91) | 0.763 | 0.09 (−12.17, 12.35) | 0.988 |
| ACE | −12.7 (−38.0, 12.5) | 0.288 | −1.67 (−9.14, 5.80) | 0.635 | 0.19 (−3.00, 3.39) | 0.903 |
| DM-ACE | −0.09 (−2.26, 2.09) | 0.932 | −1.79 (−4.44, 0.87) | 0.168 | −0.55 (−1.82, 0.71) | 0.380 |
| Ponderal index (n = 47, including 25 boys and 22 girls) | ||||||
| IMI | −0.02 (−0.08, 0.03) | 0.360 | 0.01 (−0.03, 0.04) | 0.770 | −0.02 (−0.04, 0.003) | 0.089 |
| THM | −0.05 (−0.15, 0.04) | 0.243 | 0.02 (0.13, 0.17) | 0.793 | −0.02 (−0.08, 0.04) | 0.485 |
| CLO | 0.08 (−0.09, 0.25) | 0.315 | 0.03 (−0.13, 0.18) | 0.723 | 0.05 (−0.03, 0.12) | 0.198 |
| ACE | 0.003 (−0.03, 0.03) | 0.859 | −0.02 (−0.06, 0.02) | 0.378 | 0.004 (−0.02, 0.02) | 0.656 |
| DM-ACE | −0.01 (−0.02, 0.01) | 0.371 | −0.01 (−0.03, 0.004) | 0.133 | −0.01 (−0.01, 0.002) | 0.165 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Fu, M.; Lei, B.; Shen, X.; Zhang, X.; Xu, J.; Zhang, X. Characteristics of Neonicotinoids in Colostrum from Shanghai, China (2007–2019): Concentration Levels, Temporal Trends, and Potential Health Risk. Toxics 2025, 13, 366. https://doi.org/10.3390/toxics13050366
Li K, Fu M, Lei B, Shen X, Zhang X, Xu J, Zhang X. Characteristics of Neonicotinoids in Colostrum from Shanghai, China (2007–2019): Concentration Levels, Temporal Trends, and Potential Health Risk. Toxics. 2025; 13(5):366. https://doi.org/10.3390/toxics13050366
Chicago/Turabian StyleLi, Kexin, Minghui Fu, Bingli Lei, Xiuhua Shen, Xinyu Zhang, Jun Xu, and Xiaolan Zhang. 2025. "Characteristics of Neonicotinoids in Colostrum from Shanghai, China (2007–2019): Concentration Levels, Temporal Trends, and Potential Health Risk" Toxics 13, no. 5: 366. https://doi.org/10.3390/toxics13050366
APA StyleLi, K., Fu, M., Lei, B., Shen, X., Zhang, X., Xu, J., & Zhang, X. (2025). Characteristics of Neonicotinoids in Colostrum from Shanghai, China (2007–2019): Concentration Levels, Temporal Trends, and Potential Health Risk. Toxics, 13(5), 366. https://doi.org/10.3390/toxics13050366