Continuous Glucose Monitoring in Healthy Adults—Possible Applications in Health Care, Wellness, and Sports
Abstract
:1. Introduction
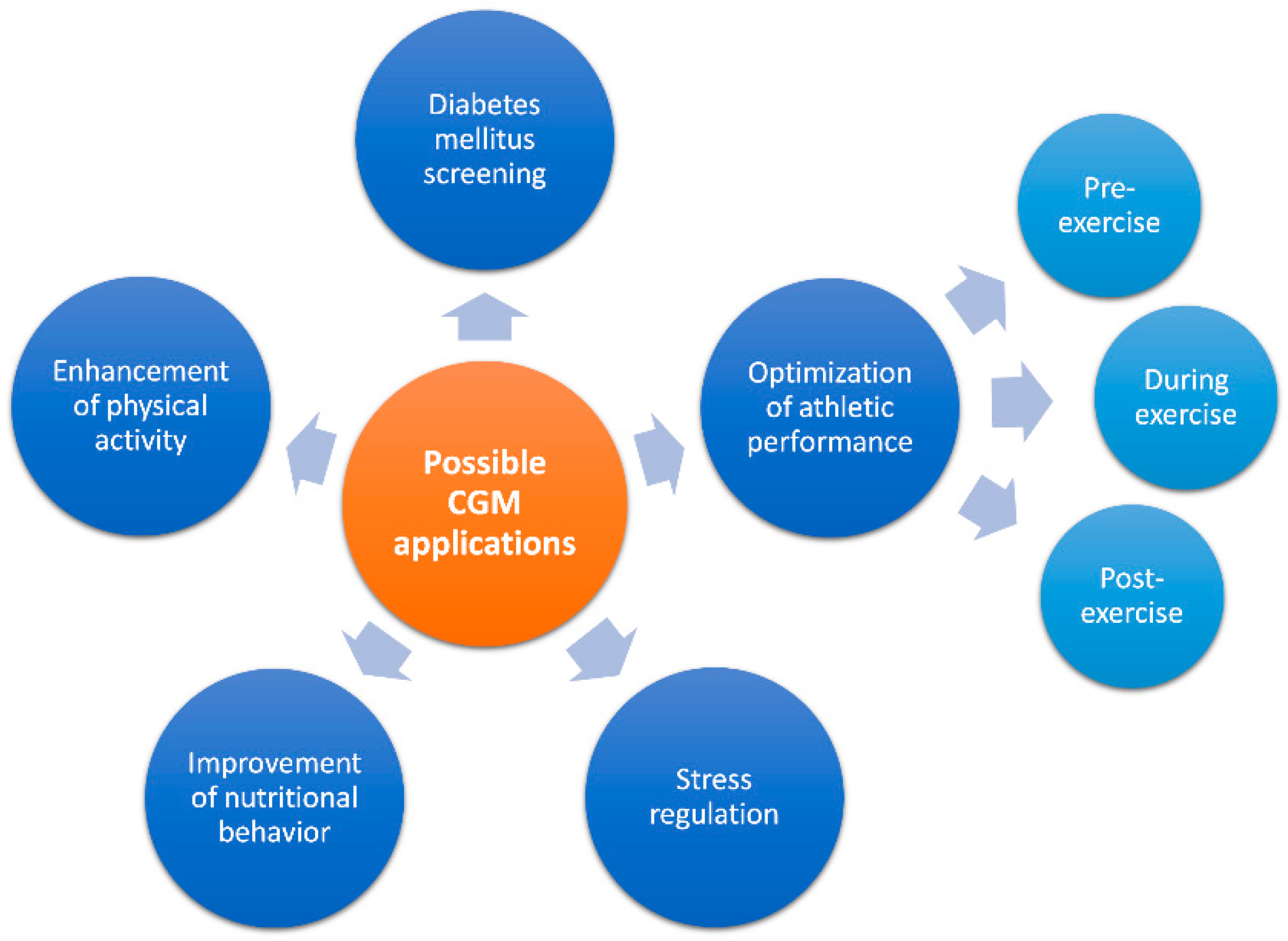
2. Possible Applications
2.1. CGM as a Screening Tool for Early Detection of Abnormal Glucose Regulation/Diabetes Mellitus
2.2. CGM for Lifestyle Optimization
2.2.1. Nutritional Behavior
2.2.2. Physical Activity
2.2.3. Stress
2.3. CGM for Optimization of Athletic Performance
3. Challenges and Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heinemann, L.; Deiss, D.; Siegmund, T.; Schlüter, S.; Naudorf, M.; Sengbusch, S.V.; Lange, K.; Freckmann, G. Glucose Measurement and Control in Patients with Type 1 or Type 2 Diabetes. Exp. Clin. Endocrinol. Diabetes 2019, 127, S8–S26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, S.F.; Foster, J.R. A History of Blood Glucose Meters and Their Role in Self-Monitoring of Diabetes Mellitus. Br. J. Biomed. Sci. 2012, 69, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Villena Gonzales, W.; Mobashsher, A.T.; Abbosh, A. The Progress of Glucose Monitoring—A Review of Invasive to Minimally and Non-Invasive Techniques, Devices and Sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamin, E.M. Self-Monitoring of Blood Glucose: The Basics. Clin. Diabetes 2002, 20, 45–47. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. 7. Diabetes Technology: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42, 71–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinemann, L. Finger Pricking and Pain: A Never Ending Story. J. Diabetes Sci. Technol. 2008, 2, 919–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olczuk, D.; Priefer, R. A History of Continuous Glucose Monitors (CGMs) in Self-Monitoring of Diabetes Mellitus. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 181–187. [Google Scholar] [CrossRef]
- Fogh-Andersen, N.; Altura, B.M.; Altura, B.T.; Siggaard-Andersen, O. Composition of Interstitial Fluid. Clin. Chem. 1995, 41, 1522–1525. [Google Scholar] [CrossRef]
- Schrangl, P.; Reiterer, F.; Heinemann, L.; Freckmann, G.; Del Re, L. Limits to the Evaluation of the Accuracy of Continuous Glucose Monitoring Systems by Clinical Trials. Biosensors 2018, 8, 50. [Google Scholar] [CrossRef] [Green Version]
- Coyle, S.; Curto, V.F.; Benito-Lopez, F.; Florea, L.; Diamond, D. Chapter 2.1—Wearable Bio and Chemical Sensors. In Wearable Sensors; Sazonov, E., Neuman, M.R., Eds.; Academic Press: Oxford, UK, 2014; pp. 65–83. ISBN 978-0-12-418662-0. [Google Scholar]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and Skeletal Muscle Glucose Uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [Green Version]
- Staal, O.M.; Hansen, H.M.U.; Christiansen, S.C.; Fougner, A.L.; Carlsen, S.M.; Stavdahl, Ø. Differences Between Flash Glucose Monitor and Fingerprick Measurements. Biosensors 2018, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Schmelzeisen-Redeker, G.; Schoemaker, M.; Kirchsteiger, H.; Freckmann, G.; Heinemann, L.; del Re, L. Time Delay of CGM Sensors: Relevance, Causes, and Countermeasures. J. Diabetes Sci. Technol. 2015, 9, 1006–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davey, R.J.; Low, C.; Jones, T.W.; Fournier, P.A. Contribution of an Intrinsic Lag of Continuous Glucose Monitoring Systems to Differences in Measured and Actual Glucose Concentrations Changing at Variable Rates in Vitro. J. Diabetes Sci. Technol. 2010, 4, 1393–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, J.I. Review of the Long-Term Implantable Senseonics Continuous Glucose Monitoring System and Other Continuous Glucose Monitoring Systems. J. Diabetes Sci. Technol. 2021, 15, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Holzer, R.; Bloch, W.; Brinkmann, C. Minimally Invasive Electrochemical Patch-Based Sensor System for Monitoring Glucose and Lactate in the Human Body—A Survey-Based Analysis of the End-User’s Perspective. Sensors 2020, 20, 5761. [Google Scholar] [CrossRef] [PubMed]
- Klonoff, D.C.; Ahn, D.; Drincic, A. Continuous Glucose Monitoring: A Review of the Technology and Clinical Use. Diabetes Res. Clin. Pract. 2017, 133, 178–192. [Google Scholar] [CrossRef]
- Reiterer, F.; Polterauer, P.; Schoemaker, M.; Schmelzeisen-Redecker, G.; Freckmann, G.; Heinemann, L.; del Re, L. Significance and Reliability of MARD for the Accuracy of CGM Systems. J. Diabetes Sci. Technol. 2017, 11, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Heinemann, L.; Schoemaker, M.; Schmelzeisen-Redecker, G.; Hinzmann, R.; Kassab, A.; Freckmann, G.; Reiterer, F.; Del Re, L. Benefits and Limitations of MARD as a Performance Parameter for Continuous Glucose Monitoring in the Interstitial Space. J. Diabetes Sci. Technol. 2020, 14, 135–150. [Google Scholar] [CrossRef]
- Freckmann, G.; Link, M.; Kamecke, U.; Haug, C.; Baumgartner, B.; Weitgasser, R. Performance and Usability of Three Systems for Continuous Glucose Monitoring in Direct Comparison. J. Diabetes Sci. Technol. 2019, 13, 890–898. [Google Scholar] [CrossRef]
- Alva, S.; Bailey, T.; Brazg, R.; Budiman, E.S.; Castorino, K.; Christiansen, M.P.; Forlenza, G.; Kipnes, M.; Liljenquist, D.R.; Liu, H. Accuracy of a 14-Day Factory-Calibrated Continuous Glucose Monitoring System With Advanced Algorithm in Pediatric and Adult Population With Diabetes. J. Diabetes Sci. Technol. 2022, 16, 70–77. [Google Scholar] [CrossRef]
- Boscari, F.; Galasso, S.; Facchinetti, A.; Marescotti, M.C.; Vallone, V.; Amato, A.M.L.; Avogaro, A.; Bruttomesso, D. FreeStyle Libre and Dexcom G4 Platinum Sensors: Accuracy Comparisons during Two Weeks of Home Use and Use during Experimentally Induced Glucose Excursions. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.B.; Gao, P.; Derdzinski, M.; Puhr, S.; Johnson, T.K.; Walker, T.C.; Graham, C. Accuracy, Utilization, and Effectiveness Comparisons of Different Continuous Glucose Monitoring Systems. Diabetes Technol. Ther. 2019, 21, 128–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freckmann, G.; Pleus, S.; Link, M.; Baumstark, A.; Schmid, C.; Högel, J.; Haug, C. Accuracy Evaluation of Four Blood Glucose Monitoring Systems in Unaltered Blood Samples in the Low Glycemic Range and Blood Samples in the Concentration Range Defined by ISO 15197. Diabetes Technol. Ther. 2015, 17, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Aleppo, G.; Webb, K. Continuous Glucose Monitoring in Clinical Practice: A Stepped Guide to Data Review and Interpretation. J. Diabetes Sci. Technol. 2019, 13, 664–673. [Google Scholar] [CrossRef]
- Scheiner, G. CGM Retrospective Data Analysis. Diabetes Technol. Ther. 2016, 18 (Suppl. 2), 14–22. [Google Scholar] [CrossRef] [Green Version]
- Shah, V.N.; DuBose, S.N.; Li, Z.; Beck, R.W.; Peters, A.L.; Weinstock, R.S.; Kruger, D.; Tansey, M.; Sparling, D.; Woerner, S.; et al. Continuous Glucose Monitoring Profiles in Healthy Nondiabetic Participants: A Multicenter Prospective Study. J. Clin. Endocrinol. Metab. 2019, 104, 4356–4364. [Google Scholar] [CrossRef]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [Green Version]
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Variation of Interstitial Glucose Measurements Assessed by Continuous Glucose Monitors in Healthy, Nondiabetic Individuals. Diabetes Care 2010, 33, 1297–1299. [Google Scholar] [CrossRef] [Green Version]
- Borg, R.; Kuenen, J.C.; Carstensen, B.; Zheng, H.; Nathan, D.M.; Heine, R.J.; Nerup, J.; Borch-Johnsen, K.; Witte, D.R.; on behalf of the ADAG Study Group. Real-Life Glycaemic Profiles in Non-Diabetic Individuals with Low Fasting Glucose and Normal HbA1c: The A1C-Derived Average Glucose (ADAG) Study. Diabetologia 2010, 53, 1608–1611. [Google Scholar] [CrossRef] [Green Version]
- Hall, H.; Perelman, D.; Breschi, A.; Limcaoco, P.; Kellogg, R.; McLaughlin, T.; Snyder, M. Glucotypes Reveal New Patterns of Glucose Dysregulation. PLoS Biol. 2018, 16, e2005143. [Google Scholar] [CrossRef] [Green Version]
- Klimontov, V.V.; Saik, O.V.; Korbut, A.I. Glucose Variability: How Does It Work? Int. J. Mol. Sci. 2021, 22, 7783. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative Stress and Metabolic Disorders: Pathogenesis and Therapeutic Strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.L.; Pyle, L.; Newnes, L.; Nadeau, K.J.; Zeitler, P.S.; Kelsey, M.M. Continuous Glucose Monitoring and Its Relationship to Hemoglobin A1c and Oral Glucose Tolerance Testing in Obese and Prediabetic Youth. J. Clin. Endocrinol. Metab. 2015, 100, 902–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acciaroli, G.; Sparacino, G.; Hakaste, L.; Facchinetti, A.; Di Nunzio, G.M.; Palombit, A.; Tuomi, T.; Gabriel, R.; Aranda, J.; Vega, S.; et al. Diabetes and Prediabetes Classification Using Glycemic Variability Indices From Continuous Glucose Monitoring Data. J. Diabetes Sci. Technol. 2018, 12, 105–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ringeval, M.; Wagner, G.; Denford, J.; Paré, G.; Kitsiou, S. Fitbit-Based Interventions for Healthy Lifestyle Outcomes: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2020, 22, e23954. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; Tan, Z.Y.A.; Cheng, L.J.; Lau, S.T. Wearable Technology-Delivered Lifestyle Intervention amongst Adults with Overweight and Obese: A Systematic Review and Meta-Regression. Int. J. Nurs. Stud. 2021, 18, 104163. [Google Scholar] [CrossRef] [PubMed]
- Jo, A.; Coronel, B.D.; Coakes, C.E.; Mainous, A.G. Is There a Benefit to Patients Using Wearable Devices Such as Fitbit or Health Apps on Mobiles? A Systematic Review. Am. J. Med. 2019, 132, 1394–1400.e1. [Google Scholar] [CrossRef]
- Tong, H.L.; Quiroz, J.C.; Kocaballi, A.B.; Fat, S.C.M.; Dao, K.P.; Gehringer, H.; Chow, C.K.; Laranjo, L. Personalized Mobile Technologies for Lifestyle Behavior Change: A Systematic Review, Meta-Analysis, and Meta-Regression. Prev. Med. 2021, 148, 106532. [Google Scholar] [CrossRef]
- Wright, E.E.; Subramanian, S. Evolving Use of Continuous Glucose Monitoring Beyond Intensive Insulin Treatment. Diabetes Technol. Ther. 2021, 23, S-12–S-18. [Google Scholar] [CrossRef]
- Ehrhardt, N.; Al Zaghal, E. Behavior Modification in Prediabetes and Diabetes: Potential Use of Real-Time Continuous Glucose Monitoring. J. Diabetes Sci. Technol. 2018, 13, 271–275. [Google Scholar] [CrossRef]
- Fechner, E.; Op ’t Eyndt, C.; Mulder, T.; Mensink, R.P. Diet-Induced Differences in Estimated Plasma Glucose Concentrations in Healthy, Non-Diabetic Adults Are Detected by Continuous Glucose Monitoring—A Randomized Crossover Trial. Nutr. Res. 2020, 80, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Dehghani Zahedani, A.; Shariat Torbaghan, S.; Rahili, S.; Karlin, K.; Scilley, D.; Thakkar, R.; Saberi, M.; Hashemi, N.; Perelman, D.; Aghaeepour, N.; et al. Improvement in Glucose Regulation Using a Digital Tracker and Continuous Glucose Monitoring in Healthy Adults and Those with Type 2 Diabetes. Diabetes Ther. 2021, 12, 1871–1886. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D.; Friedman, M.I. Hunger. DDI 1993, 11, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Ciampolini, M.; Lovell-Smith, H.D.; Kenealy, T.; Bianchi, R. Hunger Can Be Taught: Hunger Recognition Regulates Eating and Improves Energy Balance. Int. J. Gen. Med. 2013, 6, 465–478. [Google Scholar] [CrossRef] [Green Version]
- Jospe, M.R.; de Bruin, W.E.; Haszard, J.J.; Mann, J.I.; Brunton, M.; Taylor, R.W. Teaching People to Eat According to Appetite—Does the Method of Glucose Measurement Matter? Appetite 2020, 151, 104691. [Google Scholar] [CrossRef]
- Diaz, K.M.; Shimbo, D. Physical Activity and the Prevention of Hypertension. Curr. Hypertens. Rep. 2013, 15, 659–668. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.-M. Physical Activity and Cancer Prevention--Data from Epidemiologic Studies. Med. Sci. Sports Exerc. 2003, 35, 1823–1827. [Google Scholar] [CrossRef]
- Mammen, G.; Faulkner, G. Physical Activity and the Prevention of Depression: A Systematic Review of Prospective Studies. Am. J. Prev. Med. 2013, 45, 649–657. [Google Scholar] [CrossRef]
- Montesi, L.; Moscatiello, S.; Malavolti, M.; Marzocchi, R.; Marchesini, G. Physical Activity for the Prevention and Treatment of Metabolic Disorders. Intern. Emerg. Med. 2013, 8, 655–666. [Google Scholar] [CrossRef] [Green Version]
- Nunan, D.; Mahtani, K.R.; Roberts, N.; Heneghan, C. Physical Activity for the Prevention and Treatment of Major Chronic Disease: An Overview of Systematic Reviews. Syst. Rev. 2013, 2, 56. [Google Scholar] [CrossRef] [Green Version]
- Wannamethee, S.G.; Shaper, A.G. Physical Activity in the Prevention of Cardiovascular Disease. Sports Med. 2001, 31, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Sparks, J.R.; Kishman, E.E.; Sarzynski, M.A.; Davis, J.M.; Grandjean, P.W.; Durstine, J.L.; Wang, X. Glycemic Variability: Importance, Relationship with Physical Activity, and the Influence of Exercise. Sports Med. Health Sci. 2021, 3, 183–193. [Google Scholar] [CrossRef]
- Solomon, T.P.J.; Eves, F.F.; Laye, M.J. Targeting Postprandial Hyperglycemia With Physical Activity May Reduce Cardiovascular Disease Risk. But What Should We Do, and When Is the Right Time to Move? Front. Cardiovasc. Med. 2018, 5, 99. [Google Scholar] [CrossRef] [PubMed]
- Bellini, A.; Nicolò, A.; Bulzomì, R.; Bazzucchi, I.; Sacchetti, M. The Effect of Different Postprandial Exercise Types on Glucose Response to Breakfast in Individuals with Type 2 Diabetes. Nutrients 2021, 13, 1440. [Google Scholar] [CrossRef]
- Holzer, R.; Schulte-Körne, B.; Seidler, J.; Predel, H.-G.; Brinkmann, C. Effects of Acute Resistance Exercise with and without Whole-Body Electromyostimulation and Endurance Exercise on the Postprandial Glucose Regulation in Patients with Type 2 Diabetes Mellitus: A Randomized Crossover Study. Nutrients 2021, 13, 4322. [Google Scholar] [CrossRef] [PubMed]
- Little, J.P.; Jung, M.E.; Wright, A.E.; Wright, W.; Manders, R.J.F. Effects of High-Intensity Interval Exercise versus Continuous Moderate-Intensity Exercise on Postprandial Glycemic Control Assessed by Continuous Glucose Monitoring in Obese Adults. Interval Train. 2014, 01, 835–841. [Google Scholar] [CrossRef]
- Borror, A.; Zieff, G.; Battaglini, C.; Stoner, L. The Effects of Postprandial Exercise on Glucose Control in Individuals with Type 2 Diabetes: A Systematic Review. Sports Med. 2018, 48, 1479–1491. [Google Scholar] [CrossRef]
- Aqeel, M.; Forster, A.; Richards, E.A.; Hennessy, E.; McGowan, B.; Bhadra, A.; Guo, J.; Gelfand, S.; Delp, E.; Eicher-Miller, H.A. The Effect of Timing of Exercise and Eating on Postprandial Response in Adults: A Systematic Review. Nutrients 2020, 12, 221. [Google Scholar] [CrossRef] [Green Version]
- Bailey, K.J.; Little, J.P.; Jung, M.E. Self-Monitoring Using Continuous Glucose Monitors with Real-Time Feedback Improves Exercise Adherence in Individuals with Impaired Blood Glucose: A Pilot Study. Diabetes Technol. Ther. 2016, 18, 185–193. [Google Scholar] [CrossRef]
- Liao, Y.; Basen-Engquist, K.M.; Urbauer, D.L.; Bevers, T.B.; Hawk, E.; Schembre, S.M. Using Continuous Glucose Monitoring to Motivate Physical Activity in Overweight and Obese Adults: A Pilot Study. Cancer Epidemiol. Prev. Biomark. 2020, 29, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Schembre, S. Acceptability of Continuous Glucose Monitoring in Free-Living Healthy Individuals: Implications for the Use of Wearable Biosensors in Diet and Physical Activity Research. JMIR Mhealth Uhealth 2018, 6, e11181. [Google Scholar] [CrossRef] [PubMed]
- Tank, A.W.; Lee Wong, D. Peripheral and Central Effects of Circulating Catecholamines. Compr. Physiol 2015, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Di Dalmazi, G.; Pagotto, U.; Pasquali, R.; Vicennati, V. Glucocorticoids and Type 2 Diabetes: From Physiology to Pathology. J. Nutr. Metab. 2012, 2012, 525093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohleder, N. Stress and Inflammation—The Need to Address the Gap in the Transition between Acute and Chronic Stress Effects. Psychoneuroendocrinology 2019, 105, 164–171. [Google Scholar] [CrossRef]
- Zea, M.; Bellagambi, F.G.; Ben Halima, H.; Zine, N.; Jaffrezic-Renault, N.; Villa, R.; Gabriel, G.; Errachid, A. Electrochemical Sensors for Cortisol Detections: Almost There. TrAC Trends Anal. Chem. 2020, 132, 116058. [Google Scholar] [CrossRef]
- Adesida, Y.; Papi, E.; McGregor, A.H. Exploring the Role of Wearable Technology in Sport Kinematics and Kinetics: A Systematic Review. Sensors 2019, 19, 1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutz, J.; Memmert, D.; Raabe, D.; Dornberger, R.; Donath, L. Wearables for Integrative Performance and Tactic Analyses: Opportunities, Challenges, and Future Directions. Int. J. Environ. Res. Public Health 2020, 17, 59. [Google Scholar] [CrossRef] [Green Version]
- Düking, P.; Stammel, C.; Sperlich, B.; Sutehall, S.; Muniz-Pardos, B.; Lima, G.; Kilduff, L.; Keramitsoglou, I.; Li, G.; Pigozzi, F.; et al. Necessary Steps to Accelerate the Integration of Wearable Sensors Into Recreation and Competitive Sports. Curr. Sports Med. Rep. 2018, 17, 178–182. [Google Scholar] [CrossRef] [Green Version]
- Rothschild, J.A.; Kilding, A.E.; Plews, D.J. What Should I Eat before Exercise? Pre-Exercise Nutrition and the Response to Endurance Exercise: Current Prospective and Future Directions. Nutrients 2020, 12, 3473. [Google Scholar] [CrossRef]
- Arent, S.M.; Cintineo, H.P.; McFadden, B.A.; Chandler, A.J.; Arent, M.A. Nutrient Timing: A Garage Door of Opportunity? Nutrients 2020, 12, 1948. [Google Scholar] [CrossRef]
- Kloby Nielsen, L.L.; Tandrup Lambert, M.N.; Jeppesen, P.B. The Effect of Ingesting Carbohydrate and Proteins on Athletic Performance: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 1483. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R. Nutrition to Support Recovery from Endurance Exercise: Optimal Carbohydrate and Protein Replacement. Curr. Sports Med. Rep. 2015, 14, 294–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravindra, P.V.; Janhavi, P.; Divyashree, S.; Muthukumar, S.P. Nutritional Interventions for Improving the Endurance Performance in Athletes. Arch. Physiol. Biochem. 2020, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ormsbee, M.J.; Bach, C.W.; Baur, D.A. Pre-Exercise Nutrition: The Role of Macronutrients, Modified Starches and Supplements on Metabolism and Endurance Performance. Nutrients 2014, 6, 1782–1808. [Google Scholar] [CrossRef] [Green Version]
- Jeukendrup, A.E.; Killer, S.C. The Myths Surrounding Pre-Exercise Carbohydrate Feeding. ANM 2010, 57, 18–25. [Google Scholar] [CrossRef]
- Brun, J.F.; Dumortier, M.; Fedou, C.; Mercier, J. Exercise Hypoglycemia in Nondiabetic Subjects. Diabetes Metab. 2001, 27, 92–106. [Google Scholar]
- Yang, W.-H.; Park, H.; Grau, M.; Heine, O. Decreased Blood Glucose and Lactate: Is a Useful Indicator of Recovery Ability in Athletes? Int. J. Environ. Res. Public Health 2020, 17, 5470. [Google Scholar] [CrossRef]
- Evans, P.L.; McMillin, S.L.; Weyrauch, L.A.; Witczak, C.A. Regulation of Skeletal Muscle Glucose Transport and Glucose Metabolism by Exercise Training. Nutrients 2019, 11, 2432. [Google Scholar] [CrossRef] [Green Version]
- Jensen, T.E.; Richter, E.A. Regulation of Glucose and Glycogen Metabolism during and after Exercise. J. Physiol. 2012, 590, 1069–1076. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, K.; Uchiyama, N.; Kizaki, S.; Mori, E.; Nonaka, T.; Oneda, H. Application of Continuous Glucose Monitoring for Assessment of Individual Carbohydrate Requirement during Ultramarathon Race. Nutrients 2020, 12, 1121. [Google Scholar] [CrossRef] [Green Version]
- Oishi, A.; Makita, N.; Kishi, S.; Isogawa, A.; Iiri, T. Continuous Glucose Monitoring of a Runner during Five Marathons. Sci. Sports 2018, 33, 370–374. [Google Scholar] [CrossRef]
- Kulawiec, D.G.; Zhou, T.; Knopp, J.L.; Chase, J.G. Continuous Glucose Monitoring to Measure Metabolic Impact and Recovery in Sub-Elite Endurance Athletes. Biomed. Signal Processing Control. 2021, 70, 103059. [Google Scholar] [CrossRef]
- Cano, A.; Ventura, L.; Martinez, G.; Cugusi, L.; Caria, M.; Deriu, F.; Manca, A. Analysis of Sex-Based Differences in Energy Substrate Utilization during Moderate-Intensity Aerobic Exercise. Eur. J. Appl. Physiol. 2022, 122, 29–70. [Google Scholar] [CrossRef] [PubMed]
- Varlamov, O.; Bethea, C.L.; Roberts, C.T. Sex-Specific Differences in Lipid and Glucose Metabolism. Front. Endocrinol. 2015, 5, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wismann, J.; Willoughby, D. Gender Differences in Carbohydrate Metabolism and Carbohydrate Loading. J. Int. Soc. Sports Nutr. 2006, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Magkos, F.; Wang, X.; Mittendorfer, B. Metabolic Actions of Insulin in Men and Women. Nutrition 2010, 26, 686–693. [Google Scholar] [CrossRef] [Green Version]
- Horton, T.J.; Grunwald, G.K.; Lavely, J.; Donahoo, W.T. Glucose Kinetics Differ between Women and Men, during and after Exercise. J. Appl. Physiol. 2006, 100, 1883–1894. [Google Scholar] [CrossRef] [Green Version]
- Bellazzi, R.; Dagliati, A.; Sacchi, L.; Segagni, D. Big Data Technologies: New Opportunities for Diabetes Management. J. Diabetes Sci. Technol. 2015, 9, 1119–1125. [Google Scholar] [CrossRef] [Green Version]
- Rigla, M.; García-Sáez, G.; Pons, B.; Hernando, M.E. Artificial Intelligence Methodologies and Their Application to Diabetes. J. Diabetes Sci. Technol. 2018, 12, 303–310. [Google Scholar] [CrossRef]
- Kavakiotis, I.; Tsave, O.; Salifoglou, A.; Maglaveras, N.; Vlahavas, I.; Chouvarda, I. Machine Learning and Data Mining Methods in Diabetes Research. Comput. Struct. Biotechnol. J. 2017, 15, 104–116. [Google Scholar] [CrossRef]
- Contreras, I.; Vehi, J. Artificial Intelligence for Diabetes Management and Decision Support: Literature Review. J. Med. Internet Res. 2018, 20, e10775. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Jeerapan, I.; Wang, J. Wearable Chemical Sensors: Present Challenges and Future Prospects. ACS Sens. 2016, 1, 464–482. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable Non-Invasive Epidermal Glucose Sensors: A Review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, W. Wearable and Flexible Electronics for Continuous Molecular Monitoring. Chem. Soc. Rev. 2019, 48, 1465–1491. [Google Scholar] [CrossRef] [PubMed]
- Jernelv, I.L.; Milenko, K.; Fuglerud, S.S.; Hjelme, D.R.; Ellingsen, R.; Aksnes, A. A Review of Optical Methods for Continuous Glucose Monitoring. Appl. Spectrosc. Rev. 2019, 54, 543–572. [Google Scholar] [CrossRef]
- Shokrekhodaei, M.; Quinones, S. Review of Non-Invasive Glucose Sensing Techniques: Optical, Electrical and Breath Acetone. Sensors 2020, 20, 1251. [Google Scholar] [CrossRef] [Green Version]
- Ye, S.; Feng, S.; Huang, L.; Bian, S. Recent Progress in Wearable Biosensors: From Healthcare Monitoring to Sports Analytics. Biosensors 2020, 10, 205. [Google Scholar] [CrossRef]
- Gao, B.; He, Z.; He, B.; Gu, Z. Wearable Eye Health Monitoring Sensors Based on Peacock Tail-Inspired Inverse Opal Carbon. Sens. Actuators B Chem. 2019, 288, 734–741. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holzer, R.; Bloch, W.; Brinkmann, C. Continuous Glucose Monitoring in Healthy Adults—Possible Applications in Health Care, Wellness, and Sports. Sensors 2022, 22, 2030. https://doi.org/10.3390/s22052030
Holzer R, Bloch W, Brinkmann C. Continuous Glucose Monitoring in Healthy Adults—Possible Applications in Health Care, Wellness, and Sports. Sensors. 2022; 22(5):2030. https://doi.org/10.3390/s22052030
Chicago/Turabian StyleHolzer, Roman, Wilhelm Bloch, and Christian Brinkmann. 2022. "Continuous Glucose Monitoring in Healthy Adults—Possible Applications in Health Care, Wellness, and Sports" Sensors 22, no. 5: 2030. https://doi.org/10.3390/s22052030
APA StyleHolzer, R., Bloch, W., & Brinkmann, C. (2022). Continuous Glucose Monitoring in Healthy Adults—Possible Applications in Health Care, Wellness, and Sports. Sensors, 22(5), 2030. https://doi.org/10.3390/s22052030







