Impact of Microplastics on Ciprofloxacin Adsorption Dynamics and Mechanisms in Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adsorption Experiments
- (1)
- Adsorption isotherm experiments
- (2)
- Soil adsorption isotherm experiments
2.3. Analysis Methods
- (1)
- Characterization methods
- (2)
- Adsorption kinetics models
- (3)
- Adsorption models
3. Results and Discussion
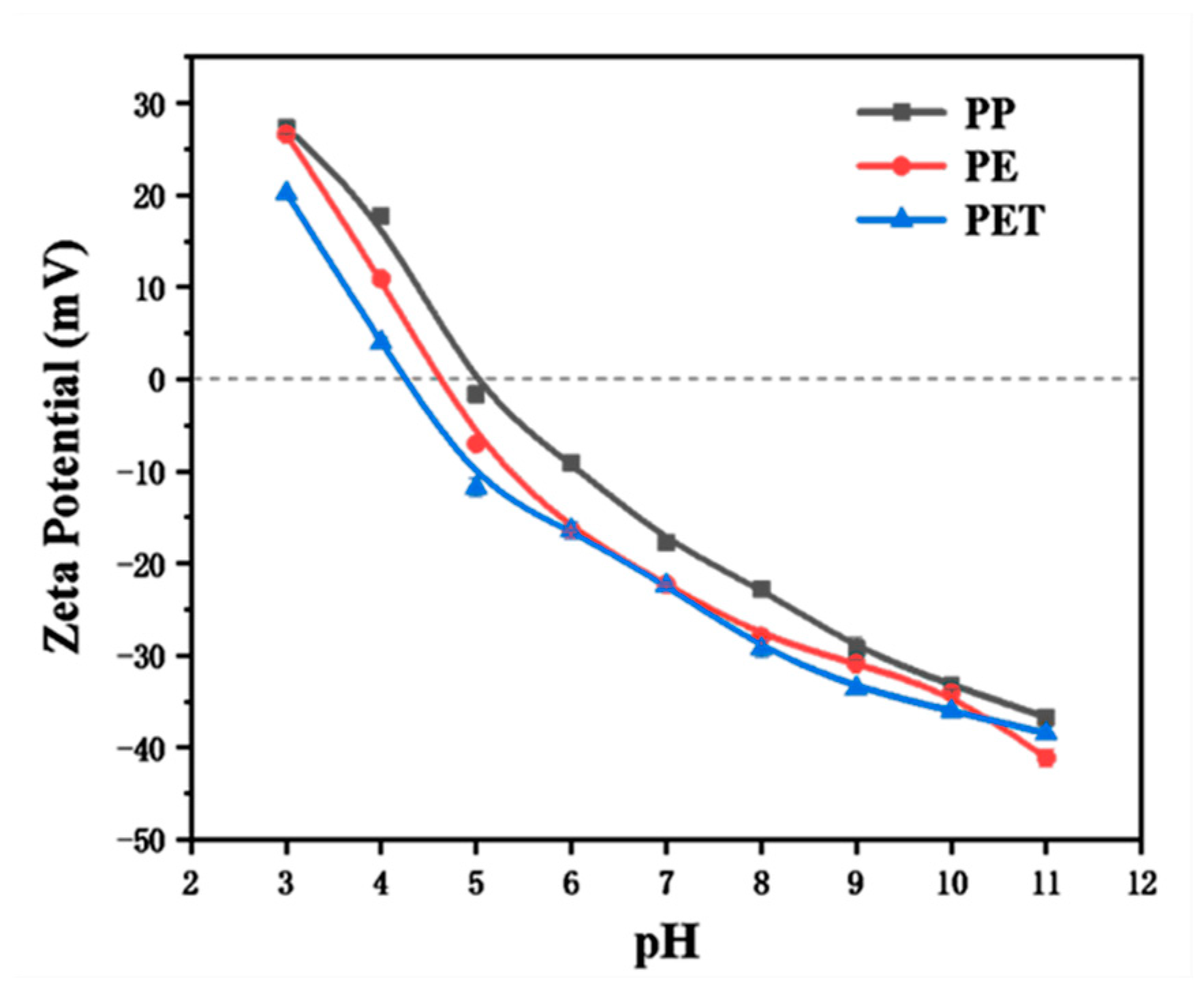
3.1. Characterization of MPs
3.2. Adsorption Behavior of CIP on Soil, Soil–MPs, and MPs
3.2.1. Adsorption Kinetics
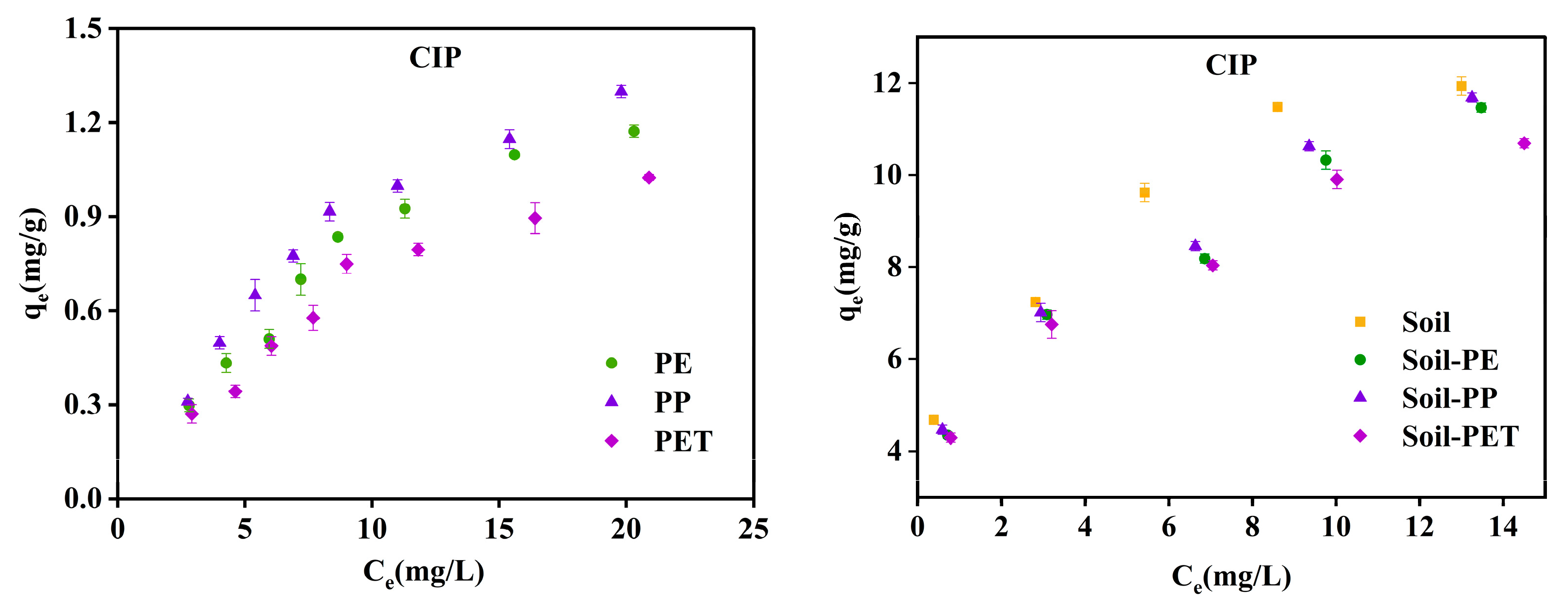
3.2.2. Adsorption Isotherms
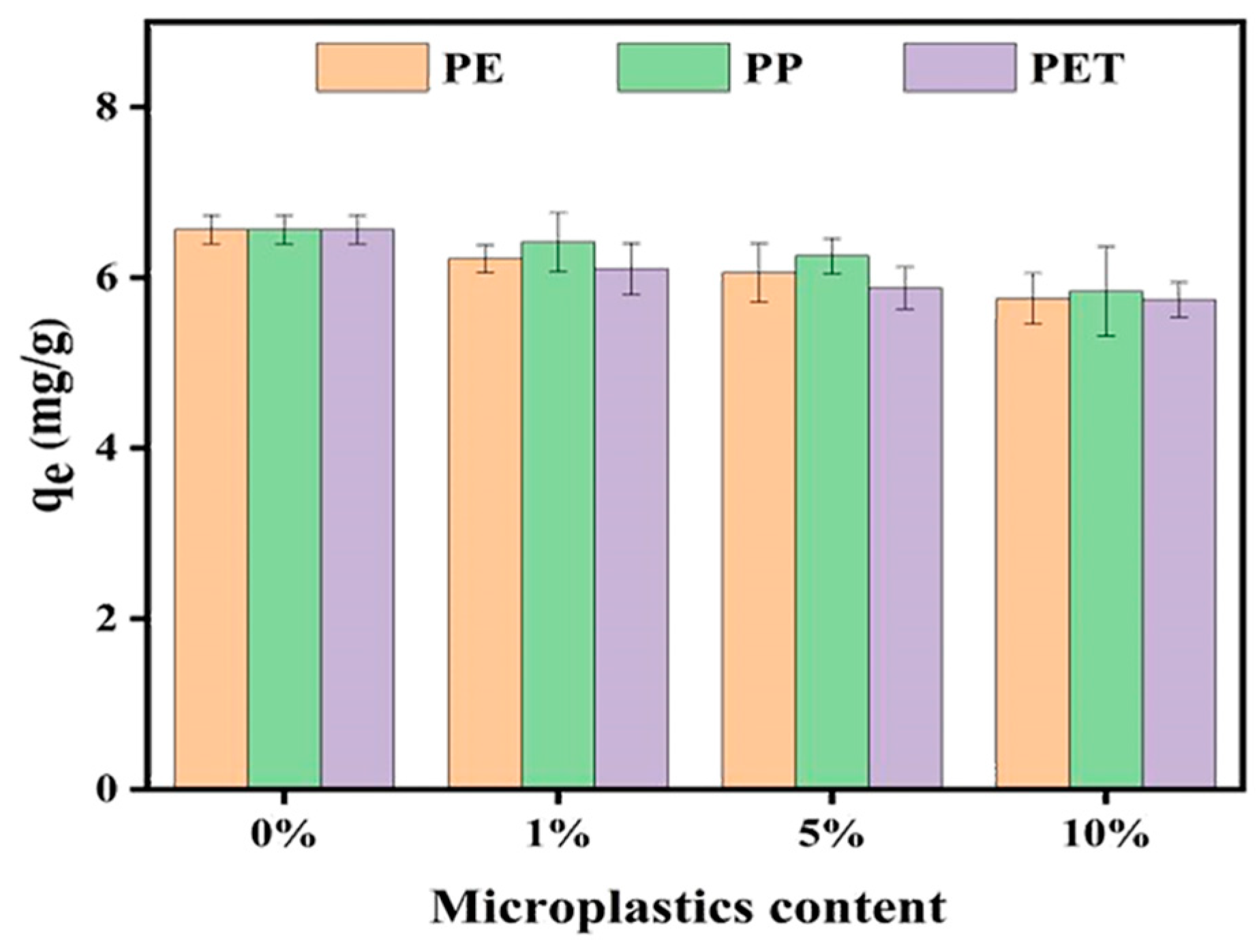
3.3. Effect of MP Dosage on CIP Adsorption in Soil
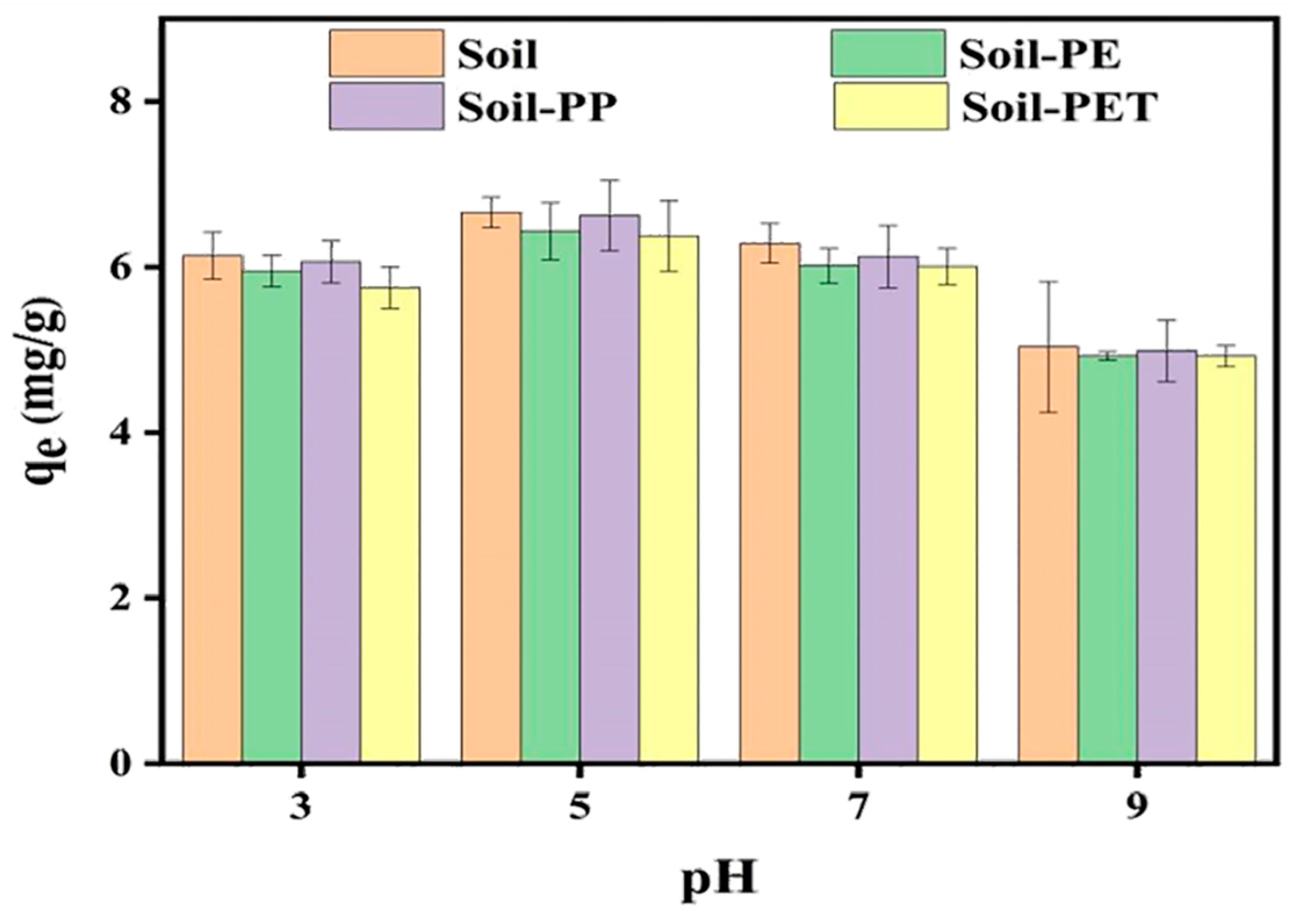
3.4. Effect of pH on CIP Adsorption onto Soil and Soil–MPs
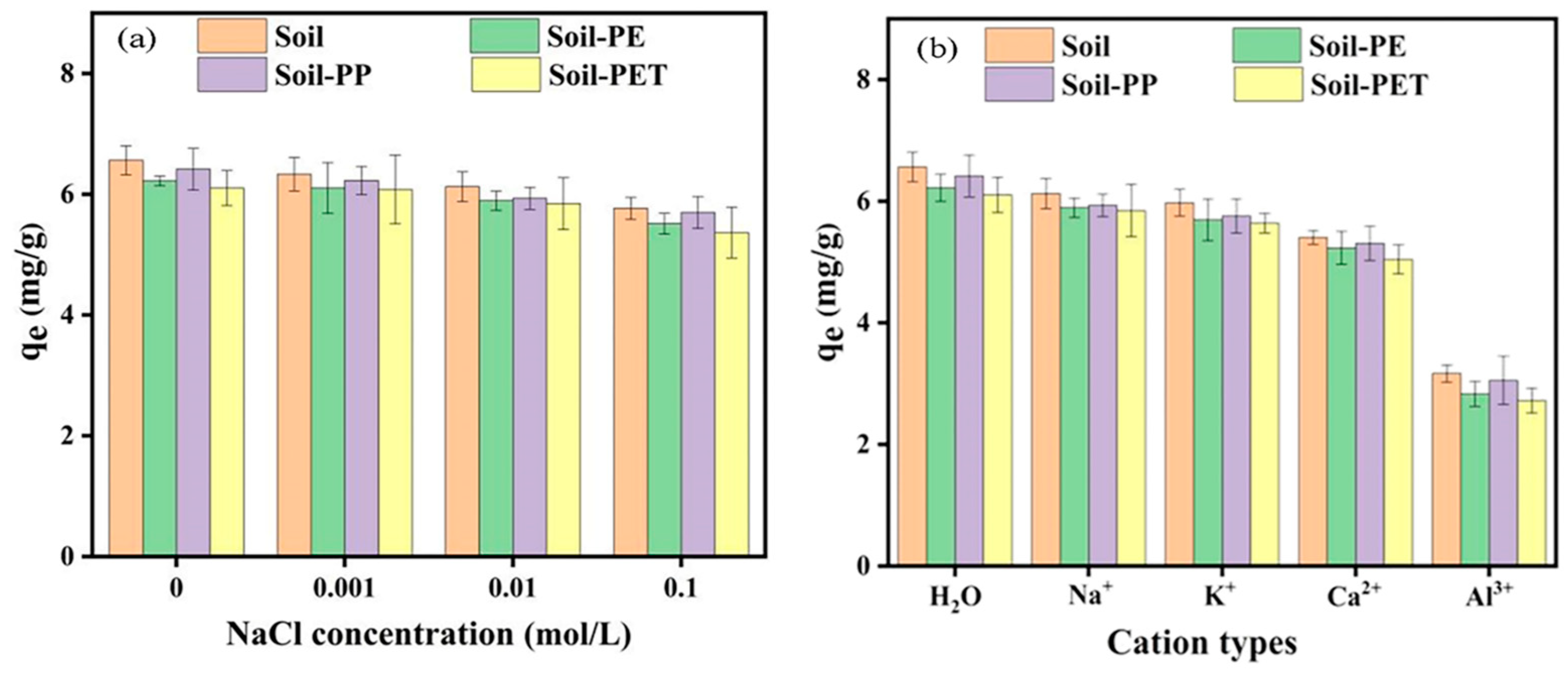
3.5. Effect of Ionic Strength and Type on CIP Adsorption onto Soil and Soil–MPs
3.6. Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiao, M.; Ying, G.G.; Singer, A.C.; Zhu, Y.G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef]
- Pan, X.; Qiang, Z.; Ben, W.; Chen, M. Simultaneous determination of three classes of antibiotics in the suspended solids of swine wastewater by ultrasonic extraction, solid-phase extraction and liquid chromatography-mass spectrometry. J. Environ. Sci. 2011, 23, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Canning-Clode, J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J. Comparative evaluation of sorption kinetics and isotherms of pyrene onto microplastics. Chemosphere 2018, 193, 567–573. [Google Scholar] [CrossRef]
- Li, X.; Mei, Q.; Chen, L.; Zhang, H.; Dong, B.; Dai, X.; He, C.; Zhou, J. Enhancement in adsorption potential of microplastics in sewage sludge for metal pollutants after the wastewater treatment process. Water Res. 2019, 157, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Razanajatovo, R.M.; Ding, J.; Zhang, S.; Jiang, H.; Zou, H. Sorption and desorption of selected pharmaceuticals by polyethylene microplastics. Mar. Pollut. Bull. 2018, 136, 516–523. [Google Scholar] [CrossRef]
- Song, W.; Fu, C.; Fang, Y.; Wang, Z.; Li, J.; Zhang, X.; Bhatt, K.; Liu, L.; Wang, N.; Liu, F.; et al. Single and combined toxicity assessment of primary or UV-aged microplastics and adsorbed organic pollutants on microalga Chlorella pyrenoidosa. Environ. Pollut. 2023, 318, 120925. [Google Scholar] [CrossRef]
- Li, N.; Zeng, Z.; Zhang, Y.; Zhang, H.; Tang, N.; Guo, Y.; Lu, L.; Li, X.; Zhu, Z.; Gao, X.; et al. Higher toxicity induced by co-exposure of polystyrene microplastics and chloramphenicol to Microcystis aeruginosa: Experimental study and molecular dynamics simulation. Sci. Total Environ. 2023, 866, 161375. [Google Scholar] [CrossRef]
- Ma, B.; Xue, W.; Ding, Y.; Hu, C.; Liu, H.; Qu, J. Removal characteristics of microplastics by Fe-based coagulants during drinking water treatment. J. Environ. Sci. 2019, 78, 267–275. [Google Scholar] [CrossRef]
- Wan, H.; Wang, J.; Zhang, W. Key influencing factors for interactions between microplastics and heavy metals, persistent organic pollutants, and antibiotics in soil. J. Agric. Resour. Environ. 2022, 39, 643–650. [Google Scholar] [CrossRef]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Li, Y.; Powell, T.; Wang, X.; Wang, G.; Zhang, P. Microplastics as contaminants in the soil environment: A mini-review. Sci. Total Environ. 2019, 691, 848–857. [Google Scholar] [CrossRef]
- Shen, X.-C.; Li, D.-C.; Sima, X.-F.; Cheng, H.-Y.; Jiang, H. The effects of environmental conditions on the enrichment of antibiotics on microplastics in simulated natural water column. Environ. Res. 2018, 166, 377–383. [Google Scholar] [CrossRef]
- Nizzetto, L.; Futter, M.; Langaas, S. Are Agricultural Soils Dumps for Microplastics of Urban Origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef]
- Rillig, M.C. Microplastic in Terrestrial Ecosystems and the Soil? Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef]
- Qian, H.; Zhang, M.; Liu, G.; Lu, T.; Qu, Q.; Du, B.; Pan, X. Effects of Soil Residual Plastic Film on Soil Microbial Community Structure and Fertility. Water Air Soil Pollut. 2018, 229, 261. [Google Scholar] [CrossRef]
- Liu, J.; Yang, H.; Meng, Q.; Feng, Q.; Yan, Z.; Liu, J.; Liu, Z.; Zhou, Z. Intergenerational and biological effects of roxithromycin and polystyrene microplastics to Daphnia magna. Aquat. Toxicol. 2022, 248, 106192. [Google Scholar] [CrossRef]
- Manoli, K.; Naziri, A.; Ttofi, I.; Michael, C.; Allan, I.J.; Fatta-Kassinos, D. Investigation of the effect of microplastics on the UV inactivation of antibiotic-resistant bacteria in water. Water Res. 2022, 222, 118906. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Adsorption and degradation of five selected antibiotics in agricultural soil. Sci. Total Environ. 2016, 545–546, 48–56. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Bermúdez-Couso, A.; Arias-Estévez, M.; Nóvoa-Muñoz, J.C.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Kinetics of tetracycline, oxytetracycline, and chlortetracycline adsorption and desorption on two acid soils. Environ. Sci. Pollut. Res. Int. 2014, 22, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Sassman, S.A.; Lee, L.S. Sorption of three tetracyclines by several soils: Assessing the role of pH and cation exchange. Environ. Sci. Technol. 2005, 39, 7452–7459. [Google Scholar] [CrossRef]
- Li, J.; YU, S.; Shen, L.; Cui, M.; Wang, Y. Influence of microplastics on sorption behaviors of oxytetracycline onto soils: A preliminary study. Environ. Chem. 2021, 40, 3133–3143. [Google Scholar] [CrossRef]
- Meng, L.; Yang, B.; Xue, N.D. A review on environmental behaviors and ecotoxicology of fluoroquinolone antibiotics. Asian J. Ecotoxicol. 2015, 10, 76–88. [Google Scholar] [CrossRef]
- Chai, T.; Cui, F.; Yin, Z.; Yang, Y.; Qiu, J.; Wang, C. Chiral PCB 91 and 149 Toxicity Testing in Embryo and Larvae (Danio rerio): Application of Targeted Metabolomics via UPLC-MS/MS. Sci. Rep. 2016, 6, 33481. [Google Scholar] [CrossRef]
- Stock, V.; Fahrenson, C.; Thuenemann, A.; Dönmez, M.H.; Voss, L.; Böhmert, L.; Braeuning, A.; Lampen, A.; Sieg, H. Impact of artificial digestion on the sizes and shapes of microplastic particles. Food Chem. Toxicol. 2020, 135, 111010. [Google Scholar]
- Guo, X.; Liu, Y.; Wang, J. Sorption of sulfamethazine onto different types of microplastics: A combined experimental and molecular dynamics simulation study. Mar. Pollut. Bull. 2019, 145, 547–554. [Google Scholar]
- Wang, C.; Zhu, L.; Song, C.; Shan, G.; Chen, P. Characterization of photocatalyst Bi3.84W0.16O6.24 and its photodegradation on bisphenol A under simulated solar light irradiation. Appl. Catal. B Environ. 2011, 105, 229–236. [Google Scholar] [CrossRef]
- Hüffer, T.; Metzelder, F.; Sigmund, G.; Slawek, S.; Schmidt, T.; Hofmann, T. Polyethylene microplastics influence the transport of organic contaminants in soil. Sci. Total Environ. 2019, 657, 242–247. [Google Scholar] [CrossRef]
- Zhang, S.; Han, B.; Sun, Y.; Wang, F. Microplastics influence the adsorption and desorption characteristics of Cd in an agricultural soil. J. Hazard. Mater. 2020, 388, 121775. [Google Scholar] [CrossRef] [PubMed]
- El-Shafey, E.S.I.; Al-Lawati, H.; Al-Sumri, A.S. Ciprofloxacin adsorption from aqueous solution onto chemically prepared carbon from date palm leaflets. J. Environ. Sci. 2012, 24, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xiong, Y.; Peng, K.; Lu, L.; Liu, J. The progress of antibiotics removal performance under the complexion effect of metal ions. Environ. Chem. 2016, 35, 133–140. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Li, H.; Li, S.; Li, Z.; Chen, S.; Liang, Y.; Li, Y.; Li, J.; Yuan, M. Impact of Microplastics on Ciprofloxacin Adsorption Dynamics and Mechanisms in Soil. Toxics 2025, 13, 294. https://doi.org/10.3390/toxics13040294
Xu Q, Li H, Li S, Li Z, Chen S, Liang Y, Li Y, Li J, Yuan M. Impact of Microplastics on Ciprofloxacin Adsorption Dynamics and Mechanisms in Soil. Toxics. 2025; 13(4):294. https://doi.org/10.3390/toxics13040294
Chicago/Turabian StyleXu, Qian, Hanbing Li, Sumei Li, Ziyi Li, Sha Chen, Yixuan Liang, Yuyang Li, Jianan Li, and Mengxin Yuan. 2025. "Impact of Microplastics on Ciprofloxacin Adsorption Dynamics and Mechanisms in Soil" Toxics 13, no. 4: 294. https://doi.org/10.3390/toxics13040294
APA StyleXu, Q., Li, H., Li, S., Li, Z., Chen, S., Liang, Y., Li, Y., Li, J., & Yuan, M. (2025). Impact of Microplastics on Ciprofloxacin Adsorption Dynamics and Mechanisms in Soil. Toxics, 13(4), 294. https://doi.org/10.3390/toxics13040294










