Effects of Environmental Non-Essential Toxic Heavy Metals on Epigenetics During Development
Abstract
:1. Introduction
2. Overview of Epigenetic Modifications
2.1. DNA Methylation
2.2. Histone Modifications
2.3. Non-Coding RNA
3. Methodology
4. Effects of Non-Essential Toxic Heavy Metals During Development on Epigenetics
4.1. Mercury
4.2. Cadmium
4.3. Arsenic
4.4. Lead
| Metals | Study Design | Samples for Exposure Assessment | Information and Number of Subjects | Study Name and Area | Target Genes and Types of Epigenetics Modifications | References |
|---|---|---|---|---|---|---|
| Hg | cohort | total Hg of maternal hair DNA from the children’s saliva | saliva from 7 years-old children (n = 406) | Seychelles Child Development Study (Seychelles) | increase in DNA methylation in GRIN2B, NR3C1 | Ulloa et al., 2021, Environ. Int. [49] |
| Hg | meta-analysis from several cohorts | total Hg in cord blood, maternal hair, or maternal blood DNA from cord or child blood | cord or child blood from 7 to 8 years-old children (n = 794) | Avon Longitudinal Study of Parents and Children (United Kingdom) Hokkaido Study on Environment and Children’s Health, Sapporo cohort (Japan) Environment and Childhood Project (Spain) KOREAN Exposome (Korea) Project VIVA (United States) Mother, Father and Child Cohort Study (Norway) Mother-Child Cohort in Crete (Greece) | increase in DNA methylation in MED31, GGH, GRK1 | Lozano et al., 2022, Environ. Res. [50] |
| Hg | cohort | Hg and miRNA from Placenta | placenta (n = 110) | National Children’s Study (United States) | decrease in miRNA in miR-151-5p, miR-10a, miR-193b, miR-1975, miR-423-5p, miR-520d-3p, miR-96, miR-526a + miR-518d-5p + miR-520c-5p, let-7 family (i.e., let-7a, let-7b, let-7c, let-7d, let-7g, and let-7i) | Li et al., 2015, Epigenetics [55] |
| Cd | cohort | Cd in maternal blood DNA in cord blood | cord blood (n = 364) | Mothers and Children’s Environmental Health multicenter prospective cohort study (Korea) | decrease in DNA methylation in ATP9A | Park et al., 2022, Environ Res [61] |
| Cd | cohort | Cd in maternal blood DNA in cord blood | cord blood (n = 319) | Newborn Epigenetic Study (United States) | increase in DNA methylation in MEG3 in male increase of DNA methylation in PEG3 in female | Vidal et al., 2015, BMC Pharmacol Toxicol [62] |
| Cd | cohort | Cd in maternal blood DNA in cord blood | cord blood (n = 19) | Newborn Epigenetic STudy (United States) | increase in DNA methylation in IGF2R, KvDMR, SNURF/SNRPN, GNASXL decrease in DNA methylation in GNASXL | Cowley et al., 2018, Environ Health Perspect [63] |
| Cd | cohort | toenail from mothers and newborns placenta | placenta (n = 94) | Rhode Island Child Health Study (United States) | decrease in DNA methylation in PCDHAC1 | Everson et al., 2016, Reprod Toxicol [64] |
| Cd | cohort | Cd in maternal blood DNA in cord blood | cord blood from 9 years-old children (n = 81) | mother–child cohort in Matlab (Bangladesh) | increase in DNA methylation in GSTT1, SAMD11 decrease in DNA methylation in AURKC, LY6G5C, TACSTD2, HLA-DQB2, NCRNA00200 | Gliga et al., 2022, Environ Int [65] |
| Cd | cohort | Cd and miRNA from Placenta | placenta (n = 110) | National Children’s Study (United States) | increase in miRNA in miR-1537 | Li et al., 2015, Epigenetics [55] |
| As | cohort | arsenic in maternal urine DNA in cord blood | infant (n = 134) | New Hampshire Birth Cohort Study (United States) | decrease in DNA methylation in ESR1, PPARGC1A | Koestler et al., 2013, Environ Health Perspect [73] |
| As | cohort | arsenic in drinking-water and urine DNA in cord blood | cord blood (n = 113) | prospective birth cohort recruited in Sirajdikhan Upazila (Bangladesh) | increase in DNA methylation in LINE-1, p16 | Kile et al., 2012, Environ Health Perspect [74] |
| As | cohort | arsenic in drinking-water DNA in cord blood | cord blood (n = 55) | Thailand | increase in DNA methylation in p53 | Intarasunanont et al., 2012, Environ Health [75] |
| As | cohort | arsenic in maternal urine or drinking water DNA in cord blood | cord blood (n = 40) | Biomarkers of Exposure to Arsenic prospective pregnancy cohort (Mexico) | increase in let-7a, miR-107, miR-126, miR-16, miR-17, miR-195, miR-20a, miR-20b, miR-26b, miR-454, miR-96, and miR-98 | Rager et al., 2014, Environ Mol Mutagen [79] |
| Pb | cohort | Pb in maternal bone DNA in cord blood | cord blood (n = 103) | Early Life Exposures in Mexico to Environmental Toxicants study (Mexico) | decrease in DNA methylation in LINE-1, Alu | Pilsner et al., 2009, Environ Health Perspect [84] |
| Pb | cohort | Pb in maternal blood DNA in cord blood | cord blood (n = 268) | Project Viva (United States) | decrease in DNA methylation in CLEC11A decrease of DNA methylation in DNHD1 in female | Wu et al., 2017, Environ Health Perspect [85] |
| Pb | cohort | Pb and miRNA from Placenta | placenta (n = 110) | National Children’s Study (United States) | decrease in miRNA in let-7f, miR-146a, miR-10a, and miR-431 increase if miRNA in miR-651 | Li et al., 2015, Epigenetics [55] |
| Metals | Study Design | Exposure Condition | Information of Samples | Target Genes and Types of Epigenetics Modifications | References |
|---|---|---|---|---|---|
| Hg | C57BL/6 mouse | pregnant mice exposed to MeHg (CH3HgOH) 0.5 mg/kg/day via drinking water from gestational day 7 until day 7 after delivery | male 12-week-old offspring | increase in DNA methylation in BDNF decrease in AcH3 in BDNF increase in H3K27me3 in BDNF | Onishchenko et al., 2008, J. Neurochem. [48] |
| Hg | C57BL/6 mouse | pregnant mice exposed to MeHg (CH3HgCl) 3 mg/kg/day via single oral administration from gestational day 12 to day 14. | fetal cortex at embryonic day 19 | increase in DNA methylation increase in DNA, AcH3K14 increase in DNMT1 increase in DNMT1, HDAC6 | Go et al., 2021, Arch Toxicol [51] |
| Hg | LUHMES cells | MeHg (CH3HgCl) 1 nM from differentiational day 2 to day 8 | cell culture | increase in DNA methylation decrease in AcH3, AcH3K14 increase in H3K27me3 increase in H3K27me3, DNMT3A, DNMT3B decrease in HDAC3, HDAC6 | Go et al., 2021, Arch Toxicol [51] |
| Hg | LUHMES cells | MeHg (CH3HgCl) 1 nM from differentiational day 2 to day 8 | cell culture | increase in H3K27me3 in TH | Go et al., 2018, Biochem Biophys Res Commun [52] |
| Hg | LUHMES cells | MeHg (CH3HgCl) 1 nM from differentiational day 2 to day 8 | cell culture | increase in DNA methylation in NR4A1 decrease in AcH3K9K9, AcH3K14 in NR4A1 increase in H3K27me3 in NR4A1 | Go et al., 2023, Toxicol Lett [53] |
| Hg | LUHMES cells | MeHg (CH3HgCl) 1 nM from differentiational day 2 to day 8 | cell culture | increase and decrease in DNA methylation in SYP, DLG4 decrease in AcH3 in SYP increase in H3K27me3 in SYP, DLG4 | Kurita et al., 2024, J Appl Toxicol [54] |
| Cd | Wistar rat | Cd (CdCl2) 10 ppm via drinking water from weaning to mating, and Cd 50 ppm via drinking water whole pregnancy period until day 20 of pregnancy | liver of pups at gestational day 20 | increase in DNA methylation in GR in male increase in DNMT3a in male | Castillo et al., 2012, PLoS One [66] |
| Cd | human cardiomyocyte differentiated from embryonic stem cells | Cd (CdCl2) 0.15–1.5 uM from differentiational day 0 to day 2 | cell culture | increase in H3K27me3 | Wu et al., 2022, Environ Health Perspect [67] |
| Cd | mouse embryonic stem cells | Cd (CdCl2) 0.16 mM for 24 h | cell culture | decrease in H3K27me1 | Gadhia et al., 2012, Toxicol Lett [68] |
| As | C3H/HeN mouse | pregnant mice exposed to As (NaAsO2) 85 ppm via drinking water from gestational day 8 to day 18. | liver of 74-week-old offspring | increase in H3K9me2 in Fabp4 increase in H3K4me3 in Slc25a30 | Nohara et al., 2012, Toxicol Sci [76] |
| As | CD-1 mouse | pregnant mice exposed to As (NaAsO2) 15 mg/L via drinking water from gestational day 1 to day 18. | fetal brain at embryonic day 18 | decrease of 5-hmC decrease in TET activity | Lv et al., 2021, Ecotoxicol Environ Saf [77] |
| As | C57BL/6 mouse | female mice exposed to As (Na3AsO4) 50 ppb via drinking water 10 days prior to mating, during gestation, and until pups were weaned at approximately postnatal day 23 | frontal cortex and dentate gyrus from 70-day-old offsprings | increase in H3K4me3 and histone methyltransferase (MLL) in male and female dentate gyrus decrease in histone demethylase (KDM5B) in male dentate gyrus increase in H3Kme3 and MLL in male frontal cortex increase in AcH3K9 and histone acetyltransferase (GCN5) in male dentate gyrus decrease in AcH3K9K9 and decrease in AcH3K9, HDAC2 in female dentate gyrus decrease of AcH3K9 and GCN5, PCAF in male frontal cortex | Tyler et al., 2015, Toxicol Appl Pharmacol [78] |
| As | mouse embryonic stem cells | As (As2O3) 0.93 mM for 24 h | cell culture | decrease in H3K27me1 | Gadhia et al., 2012, Toxicol Lett [68] |
| Pb | C57BL/6 mouse | Pb (Pb-acetate) 300 ppm via drinking water embryonic day 8.0 to 10.5 | frontal cortex from 20-weeks-old offsprings | decrease in DNA methylation in Chd7 | Hill et al., 2015, Behav Neurol [86] |
| Pb | C57BL/6 mouse | male mice exposed to 0.2% Pb (Pb-acetate) from postnatal day 1 to 20 through the drinking water of the dam. | brains at postnatal day 20, 180, 270, 540, and 700 | decrease in MeCP2, DNMT1,H3K9Ac and H3K4me2 increase in H3K27me3 | Eid et al., 2016, Alzheimers Dement (Amst) [87] |
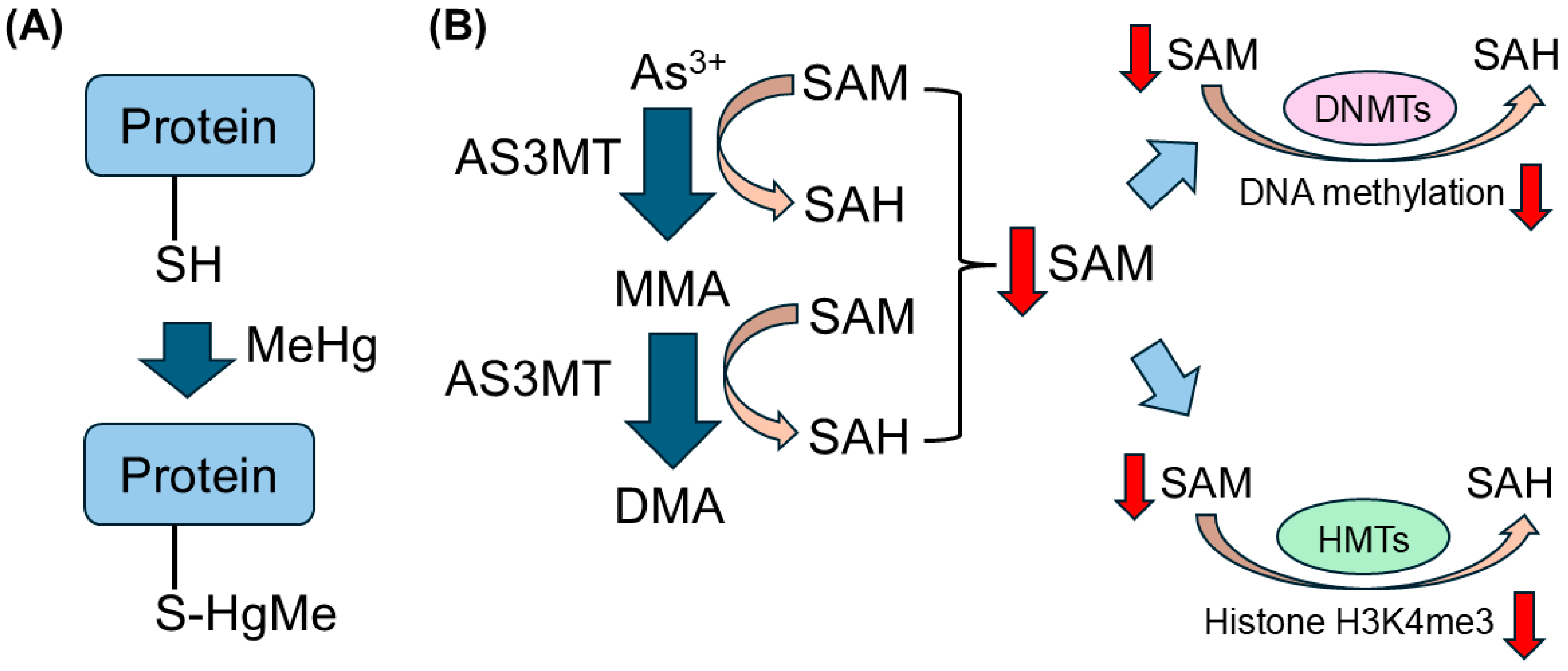
4.5. Possible Mechanisms of Non-Essential Toxic Heavy Metals on Epigenetic Modifications
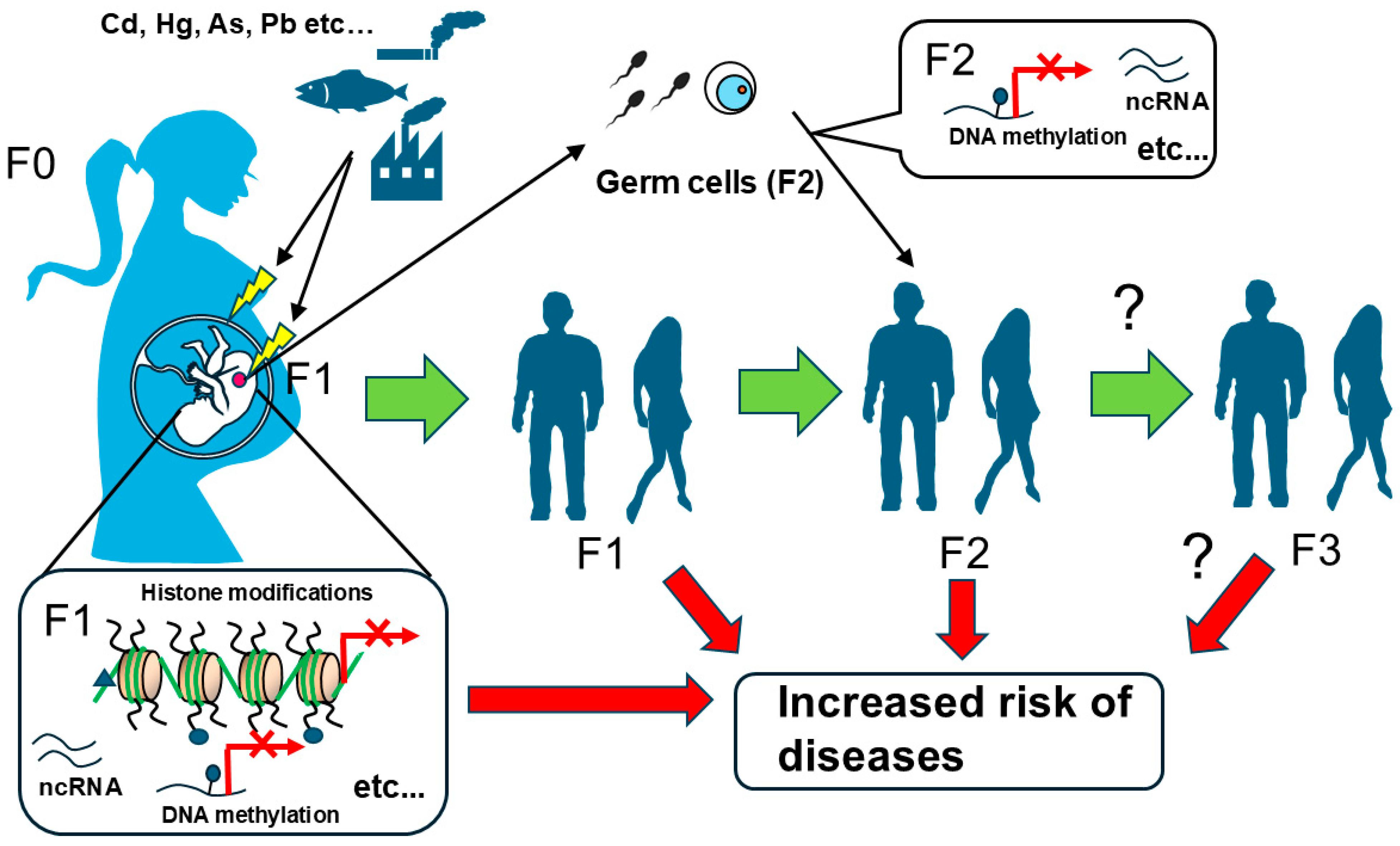
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, X.; Zhou, S.; Li, Y. An updated review on abnormal epigenetic modifications in the pathogenesis of systemic lupus erythematosus. Front. Immunol. 2024, 15, 1501783. [Google Scholar] [CrossRef] [PubMed]
- Robertson, K.D.; Wolffe, A.P. DNA methylation in health and disease. Nat. Rev. Genet. 2000, 1, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Margolis, E.T.; Gabard-Durnam, L.J. Prenatal influences on postnatal neuroplasticity: Integrating DOHaD and sensitive/critical period frameworks to understand biological embedding in early development. Infancy 2025, 30, e12588. [Google Scholar] [CrossRef] [PubMed]
- Ruden, D.M.; Singh, A.; Rappolee, D.A. Pathological epigenetic events and reversibility review: The intersection between hallmarks of aging and developmental origin of health and disease. Epigenomics 2023, 15, 741–754. [Google Scholar] [CrossRef]
- Davis, D.D.; Diaz-Castillo, C.; Chamorro-Garcia, R. Multigenerational metabolic disruption: Developmental origins and mechanisms of propagation across generations. Front. Toxicol. 2022, 4, 902201. [Google Scholar] [CrossRef]
- Ho, S.M.; Cheong, A.; Adgent, M.A.; Veevers, J.; Suen, A.A.; Tam, N.N.C.; Leung, Y.K.; Jefferson, W.N.; Williams, C.J. Environmental factors, epigenetics, and developmental origin of reproductive disorders. Reprod. Toxicol. 2017, 68, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Akhatova, A.; Jones, C.; Coward, K.; Yeste, M. How do lifestyle and environmental factors influence the sperm epigenome? Effects on sperm fertilising ability, embryo development, and offspring health. Clin. Epigenet. 2025, 17, 7. [Google Scholar] [CrossRef]
- Gevaert, A.B.; Wood, N.; Boen, J.R.A.; Davos, C.H.; Hansen, D.; Hanssen, H.; Krenning, G.; Moholdt, T.; Osto, E.; Paneni, F.; et al. Epigenetics in the primary and secondary prevention of cardiovascular disease: Influence of exercise and nutrition. Eur. J. Prev. Cardiol. 2022, 29, 2183–2199. [Google Scholar] [CrossRef] [PubMed]
- Padilla, A.; Manganaro, J.F.; Huesgen, L.; Roess, D.A.; Brown, M.A.; Crans, D.C. Targeting Epigenetic Changes Mediated by Members of the SMYD Family of Lysine Methyltransferases. Molecules 2023, 28, 2000. [Google Scholar] [CrossRef] [PubMed]
- Juan, D.; Perner, J.; Carrillo de Santa Pau, E.; Marsili, S.; Ochoa, D.; Chung, H.R.; Vingron, M.; Rico, D.; Valencia, A. Epigenomic Co-localization and Co-evolution Reveal a Key Role for 5hmC as a Communication Hub in the Chromatin Network of ESCs. Cell Rep. 2016, 14, 1246–1257. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.D.; Helin, K. Role of TET enzymes in DNA methylation, development, and cancer. Genes. Dev. 2016, 30, 733–750. [Google Scholar] [CrossRef]
- DeNizio, J.E.; Dow, B.J.; Serrano, J.C.; Ghanty, U.; Drohat, A.C.; Kohli, R.M. TET-TDG Active DNA Demethylation at CpG and Non-CpG Sites. J. Mol. Biol. 2021, 433, 166877. [Google Scholar] [CrossRef]
- Richa, R.; Sinha, R.P. Hydroxymethylation of DNA: An epigenetic marker. EXCLI J. 2014, 13, 592–610. [Google Scholar] [PubMed]
- Neri, F.; Incarnato, D.; Krepelova, A.; Dettori, D.; Rapelli, S.; Maldotti, M.; Parlato, C.; Anselmi, F.; Galvagni, F.; Oliviero, S. TET1 is controlled by pluripotency-associated factors in ESCs and downmodulated by PRC2 in differentiated cells and tissues. Nucleic Acids Res. 2015, 43, 6814–6826. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.A.; Qiu, R.; Wu, X.; Li, A.X.; Zhang, H.; Wang, J.; Jui, J.; Jin, S.G.; Jiang, Y.; Pfeifer, G.P.; et al. Dynamics of 5-hydroxymethylcytosine and chromatin marks in Mammalian neurogenesis. Cell Rep. 2013, 3, 291–300. [Google Scholar] [CrossRef]
- Breiling, A.; Lyko, F. Epigenetic regulatory functions of DNA modifications: 5-methylcytosine and beyond. Epigenetics Chromatin 2015, 8, 24. [Google Scholar] [CrossRef]
- Pastor, W.A.; Aravind, L.; Rao, A. TETonic shift: Biological roles of TET proteins in DNA demethylation and transcription. Nat. Rev. Mol. Cell Biol. 2013, 14, 341–356. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, F.; Tan, L.; Kong, L.; Xiong, L.; Deng, J.; Barbera, A.J.; Zheng, L.; Zhang, H.; Huang, S.; et al. Genome-wide regulation of 5hmC, 5mC, and gene expression by Tet1 hydroxylase in mouse embryonic stem cells. Mol. Cell 2011, 42, 451–464. [Google Scholar] [CrossRef]
- Davey, C.A.; Sargent, D.F.; Luger, K.; Maeder, A.W.; Richmond, T.J. Solvent mediated interactions in the structure of the nucleosome core particle at 1.9 a resolution. J. Mol. Biol. 2002, 319, 1097–1113. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Huisinga, K.L.; Brower-Toland, B.; Elgin, S.C. The contradictory definitions of heterochromatin: Transcription and silencing. Chromosoma 2006, 115, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Struhl, K. Histone acetylation and transcriptional regulatory mechanisms. Genes. Dev. 1998, 12, 599–606. [Google Scholar] [CrossRef]
- Zhang, T.; Cooper, S.; Brockdorff, N. The interplay of histone modifications—Writers that read. EMBO Rep. 2015, 16, 1467–1481. [Google Scholar] [CrossRef]
- Padeken, J.; Methot, S.P.; Gasser, S.M. Establishment of H3K9-methylated heterochromatin and its functions in tissue differentiation and maintenance. Nat. Rev. Mol. Cell Biol. 2022, 23, 623–640. [Google Scholar] [CrossRef] [PubMed]
- Volker-Albert, M.; Bronkhorst, A.; Holdenrieder, S.; Imhof, A. Histone Modifications in Stem Cell Development and Their Clinical Implications. Stem Cell Rep. 2020, 15, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, B.E.; Mikkelsen, T.S.; Xie, X.; Kamal, M.; Huebert, D.J.; Cuff, J.; Fry, B.; Meissner, A.; Wernig, M.; Plath, K.; et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 2006, 125, 315–326. [Google Scholar] [CrossRef]
- Delcuve, G.P.; Khan, D.H.; Davie, J.R. Roles of histone deacetylases in epigenetic regulation: Emerging paradigms from studies with inhibitors. Clin. Epigenetics 2012, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.D.; Cowley, S.M. The physiological roles of histone deacetylase (HDAC) 1 and 2: Complex co-stars with multiple leading parts. Biochem. Soc. Trans. 2013, 41, 741–749. [Google Scholar] [CrossRef]
- Friedmann, D.R.; Marmorstein, R. Structure and mechanism of non-histone protein acetyltransferase enzymes. FEBS J. 2013, 280, 5570–5581. [Google Scholar] [CrossRef]
- Kim, Y.Z. Altered histone modifications in gliomas. Brain Tumor Res. Treat. 2014, 2, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Wapenaar, H.; Dekker, F.J. Histone acetyltransferases: Challenges in targeting bi-substrate enzymes. Clin. Epigenetics 2016, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.J.; Man, N.; Tan, Y.; Nimer, S.D.; Wang, L. The Role of Histone Acetyltransferases in Normal and Malignant Hematopoiesis. Front. Oncol. 2015, 5, 108. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.P.; Umit Kaniskan, H.; Jin, J.; Gozani, O. Epigenetics and beyond: Targeting writers of protein lysine methylation to treat disease. Nat. Rev. Drug Discov. 2021, 20, 265–286. [Google Scholar] [CrossRef] [PubMed]
- Adamo, A.; Sese, B.; Boue, S.; Castano, J.; Paramonov, I.; Barrero, M.J.; Izpisua Belmonte, J.C. LSD1 regulates the balance between self-renewal and differentiation in human embryonic stem cells. Nat. Cell Biol. 2011, 13, 652–659. [Google Scholar] [CrossRef]
- Gu, B.; Lee, M.G. Histone H3 lysine 4 methyltransferases and demethylases in self-renewal and differentiation of stem cells. Cell Biosci. 2013, 3, 39. [Google Scholar] [CrossRef]
- Patel, A.; Dharmarajan, V.; Vought, V.E.; Cosgrove, M.S. On the mechanism of multiple lysine methylation by the human mixed lineage leukemia protein-1 (MLL1) core complex. J. Biol. Chem. 2009, 284, 24242–24256. [Google Scholar] [CrossRef] [PubMed]
- Klose, R.J.; Zhang, Y. Regulation of histone methylation by demethylimination and demethylation. Nat. Rev. Mol. Cell Biol. 2007, 8, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Ueda, J.; Fukuda, M.; Takeda, N.; Ohta, T.; Iwanari, H.; Sakihama, T.; Kodama, T.; Hamakubo, T.; Shinkai, Y. Histone methyltransferases G9a and GLP form heteromeric complexes and are both crucial for methylation of euchromatin at H3-K9. Genes. Dev. 2005, 19, 815–826. [Google Scholar] [CrossRef]
- Feldman, N.; Gerson, A.; Fang, J.; Li, E.; Zhang, Y.; Shinkai, Y.; Cedar, H.; Bergman, Y. G9a-mediated irreversible epigenetic inactivation of Oct-3/4 during early embryogenesis. Nat. Cell Biol. 2006, 8, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Loh, Y.H.; Zhang, W.; Chen, X.; George, J.; Ng, H.H. Jmjd1a and Jmjd2c histone H3 Lys 9 demethylases regulate self-renewal in embryonic stem cells. Genes. Dev. 2007, 21, 2545–2557. [Google Scholar] [CrossRef] [PubMed]
- Bracken, A.P.; Helin, K. Polycomb group proteins: Navigators of lineage pathways led astray in cancer. Nat. Rev. Cancer 2009, 9, 773–784. [Google Scholar] [CrossRef]
- Collinson, A.; Collier, A.J.; Morgan, N.P.; Sienerth, A.R.; Chandra, T.; Andrews, S.; Rugg-Gunn, P.J. Deletion of the Polycomb-Group Protein EZH2 Leads to Compromised Self-Renewal and Differentiation Defects in Human Embryonic Stem Cells. Cell Rep. 2016, 17, 2700–2714. [Google Scholar] [CrossRef]
- Maji, R.K.; Leisegang, M.S.; Boon, R.A.; Schulz, M.H. Revealing microRNA regulation in single cells. Trends Genet. 2025. [CrossRef]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 2019, 20, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Shang, R.; Lee, S.; Senavirathne, G.; Lai, E.C. microRNAs in action: Biogenesis, function and regulation. Nat. Rev. Genet. 2023, 24, 816–833. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, M.; Usuki, F.; Cheng, J.; Zhao, W. Prenatal low-dose methylmercury exposure impairs neurite outgrowth and synaptic protein expression and suppresses TrkA pathway activity and eEF1A1 expression in the rat cerebellum. Toxicol. Appl. Pharmacol. 2016, 298, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, M.; Usuki, F. Pregnant rats exposed to low-level methylmercury exhibit cerebellar synaptic and neuritic remodeling during the perinatal period. Arch. Toxicol. 2020, 94, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Onishchenko, N.; Karpova, N.; Sabri, F.; Castren, E.; Ceccatelli, S. Long-lasting depression-like behavior and epigenetic changes of BDNF gene expression induced by perinatal exposure to methylmercury. J. Neurochem. 2008, 106, 1378–1387. [Google Scholar] [CrossRef]
- Cediel Ulloa, A.; Gliga, A.; Love, T.M.; Pineda, D.; Mruzek, D.W.; Watson, G.E.; Davidson, P.W.; Shamlaye, C.F.; Strain, J.J.; Myers, G.J.; et al. Prenatal methylmercury exposure and DNA methylation in seven-year-old children in the Seychelles Child Development Study. Environ. Int. 2021, 147, 106321. [Google Scholar] [CrossRef] [PubMed]
- Lozano, M.; Yousefi, P.; Broberg, K.; Soler-Blasco, R.; Miyashita, C.; Pesce, G.; Kim, W.J.; Rahman, M.; Bakulski, K.M.; Haug, L.S.; et al. DNA methylation changes associated with prenatal mercury exposure: A meta-analysis of prospective cohort studies from PACE consortium. Environ. Res. 2022, 204 Pt B, 112093. [Google Scholar] [CrossRef]
- Go, S.; Kurita, H.; Hatano, M.; Matsumoto, K.; Nogawa, H.; Fujimura, M.; Inden, M.; Hozumi, I. DNA methyltransferase- and histone deacetylase-mediated epigenetic alterations induced by low-level methylmercury exposure disrupt neuronal development. Arch. Toxicol. 2021, 95, 1227–1239. [Google Scholar] [CrossRef]
- Go, S.; Kurita, H.; Matsumoto, K.; Hatano, M.; Inden, M.; Hozumi, I. Methylmercury causes epigenetic suppression of the tyrosine hydroxylase gene in an in vitro neuronal differentiation model. Biochem. Biophys. Res. Commun. 2018, 502, 435–441. [Google Scholar] [CrossRef]
- Go, S.; Masuda, H.; Tsuru, M.; Inden, M.; Hozumi, I.; Kurita, H. Exposure to a low concentration of methylmercury in neural differentiation downregulates NR4A1 expression with altered epigenetic modifications and inhibits neuronal spike activity in vitro. Toxicol. Lett. 2023, 374, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Kurita, H.; Masuda, H.; Okuda, A.; Go, S.; Ohuchi, K.; Yoshioka, H.; Fujimura, M.; Hozumi, I.; Inden, M. Epigenetic alternations in the SYP and DLG4 genes due to low-level methylmercury exposure during neuronal differentiation in vitro. J. Appl. Toxicol. 2024, 44, 1986–1996. [Google Scholar] [CrossRef]
- Li, Q.; Kappil, M.A.; Li, A.; Dassanayake, P.S.; Darrah, T.H.; Friedman, A.E.; Friedman, M.; Lambertini, L.; Landrigan, P.; Stodgell, C.J.; et al. Exploring the associations between microRNA expression profiles and environmental pollutants in human placenta from the National Children’s Study (NCS). Epigenetics 2015, 10, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Olsvik, P.A.; Williams, T.D.; Tung, H.S.; Mirbahai, L.; Sanden, M.; Skjaerven, K.H.; Ellingsen, S. Impacts of TCDD and MeHg on DNA methylation in zebrafish (Danio rerio) across two generations. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014, 165, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Camsari, C.; Folger, J.K.; Rajput, S.K.; McGee, D.; Latham, K.E.; Smith, G.W. Transgenerational Effects of Periconception Heavy Metal Administration on Adipose Weight and Glucose Homeostasis in Mice at Maturity. Toxicol. Sci. 2019, 168, 610–619. [Google Scholar] [CrossRef]
- Lawless, L.; Xie, L.; Zhang, K. The inter- and multi- generational epigenetic alterations induced by maternal cadmium exposure. Front. Cell Dev. Biol. 2023, 11, 1148906. [Google Scholar] [CrossRef] [PubMed]
- Young, J.L.; Cai, L. Implications for prenatal cadmium exposure and adverse health outcomes in adulthood. Toxicol. Appl. Pharmacol. 2020, 403, 115161. [Google Scholar] [CrossRef] [PubMed]
- Kurita, H.; Hasegawa, T.; Seko, Y.; Nagase, H.; Tokumoto, M.; Lee, J.Y.; Satoh, M. Effect of gestational cadmium exposure on fetal growth, polyubiquitinated protein and monoubiqutin levels in the fetal liver of mice. J. Toxicol. Sci. 2018, 43, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, J.; Kim, E.; Won, S.; Kim, W.J. Association between prenatal cadmium exposure and cord blood DNA methylation. Environ. Res. 2022, 212 Pt B, 113268. [Google Scholar] [CrossRef]
- Vidal, A.C.; Semenova, V.; Darrah, T.; Vengosh, A.; Huang, Z.; King, K.; Nye, M.D.; Fry, R.; Skaar, D.; Maguire, R.; et al. Maternal cadmium, iron and zinc levels, DNA methylation and birth weight. BMC Pharmacol. Toxicol. 2015, 16, 20. [Google Scholar] [CrossRef]
- Cowley, M.; Skaar, D.A.; Jima, D.D.; Maguire, R.L.; Hudson, K.M.; Park, S.S.; Sorrow, P.; Hoyo, C. Effects of Cadmium Exposure on DNA Methylation at Imprinting Control Regions and Genome-Wide in Mothers and Newborn Children. Environ. Health Perspect. 2018, 126, 037003. [Google Scholar] [CrossRef] [PubMed]
- Everson, T.M.; Armstrong, D.A.; Jackson, B.P.; Green, B.B.; Karagas, M.R.; Marsit, C.J. Maternal cadmium, placental PCDHAC1, and fetal development. Reprod. Toxicol. 2016, 65, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Gliga, A.R.; Malin Igra, A.; Hellberg, A.; Engstrom, K.; Raqib, R.; Rahman, A.; Vahter, M.; Kippler, M.; Broberg, K. Maternal exposure to cadmium during pregnancy is associated with changes in DNA methylation that are persistent at 9 years of age. Environ. Int. 2022, 163, 107188. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P.; Ibanez, F.; Guajardo, A.; Llanos, M.N.; Ronco, A.M. Impact of cadmium exposure during pregnancy on hepatic glucocorticoid receptor methylation and expression in rat fetus. PLoS ONE 2012, 7, e44139. [Google Scholar] [CrossRef]
- Wu, X.; Chen, Y.; Luz, A.; Hu, G.; Tokar, E.J. Cardiac Development in the Presence of Cadmium: An in Vitro Study Using Human Embryonic Stem Cells and Cardiac Organoids. Environ. Health Perspect. 2022, 130, 117002. [Google Scholar] [CrossRef] [PubMed]
- Gadhia, S.R.; Calabro, A.R.; Barile, F.A. Trace metals alter DNA repair and histone modification pathways concurrently in mouse embryonic stem cells. Toxicol. Lett. 2012, 212, 169–179. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, L.; Zhuang, S.; Zhang, C.; Li, Y.; Zhu, J.; Zhang, W. Cadmium exposure during prenatal development causes progesterone disruptors in multiple generations via steroidogenic enzymes in rat ovarian granulosa cells. Ecotoxicol. Environ. Saf. 2020, 201, 110765. [Google Scholar] [CrossRef] [PubMed]
- Young, J.L.; Cai, L.; States, J.C. Impact of prenatal arsenic exposure on chronic adult diseases. Syst. Biol. Reprod. Med. 2018, 64, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Monne, M.; Marobbio, C.M.T.; Agrimi, G.; Palmieri, L.; Palmieri, F. Mitochondrial transport and metabolism of the major methyl donor and versatile cofactor S-adenosylmethionine, and related diseases: A review(dagger). IUBMB Life 2022, 74, 573–591. [Google Scholar] [CrossRef] [PubMed]
- Drobna, Z.; Waters, S.B.; Devesa, V.; Harmon, A.W.; Thomas, D.J.; Styblo, M. Metabolism and toxicity of arsenic in human urothelial cells expressing rat arsenic (+3 oxidation state)-methyltransferase. Toxicol. Appl. Pharmacol. 2005, 207, 147–159. [Google Scholar] [CrossRef]
- Koestler, D.C.; Avissar-Whiting, M.; Houseman, E.A.; Karagas, M.R.; Marsit, C.J. Differential DNA methylation in umbilical cord blood of infants exposed to low levels of arsenic in utero. Environ. Health Perspect. 2013, 121, 971–977. [Google Scholar] [CrossRef]
- Kile, M.L.; Baccarelli, A.; Hoffman, E.; Tarantini, L.; Quamruzzaman, Q.; Rahman, M.; Mahiuddin, G.; Mostofa, G.; Hsueh, Y.M.; Wright, R.O.; et al. Prenatal arsenic exposure and DNA methylation in maternal and umbilical cord blood leukocytes. Environ. Health Perspect. 2012, 120, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Intarasunanont, P.; Navasumrit, P.; Waraprasit, S.; Chaisatra, K.; Suk, W.A.; Mahidol, C.; Ruchirawat, M. Effects of arsenic exposure on DNA methylation in cord blood samples from newborn babies and in a human lymphoblast cell line. Environ. Health 2012, 11, 31. [Google Scholar] [CrossRef]
- Nohara, K.; Tateishi, Y.; Suzuki, T.; Okamura, K.; Murai, H.; Takumi, S.; Maekawa, F.; Nishimura, N.; Kobori, M.; Ito, T. Late-onset increases in oxidative stress and other tumorigenic activities and tumors with a Ha-ras mutation in the liver of adult male C3H mice gestationally exposed to arsenic. Toxicol. Sci. 2012, 129, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.W.; Song, Y.P.; Zhang, Z.C.; Fan, Y.J.; Xu, F.X.; Gao, L.; Zhang, X.Y.; Zhang, C.; Wang, H.; Xu, D.X. Gestational arsenic exposure induces anxiety-like behaviors in adult offspring by reducing DNA hydroxymethylation in the developing brain. Ecotoxicol. Environ. Saf. 2021, 227, 112901. [Google Scholar] [CrossRef]
- Tyler, C.R.; Hafez, A.K.; Solomon, E.R.; Allan, A.M. Developmental exposure to 50 parts-per-billion arsenic influences histone modifications and associated epigenetic machinery in a region- and sex-specific manner in the adult mouse brain. Toxicol. Appl. Pharmacol. 2015, 288, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Rager, J.E.; Bailey, K.A.; Smeester, L.; Miller, S.K.; Parker, J.S.; Laine, J.E.; Drobna, Z.; Currier, J.; Douillet, C.; Olshan, A.F.; et al. Prenatal arsenic exposure and the epigenome: Altered microRNAs associated with innate and adaptive immune signaling in newborn cord blood. Environ. Mol. Mutagen. 2014, 55, 196–208. [Google Scholar] [CrossRef]
- Nohara, K.; Okamura, K.; Suzuki, T.; Murai, H.; Ito, T.; Shinjo, K.; Takumi, S.; Michikawa, T.; Kondo, Y.; Hata, K. Augmenting effects of gestational arsenite exposure of C3H mice on the hepatic tumors of the F(2) male offspring via the F(1) male offspring. J. Appl. Toxicol. 2016, 36, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Nohara, K.; Suzuki, T.; Okamura, K.; Kawai, T.; Nakabayashi, K. Acquired sperm hypomethylation by gestational arsenic exposure is re-established in both the paternal and maternal genomes of post-epigenetic reprogramming embryos. Epigenetics Chromatin 2025, 18, 4. [Google Scholar] [CrossRef]
- Olympio, K.P.; Goncalves, C.; Gunther, W.M.; Bechara, E.J. Neurotoxicity and aggressiveness triggered by low-level lead in children: A review. Rev. Panam. Salud Publica 2009, 26, 266–275. [Google Scholar] [CrossRef]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Pilsner, J.R.; Hu, H.; Ettinger, A.; Sanchez, B.N.; Wright, R.O.; Cantonwine, D.; Lazarus, A.; Lamadrid-Figueroa, H.; Mercado-Garcia, A.; Tellez-Rojo, M.M.; et al. Influence of prenatal lead exposure on genomic methylation of cord blood DNA. Environ. Health Perspect. 2009, 117, 1466–1471. [Google Scholar] [CrossRef]
- Wu, S.; Hivert, M.F.; Cardenas, A.; Zhong, J.; Rifas-Shiman, S.L.; Agha, G.; Colicino, E.; Just, A.C.; Amarasiriwardena, C.; Lin, X.; et al. Exposure to Low Levels of Lead in Utero and Umbilical Cord Blood DNA Methylation in Project Viva: An Epigenome-Wide Association Study. Environ. Health Perspect. 2017, 125, 087019. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.S.; Cabrera, R.; Wallis Schultz, D.; Zhu, H.; Lu, W.; Finnell, R.H.; Wlodarczyk, B.J. Autism-Like Behavior and Epigenetic Changes Associated with Autism as Consequences of In Utero Exposure to Environmental Pollutants in a Mouse Model. Behav. Neurol. 2015, 2015, 426263. [Google Scholar] [CrossRef]
- Eid, A.; Bihaqi, S.W.; Renehan, W.E.; Zawia, N.H. Developmental lead exposure and lifespan alterations in epigenetic regulators and their correspondence to biomarkers of Alzheimer’s disease. Alzheimer’s Dement. 2016, 2, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Heredia, N.; Senut, M.C.; Land, S.; Hollocher, K.; Lu, X.; Dereski, M.O.; Ruden, D.M. Multigenerational epigenetic inheritance in humans: DNA methylation changes associated with maternal exposure to lead can be transmitted to the grandchildren. Sci. Rep. 2015, 5, 14466. [Google Scholar] [CrossRef] [PubMed]
- Sobolewski, M.; Abston, K.; Conrad, K.; Marvin, E.; Harvey, K.; Susiarjo, M.; Cory-Slechta, D.A. Lineage- and Sex-Dependent Behavioral and Biochemical Transgenerational Consequences of Developmental Exposure to Lead, Prenatal Stress, and Combined Lead and Prenatal Stress in Mice. Environ. Health Perspect. 2020, 128, 27001. [Google Scholar] [CrossRef]
- Shinkai, Y.; Kimura, T.; Itagaki, A.; Yamamoto, C.; Taguchi, K.; Yamamoto, M.; Kumagai, Y.; Kaji, T. Partial contribution of the Keap1-Nrf2 system to cadmium-mediated metallothionein expression in vascular endothelial cells. Toxicol. Appl. Pharmacol. 2016, 295, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Unoki, T.; Abiko, Y.; Toyama, T.; Uehara, T.; Tsuboi, K.; Nishida, M.; Kaji, T.; Kumagai, Y. Methylmercury, an environmental electrophile capable of activation and disruption of the Akt/CREB/Bcl-2 signal transduction pathway in SH-SY5Y cells. Sci. Rep. 2016, 6, 28944. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Abiko, Y. Environmental Electrophiles: Protein Adducts, Modulation of Redox Signaling, and Interaction with Persulfides/Polysulfides. Chem. Res. Toxicol. 2017, 30, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Nakahara, K.; Ito, A.; Iijima, Y.; Nomura, R.; Kumar, A.; Fujikawa, K.; Adachi, K.; Shimada, Y.; Fujio, S.; et al. Pivotal role for S-nitrosylation of DNA methyltransferase 3B in epigenetic regulation of tumorigenesis. Nat. Commun. 2023, 14, 621. [Google Scholar] [CrossRef]
- Okuda, K.; Ito, A.; Uehara, T. Regulation of Histone Deacetylase 6 Activity via S-Nitrosylation. Biol. Pharm. Bull. 2015, 38, 1434–1437. [Google Scholar] [CrossRef]
- Wild, C.P. Complementing the genome with an “exposome”: The outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1847–1850. [Google Scholar] [CrossRef] [PubMed]
- Land, W.G. Role of Damage-Associated Molecular Patterns in Light of Modern Environmental Research: A Tautological Approach. Int. J. Environ. Res. 2020, 14, 583–604. [Google Scholar] [CrossRef] [PubMed]
- Danieli, M.G.; Casciaro, M.; Paladini, A.; Bartolucci, M.; Sordoni, M.; Shoenfeld, Y.; Gangemi, S. Exposome: Epigenetics and autoimmune diseases. Autoimmun. Rev. 2024, 23, 103584. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Fan, B.; Yang, Y.; Wang, P.; Wu, M.; Xia, H.; Syed, B.M.; Wu, H.; Liu, Q. Construction of an adverse outcome pathway framework for arsenic-induced lung cancer using a network-based approach. Ecotoxicol. Environ. Saf. 2024, 283, 116809. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.; Beaton, D.; Tollefsen, K.E.; Preston, J.; Burtt, J.J.; Leblanc, J.; Hamada, N.; Azzam, E.I.; Armant, O.; Bouffler, S.; et al. Radiation Adverse Outcome pathways (AOPs): Examining priority questions from an international horizon-style exercise. Int. J. Radiat. Biol. 2024, 100, 982–995. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Morales Valencia, M.; Yu, Y.; Ouchi, Y.; Takahashi, K.; Shokhirev, M.N.; Lande, K.; Williams, A.E.; Fresia, C.; Kurita, M.; et al. Transgenerational inheritance of acquired epigenetic signatures at CpG islands in mice. Cell 2023, 186, 715–731 e19. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurita, H.; Ohuchi, K.; Inden, M. Effects of Environmental Non-Essential Toxic Heavy Metals on Epigenetics During Development. Toxics 2025, 13, 167. https://doi.org/10.3390/toxics13030167
Kurita H, Ohuchi K, Inden M. Effects of Environmental Non-Essential Toxic Heavy Metals on Epigenetics During Development. Toxics. 2025; 13(3):167. https://doi.org/10.3390/toxics13030167
Chicago/Turabian StyleKurita, Hisaka, Kazuki Ohuchi, and Masatoshi Inden. 2025. "Effects of Environmental Non-Essential Toxic Heavy Metals on Epigenetics During Development" Toxics 13, no. 3: 167. https://doi.org/10.3390/toxics13030167
APA StyleKurita, H., Ohuchi, K., & Inden, M. (2025). Effects of Environmental Non-Essential Toxic Heavy Metals on Epigenetics During Development. Toxics, 13(3), 167. https://doi.org/10.3390/toxics13030167







