Electro-Mechanical Ionic Channel Modeling for Uterine Contractions and Oxytocin Effect during Pregnancy
Abstract
1. Introduction
2. Materials and Methods
2.1. Sodium Channel
2.2. Potassium Channel
2.3. Calcium Channel
2.4. Oxytocin Model
2.5. Force Model
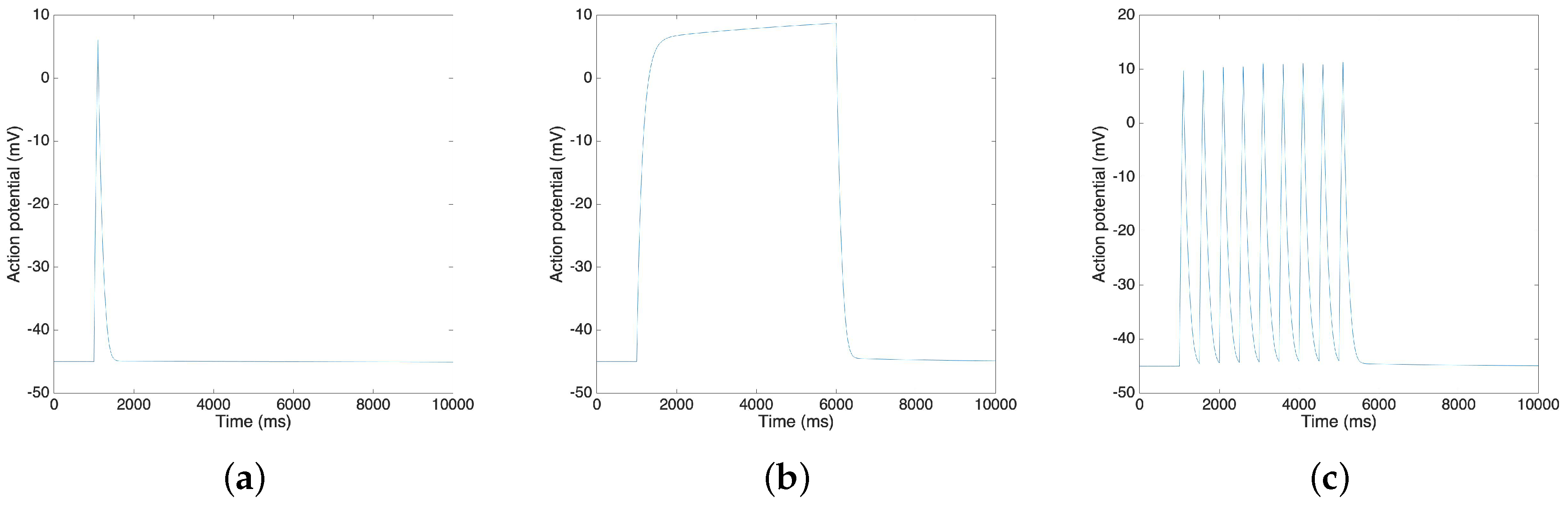
3. Results and Discussion
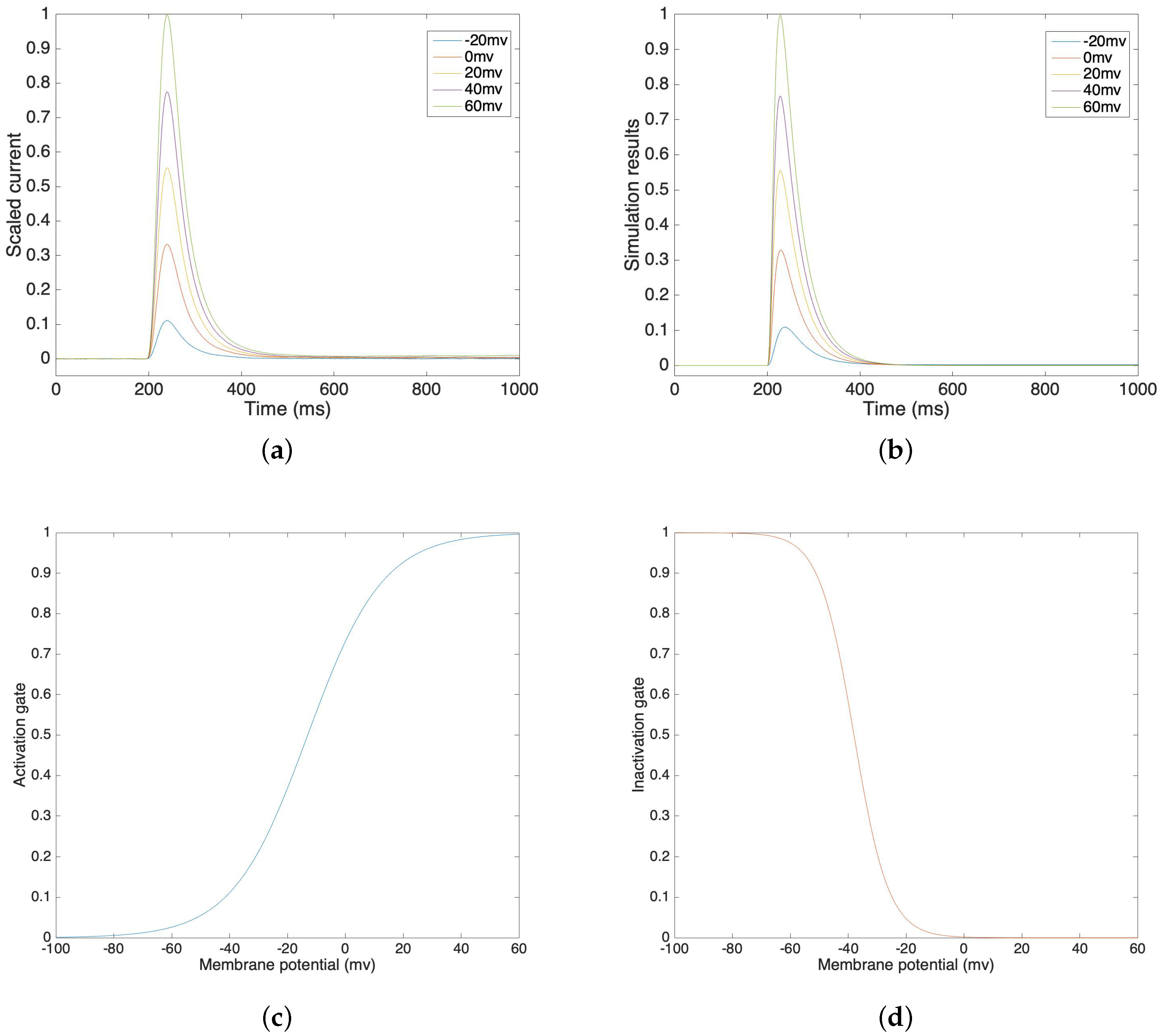
3.1. Sodium Channel
3.2. Potassium Channel
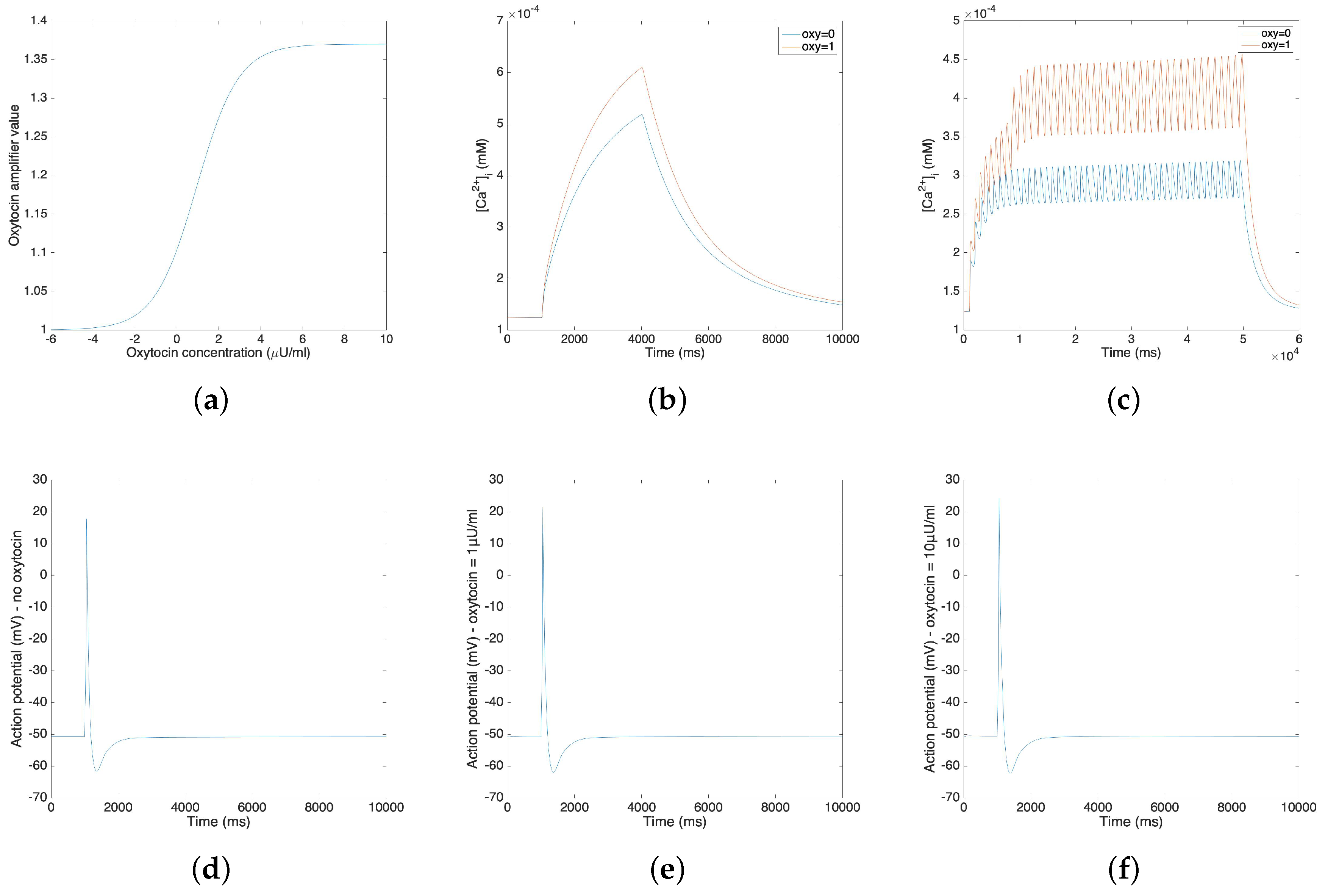
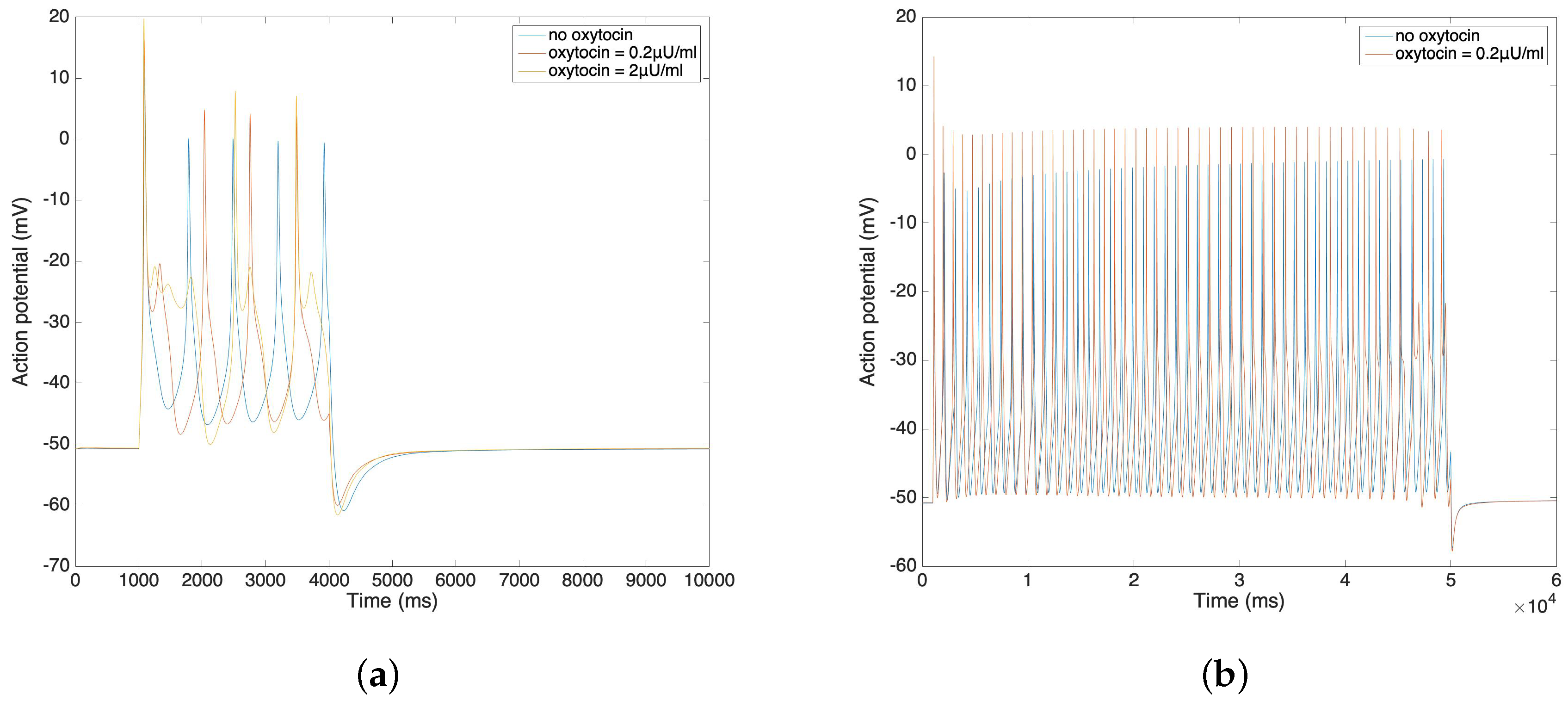
3.3. Oxytocin Model
3.4. Uterine Cell Force Model
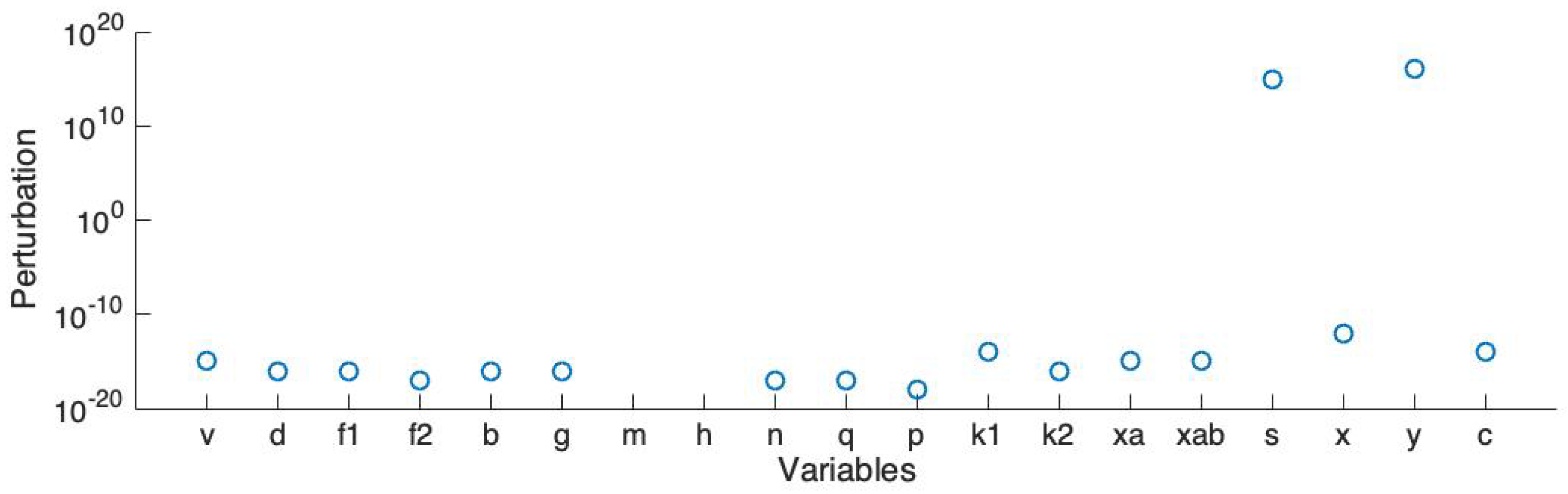
3.5. Model Simplification To Enable Large Scale Simulations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McCormick, M.C.; Litt, J.S.; Smith, V.C.; Zupancic, J.A. Prematurity: An overview and public health implications. Annu. Rev. Pub. Health 2011, 32, 367–379. [Google Scholar] [CrossRef]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef]
- El Manouni el Hassani, S.; Niemarkt, H.; Said, H.; Berkhout, D.; van Kaam, A.; van Lingen, R.; Benninga, M.; de Boer, N.; de Meij, T. Fecal volatile organic compounds in preterm infants are influenced by enteral feeding composition. Sensors 2018, 18, 3037. [Google Scholar] [CrossRef] [PubMed]
- Tamilia, E.; Taffoni, F.; Formica, D.; Ricci, L.; Schena, E.; Keller, F.; Guglielmelli, E. Technological solutions and main indices for the assessment of newborns’ nutritive sucking: A review. Sensors 2014, 14, 634–658. [Google Scholar] [CrossRef] [PubMed]
- Wilde, D.; Marshall, J. Effects of tetraethylammonium and 4-aminopyridine on the plateau potential of circular myometrium from the pregnant rat. Biol. Reprod. 1988, 38, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Okabe, K.; Soeda, H. Augmentation and suppression of action potentials by estradiol in the myometrium of pregnant rat. Can. J. Physiol. Pharmacol. 1999, 77, 447–453. [Google Scholar] [CrossRef]
- Chard, T.; Grudzinskas, J.G.; Grudzinskas, J. The Uterus; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Maffeo, C.; Bhattacharya, S.; Yoo, J.; Wells, D.; Aksimentiev, A. Modeling and simulation of ion channels. Chem. Rev. 2012, 112, 6250–6284. [Google Scholar] [CrossRef]
- Yang, J.; Clark, J.W., Jr.; Bryan, R.M.; Robertson, C. The myogenic response in isolated rat cerebrovascular arteries: Smooth muscle cell model. Med. Eng. Phys. 2003, 25, 691–709. [Google Scholar] [CrossRef]
- Yang, Z.M.; Chen, D.B.; Le, S.P.; Harper, M.J. Differential hormonal regulation of leukemia inhibitory factor (LIF) in rabbit and mouse uterus. Mol. Reprod. Dev. Inc. Gamete Res. 1996, 43, 470–476. [Google Scholar] [CrossRef]
- Tong, W.C.; Tribe, R.M.; Smith, R.; Taggart, M.J. Computational modeling reveals key contributions of KCNQ and hERG currents to the malleability of uterine action potentials underpinning labor. PLoS ONE 2014, 9, e114034. [Google Scholar] [CrossRef]
- Tong, W.C.; Choi, C.Y.; Karche, S.; Holden, A.V.; Zhang, H.; Taggart, M.J. A computational model of the ionic currents, Ca2+ dynamics and action potentials underlying contraction of isolated uterine smooth muscle. PLoS ONE 2011, 6, e18685. [Google Scholar] [CrossRef]
- Massó, P.; Callejas, A.; Melchor, J.; Molina, F.S.; Rus, G. In Vivo Measurement of Cervical Elasticity on Pregnant Women by Torsional Wave Technique: A Preliminary Study. Sensors 2019, 19, 3249. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, P.S.; Eswaran, H.; Preissl, H.; Nehorai, A. Multiscale forward electromagnetic model of uterine contractions during pregnancy. BMC Med. Phys. 2012, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tidwell, V.; La Rosa, P.S.; Wilson, J.D.; Eswaran, H.; Nehorai, A. Modeling magnetomyograms of uterine contractions during pregnancy using a multiscale forward electromagnetic approach. PLoS ONE 2016, 11, e0152421. [Google Scholar] [CrossRef]
- Zhang, M.; La Rosa, P.S.; Eswaran, H.; Nehorai, A. Estimating uterine source current during contractions using magnetomyography measurements. PLoS ONE 2018, 13, e0202184. [Google Scholar] [CrossRef]
- Eswaran, H.; Preissl, H.; Wilson, J.D.; Murphy, P.; Robinson, S.E.; Lowery, C.L. First magnetomyographic recordings of uterine activity with spatial-temporal information with a 151-channel sensor array. Am. J. Obstet. Gynecol. 2002, 187, 145–151. [Google Scholar] [CrossRef]
- Escalona-Vargas, D.; Govindan, R.B.; Furdea, A.; Murphy, P.; Lowery, C.L.; Eswaran, H. Characterizing the propagation of uterine electrophysiological signals recorded with a multi-sensor abdominal array in term‚ pregnancies. PLoS ONE 2015, 10, e0140894. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar] [CrossRef]
- Yochum, M.; Laforêt, J.; Marque, C. Uterine smooth muscle cell force generation from electrical properties. In BioMedWomen; CRC Press: Boca Raton, FL, USA, 2016; pp. 41–44. [Google Scholar]
- Hai, C.M.; Murphy, R.A. Cross-bridge phosphorylation and regulation of latch state in smooth muscle. Am. J. Physiol.-Cell Physiol. 1988, 254, C99–C106. [Google Scholar] [CrossRef]
- Mironneau, J. Effects of oxytocin on ionic currents underlying rhythmic activity and contraction in uterine smooth muscle. Pflügers Arch. 1976, 363, 113–118. [Google Scholar] [CrossRef]
- Nakao, K.; Inoue, Y.; Okabe, K.; Kawarabayashi, T.; Kitamura, K. Oxytocin enhances action potentials in pregnant human myometrium—A study with microelectrodes. Am. J. Obstet. Gynecol. 1997, 177, 222–228. [Google Scholar] [CrossRef]
- Ferreira, J.J.; Butler, A.; Stewart, R.; Gonzalez-Cota, A.L.; Lybaert, P.; Amazu, C.; Reinl, E.L.; Wakle-Prabagaran, M.; Salkoff, L.; England, S.K.; et al. Oxytocin can regulate myometrial smooth muscle excitability by inhibiting the Na+-activated K+ channel, Slo2. 1. J. Physiol. 2019, 597, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Goldman, D.E. Potential, impedance, and rectification in membranes. J. Gen. Physiol. 1943, 27, 37–60. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.W. Principles of Statistical Mechanics. In Elementary Principles in Statistical Mechanics; Charles Scribner’s Sons: New York, NY, USA, 1902. [Google Scholar]
- Yoshino, M.; Wang, S.; Kao, C. Sodium and calcium inward currents in freshly dissociated smooth myocytes of rat uterus. J. Gen. Physiol. 1997, 110, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011, 3, a003947. [Google Scholar] [CrossRef]
- Arias, F. Pharmacology of oxytocin and prostaglandins. Clin. Obstet. Gynecol. 2000, 43, 455–468. [Google Scholar] [CrossRef]
- Buford, P.S. Human Uterine Smooth Muscle Single Cell Model. Ph.D. Thesis, University of Arkansas at Little Rock, Little Rock, AR, USA, 2007. [Google Scholar]
- Gibbs, M.N.; MacKay, D.J. Variational Gaussian process classifiers. IEEE Trans. Neural Netw. 2000, 11, 1458–1464. [Google Scholar]
- Burdyga, T.; Wray, S.; Noble, K. In situ calcium signaling: No calcium sparks detected in rat myometrium. Ann. N. Y. Acad. Sci. 2007, 1101, 85–96. [Google Scholar] [CrossRef]
- Arrowsmith, S.; Wray, S. Oxytocin: Its mechanism of action and receptor signaling in the myometrium. J. Neuroendocrinol. 2014, 26, 356–369. [Google Scholar] [CrossRef]
- Zafrah, H.A.; Alotaibi, M.F. The effect of extracellular ATP on rat uterine contraction from different gestational stages and its possible mechanisms of action. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 209–217. [Google Scholar] [CrossRef]
- Rihana, S.; Santos, J.; Mondie, S.; Marque, C. Dynamical analysis of uterine cell electrical activity model. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 4179–4182. [Google Scholar]
- Ermentrout, B. Simulating, Analyzing, and Animating Dynamical Systems: A Guide to XPPAUT for Researchers and Students; SIAM: Philadelphia, PA, USA, 2002; Volume 14. [Google Scholar]
- Casteels, R.; Kuriyama, H. Membrane potential and ionic content in pregnant and non-pregnant rat myometrium. J. Physiol. 1965, 177, 263–287. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Zhang, M.; La Rosa, P.S.; Wilson, J.D.; Nehorai, A. Electro-Mechanical Ionic Channel Modeling for Uterine Contractions and Oxytocin Effect during Pregnancy. Sensors 2019, 19, 4898. https://doi.org/10.3390/s19224898
Lin Y, Zhang M, La Rosa PS, Wilson JD, Nehorai A. Electro-Mechanical Ionic Channel Modeling for Uterine Contractions and Oxytocin Effect during Pregnancy. Sensors. 2019; 19(22):4898. https://doi.org/10.3390/s19224898
Chicago/Turabian StyleLin, Yiqi, Mengxue Zhang, Patricio S. La Rosa, James D. Wilson, and Arye Nehorai. 2019. "Electro-Mechanical Ionic Channel Modeling for Uterine Contractions and Oxytocin Effect during Pregnancy" Sensors 19, no. 22: 4898. https://doi.org/10.3390/s19224898
APA StyleLin, Y., Zhang, M., La Rosa, P. S., Wilson, J. D., & Nehorai, A. (2019). Electro-Mechanical Ionic Channel Modeling for Uterine Contractions and Oxytocin Effect during Pregnancy. Sensors, 19(22), 4898. https://doi.org/10.3390/s19224898




