4′-Hydroxydehydrokawain Mitigate the Cytotoxicity of Citrinin in Porcine Intestinal Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Cell Viability
2.3. Gene Expression Profiling
2.4. Cell Cycle Analysis
2.5. Annexin-V and Propidium Iodide (PI) Staining
2.6. RT–qPCR
2.7. High-Throughput Screening
2.8. Statistics
3. Results
3.1. Citrinin Decreased the Viability of IPEC-J2 Cells
3.2. Identification and Validation of DEGs
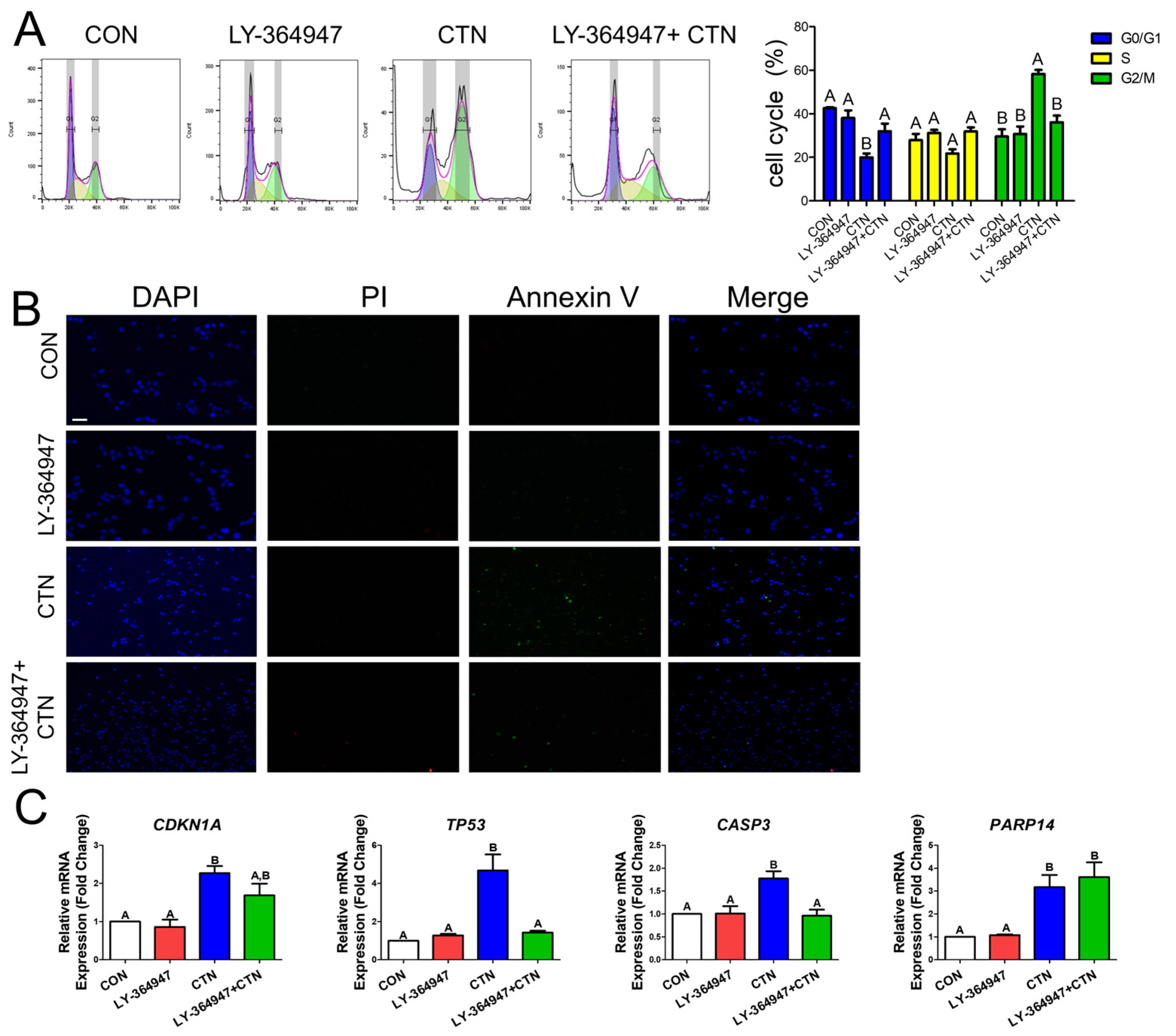
3.3. CTN Induces G2/M Phase Cell Cycle Arrest and Apoptosis in IPEC-J2 Cells
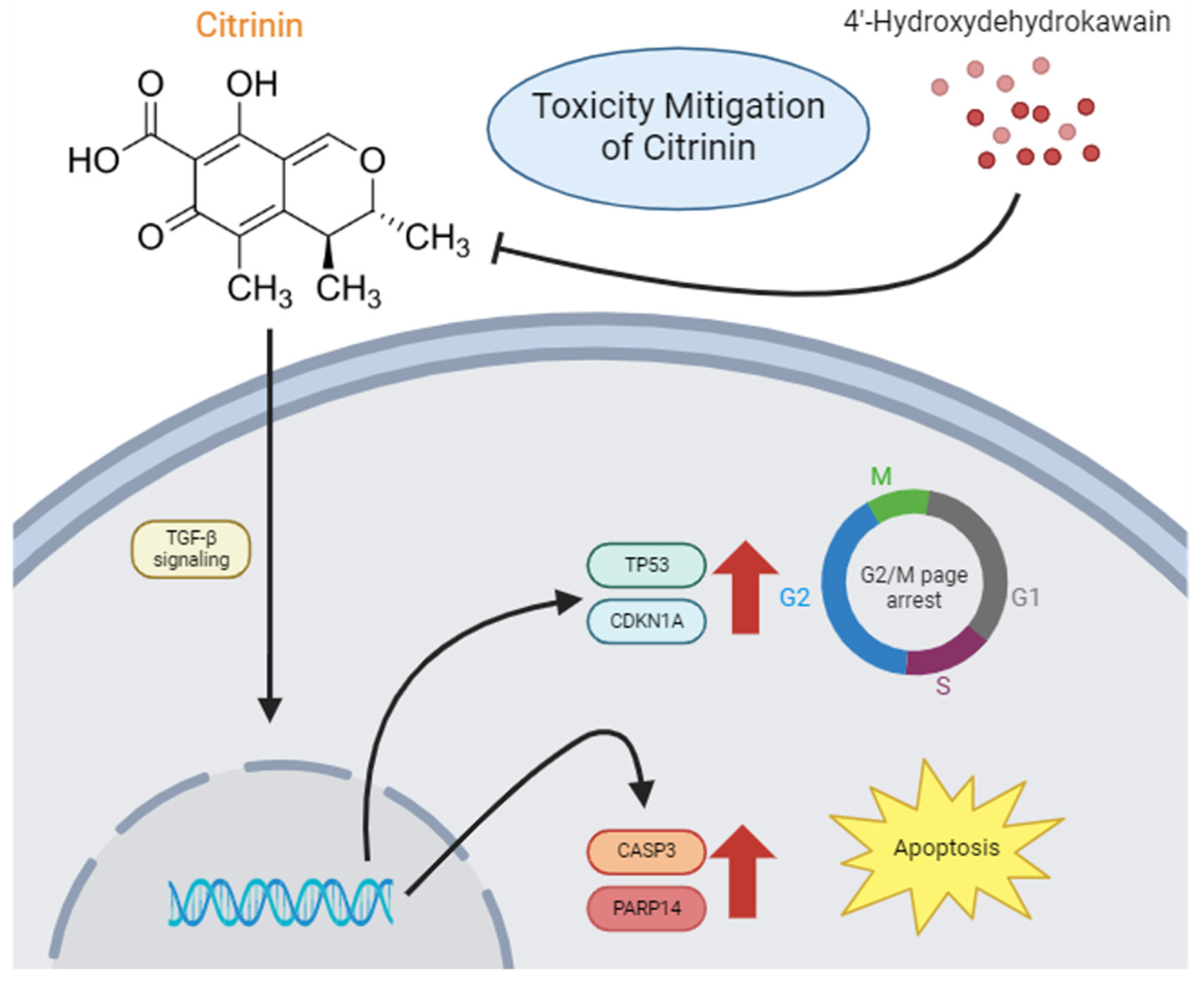
3.4. CTN Induces Apoptosis and G2/M Phase Cell Cycle Arrest Through the TGF-Beta Signaling Pathway
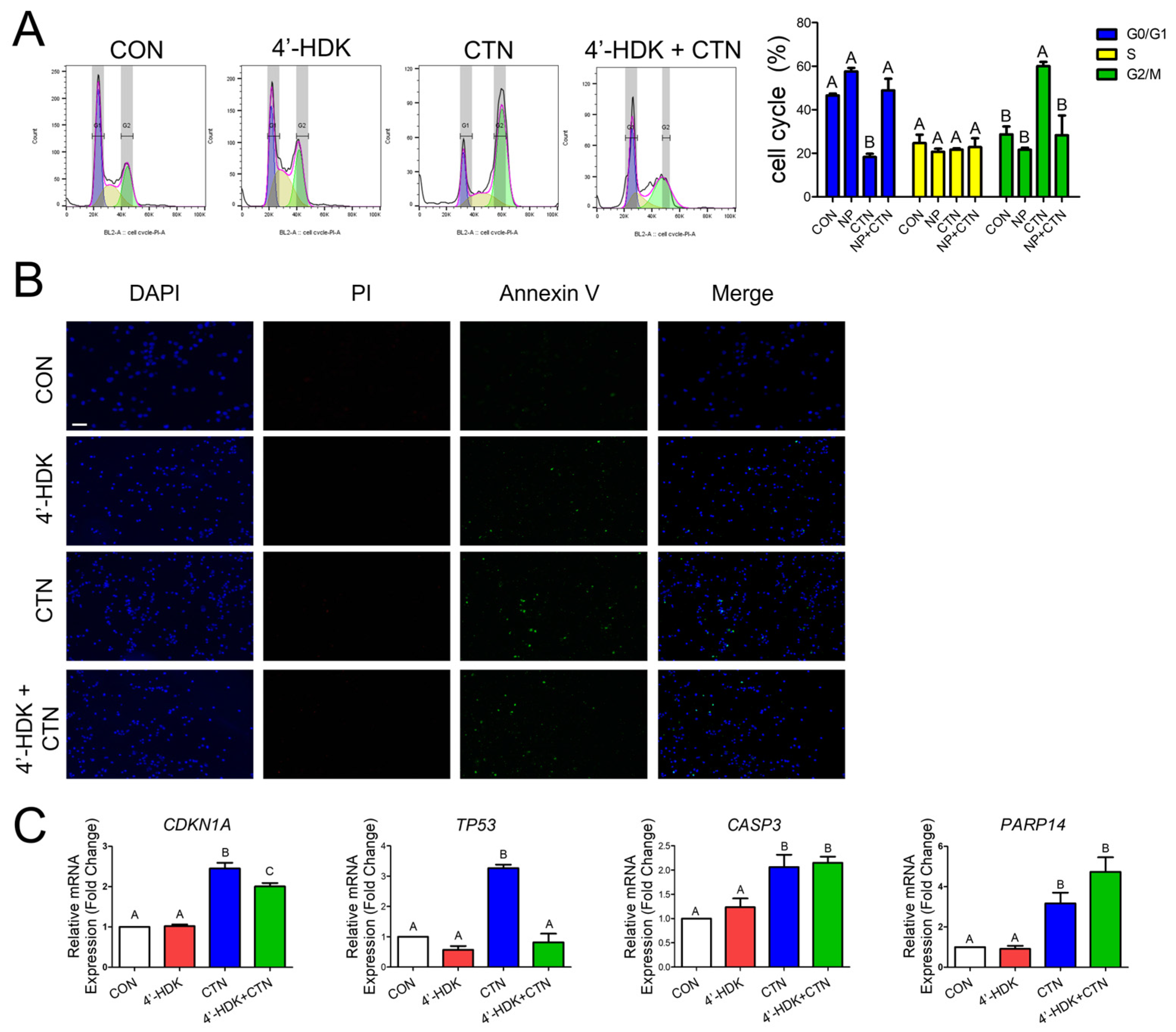
3.5. High-Throughput Screening of NPs to Assess Their Ability to Alleviate Citrinin Toxicity
3.6. 4-HDK Mitigates Citrinin-Induced Toxicity by Modulating Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CTN | Citrinin |
| IPEC-J2 | Intestinal Porcine Epithelial Cells-J2 |
| TGF-β | Transforming Growth Factor-beta |
| 4-HDK | 4′-Hydroxydehydrokawain |
| PI | Propidium iodide |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| RT-qPCR | Reverse Transcription Quantitative Polymerase Chain Reaction |
| DEG | Differentially expressed gene |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| FACS | Fluorescence-Activated Cell Sorting |
| NP | Natural product |
References
- Peraica, M.; Radić, B.; Lucić, A.; Pavlović, M. Toxic effects of mycotoxins in humans. Bull. World Health Organ. 1999, 77, 754–766. [Google Scholar] [PubMed]
- Mitchell, N.J.; Bowers, E.; Hurburgh, C.; Wu, F. Potential economic losses to the US corn industry from aflatoxin contamination. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 540–550. [Google Scholar] [CrossRef]
- Chang, C.H.; Yu, F.Y.; Wu, T.S.; Wang, L.T.; Liu, B.H. Mycotoxin citrinin induced cell cycle G2/M arrest and numerical chromosomal aberration associated with disruption of microtubule formation in human cells. Toxicol. Sci. 2011, 119, 84–92. [Google Scholar] [CrossRef]
- Gao, Z.; Xu, C.; Fan, H.; Wang, H.; Wu, Z.; Wu, S.; Bao, W. Analysis of RIOK2 Functions in Mediating the Toxic Effects of Deoxynivalenol in Porcine Intestinal Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 12712. [Google Scholar] [CrossRef] [PubMed]
- Ciacci-Zanella, J.R.; Merrill, A.H., Jr.; Wang, E.; Jones, C. Characterization of cell-cycle arrest by fumonisin B1 in CV-1 cells. Food Chem. Toxicol. 1998, 36, 791–804. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Q.; Wang, L.; Gao, X.; Zhu, W.; Mu, P.; Deng, Y. Aflatoxin B1 Induces Neurotoxicity through Reactive Oxygen Species Generation, DNA Damage, Apoptosis, and S-Phase Cell Cycle Arrest. Int. J. Mol. Sci. 2020, 21, 6517. [Google Scholar] [CrossRef] [PubMed]
- Solcan, C.; Timofte, D.; Floristean, V.C.; Carter, S.D.; Solcan, G. Ultrastructural lesions and immunohistochemical analysis of Bcl-2 protein expression in the kidney of chickens with experimental ochratoxicosis. Acta Vet. Hung. 2013, 61, 344–353. [Google Scholar] [CrossRef]
- Wang, F.; Zuo, Z.; Chen, K.; Gao, C.; Yang, Z.; Zhao, S.; Li, J.; Song, H.; Peng, X.; Fang, J.; et al. Histopathological Injuries, Ultrastructural Changes, and Depressed TLR Expression in the Small Intestine of Broiler Chickens with Aflatoxin B1. Toxins 2018, 10, 131. [Google Scholar] [CrossRef]
- Feng, G.D.; He, J.; Ao, X.; Chen, D.W. Effects of maize naturally contaminated with aflatoxin B1 on growth performance, intestinal morphology, and digestive physiology in ducks. Poult. Sci. 2017, 96, 1948–1955. [Google Scholar] [CrossRef]
- Shimizu, T.; Kinoshita, H.; Ishihara, S.; Sakai, K.; Nagai, S.; Nihira, T. Polyketide synthase gene responsible for citrinin biosynthesis in Monascus purpureus. Appl. Environ. Microbiol. 2005, 71, 3453–3457. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Aleo, M.D.; Wyatt, R.D.; Schnellmann, R.G. The role of altered mitochondrial function in citrinin-induced toxicity to rat renal proximal tubule suspensions. Toxicol. Appl. Pharmacol. 1991, 109, 455–463. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.W.G.; Islam, M.T.; Ali, E.S.; Uddin, S.J.; Santos, J.V.O.; de Alencar, M.; Júnior, A.L.G.; Paz, M.; de Brito, M.; JMC, E.S.; et al. A comprehensive review on biological properties of citrinin. Food Chem. Toxicol. 2017, 110, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.H.; Yu, F.Y.; Wu, T.S.; Li, S.Y.; Su, M.C.; Wang, M.C.; Shih, S.M. Evaluation of genotoxic risk and oxidative DNA damage in mammalian cells exposed to mycotoxins, patulin and citrinin. Toxicol. Appl. Pharmacol. 2003, 191, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Nusrat, A. The Life and Death of Epithelia During Inflammation: Lessons Learned from the Gut. Annu. Rev. Pathol. Mech. Dis. 2012, 7, 35–60. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, K.; Roth, S.; Nieuwenhuis, E.E.S.; Middendorp, S. Intestinal epithelial cell polarity defects in disease: Lessons from microvillus inclusion disease. Dis. Model. Mech. 2018, 11, dmm031088. [Google Scholar] [CrossRef]
- Rescigno, M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 2011, 32, 256–264. [Google Scholar] [CrossRef]
- Okamoto, R.; Watanabe, M. Molecular and clinical basis for the regeneration of human gastrointestinal epithelia. J. Gastroenterol. 2004, 39, 1–6. [Google Scholar] [CrossRef]
- Potten, C.S.; Gandara, R.; Mahida, Y.R.; Loeffler, M.; Wright, N.A. The stem cells of small intestinal crypts: Where are they? Cell Prolif. 2009, 42, 731–750. [Google Scholar] [CrossRef]
- Hageman, J.H.; Heinz, M.C.; Kretzschmar, K.; van der Vaart, J.; Clevers, H.; Snippert, H.J.G. Intestinal Regeneration: Regulation by the Microenvironment. Dev. Cell 2020, 54, 435–446. [Google Scholar] [CrossRef]
- Sharpless, N.E.; Sherr, C.J. Forging a signature of in vivo senescence. Nat. Rev. Cancer 2015, 15, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Bianco, G.; Fontanella, B.; Severino, L.; Quaroni, A.; Autore, G.; Marzocco, S. Nivalenol and deoxynivalenol affect rat intestinal epithelial cells: A concentration related study. PLoS ONE 2012, 7, e52051. [Google Scholar] [CrossRef]
- He, Y.; Yin, X.; Dong, J.; Yang, Q.; Wu, Y.; Gong, Z. Transcriptome Analysis of Caco-2 Cells upon the Exposure of Mycotoxin Deoxynivalenol and Its Acetylated Derivatives. Toxins 2021, 13, 167. [Google Scholar] [CrossRef]
- Yin, H.; Jiang, M.; Peng, X.; Cui, H.; Zhou, Y.; He, M.; Zuo, Z.; Ouyang, P.; Fan, J.; Fang, J. The molecular mechanism of G2M cell cycle arrest induced by AFB1 in the jejunum. Oncotarget 2016, 7, 35592–35606. [Google Scholar] [CrossRef] [PubMed]
- Doug, H.; Chen, S.X.; Xu, H.X.; Kadota, S.; Namba, T. A new antiplatelet diarylheptanoid from Alpinia blepharocalyx. J. Nat. Prod. 1998, 61, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zheng, C. Effects of Alpinetin on Intestinal Barrier Function, Inflammation and Oxidative Stress in Dextran Sulfate Sodium-Induced Ulcerative Colitis Mice. Am. J. Med. Sci. 2018, 355, 377–386. [Google Scholar] [CrossRef]
- Wang, K.; Lv, Q.; Miao, Y.M.; Qiao, S.M.; Dai, Y.; Wei, Z.F. Cardamonin, a natural flavone, alleviates inflammatory bowel disease by the inhibition of NLRP3 inflammasome activation via an AhR/Nrf2/NQO1 pathway. Biochem. Pharmacol. 2018, 155, 494–509. [Google Scholar] [CrossRef]
- Yang, J.; Dai, Y.; Xia, Y.F.; Huang, W.Z.; Wang, Z.T. Alpinia katsumadai Hayata prevents mouse sepsis induced by cecal ligation and puncture through promoting bacterial clearance and downregulating systemic inflammation. Phytother. Res. 2009, 23, 267–273. [Google Scholar] [CrossRef]
- An, W.; Zhang, Y.; Lai, H.; Zhang, Y.; Zhang, H.; Zhao, G.; Liu, M.; Li, Y.; Lin, X.; Cao, S. Alpinia katsumadai Hayata induces growth inhibition and autophagy-related apoptosis by regulating the AMPK and Akt/mTOR/p70S6K signaling pathways in cancer cells. Oncol. Rep. 2022, 48, 142. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.K.; Gupta, A.; Pandhi, S.; Sharma, N.; Sharma, B.; Mishra, S.; Arora, S.; Selvakumar, R.; Saurabh, V.; et al. Citrinin Mycotoxin Contamination in Food and Feed: Impact on Agriculture, Human Health, and Detection and Management Strategies. Toxins 2022, 14, 85. [Google Scholar] [CrossRef]
- Meerpoel, C.; Vidal, A.; di Mavungu, J.D.; Huybrechts, B.; Tangni, E.K.; Devreese, M.; Croubels, S.; De Saeger, S. Development and validation of an LC-MS/MS method for the simultaneous determination of citrinin and ochratoxin a in a variety of feed and foodstuffs. J. Chromatogr. A 2018, 1580, 100–109. [Google Scholar] [CrossRef]
- Flajs, D.; Peraica, M. Toxicological properties of citrinin. Arh. Hig. Rada Toksikol. 2009, 60, 457–464. [Google Scholar] [CrossRef]
- Zargar, S.; Wani, T.A. Food Toxicity of Mycotoxin Citrinin and Molecular Mechanisms of Its Potential Toxicity Effects through the Implicated Targets Predicted by Computer-Aided Multidimensional Data Analysis. Life 2023, 13, 880. [Google Scholar] [CrossRef]
- Nakayama, H.; Kitagawa, N.; Otani, T.; Iida, H.; Anan, H.; Inai, T. Ochratoxin A, citrinin and deoxynivalenol decrease claudin-2 expression in mouse rectum CMT93-II cells. Microscopy 2018, 67, 99–111. [Google Scholar] [CrossRef]
- Jiang, W.J.; Liu, W.; Li, Y.H.; Jiang, H.; Xu, Y.N.; Kim, N.H. Citrinin impairs pig oocyte maturation by inducing oxidative stress and apoptosis. Toxicon 2022, 205, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.F.; Wu, T.S.; Huang, Y.T.; Lin, W.J.; Yu, F.Y.; Liu, B.H. Exposure to Mycotoxin Citrinin Promotes Carcinogenic Potential of Human Renal Cells. J. Agric. Food Chem. 2023, 71, 19054–19065. [Google Scholar] [CrossRef]
- Jo, M.K.; Moon, C.M.; Jeon, H.J.; Han, Y.; Lee, E.S.; Kwon, J.H.; Yang, K.M.; Ahn, Y.H.; Kim, S.E.; Jung, S.A.; et al. Effect of aging on the formation and growth of colonic epithelial organoids by changes in cell cycle arrest through TGF-β-Smad3 signaling. Inflamm. Regen. 2023, 43, 35. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.W. Recycling the Cell Cycle: Cyclins Revisited. Cell 2004, 116, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chung, D.H.; Kim, Y.B.; Choi, Y.H.; Moon, Y. Ribotoxic mycotoxin deoxynivalenol induces G2/M cell cycle arrest via p21Cip/WAF1 mRNA stabilization in human epithelial cells. Toxicology 2008, 243, 145–154. [Google Scholar] [CrossRef]
- Denicourt, C.; Dowdy, S.F. Cip/Kip proteins: More than just CDKs inhibitors. Genes Dev. 2004, 18, 851–855. [Google Scholar] [CrossRef]
- Engeland, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Oyarbide, U.; Shah, A.N.; Amaya-Mejia, W.; Snyderman, M.; Kell, M.J.; Allende, D.S.; Calo, E.; Topczewski, J.; Corey, S.J. Loss of Sbds in zebrafish leads to neutropenia and pancreas and liver atrophy. JCI. Insight 2020, 5, e134309. [Google Scholar] [CrossRef] [PubMed]
- Bratton, S.B.; MacFarlane, M.; Cain, K.; Cohen, G.M. Protein complexes activate distinct caspase cascades in death receptor and stress-induced apoptosis. Exp. Cell Res. 2000, 256, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Chen, Q.; Shi, W.; Tang, L.; Fu, Y. PARP14 promotes the proliferation and gemcitabine chemoresistance of pancreatic cancer cells through activation of NF-κB pathway. Mol. Carcinog. 2019, 58, 1291–1302. [Google Scholar] [CrossRef] [PubMed]




| Genes | Description | Accession No. | Sequence (5’-3’) | |
|---|---|---|---|---|
| TGM2 | Transglutaminase 2 | XM_003359989.5 | Forward | CGC CTT CTC TCC GTA TGA CT |
| Reverse | TTT TGT GCT TCT TCC TGT GC | |||
| BEX5 | Brain expressed X-linked 5 | XM_005657871.3 | Forward | TAT CCT CAG CAG GTC CAC GT |
| Reverse | CTT CTT CATVTCC GCA TTT GA | |||
| FOXJ1 | Forkhead box protein J1 | XM_003357959.4 | Forward | CTG TCC TCC CCA GGT CTC TA |
| Reverse | AAA TCT CCT TGC TCC ACC AG | |||
| BCAS1 | Brain-enriched myelin- associated protein 1 | NM_001110175.1 | Forward | GCC CCC GAC AGA GAA TAA TA |
| Reverse | CAC TTG AGC ATC CAA CAT CG | |||
| CASP3 | Caspase3 | NM_214131 | Forward | CTC AGG GAG ACC TTC ACA AC |
| Reverse | GCA CGC AAA TAA AAC TGC TC | |||
| PARP14 | Poly (ADP-ribose) polymerase family member 14 | XM_021070260.1 | Forward | CCA CTC TCT GTG TTC CCG TA |
| Reverse | GGT GAG AGA CAC AAG GGC AT | |||
| CDKN1A | Cyclin-dependent kinase inhibitor 1A | XM_013977858.2 | Forward | GGT TCC CCA GTT CTA CCT CA |
| Reverse | GCG TCT CGC TTC ATC ATT TA | |||
| TP53 | Transformation-related protein 53 | NM_213824.3 | Forward | TGC TGT TTC CGT GTG TTT TT |
| Reverse | ATG GGG AGG GAG GTT ATC A | |||
| GABDH | Glyceraldehyde-3- phosphate dehydrogenase | NM_001206359 | Forward | ACA CCG AGC ATC TCC TGA CT |
| Reverse | GAC GAG GCA GGT CTC CCT AA | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.J.; Shin, S.; Lee, S.I. 4′-Hydroxydehydrokawain Mitigate the Cytotoxicity of Citrinin in Porcine Intestinal Epithelial Cells. Toxics 2025, 13, 315. https://doi.org/10.3390/toxics13040315
Lim SJ, Shin S, Lee SI. 4′-Hydroxydehydrokawain Mitigate the Cytotoxicity of Citrinin in Porcine Intestinal Epithelial Cells. Toxics. 2025; 13(4):315. https://doi.org/10.3390/toxics13040315
Chicago/Turabian StyleLim, Seung Joon, Sangsu Shin, and Sang In Lee. 2025. "4′-Hydroxydehydrokawain Mitigate the Cytotoxicity of Citrinin in Porcine Intestinal Epithelial Cells" Toxics 13, no. 4: 315. https://doi.org/10.3390/toxics13040315
APA StyleLim, S. J., Shin, S., & Lee, S. I. (2025). 4′-Hydroxydehydrokawain Mitigate the Cytotoxicity of Citrinin in Porcine Intestinal Epithelial Cells. Toxics, 13(4), 315. https://doi.org/10.3390/toxics13040315







