Abstract
Heart failure (HF) is a major health care issue, and the incidence of HF is only expected to grow further. Due to the frequent hospitalizations, HF places a major burden on the available hospital and healthcare resources. In the future, HF care should not only be organized solely at the clinical ward and outpatient clinics, but remote monitoring strategies are urgently needed to guide, monitor, and treat chronic HF patients remotely from their homes as well. The intuitiveness and relatively low costs of non-invasive remote monitoring tools make them an appealing and emerging concept for developing new medical apps and devices. The recent COVID-19 pandemic and the associated transition of patient care outside the hospital will boost the development of remote monitoring tools, and many strategies will be reinvented with modern tools. However, it is important to look carefully at the inconsistencies that have been reported in non-invasive remote monitoring effectiveness. With this review, we provide an up-to-date overview of the available evidence on non-invasive remote monitoring in chronic HF patients and provide future perspectives that may significantly benefit the broader group of HF patients.
1. Introduction
Worldwide, approximately 26 million patients are currently diagnosed with heart failure (HF), and this population is rapidly growing [1]. Several factors, including an increase in awareness, improved diagnostic techniques, improved survival of coronary artery disease, increase in the prevalence of HF-related comorbidities such as hypertension and diabetes mellitus, and an aging population, contributes to this growth [2]. HF management places a major burden on healthcare resources due to frequent hospitalizations and outpatient visits [3]. Additionally, chronic HF is associated with increased mortality and morbidity [4].
Timely detection of congestion due to HF can prevent HF-related hospitalization, reduce the overall burden on health care resources, and improve patient outcomes [5,6]. Remote monitoring could be a crucial tool for the early detection of deterioration of HF. Furthermore, remote monitoring could also be used to stratify which patients are at high risk for deterioration and need frequent follow-up or outpatient attention and those who are at low risk and require less regular follow-up. It has been shown that the uptake and titration of guideline-recommended medical HF therapy could be improved further [7,8,9,10]. Remote monitoring strategies can be used to aid clinicians in the up-titration of guideline-recommended medical HF therapy [11]. Over the last years, multiple monitoring strategies, such as non-invasive remote monitoring (consisting of structured telephone support or non-invasively monitoring of parameters including body weight, blood pressure, and heart rate), monitoring using cardiac implantable electronic devices (such as implantable cardioverter-defibrillator or cardiac resynchronization therapy devices) and invasive remote hemodynamic monitoring, have been proposed and tested [12,13,14]. Considering the surge in medical technology apps, which will be boosted by the recent COVID-19 pandemic, it is essential to have an updated overview of the available tools and their evidence. In this review, we will focus on non-invasive remote monitoring tools in HF patients. Studies investigating non-invasive remote monitoring strategies can be divided into studies that have compared usual care with (I) structured telephone support or (II) non-invasive telemonitoring. In the case of non-invasive telemonitoring, patients were instructed to measure specific parameters (such as body weight, heart rate, or blood pressure), which were automatically sent to their health care team. In 2015 a Cochrane meta-analysis assessing the effects of both non-invasive remote monitoring strategies in chronic HF patients had been updated [12]. This Cochrane review reported a significant reduction in all-cause mortality for both structured telephone support, as well as non-invasive telemonitoring (Risk Ratio (RR) 0.87 (0.77–0.98) and RR 0.80 (0.68–0.94), resp.) and a significant reduction in HF-related hospitalizations (RR 0.85 (0.77–0.93) and RR 0.71 (0.60–0.83), resp.). However, the effects were relatively small and not convincingly positive, with the vast majority of studies being negative. This is important when new apps and e-health tools are developed based on old principles. However, since then, several new studies have been published which have reported more positive results if a structured approach is used in specific populations. Therefore, this review aims to provide an overview of the currently available evidence on both non-invasive remote monitoring strategies of chronic HF patients.
2. Methods for Study Selection
We included randomized controlled trials as well as clinical studies comparing HF management delivered via structured telephone support or non-invasive home telemonitoring with usual post-discharge care for people with heart failure living within the community. We included only studies that have been published in full in the peer-reviewed literature. We excluded any studies that did not report data for any of our outcomes of interest in an extractable format (all-cause mortality, all-cause hospitalization, HF-related hospitalization, or quality of life) or those who used home visits or additional outpatient clinics. Additionally, all included studies reported data of only adult patients (aged 18 years or older) of either sex, any ethnic group, with a definitive diagnosis of HF. Patients could have been recently discharged from a cardiac clinic after an episode of decompensation or being recruited in a stable setting from outpatient clinics, as well as studies reporting data on general cardiac or chronic disorder rather than specifically HF. A combination of the following search terms were used: ‘heart failure’, ‘heart or cardiac or myocard’, and ‘failure or insufficiency or decompensation’, in combination with ‘telemedicine’, ‘telecommunication’, ‘telemonitoring’, ‘teleconsult’, ‘telehealth’, ‘home monitoring’, ‘home care’, ‘ambulatory monitoring’, ‘telehome’, ‘ehealth’ or ‘mobile health’.
We searched the MEDLINE, pubmed, database on 1 September 2020, and performed the following. All titles and abstracts were checked for relevance to the review topic by two authors, independently. In case of disagreement, a third author would check the article as well. All data relevant data were extracted from the articles.
3. Structured Telephone Support versus Usual Care
We identified 31 studies that compared structured telephone support with usual care, which included a total of 11,270 patients [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. The study characteristics, as well as the reported outcomes on all-cause mortality, all-cause hospitalization, and HF-related hospitalization rates of the five largest studies, representing 49% (5560) of all patients, will be discussed in detail below [19,21,22,23,34]. The study characteristics and outcomes of the other 26 studies will be summarized below.
3.1. Chaudhry et al. (Tele-HF Trial)
In 2010, Chaudhry et al. published the results from the Telemonitoring to Improve Heart Failure Outcomes (Tele-HF) trial, including 1653 recently hospitalized chronic (e.g., unstable) HF patients with a median age of 61 years, 58% were men, and 57% were in the New York Heart Association (NYHA) class III or higher [23]. The prescribed background was relatively low, with 79% of patients receiving a beta-blocker, 67% a renin-angiotensin system (RAS)-inhibitor, and only 33% a mineralocorticoid receptor antagonist (MRA). The patients were followed for six months. During this period, no significant differences in the all-cause mortality (odds ratio (OR) 0.98 (0.75–1.28), all-cause hospitalization (OR 1.08 (0.89–1.31)) or HF-related hospitalization rates (OR 1.04 (0.84–1.30)) were observed. Overall, a marginal, non-significant reduction in all-cause mortality and both all-cause and HF-related hospitalizations occurred more often in HF patients receiving structured telephone support.
3.2. Ferrante et al. (DIAL Trial)
The results from the Randomized Trial of Phone Intervention in Chronic Heart Failure (DIAL) were published in 2010 by Ferrante et al. [22]. They included 1518 stable chronic HF patients, with a mean age of 65 years, 71% were men, and 49% were in an NYHA class III or IV. The prescribed background was relatively low; only 62% received a beta-blocker, 93% a RAS-inhibitor, and 32% an MRA. All patients were followed for 12 months; during this period, no significant reduction in all-cause mortality (OR 0.95 (0.75–1.20)) or all-cause hospitalizations (OR 0.82 (0.66–1.01)) were observed. However, a significant reduction in the number of HF-related hospitalizations was reported (OR 0.71 (0.55–0.91)) in patients receiving structured telephone support.
3.3. Galbreath et al.
Galbreath et al. included 1069 stable chronic HF patients, with a mean age of 71 years, 71% were men, and 24% were in an NYHA class III or IV and reported their results in 2004 [34]. The background therapy was not frequently prescribed, with 47% of patients receiving a beta-blocker and 73% receiving a RAS-inhibitor. No data on MRA prescription rates were reported. The follow-up duration was 18 months, and during this period, no significant difference in the all-cause mortality rates (OR 0.70 (0.47–1.04)) was reported. The study did not report data on all-cause or HF-related hospitalization rates.
3.4. Angermann et al. (INH Study)
The results from the Interdisciplinary Network for Heart Failure (INH) study performed by Angermann et al. was published in 2012 [19]. A total of 715 unstable HF patients (mean age 69 years, 71% males and 40% in an NYHA class III or higher) were included. These patients frequently received background HF therapy; 80% received a beta-blocker, 88% a RAS-inhibitor, and 42% an MRA. A significant reduction in the all-cause mortality rates (OR 0.63 (0.42–0.96)) was observed during the six month follow-up period in patients receiving structured telephone support. No significant differences were observed in the all-cause or HF-related hospitalization rates (OR 1.14 (0.84–1.57) and OR 0.79 (0.49–1.25), respectively).
3.5. Baker et al.
In 2011, Baker et al. published the results of their study, including 605 stable chronic HF patients (mean age 61 years, 52% were men, and 31% were in an NYHA class III/IV) [21]. Many patients received HF background therapy; a beta-blocker was prescribed in 81%, RAS-inhibitor in 82%, and MRAs in 27%. The patients were followed for one month, and during this period, no significant difference in the all-cause mortality was observed (OR 0.20 (0.01–4.13)) between HF patients receiving usual care or structured telephone support. The study did not report data on all-cause or HF-related hospitalization rates.
3.6. Other Studies
A summary of the study characteristics for the 26 other structured telephone support studies is shown in Table 1. As shown, large differences in the sample sizes and patient demographics exist between the studies. Between 20 to 462 patients were included in these studies, with a mean age ranging from 57 to 76 years. The follow-up duration ranged from three to 12 months. Additionally, significant differences in the reported background therapy were reported. Between 4% to 87% of the patients with structured telephone support received a beta-blocker, 54% to 95% received a renin-angiotensin system (RAS) inhibitors, and 6% to 63% received a mineralocorticoid receptor antagonist (MRA).

Table 1.
Baseline characteristics and background HF therapy in studies investigating structured telephone support in HF patients.
Of the 26 other studies, 24 studies reported data on the all-cause mortality, these results are shown in Table 2/Figure 1 [15,17,18,20,24,25,27,28,29,30,31,32,33,35,36,37,38,39,40,41,42,43,44,45]. As shown, 13 studies did report a non-significant reduction in all-cause mortality [20,25,29,30,32,35,36,37,38,40,41,43,45], while 11 studies did not show a reduction in the all-cause mortality [15,17,18,24,27,28,31,33,39,42,44].

Table 2.
Clinical outcomes of structured telephone support studies.
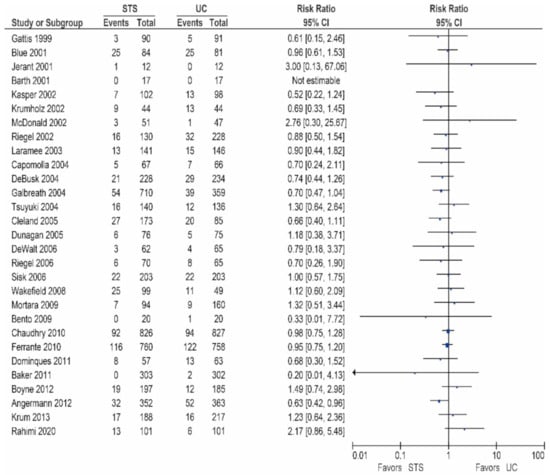
Figure 1.
All-cause mortality in patients receiving structured telephone support versus usual care.
Sixteen of the other studies reported data on all-cause hospitalization rates, as shown in Table 2/Figure 2 [15,17,18,20,24,25,27,28,29,31,32,33,35,37,38,45]. Of these studies, five demonstrated a significant reduction in the all-cause hospitalization rates [17,25,27,31,45], while five studies reported a non-significant reduction [20,28,29,32,38]. In contrast, six studies did not report a beneficial effect [15,18,24,33,35,37].
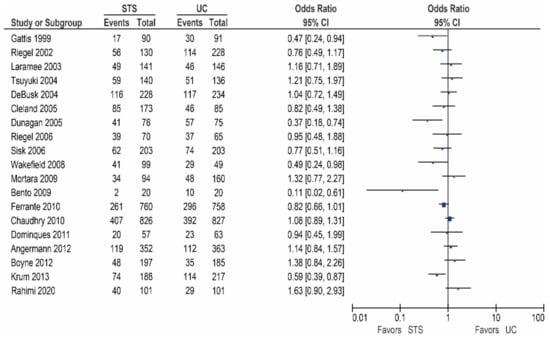
Figure 2.
All-cause hospitalization in patients receiving structured telephone support versus usual care.
Twenty-two of the 26 other studies reported data on the HF-related hospitalization rates, and are shown in Table 3/Figure 3 [15,17,18,24,27,28,29,30,31,32,33,35,36,37,38,39,40,41,42,43,44,45]. Of these studies, six reported a significant reduction in the HF-related hospitalization rates [31,36,38,39,42,45], while 13 studies showed a non-significant reduction [17,18,27,28,29,30,32,33,35,37,40,41,42]. In contrast, three studies reported no beneficial effect [15,24,44].

Table 3.
Baseline characteristics and background HF therapy in studies investigating non-invasive telemonitoring in HF patients.
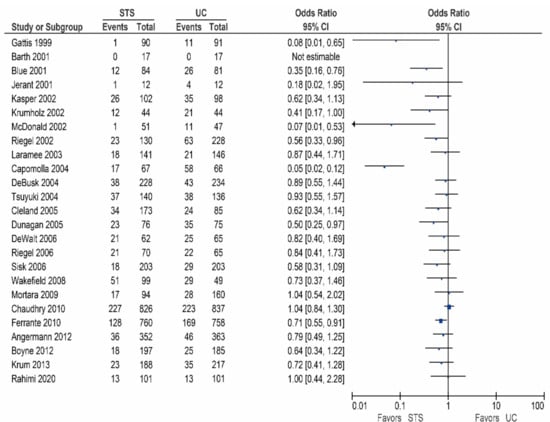
Figure 3.
Heart Failure (HF)-related hospitalizations in patients receiving structured telephone support versus usual care.
3.7. Quality of Life, Symptoms, and Functional Performance
A variety of questionnaires, including Short Form 12 Item (SF-12), Short Form 36 Item (SF-36), Minnesota Living with Heart Failure Questionnaire (MLWHFQ), EuroQol five-dimension scale (EQ-5D), Patient Health Questionnaire-9 (PHQ-9), and other tools were used to evaluate the quality of life.
Thirteen studies have assessed the effects of structured telephone support on the quality of life. The quality of life of patients who received structured telephone support improved significantly more than standard care in seven studies [19,21,22,26,28,41,45], and non-significantly in one study [27]. In contrast, five studies did not show a larger improvement in the quality of life [15,16,29,30,46].
In total, three of the five studies investigating structure telephone support reported a significantly bigger improvement of symptoms compared to the usual care [19,34,41], while two studies did not find a difference [15,32].
Of the two studies reporting functional performance data in patients receiving structured telephone support, one study demonstrated a bigger improvement in the intervention group [16], while the other study did not observe a difference [34].
4. Non-Invasive Telemonitoring versus Usual Care
In total, 30 studies investigating non-invasive telemonitoring have been identified, including a total of 8892 patients [24,32,42,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73]. The study characteristics, as well as the reported outcomes on all-cause mortality, all-cause hospitalization, and HF-related hospitalization rates of the five largest studies, representing 52% (4606) of all patients, will be discussed in detail below [24,51,52,61,64]. The study characteristics and outcomes of the other 25 studies will be summarized below [32,42,47,48,49,50,53,54,55,56,57,58,59,60,62,63,65,66,67,68,69,70,71,72,73].
4.1. Koehler et al. (TIM-HF2 Trial)
The results from the Telemedial Interventional Management in Heart Failure II (TIM-HF2) trial have been published in 2018 by Koehler et al. [51]. In this study, 1571 stable chronic HF patients (mean age 70 years, 70% was men and 48% were in an NYHA class III/IV) were randomized towards usual care or remote telemonitoring consisting of daily transfers of body weight, blood pressure, heart rate, heart rhythm, peripheral capillary oxygen saturation, and self-rated health status. Many included patients received HF background therapy; 92% received a beta-blocker, 83% a RAS-inhibitor, and 55% an MRA. The patients were followed for 12 months, and the compliance with the daily data transfer was 95% in the intervention patient group. During this period, a significant reduction in all-cause mortality (OR 0.64 (0.45–0.90) was observed in HF patients receiving non-invasive telemonitoring, while no beneficial effect on the all-cause hospitalization rates (OR 1.04 (0.84–1.29) was reported. No individual data on HF-related hospitalization were reported.
4.2. Ong et al. (BEAT-HF Trial)
In 2016 Ong et al. published the Better Effectiveness After Transition-Heart Failure (BEAT-HF) trial [52]. This study included 1437 hospitalized HF patients (median age 74 years, 54% were men and 75% was in an NYHA class III or higher) and randomized them towards usual care or remote telemonitoring, consisting of daily data transfers of blood pressure, heart rate, symptoms, and body weight in addition to health coaching telephone calls and usual care. The use of HF background therapy was relatively low; beta-blockers were prescribed to 75% of all patients, RAS-inhibitors to 56%, and only 19% received an MRA. The patients were followed for up to six months. During this period, the telemonitoring adherence was 51.7% in the patients who were remotely monitored. A non-significant reduction in all-cause mortality (OR 0.86 (0.64–1.16)) was reported, while no beneficial effect on the all-cause hospitalization rates (1.07 (0.87–1.31)) was shown in chronic HF patients receiving non-invasive telemonitoring. Data on HF-related hospitalization rates were not reported.
4.3. Koehler et al. (TIM-HF Study)
The Telemedical Interventional Monitoring in Heart Failure (TIM-HF) trial by Koehler et al., published in 2011, included 710 stable chronic HF patients (mean age 67 years, 81% was male and 50% were in NYHA class III or IV) [61]. These patients were randomized towards usual care or remote telemonitoring, consisting of electrocardiogram (ECG), blood pressure, and body weight measurements on top of usual care. Relative a high percentage of patients received HF background therapy; 93% received a beta-blocker, 95% a RAS-inhibitor, and 64% an MRA. The median follow-up was 26 months. During this period, 81% of all patients receiving telemonitoring performed at least 70% of all daily data transfers. During this period no effects on the all-cause mortality (OR 0.99 (0.65–1.48), all-cause hospitalization (OR 1.17 (0.87–1.57) or HF-related hospitalization rates (OR 0.84 (0.58–1.22)) were observed between HF patients receiving non-invasive telemonitoring or usual care.
4.4. Mortara et al. (HHH Study)
Mortara et al. published the results from the Home of Hospital in Heart Failure (HHH) in 2009 [24]. In total 461 stable chronic HF patients (mean age 60 years, 85% were men and 40% was in NYHA class III/IV) were included and randomized towards usual care or telemonitoring, consisting of blood pressure, body weight, heart rate, and signs and symptoms measurements on top of usual care. Many patients received HF background therapy, with 87% of patients receiving a beta-blocker and RAS-inhibitor. Patients were followed for 12 months, during this period no reduction in all-cause mortality (OR 1.44 (0.54–3.87)), all-cause hospitalizations (OR 1.24 (0.73–2.10)) or HF-related hospitalizations (OR 1.02 (0.53–1.96)) were observed in patients receiving non-invasive telemonitoring.
4.5. Giordano et al.
In 2009, Giordano et al. published the results from their study, including 460 unstable chronic HF patients (mean age 57 years, 85% were men, and 40% was in an NYHA class III or higher) [64]. The use of background HF therapy was relatively low; 72% of patients received a beta-blocker, 94% a RAS-inhibitor, and 62% an MRA. The patients randomized towards telemonitoring received regular remote monitoring using ECG on top of usual care. The follow-up period was 12 months, during which a significant reduction in the all-cause mortality (OR 0.39 (0.18–0.82)), all-cause hospitalization (OR 0.57 (0.39–0.84)), and HF-related hospitalization rates (OR 0.49 (0.32–0.76)) were observed in chronic HF patients receiving non-invasive telemonitoring compared to patients receiving usual care.
4.6. Other Studies
A summary of the study characteristics for the 25 other structured telephone support studies is shown in Table 3. As shown, large differences in the sample sizes and patient demographics exist between the studies. Between 20 to 426 patients were included in these studies, with a mean age ranging from 54 to 82 years. The follow-up duration ranged from one to 48 months. Additionally, significant differences in the reported background therapy were reported. Between 38% to 98% of the patients with structured telephone support received a beta-blocker, 66% to 100% received a renin-angiotensin system (RAS) inhibitors, and 21% to 58% received a mineralocorticoid receptor antagonist (MRA).
Twelve of these other studies reported data on monitoring adherence, ranging from 46% up to 95% [47,48,50,54,55,56,59,60,63,65,69,73]. Importantly to note, the monitoring strategy’s adherence decreased when the patients had to perform multiple measurements [48,50,73]. Additionally, the adherence decreased over time [48,50,55,56]. Surprisingly, the adherence also decreased in the weeks after hospitalization [47]. These results highlight that some studies’ adherence was far from optimal and could be even lower in a ‘real world’ setting. Treating clinicians should reinforce the importance of adherence to the monitoring strategies by the patients to optimize the effects of non-invasive remote monitoring strategies.
Of the 25 other studies, 20 studies reported data on the all-cause mortality, these results are shown in Table 4/Figure 4 [32,42,48,53,54,55,56,58,59,60,62,63,65,66,67,68,69,70,71,73]. As shown, two studies reported a significant reduction [59,71], ten studies did report a non-significant reduction in all-cause mortality [32,54,55,58,60,62,63,67,70,73], while eight studies did not show a reduction in the all-cause mortality [42,48,53,56,65,66,68,69].

Table 4.
Clinical outcomes of non-invasive telemonitoring studies.
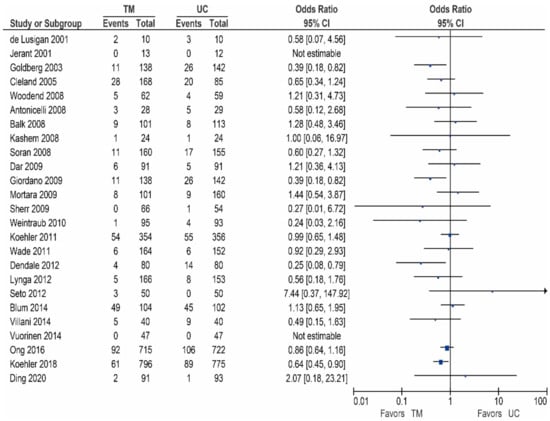
Figure 4.
All-cause mortality in patients receiving non-invasive telemonitroing versus usual care.
Seventeen of the other studies reported data on all-cause hospitalization rates, as shown in Table 4/Figure 5 [32,42,48,53,55,56,57,58,59,60,62,65,66,67,70,71]. Of these studies, two demonstrated a significant reduction in the all-cause hospitalization rates [63,70], while seven studies reported a non-significant reduction [32,42,53,58,59,62,65]. In contrast, eight studies did not report a beneficial effect [48,55,56,57,60,66,70,71].
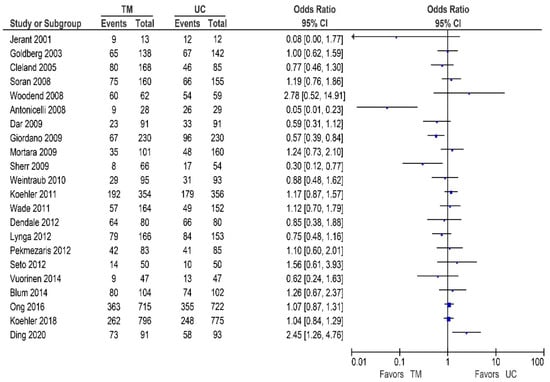
Figure 5.
All-cause hospitalization in patients receiving non-invasive telemonitoring versus usual care.
Thirteen of the 25 other studies reported data on the HF-related hospitalization rates, and are shown in Table 4/Figure 6 [32,42,48,53,54,57,59,62,63,65,67,68,72]. Of these studies, three reported a significant reduction in the HF-related hospitalization rates [54,59,68], while nine studies showed a non-significant reduction [32,42,53,57,62,63,65,67,72]. In contrast, only one study reported no beneficial effect [48].
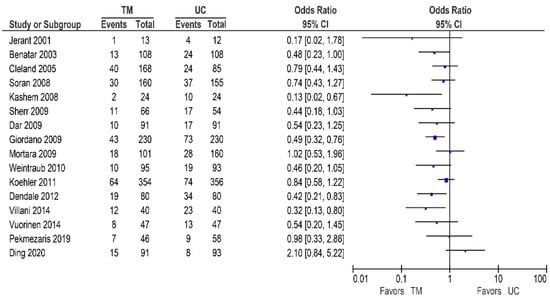
Figure 6.
HF-related hospitalizations in patients receiving non-invasive telemonitoring versus usual care.
4.7. Quality of Life, Symptoms, and Functional Performance
Seventeen studies investigating non-invasive telemonitoring reported data on the quality of life. Seven of these studies reported a significantly larger improvement in the quality of life compared to the standard care [48,52,54,56,61,67,70], while four studies demonstrated a non-significant difference [60,71,72,73]. In total, six studies did not demonstrate a beneficial effect on the quality of life [49,51,55,65,66,69].
Two of the five studies including telemonitoring strategies reported a significantly larger improvement of symptoms compared to the standard care [54,63], while three studies did not demonstrate a beneficial effect of non-invasive telemonitoring [32,56,61].
Only one study investigated the effect of non-invasive telemonitoring on functional performance and did not found a beneficial effect of this intervention [48].
5. Overview of Available Studies for Non-Invasive Remote Monitoring in Heart Failure Management: Clinical Interpretations
As shown above, considerable heterogeneity has been observed in the reported results of all-cause mortality, all-cause hospitalization, HF-related hospitalization, and quality of life for both structured telephone support and non-invasive telemonitoring. Considering all the published results, the following overall effects could be observed.
5.1. Structured Telephone Support
Based on all the published results, structured telephone support appears to have a small beneficial effect on all-cause mortality and all-cause hospitalization rates, although it might not be significant. In contrast, a more clear beneficial impact on the HF-related hospitalization rates has been observed. Additionally, this remote monitoring strategy could improve the quality of life. However, large heterogeneity has been observed between the published studies. Several reasons might explain the observed differences. Firstly, the number of included patients and the study design different significantly between the studies. Additionally, we observed a clear association between the year of publication and the treatment effect, with older studies showing more often a beneficial effect. Finally, the heterogeneity might be explained by differences in the follow-up period. Studies using a shorter follow-up period were more likely to demonstrate a beneficial effect. The reasons for the inconsistency in the reported results are discussed in more detail down below.
5.2. Non-Invasive Telemonitoring
Overall, non-invasive telemonitoring strategies might significantly reduce all-cause mortality and HF-related hospitalization rates. In contrast, no significant reduction in the all-cause hospitalization rates has been observed. The quality of life, symptom burden, and functional performance improved in patients who received non-invasive telemonitoring. However, significant heterogeneity has been observed in the published results. Studies demonstrating a beneficial effect were more likely to include patients who were recently hospitalized for HF, representing patients with unstable HF. Additionally, studies including a more extensive remote monitoring strategy, using multiple parameters, more often reported a beneficial effect. Additionally, more recent published studies less often demonstrated a beneficial effect, compared to older studies. These reasons for inconsistency in the reported results are discussed in more detail down below.
6. Reasons for Inconsistent Results
As demonstrated, large heterogeneity in the reported effects of non-invasive remote monitoring strategies on all-cause mortality, all-cause hospitalization, and HF-related hospitalizations exists. Several differences in study design and patient characteristics might explain the inconsistency in the reported results. So was the percentage of studies that included patients who were hospitalized due to HF (e.g., unstable HF patients) lower in studies who did not report a beneficial effect of structured telephone support or non-invasive remote monitoring on the all-cause mortality, all-cause hospitalization, or HF-related hospitalization rates. Hospitalization for HF is associated with an increased risk for mortality as well as rehospitalizations [5,74]. In these unstable HF patients, non-invasive remote monitoring has the largest potential to improve their clinical outcomes and reduce the HF-related hospitalization rates. In contrast, stable HF patients might already be in an ideal clinical condition, and adding non-invasive remote monitoring would not lead to further optimization of their condition.
It has been suggested that the more recently published non-invasive remote monitoring studies would have a reduced benefit in preventing all-cause mortality, all-cause hospitalization, and HF-related hospitalization rates [12]. We showed similar results, with most studies that showed no beneficial effects of non-invasive remote monitoring were published in 2008 or more recent. Over the last decades, cardiac imaging, diagnostic testing, pharmacological treatment, and device therapy have evolved continuously [75]. Results from earlier perform studies might not reflect the current state of HF care, as indicated by the lower uptake of the guideline-recommended background therapy in the earlier published studies. This could have significantly impacted the results, with less optimized patients included in the earlier studies and more optimized patients in the later studies.
Additionally, the follow-up duration in the studies ranged from one month up to four years. We observed that most studies that demonstrated a reduction of all-cause mortality had a follow-up period of six months or shorter. Studies with a follow-up period longer than six months reported more often no difference in the all-cause mortality. Similar results were found in the most recent Cochrane review [12]. These results indicate that non-invasive remote monitoring might improve the clinical outcomes in the short term, but that long term survival remains unaffected. Structured telephone support studies with a follow-up period of six months or shorter reported more often a beneficial effect on the hospitalization rates, while studies with a longer follow-up demonstrated more often no reduction.
In contrast, such difference was not found in studies analyzing non-invasive remote telemonitoring, as also have been demonstrated by the most recent Cochrane meta-analysis [12]. Many structured telephone support monitoring strategies focused on patient education. This could help maintain an optimal clinical state during the short term but would be ineffective in detecting an upcoming deterioration of HF in time. Especially since the interval of the telephone calls was only once every two weeks or even less often. In contrast, patients receiving non-invasive remote telemonitoring were instructed to take daily readings. Therefore, in these patients, signals of imminent HF deterioration could be detected in time, and hospitalization might be prevented, even during a longer follow-up period.
Finally, the variables included in the non-invasive remote telemonitoring studies varied widely. This limits the comparability of the studies and might explain some of the observed inconsistent results. Furthermore, in some studies, adherence to the telemonitoring strategy declined rapidly, reducing these monitoring strategies’ effectiveness. Improving patient participation in the monitoring strategies, ensuring that the patient performs the monitoring readings daily regardless of their situation, is crucial for developing an effective remote monitoring strategy.
7. Future Perspectives
Currently, the 2016 European Society of Cardiology (ESC) Guidelines for the diagnosis and treatment of acute and chronic heart failure does not provide any recommendation for the use of non-invasive remote monitoring [76]. In contrast, the use of invasive remote monitoring of pulmonary artery pressures or multiparameter monitoring based on implantable cardioverter-defibrillator may be considered (Class IIb recommendation) [76]. In contrast, the 2013 American College of Cardiology (ACC) and American Heart Association (AHA) Guideline for the management of heart failure recommended using effective systems to coordinate HF care to provide the guideline-recommended medical therapy and prevent hospitalizations (class I recommendation), although remote monitoring is not explicitly recommended [77]. However, in the most recent updated ACC/AHA guideline of 2017, no new recommendation on remote monitoring has been included [78]. Since more evidence shows a beneficial effect of remote monitoring and provides incremental information that could be used in the titration of HF treatment, we expect that in the newest guidelines of both the ESC and ACC/AHA, remote monitoring of chronic HF patients will receive a more specific positive recommendation. Since the more recent published studies on non-invasive remote monitoring strategies have shown less often a beneficial effect on preventing HF-related hospitalizations, this strategy might be more indicated to be used in less symptomatic HF patients to guide the titration of their HF therapy. In contrast, more invasive remote monitoring strategies could be recommended in more symptomatic HF patients.
The additional information, provided by non-invasive remote monitoring strategies, can be used to prevent HF decompensations, and alert treating clinicians for an imminent HF-related hospitalization. Currently, most studies investigating non-invasive remote monitoring have focused on this aspect. However, these strategies are limited due to their reactive design, leaving only a short period for the treating clinicians to react and prevent HF-related hospitalizations [6]. Instead, an active strategy could be used as well. Non-invasive remote monitoring could also be used to assess an ideal target for each patient. The provided feedback could guide the medical therapy to reach and maintain the patients as close to this target as possible, improving their clinical status, keeping them as stable as possible, and potentially allowing for cardiac remodeling and improving their survival [79]. Both a reactive and active strategy should not exclude each other and should be incorporated into one remote monitoring system. Reacting to imminent HF deterioration should be ideally coordinated from a central national center, allowing for timely intervention. In contrast, the active strategy, consisting of optimizing the HF therapy, should be managed by the local health care teams, who are in close contact with the patients [80].
Furthermore, the efficacy of newly introduced HF drugs, as well as invasive procedures (such as valvular interventions) could be monitored and analyzed using remote monitoring strategies. The feedback provided by these remote monitoring strategies can be used to better understand the (lack of) effects of these new treatment options. Additionally, the effects of treatment changes can be used to determine whether more invasive interventions are indicated.
Recently, studies investigating invasive remote monitoring strategies have shown a beneficial effect in more symptomatic patients [81,82,83,84]. However, these invasive strategies are limited due to their higher costs and its invasive nature. We believe that these invasive strategies should only be used in more symptomatic and more ill patients. Non-invasive remote monitoring strategies are easier to be widely implemented and should be used to monitor less symptomatic chronic HF patients.
In the future, the results from studies investigating remote monitoring in chronic HF patients should be analyzed by a trans-disciplinary team. Thereby, new technologies, such as artificial intelligence and machine learning, could be used to determine effective remote monitoring strategies and could highlight new inroads for further studies.
8. Conclusions
Despite some inconsistency in the reported results on the effectiveness of non-invasive remote monitoring in chronic HF patients, the overall combined results demonstrated a small beneficial effect on the overall survival, HF-related hospitalizations, and adherence to the guideline-recommended pharmacological therapy. Due to its simplicity, non-invasive nature, and relatively low costs, non-invasive remote monitoring is desirable and to be recommended in lower risk or less symptomatic chronic HF patients. As the volume of HF patients is very high, the impact of non-invasive remote monitoring strategies could have a large impact at not too high costs. More symptomatic and complex higher risk HF patients would likely benefit more from invasive remote monitoring strategies, but at a higher cost.
Author Contributions
J.F.V.: Writing—Original Draft Preparation and Visualization; S.P.R.: Writing—Review and Editing; P.H.: Writing—Review and Editing; J.J.B.: Writing—Review and Editing, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
All authors had no conflict of interest.
References
- Ponikowski, P.; Anker, S.D.; AlHabib, K.F.; Cowie, M.R.; Force, T.L.; Hu, S.; Jaarsma, T.; Krum, H.; Rastogi, V.; Rohde, L.E.; et al. Heart failure: Preventing disease and death worldwide. ESC Heart Fail. 2014, 1, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Najafi, F.; Jamrozik, K.; Dobson, A.J. Understanding the ’epidemic of heart failure’: A systematic review of trends in deter-minants of heart failure. Eur. J. Heart Fail. 2009, 11, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.L.; Tong, X.; King, R.J.; Loustalot, F.; Hong, Y.; Ritchey, M.D. National Burden of Heart Failure Events in the United States, 2006 to 2014. Circ. Hear. Fail. 2018, 11, e004873. [Google Scholar] [CrossRef] [PubMed]
- Farré, N.; Vela, E.; Clèries, M.; Bustins, M.; Cainzos-Achirica, M.; Enjuanes, C.; Moliner, P.; Ruiz, S.; Verdú-Rotellar, J.M.; Comín-Colet, J. Real world heart failure epidemiology and outcome: A population-based analysis of 88,195 patients. PLoS ONE 2017, 12, e0172745. [Google Scholar] [CrossRef] [PubMed]
- Buddeke, J.; Valstar, G.B.; Van Dis, I.; Visseren, F.L.J.; Rutten, F.H.; Ruijter, H.M.D.; Vaartjes, I.; Bots, M.L. On behalf of the Queen of Hearts and RECONNECT investigators Mortality after hospital admission for heart failure: Improvement over time, equally strong in women as in men. BMC Public Health 2020, 20, 36. [Google Scholar] [CrossRef]
- Abraham, W.T. The Role of Implantable Hemodynamic Monitors to Manage Heart Failure. Cardiol. Clin. 2017, 35, 273–279. [Google Scholar] [CrossRef]
- Brugts, J.J.; Linssen, G.C.M.; Hoes, A.W.; Brunner-La Rocca, H.P. CHECK-HF investigators. Real-world heart failure manage-ment in 10,910 patients with chronic heart failure in the Netherlands: Design and rationale of the Chronic Heart failure ESC guideline-based Cardiology practice Quality project (CHECK-HF) registry. Neth Heart J. 2018, 26, 272–279. [Google Scholar] [CrossRef]
- Brunner-La Rocca, H.P.; Linssen, G.C.; Smeele, F.J.; van Drimmelen, A.A.; Schaafsma, H.J.; Westendorp, P.H.; Rademaker, P.C.; van de Kamp, H.J.; Hoes, A.W.; Brugts, J.J. Contemporary Drug Treatment of Chronic Heart Failure With Reduced Ejection Fraction: The CHECK-HF Registry. JACC Heart Fail. 2019, 7, 13–21. [Google Scholar] [CrossRef]
- Veenis, J.F.; Rocca, H.-P.B.-L.; Linssen, G.C.; Geerlings, P.R.; Van Gent, M.W.; Aksoy, I.; Oosterom, L.; Moons, A.H.; Hoes, A.W.; Brugts, J.J.; et al. Age differences in contemporary treatment of patients with chronic heart failure and reduced ejection fraction. Eur. J. Prev. Cardiol. 2019, 26, 1399–1407. [Google Scholar] [CrossRef]
- Greene, S.J.; Butler, J.; Albert, N.M.; DeVore, A.D.; Sharma, P.P.; Duffy, C.I.; Hill, C.L.; McCague, K.; Mi, X.; Patterson, J.H.; et al. Medical Therapy for Heart Failure With Reduced Ejection Fraction: The CHAMP-HF Registry. J. Am. Coll. Cardiol. 2018, 72, 351–366. [Google Scholar] [CrossRef]
- Smeets, C.J.; Storms, V.; Vandervoort, P.M.; Dreesen, P.; Vranken, J.; Houbrechts, M.; Goris, H.; Grieten, L.; Dendale, P. A Novel Intelligent Two-Way Communication System for Remote Heart Failure Medication Uptitration (the CardioCoach Study): Randomized Controlled Feasibility Trial. JMIR Cardio 2018, 2, e8. [Google Scholar] [CrossRef] [PubMed]
- Inglis, S.C.; Clark, R.A.; Dierckx, R.; Prieto-Merino, D.; Cleland, J.G. Structured telephone support or non-invasive telemonitor-ing for patients with heart failure. Cochrane Database Syst. Rev. 2015, 10, CD007228. [Google Scholar]
- Alotaibi, S.; Hernandez-Montfort, J.; Ali, O.E.; El-Chilali, K.; Perez, B.A. Remote monitoring of implantable cardiac devices in heart failure patients: A systematic review and meta-analysis of randomized controlled trials. Hear. Fail. Rev. 2020, 25, 469–479. [Google Scholar] [CrossRef]
- Abraham, W.T.; Stevenson, L.W.; Bourge, R.C.; Lindenfeld, J.A.; Bauman, J.G.; Adamson, P.B. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: Complete follow-up results from the CHAMPION randomised trial. Lancet 2016, 387, 453–461. [Google Scholar] [CrossRef]
- Rahimi, K.; Nazarzadeh, M.; Pinho-Gomes, A.-C.; Woodward, M.; Salimi-Khorshidi, G.; Ohkuma, T.; Fitzpatrick, R.; Tarassenko, L.; Denis, M.; Cleland, J.; et al. Home monitoring with technology-supported management in chronic heart failure: A randomised trial. Heart 2020, 106, 1573–1578. [Google Scholar] [PubMed]
- Gingele, A.J.; Ramaekers, B.; Rocca, H.P.B.-L.; De Weerd, G.; Kragten, J.; Van Empel, V.; Van Der Weg, K.; Vrijhoef, H.J.M.; Gorgels, A.; Cleuren, G.; et al. Effects of tailored telemonitoring on functional status and health-related quality of life in patients with heart failure. Neth. Hear. J. 2019, 27, 565–574. [Google Scholar] [CrossRef]
- Krum, H.; Forbes, A.; Yallop, J.; Driscoll, A.; Croucher, J.; Chan, B.; Clark, R.; Davidson, P.M.; Huynh, L.; Kasper, E.K.; et al. Telephone Support to Rural and Remote Patients with Heart Failure: The Chronic Heart Failure Assessment by Telephone (CHAT) study. Cardiovasc. Ther. 2013, 31, 230–237. [Google Scholar] [CrossRef]
- Boyne, J.J.; Vrijhoef, H.J.; Crijns, H.J.; De Weerd, G.; Kragten, J.; Gorgels, A.P. TEHAF investigators. Tailored telemonitoring in patients with heart failure: Results of a multicentre ran-domized controlled trial. Eur. J. Heart Fail. 2012, 14, 791–801. [Google Scholar] [CrossRef]
- Angermann, C.E.; Störk, S.; Gelbrich, G.; Faller, H.; Jahns, R.; Frantz, S.; Loeffler, M.; Ertl, G. Mode of action and effects of standardized collaborative disease management on mortality and morbidity in patients with systolic heart failure: The Interdisciplinary Network for Heart Failure (INH) study. Circ. Heart Fail. 2012, 5, 25–35. [Google Scholar] [CrossRef]
- Domingues, F.B.; Clausell, N.; Aliti, G.B.; Dominguez, D.R.; Rabelo, E.R. Education and telephone monitoring by nurses of patients with heart failure: Randomized clinical trial. Arq. Bras. de Cardiol. 2011, 96, 233–239. [Google Scholar] [CrossRef]
- Baker, D.W.; DeWalt, D.A.; Schillinger, D.; Hawk, V.; Ruo, B.; Bibbins-Domingo, K.; Weinberger, M.; Macabasco-O’Connell, A.; Grady, K.L.; Holmes, G.M.; et al. The Effect of Progressive, Reinforcing Telephone Education and Counseling Versus Brief Educational Intervention on Knowledge, Self-Care Behaviors and Heart Failure Symptoms. J. Card. Fail. 2011, 17, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, D.; Varini, S.; Macchia, A.; Soifer, S.; Badra, R.; Nul, D.; Grancelli, H.; Doval, H. GESICA Investigators. Long-term results after a telephone intervention in chronic heart failure: DIAL (Ran-domized Trial of Phone Intervention in Chronic Heart Failure) follow-up. J. Am. Coll. Cardiol. 2010, 56, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.I.; Mattera, J.A.; Curtis, J.P.; Spertus, J.A.; Herrin, J.; Lin, Z.; Phillips, C.O.; Hodshon, B.V.; Cooper, L.S.; Krumholz, H.M. Telemonitoring in Patients with Heart Failure. New Engl. J. Med. 2010, 363, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Mortara, A.; Pinna, G.D.; Johnson, P.; Maestri, R.; Capomolla, S.; La Rovere, M.T.; Ponikowski, P.; Tavazzi, L.; Sleight, P. On behalf of the HHH Investigators Home telemonitoring in heart failure patients: The HHH study (Home or Hospital in Heart Failure). Eur. J. Hear. Fail. 2009, 11, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Bento, V.F.; Brofman, P.R. Impact of the nursing consultation on the frequency of hospitalizations in patients with heart failure in Curitiba, Parana State. Arq. Bras Cardiol. 2009, 92, 454–460. [Google Scholar]
- Brandon, A.F.; Schuessler, J.B.; Ellison, K.J.; Lazenby, R.B. The effects of an advanced practice nurse led telephone intervention on outcomes of patients with heart failure. Appl. Nurs. Res. 2009, 22, e1–e7. [Google Scholar] [CrossRef]
- Wakefield, B.J.; Ward, M.M.; Holman, J.E.; Ray, A.; Scherubel, M.; Burns, T.L.; Kienzle, M.G.; Rosenthal, G.E. Evaluation of home telehealth following hospitalization for heart failure: A ran-domized trial. Telemed. J. E Health. 2008, 14, 753–761. [Google Scholar] [CrossRef]
- Sisk, J.E.; Hebert, P.L.; Horowitz, C.R.; McLaughlin, M.A.; Wang, J.J.; Chassin, M.R. Effects of nurse management on the quality of heart failure care in minority communities: A randomized trial. Ann. Intern. Med. 2006, 145, 273–283. [Google Scholar] [CrossRef]
- Riegel, B.; Carlson, B.; Glaser, D.; Romero, T. Randomized Controlled Trial of Telephone Case Management in Hispanics of Mexican Origin with Heart Failure. J. Card. Fail. 2006, 12, 211–219. [Google Scholar] [CrossRef]
- DeWalt, D.A.; Malone, R.M.; Bryant, M.; Kosnar, M.C.; Corr, K.; Rothman, R.L.; Sueta, C.; Pignone, M.P. A heart failure self-management program for patients of all literacy levels: A randomized, controlled trial [ISRCTN11535170]. BMC Health Serv. Res. 2006, 6, 30. [Google Scholar] [CrossRef]
- Dunagan, W.C.; Littenberg, B.; Ewald, G.A.; Jones, C.A.; Emery, V.B.; Waterman, B.M.; Silverman, D.C.; Rogers, J.G. Randomized trial of a nurse-administered, telephone-based disease manage-ment program for patients with heart failure. J. Card Fail. 2005, 11, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.; Louis, A.; Rigby, A.; Janssens, U.; Balk, A. Noninvasive Home Telemonitoring for Patients With Heart Failure at High Risk of Recurrent Admission and Death: The Trans-European Network–Home-Care Management System (TEN-HMS) Study. ACC Curr. J. Rev. 2005, 14, 37. [Google Scholar] [CrossRef]
- Tsuyuki, R.T.; Fradette, M.; Johnson, J.A.; Bungard, T.J.; Eurich, D.T.; Ashton, T.; Gordon, W.; Ikuta, R.; Kornder, J.; Mackay, E.; et al. A multicenter disease management program for hospitalized patients with heart failure. J. Card. Fail. 2004, 10, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Galbreath, A.D.; Krasuski, R.A.; Smith, B.; Stajduhar, K.; Kwan, M.D.; Ellis, R.; Freeman, G.L. Long-Term Healthcare and Cost Outcomes of Disease Management in a Large, Randomized, Community-Based Population With Heart Failure. Circulation 2004, 110, 3518–3526. [Google Scholar] [CrossRef] [PubMed]
- De Busk, R.; Miller, N.; Parker, K. Care management for low-risk patients with heart failure: A randomized, controlled trial. ACC Curr. J. Rev. 2005, 14, 34–35. [Google Scholar] [CrossRef]
- Capomolla, S.; Pinna, G.; La Rovere, M.T.; Maestri, R.; Ceresa, M.; Ferrari, M.; Febo, O.; Caporotondi, A.; Guazzotti, G.; Lenta, F.; et al. Heart failure case disease management program: A pilot study of home tele-monitoring versus usual care. Eur. Heart J. Suppl. 2004, 6, F91–F98. [Google Scholar] [CrossRef]
- Laramee, A.S.; Levinsky, S.K.; Sargent, J.; Ross, R.; Callas, P. Case management in a heterogeneous congestive heart failure population: A randomized controlled trial. Arch. Intern. Med. 2003, 163, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Riegel, B.; Carlson, B.; Kopp, Z.; LePetri, B.; Glaser, D.; Unger, A. Effect of a Standardized Nurse Case-Management Telephone Intervention on Resource Use in Patients With Chronic Heart Failure. Arch. Intern. Med. 2002, 162, 705–712. [Google Scholar] [CrossRef]
- McDonald, K.; Ledwidge, M.; Cahill, J.; Quigley, P.; Maurer, B.; Travers, B.; Ryder, M.; Kieran, E.; Timmons, L.; Ryan, E. Heart failure management: Multidisciplinary care has intrinsic benefit above the optimization of medical care. J. Card. Fail. 2002, 8, 142–148. [Google Scholar] [CrossRef]
- Krumholz, H.M.; Amatruda, J.; Smith, G.L.; Mattera, J.A.; Roumanis, S.A.; Radford, M.J.; Crombie, P.; Vaccarino, V. Randomized trial of an education and support intervention to prevent read-mission of patients with heart failure. J. Am. Coll. Cardiol. 2002, 39, 83–89. [Google Scholar] [CrossRef]
- Kasper, E.K.; Gerstenblith, G.; Hefter, G.; Van Anden, E.; Brinker, J.A.; Thiemann, D.R.; Terrin, M.; Forman, S.; Gottlieb, S.H. A randomized trial of the efficacy of multidisciplinary care in heart failure out-patients at high risk of hospital readmission. J. Am. Coll. Cardiol. 2002, 39, 471–480. [Google Scholar] [CrossRef]
- Jerant, A.F.; Azari, R.; Nesbitt, T.S. Reducing the cost of frequent hospital admissions for congestive heart failure: A random-ized trial of a home telecare intervention. Med. Care 2001, 39, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Blue, L.; Lang, E.; McMurray, J.J.; Davie, A.P.; McDonagh, T.A.; Murdoch, D.R.; Petrie, M.C.; Connolly, E.; Norrie, J.; Round, C.E.; et al. Randomised controlled trial of specialist nurse intervention in heart failure. BMJ 2001, 323, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Barth, V. A Nurse-Managed Discharge Program for Congestive Heart Failure Patients: Outcomes and Costs. Home Health Care Manag. Pr. 2001, 13, 436–443. [Google Scholar] [CrossRef]
- Gattis, W.A.; Hasselblad, V.; Whellan, D.J.; O’Connor, C.M. Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team: Results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) Study. Arch Intern Med. 1999, 159, 1939–1945. [Google Scholar] [CrossRef]
- Smith, B.; Forkner, E.; Zaslow, B.; Krasuski, R.A.; Stajduhar, K.; Kwan, M.; Ellis, R.; Galbreath, A.D.; Freeman, G.L. Disease management produces limited quality-of-life improvements in patients with congestive heart failure: Evidence from a randomized trial in community-dwelling patients. Am. J. Manag. Care 2005, 11, 701–713. [Google Scholar]
- Haynes, S.C.; Tancredi, D.J.; Tong, K.; Hoch, J.S.; Ong, M.K.; Ganiats, T.G.; Evangelista, L.S.; Black, J.T.; Auerbach, A.; Romano, P.S. The Effect of Rehospitalization and Emergency Department Visits on Subsequent Adherence to Weight Telemonitoring. J. Cardiovasc. Nurs. 2020. [Google Scholar] [CrossRef]
- Ding, H.; Jayasena, R.; Chen, S.H.; Maiorana, A.; Dowling, A.; Layland, J.; Good, N.; Karunanithi, M.; Edwards, I. The Effects of Telemonitoring on Patient Compliance With Self-Management Recom-mendations and Outcomes of the Innovative Telemonitoring Enhanced Care Program for Chronic Heart Failure: Random-ized Controlled Trial. J. Med. Internet Res. 2020, 22, e17559. [Google Scholar] [CrossRef]
- Pekmezaris, R.; Nouryan, C.N.; Schwartz, R.; Castillo, S.; Makaryus, A.N.; Ahern, D.; Akerman, M.B.; Lesser, M.L.; Bauer, L.; Murray, L.; et al. A Randomized Controlled Trial Comparing Telehealth Self-Management to Standard Outpatient Management in Underserved Black and Hispanic Patients Living with Heart Failure. Telemed. e-Health 2019, 25, 917–925. [Google Scholar] [CrossRef]
- Park, C.; Otobo, E.; Ullman, J.; Rogers, J.; Fasihuddin, F.; Garg, S.; Kakkar, S.; Goldstein, M.; Chandrasekhar, S.V.; Pinney, S.; et al. Impact on Readmission Reduction Among Heart Failure Patients Using Digital Health Monitoring: Feasibility and Adoptability Study. JMIR Med. Informatics 2019, 7, e13353. [Google Scholar] [CrossRef]
- Koehler, F.; Koehler, K.; Deckwart, O.; Prescher, S.; Wegscheider, K.; Kirwan, B.-A.; Winkler, S.; Vettorazzi, E.; Bruch, L.; Oeff, M.; et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): A randomised, controlled, parallel-group, unmasked trial. Lancet 2018, 392, 1047–1057. [Google Scholar] [CrossRef]
- Ong, M.K.; Romano, P.S.; Edgington, S.; Aronow, H.U.; Auerbach, A.D.; Black, J.T.; De Marco, T.; Escarce, J.J.; Evangelista, L.S.; Hanna, B.; et al. Effectiveness of Remote Patient Monitoring After Discharge of Hospitalized Pa-tients With Heart Failure: The Better Effectiveness After Transition—Heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA Intern Med. 2016, 176, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Vuorinen, A.L.; Leppänen, J.; Kaijanranta, H.; Kulju, M.; Heliö, T.; van Gils, M.; Lähteenmäki, J. Use of home telemonitoring to support multidisciplinary care of heart fail-ure patients in Finland: Randomized controlled trial. J. Med. Internet Res. 2014, 16, e282. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.; Malfatto, G.; Compare, A.; Della Rosa, F.; Bellardita, L.; Branzi, G.; Molinari, E.; Parati, G. Clinical and psychological telemonitoring and telecare of high risk heart failure patients. J. Telemed. Telecare 2014, 20, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Gottlieb, S.S. The effect of a randomized trial of home telemonitoring on medical costs, 30-day readmissions, mortality, and health-related quality of life in a cohort of community-dwelling heart failure patients. J. Card Fail. 2014, 20, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Seto, E.; Leonard, K.J.; Cafazzo, J.A.; Barnsley, J.; Masino, C.; Ross, H.J. Mobile Phone-Based Telemonitoring for Heart Failure Management: A Randomized Controlled Trial. J. Med. Internet Res. 2012, 14, e31. [Google Scholar] [CrossRef]
- Pekmezaris, R.; Mitzner, I.; Pecinka, K.R.; Nouryan, C.N.; Lesser, M.L.; Siegel, M.; Swiderski, J.W.; Moise, G.; Younker Sr, R.; Smolich, K. The impact of remote patient monitoring (telehealth) upon Medicare beneficiar-ies with heart failure. Telemed. J. E Health. 2012, 18, 101–108. [Google Scholar] [CrossRef]
- Lynga, P.; Persson, H.; Hägg-Martinell, A.; Hägglund, E.; Hagerman, I.; Langius-Eklöf, A.; Rosenqvist, M. Weight monitoring in patients with severe heart failure (WISH). A randomized controlled trial. Eur. J. Hear. Fail. 2012, 14, 438–444. [Google Scholar] [CrossRef]
- Dendale, P.; De Keulenaer, G.; Troisfontaines, P.; Weytjens, C.; Mullens, W.; Elegeert, I.; Ector, B.; Houbrechts, M.; Willekens, K.; Hansen, D. Effect of a telemonitoring-facilitated collaboration between general practitioner and heart failure clinic on mortality and rehospitalization rates in severe heart failure: The TEMA-HF 1 (TEle-monitoring in the MAnagement of Heart Failure) study. Eur. J. Heart Fail. 2012, 14, 333–340. [Google Scholar] [CrossRef]
- Wade, M.J.; Desai, A.S.; Spettell, C.; Snyder, A.D.; McGowan-Stackewicz, V.; Kummer, P.J.; MacCoy, M.C.; Krakauer, R.S. Telemonitoring with case management for seniors with heart failure. Am. J. Manag. Care 2011, 17, 71–79. [Google Scholar]
- Koehler, F.; Winkler, S.; Schieber, M.; Sechtem, U.; Stangl, K.; Böhm, M.; Boll, H.; Baumann, G.; Honold, M.; Koehler, K.; et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: The telemedical interventional monitoring in heart failure study. Circulation 2011, 123, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, A.; Gregory, D.; Patel, A.R.; Levine, D.; Venesy, D.; Perry, K.; Delano, C.; Konstam, M.A. A Multicenter Randomized Controlled Evaluation of Automated Home Monitoring and Telephonic Disease Management in Patients Recently Hospitalized for Congestive Heart Failure: The SPAN-CHF II Trial. J. Card. Fail. 2010, 16, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Scherr, D.; Kastner, P.; Kollmann, A.; Hallas, A.; Auer, J.; Krappinger, H.; Schuchlenz, H.; Stark, G.; Grander, W.; Jakl, G.; et al. Effect of home-based telemonitoring using mobile phone technology on the out-come of heart failure patients after an episode of acute decompensation: Randomized controlled trial. J. Med. Internet Res. 2009, 11, e34. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Scalvini, S.; Zanelli, E.; Corrà, U.; Longobardi, G.L.; Ricci, V.; Baiardi, P.; Glisenti, F. Multicenter randomised trial on home-based telemanagement to prevent hospital readmission of patients with chronic heart failure. Int. J. Cardiol. 2009, 131, 192–199. [Google Scholar] [CrossRef]
- Dar, O.; Riley, J.; Chapman, C.; Dubrey, S.W.; Morris, S.; Rosen, S.D.; Roughton, M.; Cowie, M.R. A randomized trial of home telemonitoring in a typical elderly heart failure population in North West London: Results of the Home-HF study. Eur. J. Hear. Fail. 2009, 11, 319–325. [Google Scholar] [CrossRef]
- Woodend, A.K.; Sherrard, H.; Fraser, M.; Stuewe, L.; Cheung, T.; Struthers, C. Telehome monitoring in patients with cardiac disease who are at high risk of readmission. Hear. Lung 2008, 37, 36–45. [Google Scholar] [CrossRef]
- Soran, O.Z.; Piña, I.L.; Lamas, G.A.; Kelsey, S.F.; Selzer, F.; Pilotte, J.; Lave, J.R.; Feldman, A.M. A Randomized Clinical Trial of the Clinical Effects of Enhanced Heart Failure Monitoring Using a Computer-Based Telephonic Monitoring System in Older Minorities and Women. J. Card. Fail. 2008, 14, 711–717. [Google Scholar] [CrossRef]
- Kashem, A.; Droogan, M.T.; Santamore, W.P.; Wald, J.W.; Bove, A.A. Managing heart failure care using an internet-based tele-medicine system. J. Card. Fail. 2008, 14, 121–126. [Google Scholar] [CrossRef]
- Balk, A.H.; Davidse, W.; Van Dommelen, P.; Klaassen, E.; Caliskan, K.; Van Der Burgh, P.; Leenders, C.M. Tele-guidance of chronic heart failure patients enhances knowledge about the disease. A multi-centre, randomised controlled study. Eur. J. Hear. Fail. 2008, 10, 1136–1142. [Google Scholar] [CrossRef]
- Antonicelli, R.; Testarmata, P.; Spazzafumo, L.; Gagliardi, C.; Valentini, M.; Olivieri, F.; Bilo, G.; Parati, G. Impact of telemonitoring at home on the management of elderly patients with congestive heart failure. J. Telemed. Telecare 2008, 14, 300–305. [Google Scholar] [CrossRef]
- Goldberg, L.R.; Piette, J.D.; Walsh, M.N.; Frank, T.; Jaski, B.; Smith, A.L.; Rodriguez, R.; Mancini, D.M.; Hopton, L.; Orav, E.; et al. Randomized trial of a daily electronic home monitoring system in patients with advanced heart failure: The Weight Monitoring in Heart Failure (WHARF) trial. Am. Hear. J. 2003, 146, 705–712. [Google Scholar] [CrossRef]
- Benatar, D.; Bondmass, M.; Ghitelman, J.; Avitall, B. Outcomes of chronic heart failure. Arch. Intern. Med. 2003, 163, 347–352. [Google Scholar] [CrossRef] [PubMed]
- De Lusignan, S.; Wells, S.; Johnson, P.; Meredith, K.; Leatham, E. Compliance and effectiveness of 1 year’s home telemonitor-ing. The report of a pilot study of patients with chronic heart failure. Eur. J. Heart Fail. 2001, 3, 723–730. [Google Scholar] [CrossRef]
- Goldgrab, D.; Balakumaran, K.; Kim, M.J.; Tabtabai, S.R. Updates in heart failure 30-day readmission prevention. Hear. Fail. Rev. 2018, 24, 177–187. [Google Scholar] [CrossRef]
- Choi, H.-M.; Park, M.-S.; Youn, J.-C. Update on heart failure management and future directions. Korean J. Intern. Med. 2019, 34, 11–43. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardi-ology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013, 128, 1810–1852. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, e137–e161. [Google Scholar] [CrossRef]
- Pellicori, P.; Cleland, J.G.F.; Zhang, J.; Kallvikbacka-Bennett, A.; Urbinati, A.; Shah, P.; Kazmi, S.; Clark, A.L. Cardiac Dysfunction, Congestion and Loop Diuretics: Their Relationship to Prognosis in Heart Failure. Cardiovasc. Drugs Ther. 2016, 30, 599–609. [Google Scholar] [CrossRef]
- Cleland, J.; Clark, R.A.; Pellicori, P.; Inglis, S.C.; Rn, S.C.I. Caring for people with heart failure and many other medical problems through and beyond the COVID -19 pandemic: The advantages of universal access to home telemonitoring. Eur. J. Hear. Fail. 2020, 22, 995–998. [Google Scholar] [CrossRef]
- Shavelle, D.M.; Desai, A.S.; Abraham, W.T.; Bourge, R.C.; Raval, N.; Rathman, L.D.; Heywood, J.T.; Jermyn, R.A.; Pelzel, J.; Jonsson, O.T.; et al. Lower Rates of Heart Failure and All-Cause Hospitalizations During Pulmo-nary Artery Pressure-Guided Therapy for Ambulatory Heart Failure: One-Year Outcomes From the CardioMEMS Post-Approval Study. Circ. Heart Fail. 2020, 13, e006863. [Google Scholar] [CrossRef] [PubMed]
- Givertz, M.M.; Stevenson, L.W.; Costanzo, M.R.; Bourge, R.C.; Bauman, J.G.; Ginn, G.; Abraham, W.T. Pulmonary Artery Pressure-Guided Management of Patients With Heart Failure and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2017, 70, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Heywood, J.T.; Jermyn, R.; Shavelle, D.; Abraham, W.T.; Bhimaraj, A.; Bhatt, K.; Sheikh, F.; Eichorn, E.; Lamba, S.; Bharmi, R.; et al. Impact of Practice-Based Management of Pulmonary Artery Pressures in 2000 Patients Implanted With the CardioMEMS Sensor. Circulation 2017, 135, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Angermann, C.E.; Assmus, B.; Anker, S.D.; Asselbergs, F.W.; Brachmann, J.; Brett, M.E.; Brugts, J.J.; Ertl, G.; Ginn, G.; Hilker, L.; et al. Pulmonary artery pressure-guided therapy in ambulatory patients with symp-tomatic heart failure: The CardioMEMS European Monitoring Study for Heart Failure (MEMS-HF). Eur. J. Heart Fail. 2020, 22, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).