Hsa_circ_0001944 Regulates FXR/TLR4 Pathway and Ferroptosis to Alleviate Nickel Oxide Nanoparticles-Induced Collagen Formation in LX-2 Cells
Abstract
1. Introduction
2. Material and Methods
2.1. Characterization of NiONPs and Sample Preparation
2.2. Construction of Animal Models
2.3. Transcriptome Sequencing of Liver Tissue
2.4. Bioinformatic Prediction
2.5. Cell Culture
2.6. Treatment of LX-2 Cells
2.6.1. Establishment of Collagen Deposition Model
2.6.2. Drug Treatment
2.6.3. Overexpression of circ_0001944
2.7. Cell Migration Assay
2.8. Cell Cycle Detection
2.9. Real-Time Fluorescent Quantitative PCR (RT-qPCR)
2.10. Western Blotting
2.11. Fluorescent Probe
2.12. Determination of Intracellular Iron Ions
2.13. Statistical Analysis
3. Results
3.1. NiONPs Affected the FXR/TLR4 Pathway, Ferroptosis and Inflammation in Rat Liver Tissue
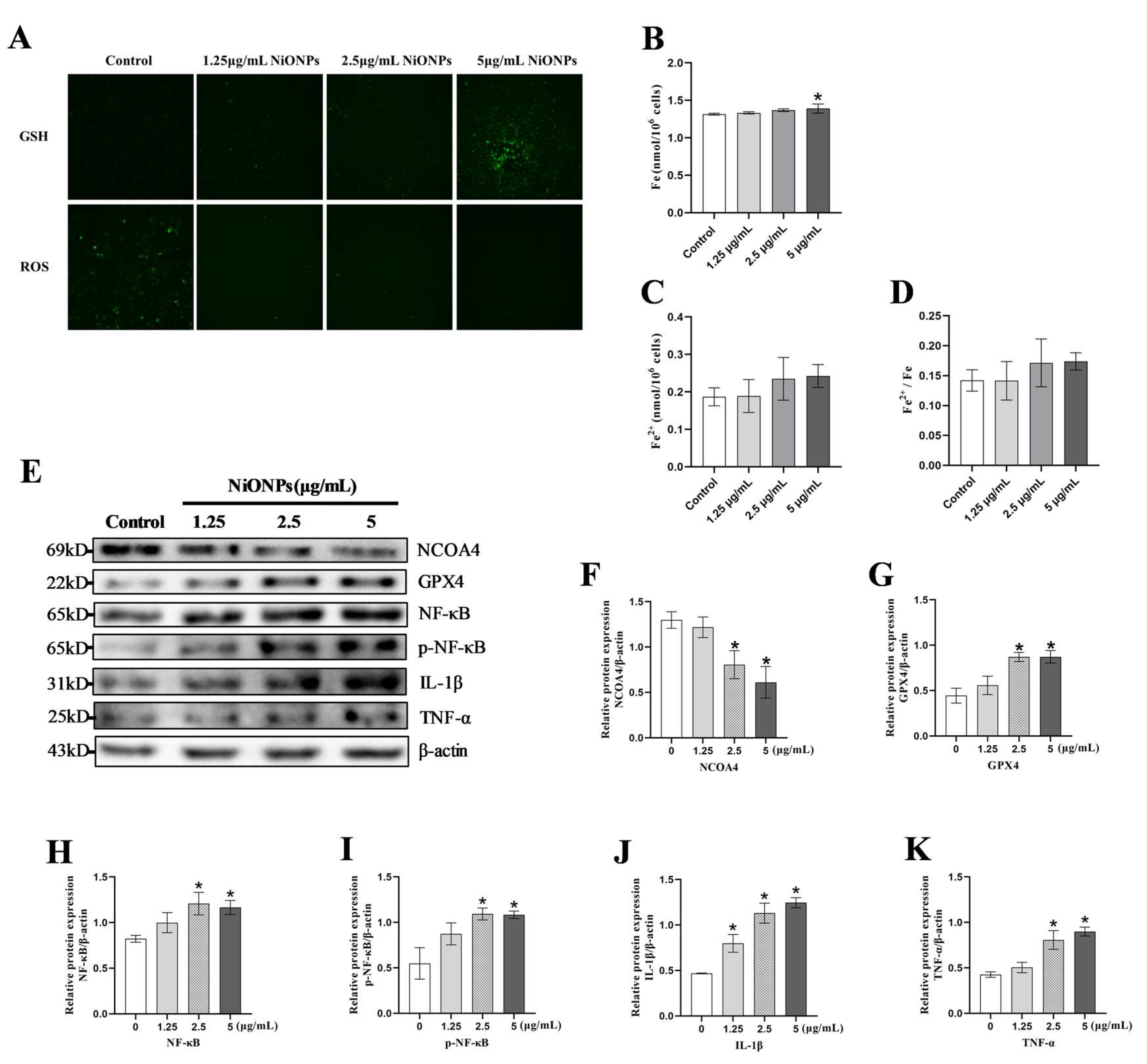
3.2. NiONPs Caused Collagen Deposition and the Changes in FXR/TLR4 Signaling Pathway, Ferroptosis and Inflammation in LX-2 Cells
3.3. Ferroptosis Alleviated the Excessive Deposition of Collagen in LX-2 Cells Treated with NiONPs
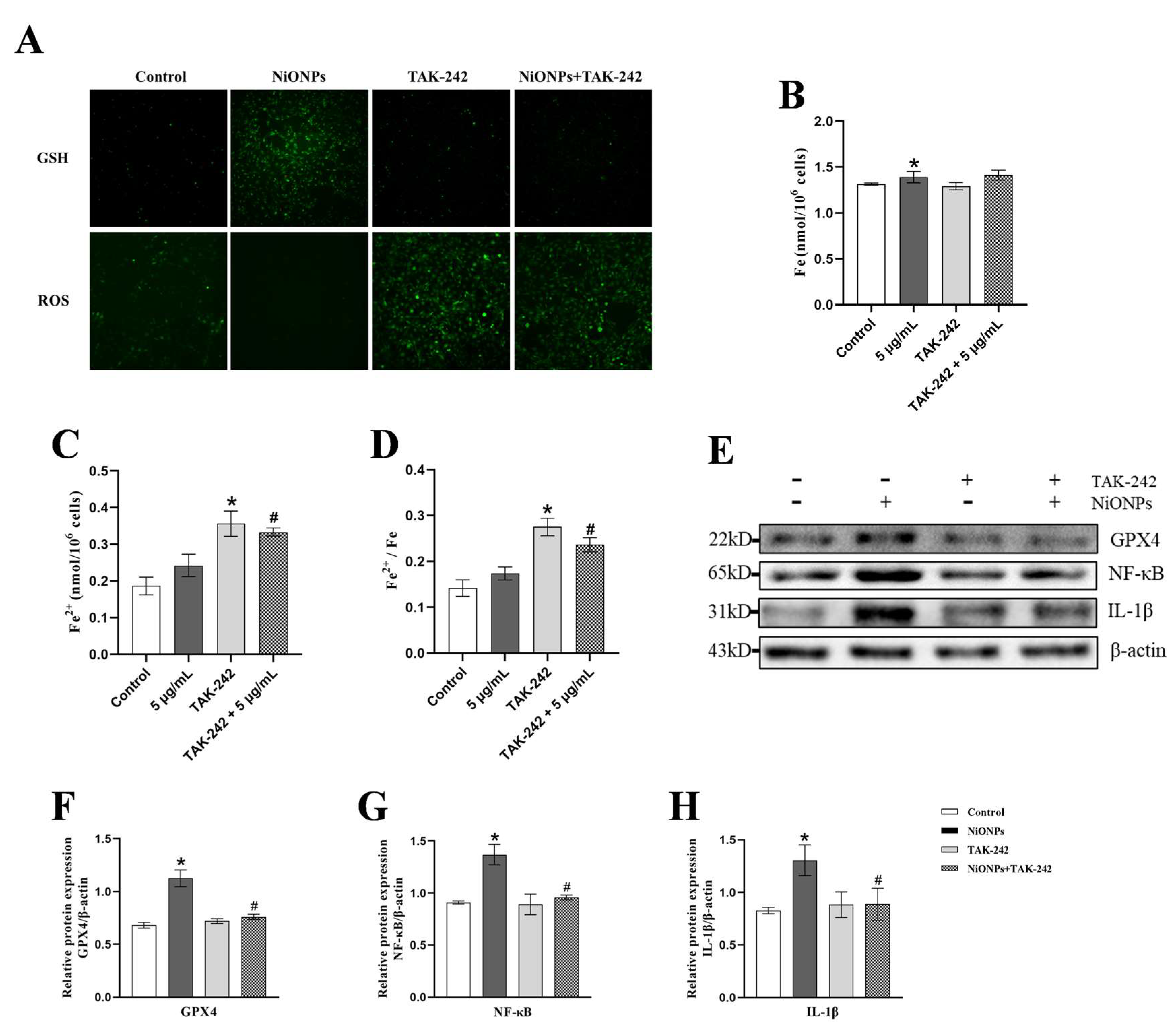
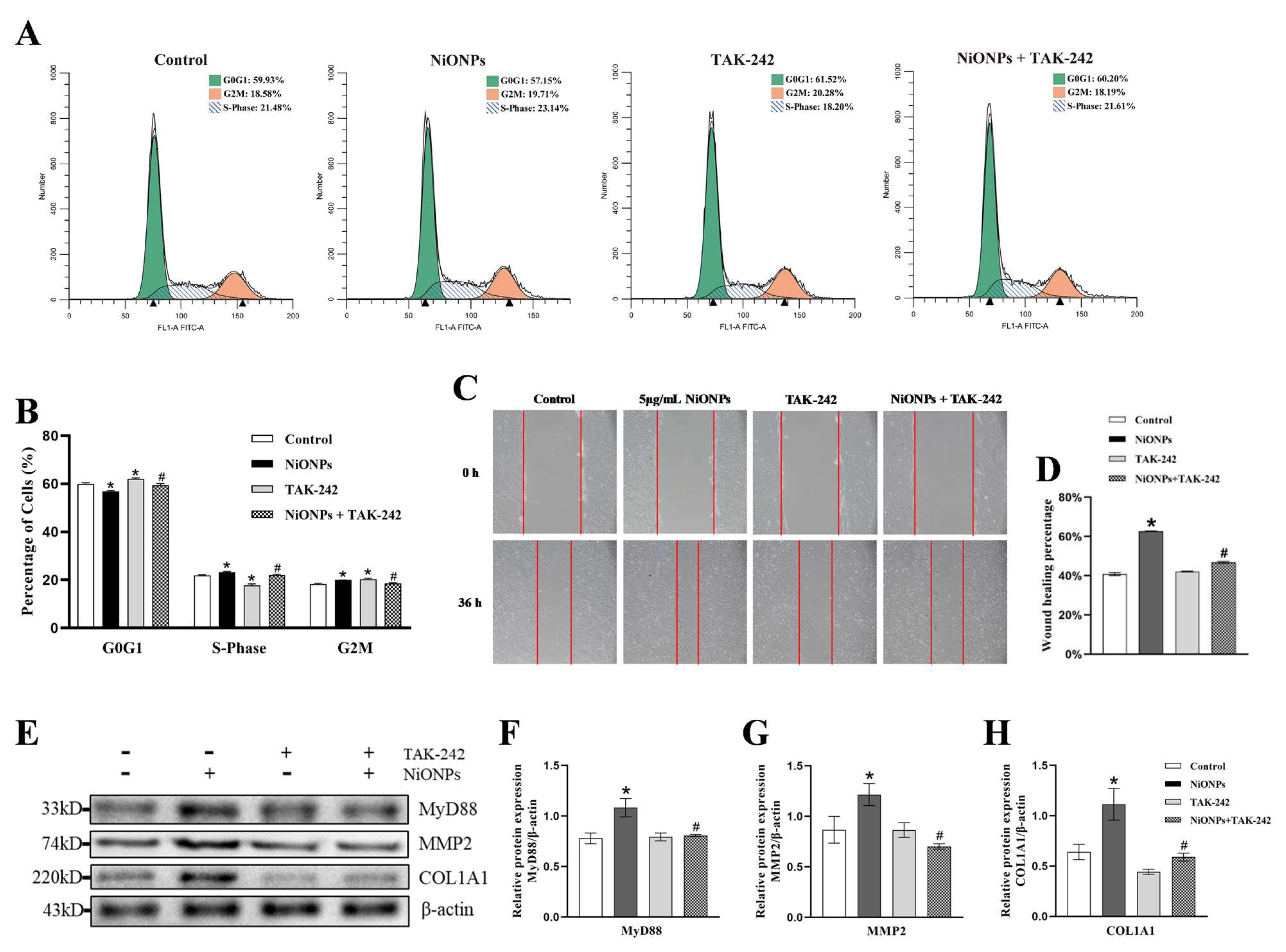
3.4. Inhibition of TLR4 Signaling Pathway Alleviated the NiONPs-Induced Collagen Deposition and Increased the Ferroptosis Features
3.5. Activation of FXR Signaling Alleviated the NiONPs-Induced Collagen Deposition Through Inhibited TLR4 and Increased the Ferroptosis Features
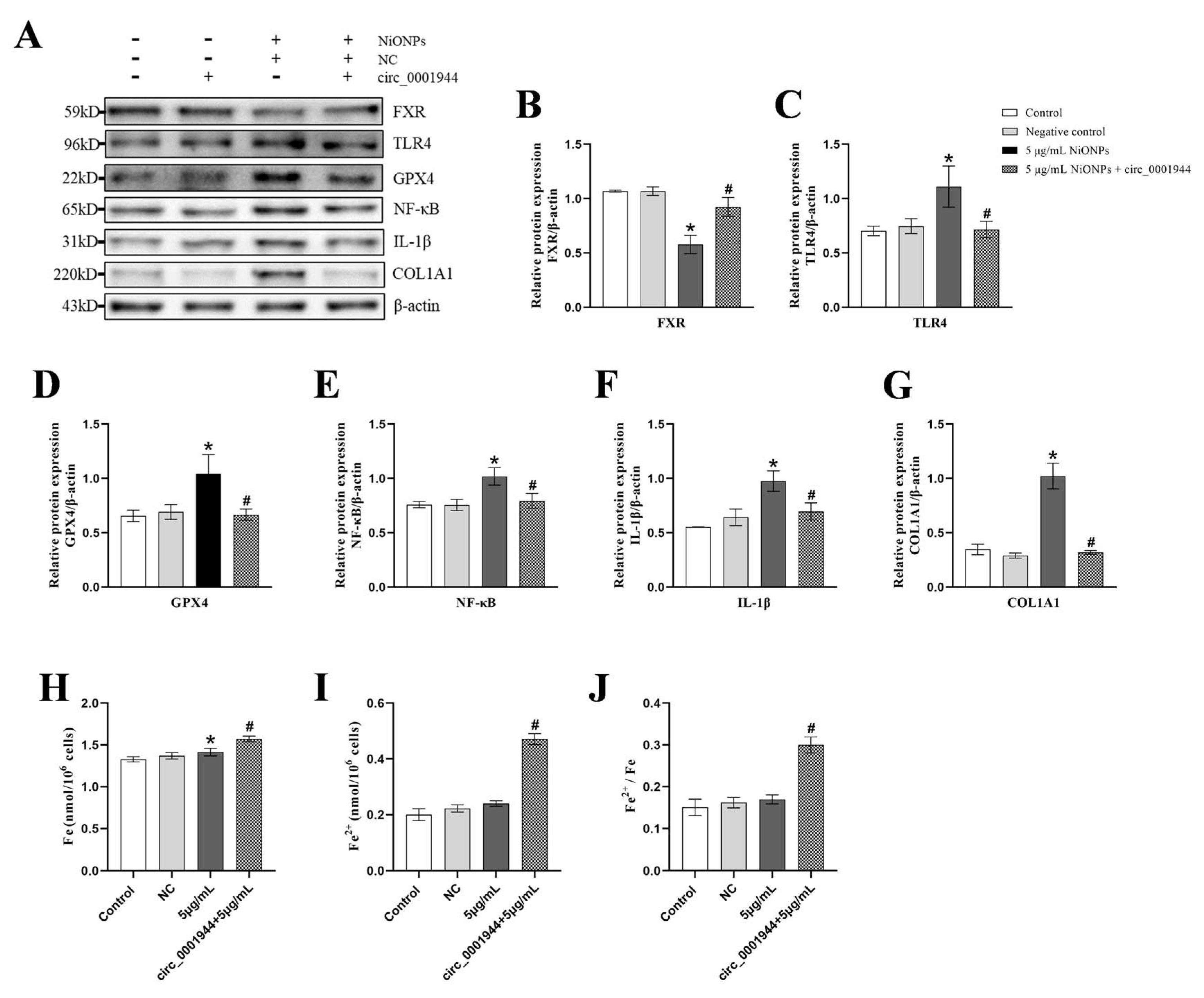
3.6. Overexpression of hsa_circ_0001944 Alleviated the NiONPs-Induced Collagen Deposition Through Regulated FXR/TLR4 Signaling Pathway and Ferroptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Narender, S.S.; Varma, V.V.S.; Srikar, C.S.; Ruchitha, J.; Varma, P.A.; Praveen, B.V.S. Nickel Oxide Nanoparticles: A Brief Review of Their Synthesis, Characterization, and Applications. Chem. Eng. Technol. 2022, 45, 397–409. [Google Scholar] [CrossRef]
- Future Markets, I. The Global Nanotechnology and Nanomaterials Market 2024–2035; Future Markets Inc.: 2024. Available online: https://www.futuremarketsinc.com/the-global-nanotechnology-and-nanomaterials-market-2024-2035/ (accessed on 29 March 2025).
- Ji, Y.; Wang, Y.; Wang, X.; Lv, C.; Zhou, Q.; Jiang, G.; Yan, B.; Chen, L. Beyond the promise: Exploring the complex interactions of nanoparticles within biological systems. J. Hazard. Mater. 2024, 468, 133800. [Google Scholar] [CrossRef]
- Fidan, E.B.; Bali, E.B.; Apaydin, F.G. Comparative study of nickel oxide and nickel oxide nanoparticles on oxidative damage, apoptosis and histopathological alterations in rat lung tissues. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2024, 83, 127379. [Google Scholar] [CrossRef]
- Liu, F.; Chang, X.; Tian, M.; Zhu, A.; Zou, L.; Han, A.; Su, L.; Li, S.; Sun, Y. Nano NiO induced liver toxicity via activating the NF-κB signaling pathway in rats. Toxicol. Res. 2017, 6, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chang, X.; Wang, H.; Liu, Y.; Wang, X.; Wu, M.; Zhan, H.; Li, S.; Sun, Y. TGF-β1 mediated Smad signaling pathway and EMT in hepatic fibrosis induced by Nano NiO in vivo and in vitro. Environ. Toxicol. 2020, 35, 419–429. [Google Scholar] [CrossRef]
- Kong, M.; Zhou, J.; Kang, A.; Kuai, Y.; Xu, H.; Li, M.; Miao, X.; Guo, Y.; Fan, Z.; Xu, Y.; et al. Histone methyltransferase Suv39h1 regulates hepatic stellate cell activation and is targetable in liver fibrosis. Gut 2024, 73, 810–824. [Google Scholar] [CrossRef]
- Wu, K.; Liu, Y.; Xia, J.; Liu, J.; Wang, K.; Liang, H.; Xu, F.; Liu, D.; Nie, D.; Tang, X.; et al. Loss of SLC27A5 Activates Hepatic Stellate Cells and Promotes Liver Fibrosis via Unconjugated Cholic Acid. Adv. Sci. 2024, 11, e2304408. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zheng, J.; Liu, H.; Gao, Q.; Yang, M.; Tang, J.; Wang, H.; Li, S.; Sun, Y.; Chang, X. Whole-transcriptome sequencing revealed differentially expressed mRNAs and non-coding RNAs played crucial roles in NiONPs-induced liver fibrosis. Ecotoxicol. Environ. Saf. 2022, 248, 114308. [Google Scholar] [CrossRef]
- Horn, P.; Tacke, F. Metabolic reprogramming in liver fibrosis. Cell Metab. 2024, 36, 1439–1455. [Google Scholar] [CrossRef]
- Liu, R.; Li, Y.; Zheng, Q.; Ding, M.; Zhou, H.; Li, X. Epigenetic modification in liver fibrosis: Promising therapeutic direction with significant challenges ahead. Acta Pharm. Sin. B 2024, 14, 1009–1029. [Google Scholar] [CrossRef]
- Ding, C.; Wang, Z.; Dou, X.; Yang, Q.; Ning, Y.; Kao, S.; Sang, X.; Hao, M.; Wang, K.; Peng, M.; et al. Farnesoid X receptor: From Structure to Function and Its Pharmacology in Liver Fibrosis. Aging Dis. 2024, 15, 1508–1536. [Google Scholar]
- Chen, J.; Li, X.; Ge, C.; Min, J.; Wang, F. The multifaceted role of ferroptosis in liver disease. Cell Death Differ. 2022, 29, 467–480. [Google Scholar]
- Yuan, S.; Wei, C.; Liu, G.; Zhang, L.; Li, J.; Li, L.; Cai, S.; Fang, L. Sorafenib attenuates liver fibrosis by triggering hepatic stellate cell ferroptosis via HIF-1α/SLC7A11 pathway. Cell Prolif. 2022, 55, e13158. [Google Scholar] [PubMed]
- Zhang, Z.; Guo, M.; Li, Y.; Shen, M.; Kong, D.; Shao, J.; Ding, H.; Tan, S.; Chen, A.; Zhang, F.; et al. RNA-binding protein ZFP36/TTP protects against ferroptosis by regulating autophagy signaling pathway in hepatic stellate cells. Autophagy 2020, 16, 1482–1505. [Google Scholar] [PubMed]
- Liu, G.; Wei, C.; Yuan, S.; Zhang, Z.; Li, J.; Zhang, L.; Wang, G.; Fang, L. Wogonoside attenuates liver fibrosis by triggering hepatic stellate cell ferroptosis through SOCS1/P53/SLC7A11 pathway. Phytother. Res. PTR 2022, 36, 4230–4243. [Google Scholar]
- Yu, S.; Liu, F.; Wang, C.; Zhang, J.; Zhu, A.; Zou, L.; Han, A.; Li, J.; Chang, X.; Sun, Y. Role of oxidative stress in liver toxicity induced by nickel oxide nanoparticles in rats. Mol. Med. Rep. 2018, 17, 3133–3139. [Google Scholar] [CrossRef] [PubMed]
- Soleiman-Meigooni, S.; Yarahmadi, A.; Kheirkhah, A.H.; Afkhami, H. Recent advances in different interactions between toll-like receptors and hepatitis B infection: A review. Front. Immunol. 2024, 15, 1363996. [Google Scholar]
- Hu, L.; Cheng, Z.; Chu, H.; Wang, W.; Jin, Y.; Yang, L. TRIF-dependent signaling and its role in liver diseases. Front. Cell Dev. Biol. 2024, 12, 1370042. [Google Scholar]
- Tang, Y.L.; Zhu, L.; Tao, Y.; Lu, W.; Cheng, H. Role of targeting TLR4 signaling axis in liver-related diseases. Pathol. Res. Pract. 2023, 244, 154410. [Google Scholar]
- Jing, X.; Zhou, G.; Zhu, A.; Jin, C.; Li, M.; Ding, K. RG-I pectin-like polysaccharide from Rosa chinensis inhibits inflammation and fibrosis associated to HMGB1/TLR4/NF-κB signaling pathway to improve non-alcoholic steatohepatitis. Carbohydr. Polym. 2024, 337, 122139. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Fan, M.; Guan, Y.; Zhang, W.; Huang, F.; Zhang, Z.; Li, X.; Yuan, B.; Liu, W.; et al. Reactive oxygen species regulation by NCF1 governs ferroptosis susceptibility of Kupffer cells to MASH. Cell Metab. 2024, 36, 1745–1763.e6. [Google Scholar]
- Zhou, Y.; Pang, N.; Li, W.; Li, Q.; Luo, J.; Gu, Y.; Hu, Q.; Ding, Y.J.; Sun, Y.; Pan, J.; et al. Inhibition of ethanol-induced eNAMPT secretion attenuates liver ferroptosis through BAT-Liver communication. Redox Biol. 2024, 75, 103274. [Google Scholar] [CrossRef]
- Li, D.; Tian, L.; Nan, P.; Zhang, J.; Zheng, Y.; Jia, X.; Gong, Y.; Wu, Z. CerS6 triggered by high glucose activating the TLR4/IKKβ pathway regulates ferroptosis of LO2 cells through mitochondrial oxidative stress. Mol. Cell. Endocrinol. 2023, 572, 111969. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Zhou, H.; Liu, Y.; Liu, J.; Guo, Y.; Zhou, H. Roles of nuclear receptors in hepatic stellate cells. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Schwabl, P.; Hambruch, E.; Budas, G.R.; Supper, P.; Burnet, M.; Liles, J.T.; Birkel, M.; Brusilovskaya, K.; Königshofer, P.; Peck-Radosavljevic, M.; et al. The Non-Steroidal FXR Agonist Cilofexor Improves Portal Hypertension and Reduces Hepatic Fibrosis in a Rat NASH Model. Biomedicines 2021, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; Ding, Y.; Shi, X.; Ren, H. Antibiotic pretreatment attenuates liver ischemia-reperfusion injury by Farnesoid X receptor activation. Cell Death Dis. 2022, 13, 484. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, P.; Sun, Z.; Lu, G.; Cao, Q.; Chen, Y.; Wu, W.; Zhang, J.; Zhuang, C.; Sheng, C.; et al. A combined nanotherapeutic approach targeting farnesoid X receptor, ferroptosis, and fibrosis for nonalcoholic steatohepatitis treatment. Acta Pharm. Sin. B 2024, 14, 2228–2246. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, X.; Wang, X.; Zhan, H.; Gao, Q.; Yang, M.; Liu, H.; Li, S.; Sun, Y. A metabolomic-based study on disturbance of bile acids metabolism induced by intratracheal instillation of nickel oxide nanoparticles in rats. Toxicol. Res. 2021, 10, 579–591. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Cai, Z.R.; Liu, J.; Wang, D.S.; Ju, H.Q.; Xu, R.H. Circular RNA: Metabolism, functions and interactions with proteins. Mol. Cancer 2020, 19, 172. [Google Scholar] [CrossRef]
- Chen, L.L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 2020, 21, 475–490. [Google Scholar] [CrossRef]
- Misir, S.; Wu, N.; Yang, B.B. Specific expression and functions of circular RNAs. Cell Death Differ. 2022, 29, 481–491. [Google Scholar] [PubMed]
- Liu, W.; Feng, R.; Li, X.; Li, D.; Zhai, W. TGF-β- and lipopolysaccharide-induced upregulation of circular RNA PWWP2A promotes hepatic fibrosis via sponging miR-203 and miR-223. Aging 2019, 11, 9569–9580. [Google Scholar]
- Yang, Y.R.; Hu, S.; Bu, F.T.; Li, H.; Huang, C.; Meng, X.M.; Zhang, L.; Lv, X.W.; Li, J. Circular RNA CREBBP Suppresses Hepatic Fibrosis Via Targeting the hsa-miR-1291/LEFTY2 Axis. Front. Pharmacol. 2021, 12, 741151. [Google Scholar]
- Zheng, Y.L.; Zhang, H.C.; Tian, D.H.; Duan, D.C.; Dai, F.; Zhou, B. Rational design of an ESIPT-based fluorescent probe for selectively monitoring glutathione in live cells and zebrafish. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 238, 118429. [Google Scholar]
- Li, G.; Zhou, C.; Wang, L.; Zheng, Y.; Zhou, B.; Li, G.; Ma, Z.; Sun, P.; Deng, Y.; Su, L.; et al. MitoCur-1 induces ferroptosis to reverse vemurafenib resistance in melanoma through inhibition of USP14. Pigment Cell Melanoma Res. 2024, 37, 316–328. [Google Scholar] [PubMed]
- Liu, L.; Zhang, W.G.; Hu, Y.J.; Ma, L.L.; Xu, X.S. Downregulation of miR-1225-5p is pivotal for proliferation, invasion, and migration of HCC cells through NFκB regulation. J. Clin. Lab. Anal. 2020, 34, e23474. [Google Scholar]
- Zhang, D.; Zhang, Y.; Zhang, X.W.; Zhai, H.J.; Sun, X.L.; Li, Y.M. Circ_0091579 Serves as a Tumor-Promoting Factor in Hepatocellular Carcinoma Through miR-1225-5p/PLCB1 Axis. Dig. Dis. Sci. 2022, 67, 585–597. [Google Scholar] [PubMed]
- Tong, S.; Li, H.; Wang, L.; Tudi, M.; Yang, L. Concentration, Spatial Distribution, Contamination Degree and Human Health Risk Assessment of Heavy Metals in Urban Soils across China between 2003 and 2019-A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 3099. [Google Scholar] [CrossRef]
- Wang, S.; Lyu, Y.; Ji, S.; Liu, N.; Wu, B.; Zhao, F.; Li, Z.; Qu, Y.; Zhu, Y.; Xie, L.; et al. Heavy metals and metalloids exposure and liver function in Chinese adults—A nationally representative cross-sectional study. Environ. Res. 2024, 252 Pt 2, 118653. [Google Scholar]
- Li, J.; Chen, C.; Xia, T. Understanding Nanomaterial-Liver Interactions to Facilitate the Development of Safer Nanoapplications. Adv. Mater. 2022, 34, e2106456. [Google Scholar]
- Trivedi, P.; Wang, S.; Friedman, S.L. The Power of Plasticity-Metabolic Regulation of Hepatic Stellate Cells. Cell Metab. 2021, 33, 242–257. [Google Scholar] [PubMed]
- Yu, Y.; Jiang, L.; Wang, H.; Shen, Z.; Cheng, Q.; Zhang, P.; Wang, J.; Wu, Q.; Fang, X.; Duan, L.; et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood 2020, 136, 726–739. [Google Scholar] [PubMed]
- Wang, C.; Su, Z.; Xu, J.H.; Ko, C.Y. Danshensu attenuated lipopolysaccharide-induced LX-2 and T6 cells activation through regulation of ferroptosis. Food Sci. Nutr. 2023, 11, 344–349. [Google Scholar]
- Huang, S.; Wang, Y.; Xie, S.; Lai, Y.; Mo, C.; Zeng, T.; Kuang, S.; Zhou, C.; Zeng, Z.; Chen, Y.; et al. Isoliquiritigenin alleviates liver fibrosis through caveolin-1-mediated hepatic stellate cells ferroptosis in zebrafish and mice. Phytomed. Int. J. Phytother. Phytopharm. 2022, 101, 154117. [Google Scholar] [CrossRef]
- Zhang, K.K.; Wan, J.Y.; Chen, Y.C.; Cheng, C.H.; Zhou, H.Q.; Zheng, D.K.; Lan, Z.X.; You, Q.H.; Sun, J. Polystyrene nanoplastics exacerbate aflatoxin B1-induced hepatic injuries by modulating the gut-liver axis. Sci. Total Environ. 2024, 935, 173285. [Google Scholar]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar]
- Takimoto, Y.; Chu, P.S.; Nakamoto, N.; Hagihara, Y.; Mikami, Y.; Miyamoto, K.; Morikawa, R.; Teratani, T.; Taniki, N.; Fujimori, S.; et al. Myeloid TLR4 signaling promotes post-injury withdrawal resolution of murine liver fibrosis. iScience 2023, 26, 106220. [Google Scholar] [PubMed]
- Yong, Q.; Huang, C.; Chen, B.; An, J.; Zheng, Y.; Zhao, L.; Peng, C.; Liu, F. Gentiopicroside improves NASH and liver fibrosis by suppressing TLR4 and NLRP3 signaling pathways. Biomed. Pharmacother. = Biomed. Pharmacother. 2024, 177, 116952. [Google Scholar]
- Tacke, F.; Puengel, T.; Loomba, R.; Friedman, S.L. An integrated view of anti-inflammatory and antifibrotic targets for the treatment of NASH. J. Hepatol. 2023, 79, 552–566. [Google Scholar]
- Azizsoltani, A.; Hatami, B.; Zali, M.R.; Mahdavi, V.; Baghaei, K.; Alizadeh, E. Obeticholic acid-loaded exosomes attenuate liver fibrosis through dual targeting of the FXR signaling pathway and ECM remodeling. Biomed. Pharmacother. = Biomed. Pharmacother. 2023, 168, 115777. [Google Scholar]
- Tschuck, J.; Theilacker, L.; Rothenaigner, I.; Weiß, S.A.I.; Akdogan, B.; Lam, V.T.; Müller, C.; Graf, R.; Brandner, S.; Pütz, C.; et al. Farnesoid X receptor activation by bile acids suppresses lipid peroxidation and ferroptosis. Nat. Commun. 2023, 14, 6908. [Google Scholar] [PubMed]
- Chien, Y.; Tsai, P.H.; Lai, Y.H.; Lu, K.H.; Liu, C.Y.; Lin, H.F.; Huang, C.S.; Wu, W.W.; Wang, C.Y. CircularRNA as novel biomarkers in liver diseases. J. Chin. Med. Assoc. JCMA 2020, 83, 15–17. [Google Scholar]
- Yuan, S.; Liu, J.; Yang, L.; Zhang, X.; Zhuang, K.; He, S. Knockdown of circ_0044226 promotes endoplasmic reticulum stress-mediated autophagy and apoptosis in hepatic stellate cells via miR-4677-3p/SEC61G axis. J. Bioenerg. Biomembr. 2024, 56, 261–271. [Google Scholar] [PubMed]
- Zhou, Z.; Zhang, R.; Li, X.; Zhang, W.; Zhan, Y.; Lang, Z.; Tao, Q.; Yu, J.; Yu, S.; Yu, Z.; et al. Circular RNA cVIM promotes hepatic stellate cell activation in liver fibrosis via miR-122-5p/miR-9-5p-mediated TGF-β signaling cascade. Commun. Biol. 2024, 7, 113. [Google Scholar]
- Nokkeaw, A.; Thamjamrassri, P.; Tangkijvanich, P.; Ariyachet, C. Regulatory Functions and Mechanisms of Circular RNAs in Hepatic Stellate Cell Activation and Liver Fibrosis. Cells 2023, 12, 378. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, B.; Chen, S.; Zuo, T.; Zhang, W.; Cheng, Z.; Fu, J.; Gong, J. Hsa_circ_0009096/miR-370-3p modulates hepatic stellate cell proliferation and fibrosis during biliary atresia pathogenesis. PeerJ 2024, 12, e17356. [Google Scholar]






| Gene Names | Category | Primer Sequence (5′-3′) |
|---|---|---|
| hsa-miR-1225-5p | miRNA | F-AATGTCGTGGGTACGGCCCA R-ATCCAGTGCAGGGTCCGAGG RT-GTCGTATCCAGTGCAGGGTCCGAG GTATTCGCACTGGATACGACCCCCCCAC |
| hsa-miR-421 | miRNA | F-CGCGGCCATCAACAGACATTAAT R-ATCCAGTGCAGGGTCCGAGG RT-GTCGTATCCAGTGCAGGGTCCGAG GTATTCGCACTGGATACGACGCGCCC |
| hsa-miR-137-3p | miRNA | F-CGCGCGTTATTGCTTAAGAATAC R-ATCCAGTGCAGGGTCCGAGG RT-GTCGTATCCAGTGCAGGGTCCGAG GTATTCGCACTGGATACGACCTACGC |
| hsa_circ_0001944 | circRNA | F-GAGAGGAGATACTTTATGAGGAGACTAAGG R-GCAAGCCAGGTACAGTCTTGTG |
| hsa_circ_0101802 | circRNA | F-GAAGAATGTGTCCAGCTACCCA R-CTGCTTTCTCTCTTCTTCTGCC |
| hsa_circ_0072088 | circRNA | F-ATGGTCTGCAGTCCTGTGTG R-TGGATAAATGGTGGCATGTTT |
| hsa_circ_0075048 | circRNA | F-ATGAAGATCCCGCTGAACAA R-CAGACTGACGTCGATCTTGC |
| GAPDH | mRNA | F-TATGACAACAGCCTCAAGAT R-AGTCCTTCCACGATACCA |
| U6 | snRNA | F-CTCGCTTCGGCAGCACA R-AACGCTTCACGAATTTGCGT |
| Name | Catalog Number | Dilution Ratio | Source |
|---|---|---|---|
| NR1H4 (FXR) | #31507 | 1:1000 | Signalway Antibody |
| NR0B2 (SHP) | #32460 | 1:1000 | Signalway Antibody |
| TLR4 | #35463 | 1:1000 | Signalway Antibody |
| MyD88 | #32107 | 1:1000 | Signalway Antibody |
| NCOA4 | #32981 | 1:1000 | Signalway Antibody |
| GPX4 | #32506 | 1:1000 | Signalway Antibody |
| NF-κB p65 | #6956 | 1:1000 | Cell Signaling Technology |
| p-NF-κB p65 | #3033 | 1:1000 | Cell Signaling Technology |
| IL-1β | #12242 | 1:1000 | Cell Signaling Technology |
| TNF-α | #3707 | 1:1000 | Cell Signaling Technology |
| MMP2 | #29090 | 1:1000 | Signalway Antibody |
| COL1A1 | #81375 | 1:1000 | Cell Signaling Technology |
| β-actin | #21338 | 1:5000 | Signalway Antibody |
| Goat Anti-Rabbit IgG Secondary Antibody HRP Conjugated | #L3012 | 1:10,000 | Signalway Antibody |
| Goat Anti-Mouse IgG Secondary Antibody HRP Conjugated | #L3032 | 1:10,000 | Signalway Antibody |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Chen, Q.; Ma, L.; Li, G.; Kang, X.; Tang, J.; Wang, H.; Li, S.; Sun, Y.; Chang, X. Hsa_circ_0001944 Regulates FXR/TLR4 Pathway and Ferroptosis to Alleviate Nickel Oxide Nanoparticles-Induced Collagen Formation in LX-2 Cells. Toxics 2025, 13, 265. https://doi.org/10.3390/toxics13040265
Zhou H, Chen Q, Ma L, Li G, Kang X, Tang J, Wang H, Li S, Sun Y, Chang X. Hsa_circ_0001944 Regulates FXR/TLR4 Pathway and Ferroptosis to Alleviate Nickel Oxide Nanoparticles-Induced Collagen Formation in LX-2 Cells. Toxics. 2025; 13(4):265. https://doi.org/10.3390/toxics13040265
Chicago/Turabian StyleZhou, Haodong, Qingyang Chen, Lijiao Ma, Gege Li, Xi Kang, Jiarong Tang, Hui Wang, Sheng Li, Yingbiao Sun, and Xuhong Chang. 2025. "Hsa_circ_0001944 Regulates FXR/TLR4 Pathway and Ferroptosis to Alleviate Nickel Oxide Nanoparticles-Induced Collagen Formation in LX-2 Cells" Toxics 13, no. 4: 265. https://doi.org/10.3390/toxics13040265
APA StyleZhou, H., Chen, Q., Ma, L., Li, G., Kang, X., Tang, J., Wang, H., Li, S., Sun, Y., & Chang, X. (2025). Hsa_circ_0001944 Regulates FXR/TLR4 Pathway and Ferroptosis to Alleviate Nickel Oxide Nanoparticles-Induced Collagen Formation in LX-2 Cells. Toxics, 13(4), 265. https://doi.org/10.3390/toxics13040265






